Abstract
Although the mechanism underlying the effect of androgen on BP and cardiovascular disease is not well understood, recent studies suggest that 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE), a primary cytochrome P450 4 (Cyp4)–derived eicosanoid, may mediate androgen-induced hypertension. Here, treatment of normotensive mice with 5α-dihydrotestosterone increased BP and induced both Cyp4a12 expression and 20-HETE levels in preglomerular microvessels. Administration of a 20-HETE antagonist prevented and reversed the effects of dihydrotestosterone on BP. Cyp4a14(−/−) mice, which exhibit androgen-sensitive hypertension in the male mice, produced increased levels of vascular 20-HETE; furthermore, administration of a 20-HETE antagonist normalized BP. To examine whether androgen-independent increases in 20-HETE are sufficient to cause hypertension, we studied Cyp4a12-transgenic mice, which express the CYP4A12–20-HETE synthase under the control of a doxycycline-sensitive promoter. Administration of doxycycline increased BP by 40%, and administration of a 20-HETE antagonist prevented this increase. Levels of CYP4A12 and 20-HETE in preglomerular microvessels of doxycycline-treated transgenic mice approximately doubled, correlating with increased 20-HETE–dependent sensitivity to phenylephrine-mediated vasoconstriction and with decreased acetylcholine-mediated vasodilation in the renal microvasculature. We observed a similar contribution of 20-HETE to myogenic tone in the mesenteric microvasculature. Taken together, these results suggest that 20-HETE both mediates androgen-induced hypertension and can cause hypertension independent of androgen.
The ω-hydroxylation of arachidonic acid (AA) to 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) is catalyzed by members of the cytochrome P450 4 (CYP4) gene family and regulated by factors such as age, sex hormones, and dietary lipids.1,2 CYP4 expression and 20-HETE synthesis have been implicated in the regulation of vascular and tubular function and the development of hypertension in experimental models.3–5 Studies demonstrating that 20-HETE is a vasoconstrictor6–8 suggest that increased 20-HETE synthesis and/or effects in the renal vasculature underlies its prohypertensive property.9–12 This notion has been substantiated by several reports showing the following: (1) the synthesis of and vascular reactivity to 20-HETE are significantly higher in spontaneously hypertensive rats (SHRs),13,14 (2) inhibition of vascular 20-HETE synthesis by CYP4A2 antisense oligonucleotides decreases BP in SHRs,15,16 and (3) endothelial-specific transduction of CYP4A2 cDNA increases vascular 20-HETE synthesis and causes hypertension.17–19
The aforementioned studies, along with clinical reports indicating an association between 20-HETE and hypertension in humans,20,21 prompted studies to identify the putative 20-HETE synthase hypertensive gene. The murine Cyp4a genes include Cyp4a10, Cyp4a12, and Cyp4a14. There are two Cyp4a12 genes, Cyp4a12a and Cyp4a12b, the product of a tandem 100-kb duplication within the Cyp4abx cluster.22 Cyp4a10 is expressed in both male and female mice, whereas Cyp4a12a is male specific and androgen regulated and Cyp4a14 is highly expressed in female mice.23–25 Among these CYP4A proteins, only CYP4A12 exhibits significant 20-HETE synthase activity.23,24,26 Interestingly, Cyp4a14(−/−) mice display male-specific hypertension that is associated with androgen-driven increases in CYP4A12 expression and 20-HETE biosynthesis.23 Indeed, androgen administration to male or female rats or mice causes hypertension.23,27–29 Moreover, the hypertensive response to androgen has been associated with increases in CYP4A827,29 and CYP4A1223,24 expression in rats and mice, respectively, as well as 20-HETE production, most prominently within the vasculature.27,29 Importantly, the androgen-induced increases in BP in rats are abrogated by administration of an inhibitor of 20-HETE synthesis or a 20-HETE antagonist,27,30 suggesting that 20-HETE mediates, at least in part, the hypertensive effect of androgen. However, the relative contribution of upregulating the expression of CYP4A12 and the production of 20-HETE in renal vascular and tubular structures to androgen-induced hypertension as opposed to other androgen-dependent actions such as activation of the renin-angiotensin system31 and upregulation of sodium transporters32 is unclear. This study aimed to address this issue by developing transgenic mice in which the expression of the CYP4A12–20-HETE synthase is under the control of an androgen-independent, tetracycline (doxycycline [DOX])–sensitive promoter. Here we show that androgen and/or DOX-mediated induction of CYP4A12 expression leads to 20-HETE–dependent vascular dysfunction and hypertension.
Results
20-HETE Mediates Androgen-Driven Hypertension
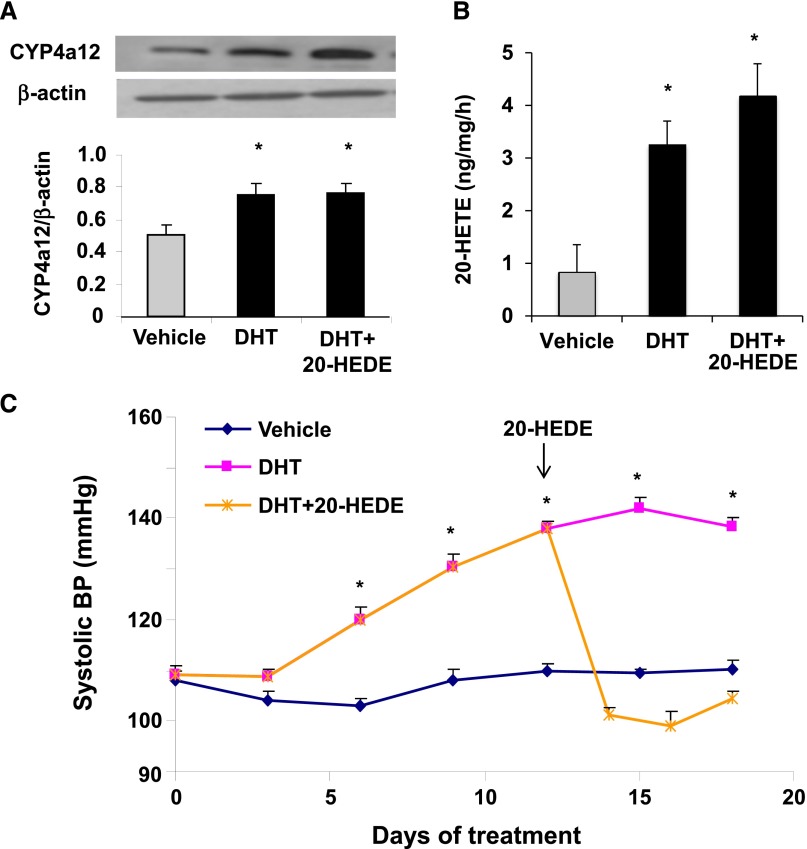
Androgen has been shown to induce CYP4A12 in mice but the relationship between androgen-mediated CYP4A12 induction, 20-HETE synthesis, and hypertension has not been established. As seen in Figure 1, A and B, after 17 days of treatment with androgen (5α-dihydrotestosterone [DHT]), mouse renal preglomerular microvessels (PGMVs) showed a 40% increase in CYP4A12 levels and a 3- to 4-fold increase in endogenous 20-HETE production. Moreover, within the first 9 days of androgen treatment, systolic BP of DHT-treated mice increased significantly (137.6±0.7 mmHg versus 109.9±0.5 mmHg for DHT and vehicle-treated mice, respectively) and reached 141.3±0.6 mmHg by day 15. Importantly, addition of 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (20-HEDE), a 20-HETE antagonist, at day 13 of the above regimen, rapidly normalized the BP of DHT-treated mice, indicating that the pressure effects of DHT are 20-HETE mediated (Figure 1C). Control experiments showed that 20-HEDE did not affect CYP4A12 expression nor did it change 20-HETE levels in the renal PGMVs (Figure 1, A and B).
Figure 1.
Androgen-driven 20-HETE–dependent hypertension in mice. Mice are treated with either vehicle (placebo pellet) or DHT for 18 days. A subgroup of DHT-treated mice is administered 20-HEDE (10 mg/kg per day) starting at day 12 of treatment. (A) CYP4A12 protein levels in renal PGMVs. (B) 20-HETE levels in renal PGMV. (C) Systolic BP (n=4–6). *P<0.05 versus vehicle.
Cyp4a14(−/−) Mice Display 20-HETE–Dependent Male-Specific Hypertension
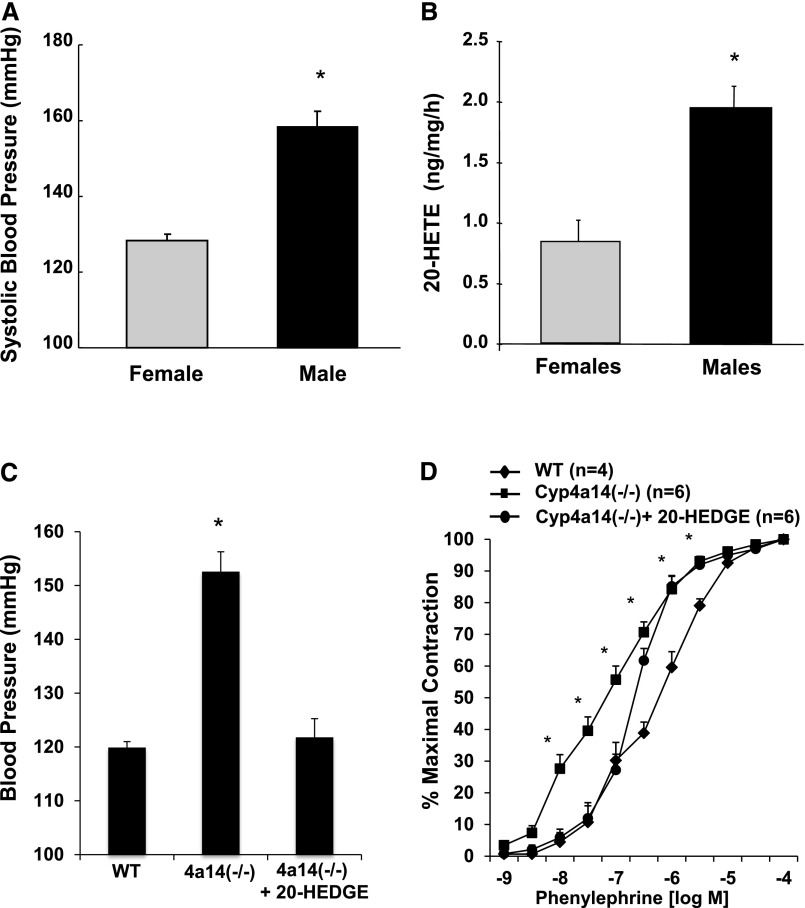
Previous studies by Holla et al.23 showed that Cyp4a14(−/−) mice displayed male-specific hypertension that was associated with increases in plasma androgen levels and renal CYP4A12 expression, and suggested that an increase in 20-HETE biosynthetic capacity contributed to the hypertension in these mice. As seen in Figure 2, A and B, BP as well as renal vascular production of 20-HETE were sex dependent, and importantly, administration of the 20-HETE antagonist, N-(20-hydroxyeicosa-6(Z),15(Z)-dienoyl)glycine (20-HEDGE), lowered the BP of male Cyp4a14(−/−) mice (Figure 2C), suggesting that the hypertension in these mice is mediated by 20-HETE. In addition, as seen in Figure 2D, renal interlobar arteries from Cyp4a14(−/−) mice displayed increased sensitivity to phenylephrine-induced constriction as evidenced by a significant decrease in the phenylephrine EC50 (0.72±0.14 and 0.11±0.01 µM in wild-type mice and Cyp4a14(−/−), respectively; P<0.05). This increased sensitivity to phenylephrine was attenuated (P<0.05) by treatment with 20-HEDGE (EC50=0.28±0.05 µM), suggesting that 20-HETE contributed to increased vascular reactivity in these mice.
Figure 2.
BP elevation in Cyp4a14(−/−) male mice is 20-HETE dependent. (A) Systolic BP in male and female Cyp4a14(−/−) mice (n=4–6). *P<0.05 versus female mice. (B) 20-HETE levels in renal PGMV from male and female Cyp4a14(−/−) mice (n=4). *P<0.05 versus female mice. (C) Systolic BP in wild-type (WT) and Cyp4a14(−/−) mice treated with and without 20-HEDGE (10 mg/kg per day) for 12 days (n=4–6). (D) Cumulative concentration-response curve to phenylephrine (10−9 to 10−4 M) in PGMVs from WT and Cyp4a14(−/−) mice treated with and without 20-HEDGE for 12 days. *P<0.05 versus WT; #P<0.05 versus Cyp4a14(−/−).
Characterization of Cyp4a12tg Mice
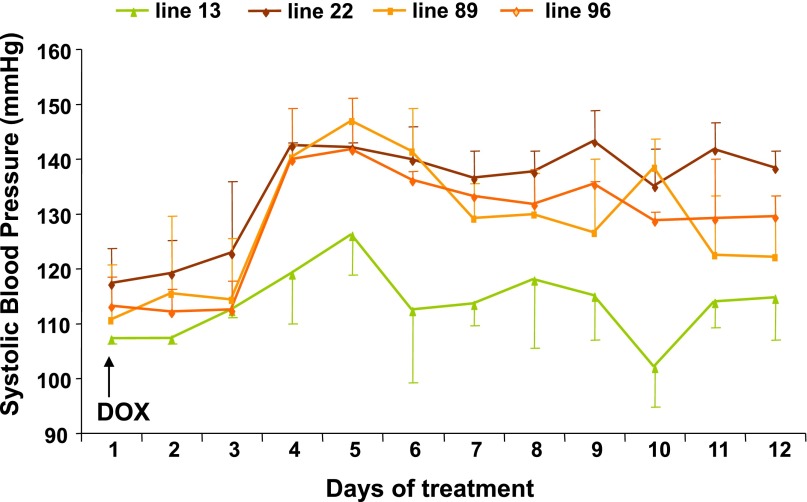
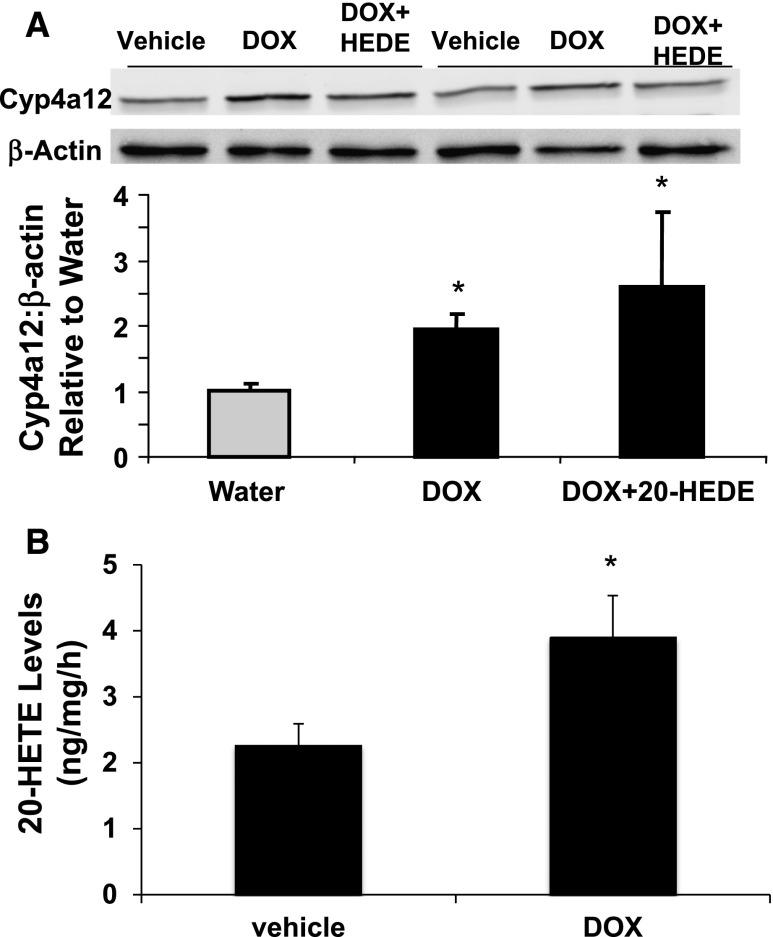
To examine the roles played by the CYP4A12–20-HETE synthase in androgen-sensitive hypertension, transgenic mice overexpressing CYP4A12 under the control of a tetracycline-sensitive promoter (Tet-on) were developed. Inasmuch as transgene copy number and insertion site(s) influence their expression level and/or could alter the expression, integrity, or functional properties of alternate unknown genes, several founder lines were characterized for their transgene relative copy numbers (Supplemental Figure 1) and BP responses to DOX administration. As seen in Figure 3, lines 22, 89, and 96, which express the rtTA-M2 and Cyp4a12 transgenes, showed BP increases in response to DOX. In contrast, the BP of the DOX-treated line 13 mice remained more or less normal (Figure 3). RT-PCR analysis of liver and kidney RNAs from mice of lines 13 and 89 indicated that DOX increased Cyp4a12 mRNA levels only in mice from line 89 (Figure 4, A and B). Likewise, DOX increased the levels of CYP4A12 protein (Figure 4C) and the microsomal CYP4A12 activity as measured by conversion of AA to 20-HETE (Figure 4D), only in kidneys of line 89 mice. Immunofluorescence of kidney sections from DOX-treated Cyp4a12tg mice showed CYP4A12 expression in all kidney structures with intense signals in the vasculature (Figure 4E, panel a). Additional costaining with CD31 antibodies indicated substantial expression of CYP4A12 in kidney vessels that appeared to be localized to the smooth muscle layer (Figure 4E, panels d–f). Lastly, as seen in Figure 5A, DOX treatment increased CYP4A12 levels in renal PGMV by 2-fold. This was associated with a 2-fold increase in endogenous production of 20-HETE (Figure 5B). Mice from line 89 were used for all subsequent experiments as the Cyp4a12tg mice. Both male and female CYP4a12tg mice displayed DOX-sensitive hypertension that was associated with CYP4A12 induction and increased 20-HETE production.
Figure 3.
BP measurements in four different lines of Cyp4a12tg mice after administration of DOX. Systolic BP is measured by tail-cuff after placing mice on DOX (1 mg/ml in drinking water). Line 13 mice express the rtTA-M2 transgene only, whereas mice of lines 22, 89, and 96 express both the rtTA-M2 and the Cyp4a12 transgenes. Relative copy numbers of the rtTA-M2/Cyp4a12 transgenes for lines 13, 22, 89, and 96 are 0/2, 2/1.5, 6/5, and 5/6, respectively.
Figure 4.
DOX-increased expression of CYP4A12 in Cyp4a12tg mice. Relative expression of Cyp4a12 in the liver (A) and kidney (B) of line 13 and line 89 (Cyp4a12tg) DOX-treated mice. (C) Western blot of CYP4A12 in kidney of line 13 and line 89 (Cyp4a12tg) DOX-treated mice. (D) 20-HETE synthase activity of microsomal fractions isolated from the kidneys of control and DOX-treated mice expressing only the rTA-M2 gene (line 13) or the combination of the Cyp4a12 and rTA-Ms transgenes (line 89). Activity is determined by measuring the conversion of 14C-labeled AA (100 µM) to 20-HETE. (E) CYP4A12 (red) and CD31 (green) immunofluorescence in kidney sections from DOX-treated and untreated Cyp4a12tg mice. Panels a and d–f are from DOX-treated Cyp4a12tg mice; panels b and c are from untreated Cyp4a12tg mice. Original magnification, ×100 in E, panels a and b; ×200 in E, panel c; ×400 in E, panels d–f.
Figure 5.
DOX-increased expression of CYP4A12 and 20-HETE production in PGMV from Cyp4a12tg mice. (A) A representative Western blot and densitometry analysis of CYP4A12 and (B) 20-HETE levels in PGMV from Cyp4a12tg mice treated with and without DOX and 20-HEDE for 14 days (n=4). *P<0.05 versus water.
Blockade of 20-HETE Action Prevents DOX-Induced Hypertension in Cyp4a12tg Mice
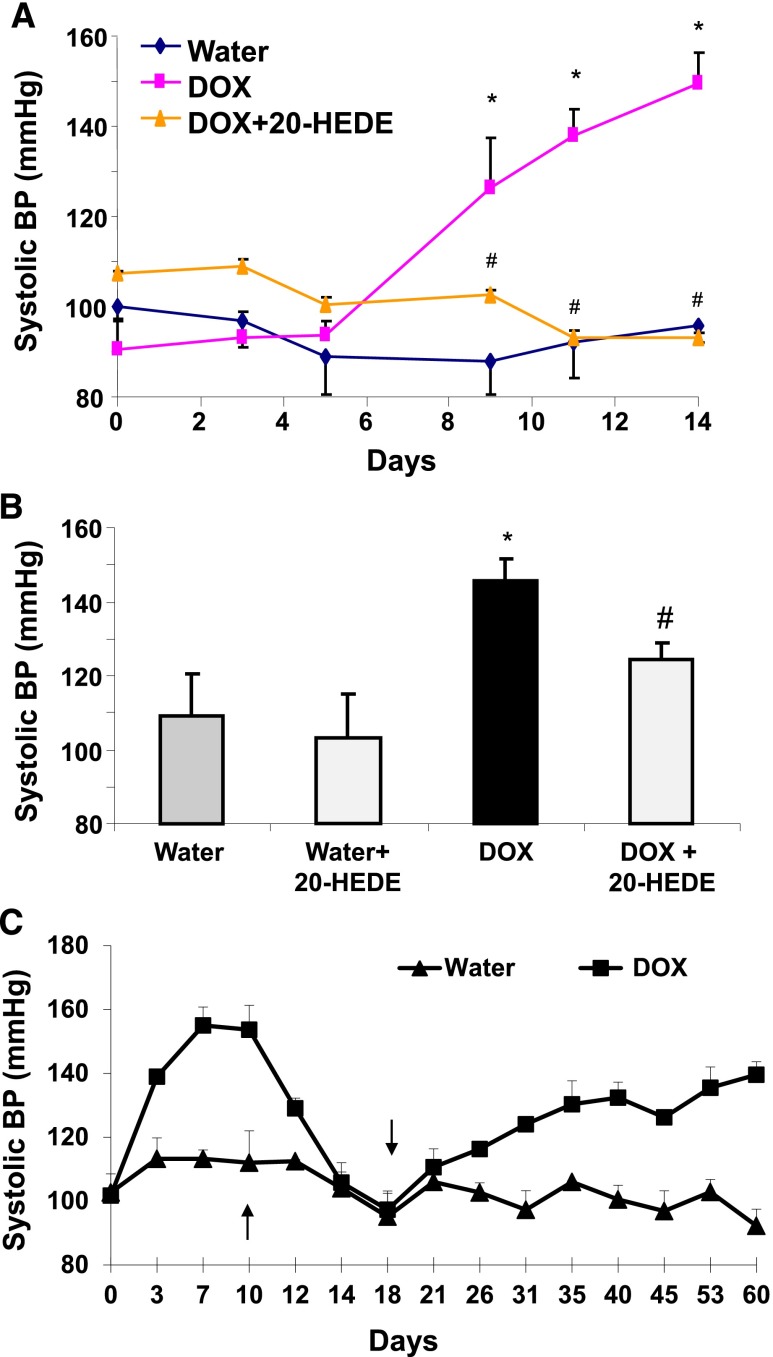
To determine whether the DOX-induced increase in 20-HETE contributes to the hypertension, 20-HEDE was either coadministered with DOX or administered to Cyp4a12tg mice treated with DOX after 10 days. Cotreatment with 20-HEDE prevented the BP increase in DOX-treated Cyp4a12tg mice (93.0±1.6 mmHg versus 145±5.9 mmHg) (Figure 6A). When administered after 10 days of DOX treatment, 20-HEDE significantly attenuated the BP increase (124.4±4.3 mmHg) (Figure 6B). On the other hand, 20-HEDE had no effect on the BP of Cyp4a12tg mice without DOX treatment (Figure 6A). Moreover, cessation of DOX administration resulted in a rapid decrease in BP, which returned to baseline within 5 days. BP began to rise once these mice were again placed on DOX treatment (Figure 6C).
Figure 6.
20-HETE antagonist attenuates DOX-induced increase in BP in Cyp4a12tg mice. (A) Systolic BP in Cyp4a12tg treated with and without DOX in the presence and absence of 20-HEDE. (B) Systolic BP in Cyp4a12tg mice treated with DOX for 10 days followed by cotreatment with 20-HEDE or vehicle for 5 additional days. (C) Systolic BP in Cyp4a12tg mice treated with DOX for 9 days and switched to water; at day 18 DOX is added back to the drinking water. Results are mean ± SE (n=4–6). *P<0.05 versus vehicle; #P<0.05 versus DOX.
Cyp4a12tg Mice Display 20-HETE–Dependent Increased Vascular Reactivity and Myogenic Response
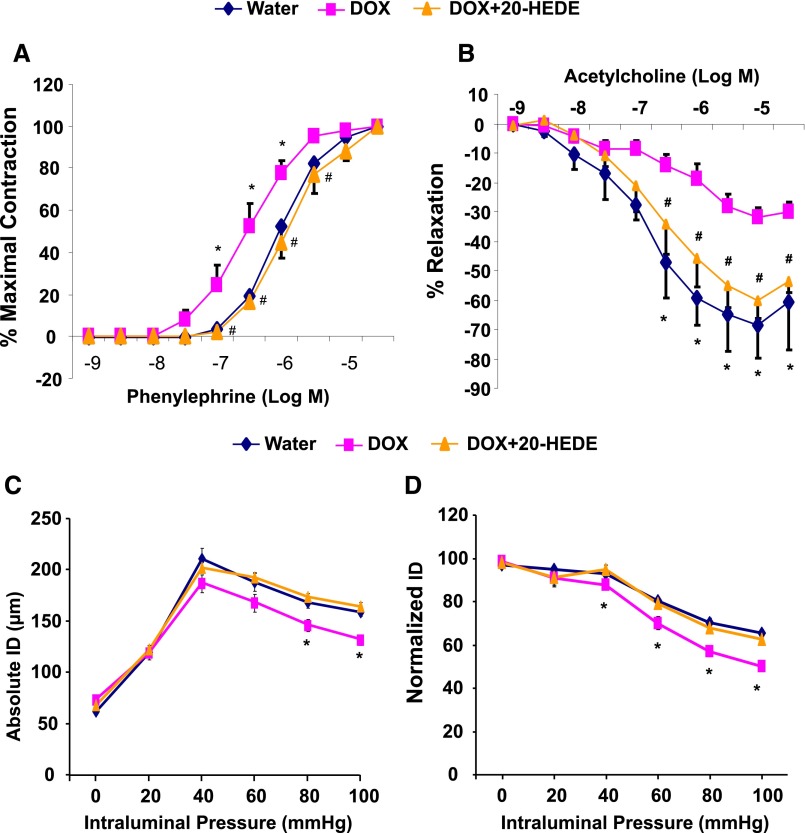
Renal interlobar arteries from mice treated with DOX for 14 days displayed a significant increase in sensitivity to phenylephrine-induced vasoconstriction as evidenced by a reduction in EC50 to phenylephrine (from 1.30±0.10 to 0.37±0.02 µM). This increase in sensitivity was attenuated in arteries from mice cotreated with DOX and 20-HEDE (EC50=1.60±0.10 µM) (Figure 7A). In addition, endothelial dysfunction measured as impaired relaxing responses to acetylcholine was observed in DOX-treated mice. The relaxation response to 10 µM acetylcholine in arteries from DOX-treated Cyp4a12tg mice was 31.7%±3.2% (compared with 68.7%±10.9% in arteries from vehicle-treated Cyp4a12tg mice); this impaired relaxing response was attenuated in arteries from DOX-treated Cyp4a12tg mice that were coadministered 20-HEDE (60.4%±5.9% relaxation) (Figure 7B).
Figure 7.
Vascular function in microvessels from Cyp4a12tg mice. Cumulative concentration-response curve to phenylephrine (A) and acetylcholine (B) in renal interlobar arteries. Effect of stepwise increments in intraluminal pressure on the absolute (C) and the normalized (D) internal diameter (ID) in third branch of mesenteric arteries (n=4–6). *P<0.05 versus water; #P<0.05 versus DOX.
A similar contribution of 20-HETE to vascular tone was also seen in the mesenteric microcirculation of Cyp4a12tg mice. As shown in Figure 7, C and D, the myogenic tone of the third branch of the mesenteric arteries (approximately 60–80 µm) significantly increased in DOX-treated as compared with nontreated Cyp4a12tg mice. Moreover, DOX-induced increase in myogenic response was abrogated by cotreatment with 20-HEDE, suggesting that 20-HETE contributed to increased vascular tone in the mesenteric microcirculation of the Cyp4a12tg mice.
Discussion
Epidemiologic, clinical, and experimental studies have shown that androgen is an important determinant of sex-specific differences in arterial BP; men aged <60 years have higher BP than women of the same age.33–35 Androgen has been suggested to contribute to hypertension in postmenopausal women and in several experimental models.36–40 The postulated mechanisms for androgen modulation of BP are numerous and include stimulation of renal prohypertensive processes involving the renin-angiotensin-aldosterone system, increases in proximal tubular reabsorption, and amplification of vascular tone through an upregulation of vasoconstrictor autacoids.31,32,41,42 20-HETE has been recognized as a prohypertensive lipid autacoid43 and several studies,23,27,29,30 including this study, identified 20-HETE as a potential mediator of androgen-induced hypertension. Given the multiple actions of androgen on several prohypertensive systems, it is difficult to separate 20-HETE’s contribution to the BP increase from that of androgen.
The Cyp4a12tg mice, which overexpress the Cyp4a12 gene under the control of the nonmammalian, endogenous factor–independent tetracycline-sensitive promoter, provided a model to assess the contribution of 20-HETE to hypertension in the absence of androgen. In these mice, liver and kidney CYP4A12 expression is rapidly induced upon the addition of DOX to the drinking water and immunohistochemistry analysis of kidney sections indicated that CYP4A12 expression is localized not only to tubular structures but also to the vasculature. Moreover, renal microsomes and PGMVs harvested from DOX-treated Cyp4a12tg mice displayed higher levels of CYP4A12 protein and produced more 20-HETE. More importantly, the DOX-treated Cyp4a12tg mice were hypertensive and showed 20-HETE–dependent increases in vascular reactivity and endothelial dysfunction, along with increased myogenic responses.
The increase in BP in DOX-treated Cyp4a12tg mice, notwithstanding the drawbacks of the tail-cuff plethysmography method, was comparable regarding the time and magnitude of the response to that observed in DHT-treated mice; in both, BP increased significantly at day 5–6, reaching 140–150 mmHg. In addition, in both models, the BP increases were 20-HETE dependent because a 20-HETE antagonist normalized BP. The demonstration of increased vascular reactivity of the renal interlobar arteries and myogenic tone of the third branch of the mesenteric arteries that was abrogated by 20-HETE antagonist suggests that hypertension in this model is a consequence of increased vascular tone brought about by increased 20-HETE production in renal and extrarenal microvasculature. However, the contribution of tubular CYP4A12 expression to setting the level of BP in this model cannot be excluded. Indeed, immunofluorescence showed a substantial CYP4A12 expression in tubular structures and kidney microsomes from DOX-treated Cyp4a12tg mice displayed high capacity to produce 20-HETE, suggesting the possibility that tubular actions of 20-HETE also contributed to the hypertension in this model. In this regard, Quigley and co-workers,32,42 demonstrated that hypertension in androgen-treated rats and in Cyp4a14(−/−) mice is associated with androgen-driven increases in sodium reabsorption at the level of the proximal tubule, a major site of 20-HETE synthesis along the nephron.44,45 It is possible that chronic upregulation of 20-HETE leads to enhanced tubular reabsorption even though 20-HETE acutely inhibits sodium transport in both the proximal tubule46 and the medullary thick ascending loop of Henle.47,48 Taken together, the mechanism underlying 20-HETE contribution to hypertension in these models may be a combination of elevated peripheral vascular resistance coupled with 20-HETE–dependent increase in tubular sodium reabsorption. This notion is supported by findings by Fidelis et al.,49 who showed that increased renal vasoconstriction and reduced vasodilation along with diminished capacity to excrete sodium contributed to the hypertension in the Cyp4a14(−/−) mice. The reports of Quigley et al.42 that deletion of Cyp4a14 had no effect on vascular reactivity of the afferent arterioles and Holla et al.23 of loss of myogenic tone of the afferent arteriole also suggest resetting of the pressure natriuresis independent of elevated renal vascular tone similar to what was reported in the SHR.50,51 Taken together, data presented here indicate that these models have a 20-HETE–dependent hypertension driven by increased renal and peripheral vascular reactivity. The exact mechanism of the role of the kidney in this model remains to be determined.
The finding that high BP is driven by increasing the expression and activity of the CYP4A12–20-HETE pathway has significant implications to human hypertension. In humans, two CYP isoforms have been identified to be the major 20-HETE synthases, CYP4A11 and CYP4F2.52,53 The T8590C CYP4A11 polymorphism has been shown to be associated with hypertension.52,54 A study by Ward et al.21 showed a positive association between the V433M CYP4F2 polymorphism and both BP and urinary 20-HETE, and a recent study by Hu et al.55 showed increased central and peripheral arterial stiffness that was correlated with increased urinary 20-HETE in patients with the V433M CYP4F2 polymorphism. Thus, the CYP4F2 polymorphism, which results in a mutation that renders the CYP4F2 enzyme inactive,56 is associated with increased levels of 20-HETE in humans. This observation suggests that the V433M CYP4F2 polymorphism is somewhat analogous to deletion of the Cyp4a14 in mice; in both, there is an increase in 20-HETE levels. Interestingly, a recent study by Fava et al.57 demonstrated that diastolic BP is higher only in male carriers of the CYP4F2 V433M allele. In addition, transgenic mice expressing the human CYP4F2 in the kidney have elevated systolic BP associated with increased levels of 20-HETE.58
Although the relationship between androgen, 20-HETE, and hypertension in animal models is well studied, this relationship and its association with either CYP4A11 or CYP4F2 expression in humans are yet to be established. Androgen has been implicated as the driving force of sex-dependent differences in BP and the hypertension frequently seen in menopausal women. These studies suggested that targeting androgen synthesis or action may have therapeutic values.34 However, androgen actions are diverse and recent studies suggest that testosterone has beneficial effects on several cardiovascular risk factors, including suppression of the inflammatory response and improving patient symptoms and well-being. Low testosterone in men is a risk factor for cardiovascular/metabolic disease and is associated independently with visceral obesity, insulin resistance, hyperglycemia, hypertension, and dyslipidemia.41,59 These opposing effects of androgen call for a better understanding of its mechanism of action in distinct pathophysiologic conditions. Our studies identifying 20-HETE as the mediator of androgen-induced hypertension suggest that targeting 20-HETE may spare the beneficial effects of androgen while abrogating androgen-dependent vascular dysfunction and hypertension.
Concise Methods
Animal Experimentation
A full description of vector constructions, verification, and production of transgenic mice is provided in the Supplemental Material. Cyp4a12 transgenic mice were produced on a B6D2 background (a cross between female C57BL/6J and male DBA/2J). All experiments were performed following an Institutional Animal Care and Use Committee–approved protocol in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Male and female Cyp4a14(−/−)23 (129/Sv background) were used between 8 and 14 weeks of age. Cyp4a12tg mice were administered DOX (1 mg/ml) in their drinking water for 15–60 days. In some experiments, mice were administered the 20-HETE antagonists, 20-HEDE or 20-HEDGE (10 mg/kg of body weight per day in 5% ethanol in saline, intraperitoneally).18,30 C57BL/6 mice were implanted with pellets containing placebo or DHT (5 mg/kg per day for 14 days; Innovative Research of America, Sarasota, FL). BP was measured by the tail-cuff method. At the end of the experiments, mice were anesthetized, kidneys were removed, and renal PGMVs or mesenteric arteries were microdissected for biochemical and functional analyses.
Measurements of 20-HETE
Renal microsomal 20-HETE synthesis from AA was measured as previously described.29 PGMVs were isolated from mice and incubated in oxygenated Krebs bicarbonate buffer, pH 7.4, with 1 mM nicotinamide adenine dinucleotide phosphate for 1 hour at 37°C with gentle shaking. Deuterated 20-HETE was added as an internal standard and 20-HETE was extracted and quantified by liquid chromatography with tandem mass spectrometry (Applied Biosystems, Foster City, CA) as previously described.17
Vascular Function
Wire myograph was used to measure constrictor responses to phenylephrine (10−9 to 5×10−5 M) and relaxation to acetylcholine (10−9 to 5×10−5 M) of renal interlobar arteries (approximately 100 µm diameter) as previously described.30 Myogenic responses were measured in freshly isolated segments from the third branch of mesenteric arteries (1–2 mm length) mounted between two micropipettes in the chamber (1 ml) of a pressure myograph as previously described.60
Statistical Analyses
The data are presented as mean ± SE. Statistical significance (P<0.05) between the experimental groups was determined by the Fisher method of analysis for multiple comparisons. For comparison between treatment groups, the null hypothesis was tested by a single-factor ANOVA (Dunnett’s multiple comparison test) for multiple groups or unpaired t test for two groups.We thank Dr. Alberto Nasjletti and Dr. Tanush Gupta for their insightful comments in preparing this manuscript.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants ( HL097402 to C.-C.W., GM31278 to J.R.F., DK038226 to J.H.C., HL034300 to M.L.S.), a Diversity Supplement Award (HL34300-26A1S1 to V.G.), an American Heart Association predoctoral fellowship (0715781T to J.C.), and a Robert A. Welch Foundation grant (GL625910 to J.R.F.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070714/-/DCSupplemental.
References
- 1.Simpson AE: The cytochrome P450 4 (CYP4) family. Gen Pharmacol 28: 351–359, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Hardwick JP: Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol 75: 2263–2275, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML: Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science 243: 388–390, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Escalante B, Sacerdoti D, Davidian MM, LaniadoSchwartzman M, McGiff JC: Chronic treatment with tin normalizes blood pressure in spontaneously hypertensive rats. Hypertension 17: 776–779, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Da Silva JL, Tiefenthaler M, Park E, Escalante B, Schwartzman ML, Abraham NG: Tin mediated heme oxygenase gene activation and cytochrome P450 arachidonate hydroxylase inhibition in spontaneously hypertensive rats. Am J Med Sci 307: 173–181, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Escalante B, Omata K, Sessa W, Lee SG, Falck JR, Schwartzman ML: 20-hydroxyeicosatetraenoic acid is an endothelium-dependent vasoconstrictor in rabbit arteries. Eur J Pharmacol 235: 1–7, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Schwartzman MLFalck JR, Yadagiri P, Escalante B: Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygenase: Formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem 264: 11658–11662, 1989 [PubMed] [Google Scholar]
- 8.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BSS, Harder DR, Roman RJ: 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res 72: 126–136, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ: Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283: R60–R68, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Moreno C, Maier KG, Hoagland KM, Yu M, Roman RJ: Abnormal pressure-natriuresis in hypertension: Role of cytochrome P450 metabolites of arachidonic acid. Am J Hypertens 14: 90S–97S, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Muthalif MM, Benter IF, Khandekar Z, Gaber L, Estes A, Malik S, Parmentier JH, Manne V, Malik KU: Contribution of Ras GTPase/MAP kinase and cytochrome P450 metabolites to deoxycorticosterone-salt-induced hypertension. Hypertension 35: 457–463, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Muthalif MM, Karzoun NA, Gaber L, Khandekar Z, Benter IF, Saeed AE, Parmentier JH, Estes A, Malik KU: Angiotensin II-induced hypertension: Contribution of Ras GTPase/Mitogen-activated protein kinase and cytochrome P450 metabolites. Hypertension 36: 604–609, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Imig JD, Falck JR, Gebremedhin D, Harder DR, Roman RJ: Elevated renovascular tone in young spontaneously hypertensive rats: Role of cytochrome P450. Hypertension 22: 357–364, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Wang MH, Krishna UM, Falck JR, Laniado-Schwartzman M, Nasjletti A: Modulation by 20-HETE of phenylephrine-induced mesenteric artery contraction in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension 38: 1311–1315, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Wang MH, Guan H, Nguyen X, Zand B, Nasjletti A, Laniado-Schwartzman M: Contribution of cytochrome P450 4A1 and 4A2 to vascular 20-hydroxyeicosatetraenoic acid synthesis in the rat kidney. Am J Physiol 276: F246–F253, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Wang MH, Zhang F, Marji J, Zand BA, Nasjletti A, Laniado-Schwartzman M: CYP4A1 antisense oligonucleotide reduces mesenteric vascular reactivity and blood pressure in SHR. Am J Physiol Regul Integr Comp Physiol 280: R255–R261, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M: Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol 297: F875–F884, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML: CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension 56: 871–878, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M: Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res 98: 962–969, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ward NC, Puddey IB, Hodgson JM, Beilin LJ, Croft KD: Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med 38: 1032–1036, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD: A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 51: 1393–1398, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW: Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14: 1–18, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH: Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A 98: 5211–5216, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH: Mouse Cyp4a isoforms: Enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J 403: 109–118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heng YM, Kuo CS, Jones PS, Savory R, Schulz RM, Tomlinson SR, Gray TJ, Bell DR: A novel murine P-450 gene, Cyp4a14, is part of a cluster of Cyp4a and Cyp4b, but not of CYP4F, genes in mouse and humans. Biochem J 325: 741–749, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Jr, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH: Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest 116: 1696–1702, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML: Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50: 123–129, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Singh H, Schwartzman ML: Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep 60: 29–37, 2008 [PubMed] [Google Scholar]
- 29.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH: Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol 284: R1055–R1062, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Wu CC, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML: Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension 57: 788–794, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kienitz T, Quinkler M: Testosterone and blood pressure regulation. Kidney Blood Press Res 31: 71–79, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Quigley R: Androgens stimulate proximal tubule transport. Gend Med 5[Suppl A]: S114–S120, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D: Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 25: 305–313, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Reckelhoff JF: Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Reckelhoff JF, Fortepiani LA: Novel mechanisms responsible for postmenopausal hypertension. Hypertension 43: 918–923, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Yanes LL, Lima R, Moulana M, Romero DG, Yuan K, Ryan MJ, Baker R, Zhang H, Fan F, Davis DD, Roman RJ, Reckelhoff JF: Postmenopausal hypertension: Role of 20-HETE. Am J Physiol Regul Integr Comp Physiol 300: R1543–R1548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reckelhoff JF, Zhang H, Srivastava K: Gender differences in development of hypertension in spontaneously hypertensive rats: Role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Crofton JT, Share L: Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension 29: 494–499, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Ashton N, Balment RJ: Sexual dimorphism in renal function and hormonal status of New Zealand genetically hypertensive rats. Acta Endocrinol (Copenh) 124: 91–97, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Crofton JT, Ota M, Share L: Role of vasopressin, the renin-angiotensin system and sex in Dahl salt-sensitive hypertension. J Hypertens 11: 1031–1038, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Liu PY, Death AK, Handelsman DJ: Androgens and cardiovascular disease. Endocr Rev 24: 313–340, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Quigley R, Chakravarty S, Zhao X, Imig JD, Capdevila JH: Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiol 113: 23–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams JM, Murphy S, Burke M, Roman RJ: 20-hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omata K, Abraham NG, Schwartzman ML: Renal cytochrome P-450-arachidonic acid metabolism: Localization and hormonal regulation in SHR. Am J Physiol 262: F591–F599, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Ito O, Alonso-Galicia M, Hopp KA, Roman RJ: Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol 274: F395–F404, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Quigley R, Baum M, Reddy KM, Griener JC, Falck JR: Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am J Physiol Renal Physiol 278: F949–F953, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escalante B, Erlij D, Falck JR, McGiff JC: Cytochrome P-450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol 266: C1775–C1782, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Yu M, Lopez B, Dos Santos EA, Falck JR, Roman RJ: Effects of 20-HETE on Na+ transport and Na+ -K+ -ATPase activity in the thick ascending loop of Henle. Am J Physiol Regul Integr Comp Physiol 292: R2400–R2405, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Fidelis P, Wilson L, Thomas K, Villalobos M, Oyekan AO: Renal function and vasomotor activity in mice lacking the Cyp4a14 gene. Exp Biol Med (Maywood) 235: 1365–1374, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Yip KP, Tse CM, McDonough AA, Marsh DJ: Redistribution of Na+/H+ exchanger isoform NHE3 in proximal tubules induced by acute and chronic hypertension. Am J Physiol 275: F565–F575, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Roman RJ: Altered pressure-natriuresis relationship in young spontaneously hypertensive rats. Hypertension 9: III131–III136, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O’Donnell CJ, Brown NJ, Waterman MR, Capdevila JH: Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation 111: 63–69, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK: Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem 275: 4118–4126, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Gainer JV, Lipkowitz MS, Yu C, Waterman MR, Dawson EP, Capdevila JH, Brown NJ, AASK Study Group : Association of a CYP4A11 variant and blood pressure in black men. J Am Soc Nephrol 19: 1606–1612, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu BC, Li Y, Li FH, Zhang Y, Sheng CS, Fan HQ, Wang JG: Peripheral and central augmentation indexes in relation to the CYP4F2 polymorphisms in Chinese. J Hypertens 29: 501–508, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Stec DE, Roman RJ, Flasch A, Rieder MJ: Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics 30: 74–81, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Fava C, Montagnana M, Danese E, Sjögren M, Almgren P, Guidi GC, Hedblad B, Engström G, Minuz P, Melander O: The functional variant V433M of the CYP4F2 and the metabolic syndrome in Swedes. Prostaglandins Other Lipid Mediat 98: 31–36, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Zhao Y, Wang L, Yang X, Zheng Z, Zhang Y, Chen F, Liu H: Overexpression of cytochrome P450 4F2 in mice increases 20-hydroxyeicosatetraenoic acid production and arterial blood pressure. Kidney Int 75: 1288–1296, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Jones TH: Testosterone deficiency: A risk factor for cardiovascular disease? Trends Endocrinol Metab 21: 496–503, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Zhang F, Wang MH, Wang JS, Zand B, Gopal VR, Falck JR, Laniado-Schwartzman M, Nasjletti A: Transfection of CYP4A1 cDNA decreases diameter and increases responsiveness of gracilis muscle arterioles to constrictor stimuli. Am J Physiol Heart Circ Physiol 287: H1089–H1095, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.