Abstract
Nearly all renal tubular epithelial cells express insulin receptor. The insulin receptor in the distal tubule appears to modulate BP, but the role of the insulin receptor in the proximal tubule is unknown. Here, we selectively knocked out the insulin receptor from the proximal tubules of mice. Western blotting confirmed a two- to three-fold reduction in renal cortical homogenate insulin receptor-β among knockout mice compared with wild-type littermates. Young knockout mice exhibited a mildly diabetic phenotype, evidenced by higher fasting plasma glucose levels than wild-type mice. Assessments by hyperinsulinemic-euglycemic clamp and a glucose tolerance test revealed no differences in insulin sensitivity or overt pancreatic function, respectively. Renal cortical mRNA expression and enzyme activity of glucose-6-phosphatase, which catalyzes the final step of glucose production, were significantly higher in knockout mice. Taken together, these results support a role for insulin receptor in the proximal tubule in the modulation of systemic glucose levels. Downregulation of the insulin receptor in the proximal tubule, which occurs in insulin-resistant states, may promote hyperglycemia through enhanced gluconeogenesis.
The insulin receptor (IR) is expressed in nearly all absorptive epithelial cells along the renal tubule, suggesting that it may have an important role in renal metabolism.1 We recently reported that knockout of the IR from the distal renal tubule in mice increases BP and reduces urine nitrates plus nitrites, suggesting a previously unappreciated role for distal tubule IR in BP control.2 However, the role of the IR in the proximal tubule segment is unclear. The renal proximal tubular epithelial cell has a variety of physiologic functions, such as reabsorption of solute from glomerular ultrafiltrate and glucose production (gluconeogenesis). Insulin has been shown to affect both metabolic and transport functions of the proximal tubule.
The kidney contributes substantially (up to 25%) to systemic glucose levels, especially under fasting conditions.3,4 Previous studies evaluating renal glucose release and uptake, as well as the participation of gluconeogenic substrates in renal gluconeogenesis, have been thoroughly reviewed by Cano.5 These studies highlighted the significance and magnitude of renal glucose production and also suggested a greater sensitivity of renal glucose release toward hormone action compared with hepatic glucose release. However, whether reduced insulin receptor signaling affects these vital functions of the proximal tubule is not clear. This is particularly relevant because we have recently shown reduced IR protein expression in kidneys (as well as reduced levels of tyrosine phosphorylated IR) in insulin-resistant rat models.6 To examine the potentially important role of insulin in the proximal tubule cells, we generated mice with targeted deletion of IR from the proximal tubule (knockout [KO] mice). We found that KO mice exhibited a mildly diabetic phenotype (i.e., elevated fasting glucose levels). Furthermore, we found no evidence of defective pancreatic function or impaired glucose clearance in these mice. Significantly increased glucose-6-phosphatase (G6Pase), an enzyme critical for renal gluconeogenesis, in the renal cortex of KO mice suggested enhanced renal gluconeogenesis as a candidate mechanism for hyperglycemia in these mice. Furthermore, these findings support an important role for the IR in the proximal tubule, in regulating systemic glucose levels.
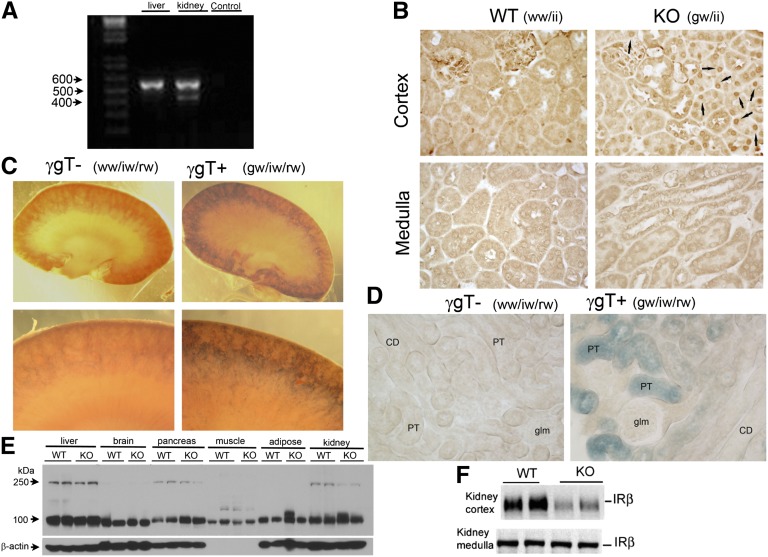
We used a Cre-loxP recombination strategy, as described previously,2 to generate KO mice with targeted deletion of proximal tubule IR. Cre-recombinase was driven by the type 1 γ-glutamyltransferase promoter, a proximal tubule–specific promoter (γGT-Cre). WT littermates used as controls in our experiments were homozygous for IRloxP and Cre negative. The KO mice were homozygous for IRloxP and heterozygous for Cre. RT-PCR revealed that KO mice had recombination in the kidney, but not in the liver (the other major gluconeogenic organ) (Figure 1A). In addition, expression of Cre-recombinase was assessed in the mice by immunoperoxidase labeling using a Cre-specific antibody (Figure 1B). Cre staining was found in the nucleus of renal cortical proximal tubule cells in the KO mice, but not in WT littermates, nor in any cells of the renal medulla (Figure 1B). Previously it was shown that in these γGT Cre-expressing mice, the γGT promoter expressed transcripts encoding Cre recombinase only in the kidney cortex, not in the brain, liver, spleen, muscle, lung, or adrenal gland.7 This was critical because the presence of IR is clearly important in these tissues for the normal use of glucose and maintenance of glucose homeostasis.8–10 Furthermore, it was shown that expression of the γGT promoter occurs in late kidney development, beginning around postpartum day 14 when nephrogenesis is almost complete.7
Figure 1.
Selective deletion of the insulin receptor from the proximal tubules of KO mice. (A) RT-PCR analysis of RNA prepared from liver and whole kidney of KO mice to study IR expression. Water is used for negative control. Presence of a smaller band of 435 bp in the kidney lane indicates that KO mice had recombination in kidney, but not in the liver, the other major gluconeogenic organ (absence of 435-bp band). (B) Expression of Cre (nuclear staining indicated by arrows) in KO mouse by immunohistochemistry using anti-Cre antibody. Cre staining is not found at all in the medulla, and only in the proximal tubule cells of the cortex in the KO mice (100×). (C). β-galactosidase activity resulted in a purple precipitant in LacZ reporter/γGT-Cre double heterozygotes, illustrating activity of Cre-recombinase to cleave lox P sites in the proximal tubule. The purple precipitant was absent in WT littermates that did not inherit γGT-Cre but were heterozygous for LacZ reporter. The kidney sections were photographed using a Zeiss dissecting scope and a Canon A590 camera with a fitted objective at 1.2× and 5× magnification. (D) A 400× image of sliced and mounted kidney cortex showing reporter staining only in the proximal tubule (PT) in IR KO mice. The genotype ww/iw/rw means that mice did not inherit the γGT transgene and were heterozygous for floxed IR and heterozygous for the reporter transgene; the genotype gw/iw/rw means that mice did inherit the γGT transgene and were heterozygous for floxed IR and the reporter transgene. These mice were littermate offspring from crossing a female KO mouse with a reporter male. CD, collecting duct; glm, glomerulus. (E) Protein expression of IR was not reduced in any other major insulin-sensitive tissue, including the liver, brain, adipose tissue, skeletal muscle, and pancreas in the KO mice by Western blotting. (F) KO mice had significantly less IR-β subunit in their kidney cortex homogenate relative to WT mice; however, no difference was found in the renal-medullary expression between the genotype (n=8/genotype; P=0.03).
To confirm localization of the IR KO to the proximal tubule, we crossed female KO mice with male mice homozygous for a LacZ reporter gene flanked by loxP sites. Treatment of the kidney with β-galactosidase substrate revealed increased blue color in the cortical proximal tubule cells of the γGT-positive mice, indicating active Cre-recombinase activity in the proximal tubule, as would be expected for a γGT promoter–driven Cre-recombinase (Figure 1, C and D). No blue color was seen in kidneys harvested from littermates containing the reporter construct but negative for γGT-Cre recombinase.
Western blot analysis showed that the protein expression of the insulin receptor was not reduced in any other major insulin-sensitive tissue, including the liver, brain, adipose tissue, skeletal muscle, and pancreas in the KO mice, compared with WT littermates (Figure 1E). In fact, pancreatic IR may have been slightly increased in the KO mice. Whole kidney homogenization did not reveal significantly reduced IR in the KO versus WT mice, probably because of the inclusion of the medulla; however, when the cortex was separated from the medulla (Figure 1F), we found a significant three- to four-fold reduction in band density of IR (β-subunit) in the KO mice.
To determine how the lack of insulin receptor affected glucose metabolism in the KO mice, plasma glucose was measured in tail blood of mice fasted for 8 hours. We found that KO mice had significantly higher fasting plasma glucose levels (mean ± SEM, 10.5±1.1 mmol/L) relative to WT littermates (7.8±0.2 mmol/L; P=0.03) (Figure 2A). Thus, the absence of the insulin receptor in the proximal tubule resulted in a mildly diabetic phenotype. However, IR deletion in the proximal tubule did not seem to affect body weight, 24-hour sodium, or potassium excretion or urine volume (Table 1).
Figure 2.
KO mice exhibited a mildly diabetic phenotype with no signs of impaired insulin sensitivity or overt pancreatic function. (A) Fasting plasma glucose levels in tail blood of 8-hour-fasted KO mice relative to WT littermates. (B) Plasma glucose clearance was measured in response to intraperitoneal glucose (5 ml of 20% dextrose/kg body weight). (C) Plasma insulin levels before and 20 minutes after intraperitoneal glucose injection (n=8/genotype). (D) Measurement of insulin sensitivity in mice using hyperinsulinemic-euglycemic clamp. *P<0.05 between the two genotypes by unpaired t test.
Table 1.
Physiologic and metabolic data in KO mice and their WT littermates under normal dietary conditions
| Variable | WT Mice | KO Littermates |
|---|---|---|
| Body weight (g) | 33.3±1.0 | 32.2±0.8 |
| 24-hr urine volume (ml) | 1.8±0.23 | 1.7±0.38 |
| Urinary sodium (μmol/d) | 67.3±12.7 | 64.5±15.0 |
| Urinary potassium (μmol/d) | 51.8±7.8 | 56.3±9.5 |
Values are the mean ± SEM.
Further experiments were performed to test whether defective plasma glucose clearance or pancreatic insulin secretion contributed to higher fasting glucose levels in these KO mice. The glucose tolerance test revealed that the glucose tolerance curve was shifted upward; however, the KO mice appeared to clear glucose in a fashion similar to that of the WT mice (with no difference in the slopes between any two time points; Figure 2B). To assess whether insulin secretion was impaired in the KO mice, insulin levels in response to exogenous glucose (intraperitoneally administered) were determined (Figure 2C). Baseline plasma insulin levels (0 minutes) were not significantly different in KO mice (Figure 2C) and were similarly increased in both genotypes in response to exogenous glucose approximately 20 minutes after an intraperitoneal glucose injection (Figure 2C). Furthermore, both genotypes were equally sensitive to the administered insulin with regard to the level of glucose we needed to infuse to keep the mice euglycemic, as indicated by the hyperinsulinemic-euglycemic clamp experiment (Figure 2D). These observations suggest that targeted deletion of the insulin receptor from the proximal tubule cells did not affect whole-body insulin sensitivity or glucose tolerance.
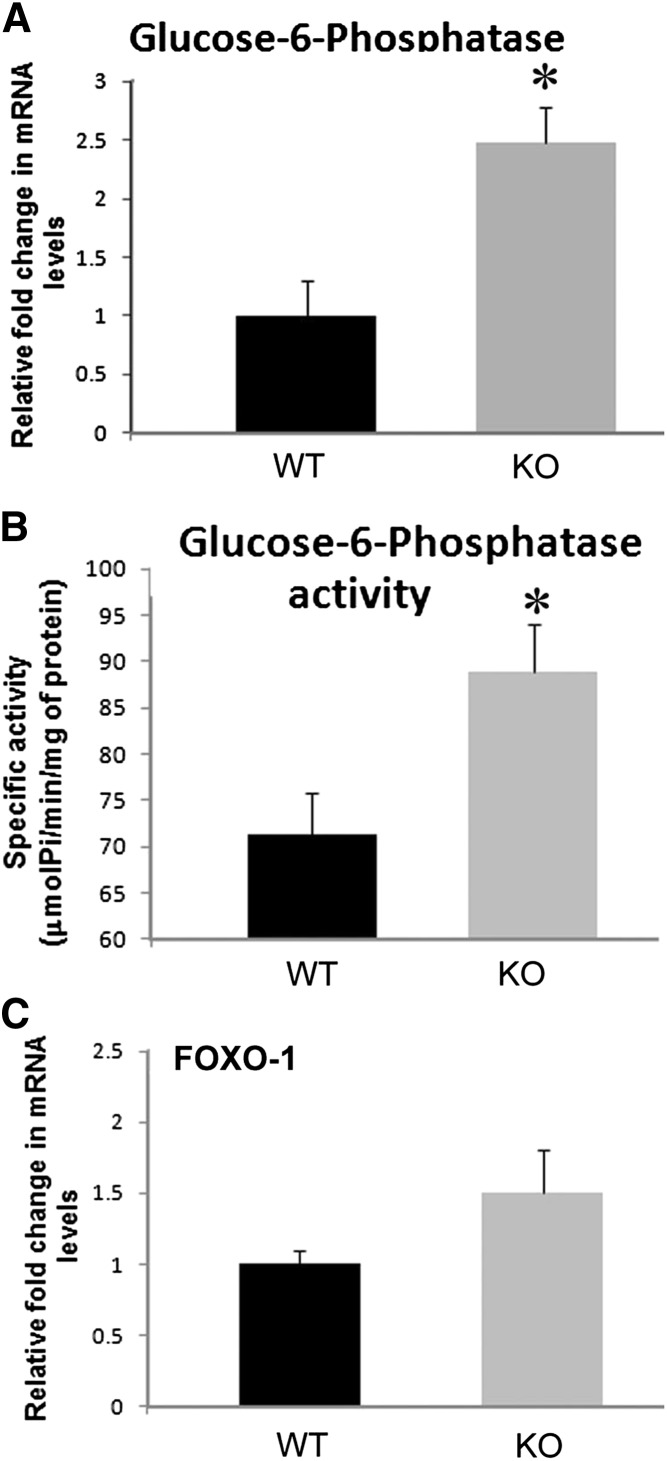
We next tested whether altered gluconeogenesis may have contributed to the elevated fasting glucose in the KO mice. The kidney cortex expresses G6Pase and contributes significantly to blood glucose via its gluconeogenic pathway.11–13 Furthermore, insulin has an inhibitory effect on renal gluconeogenesis.3 Therefore, we hypothesized that in the KO mice, insulin may have affected systemic glucose levels via regulating renal gluconeogenesis. As is seen in the liver, insulin may suppress expression or activity of gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase and G6Pase, thereby inhibiting gluconeogenesis in kidney. This suppression of gluconeogenic genes in the liver occurs by the activation of the IR and downstream signaling by phosphoinositide-3 kinase.14,15 However, the role of reduced IR expression in the proximal tubule in renal gluconeogenesis has not been as carefully studied. We found a 2.5-fold increase in G6Pase transcript levels in the renal cortex of KO relative to WT littermates using real-time RT-PCR (P=0.02; n=7) (Figure 3A). Before the measurement of G6Pase mRNA levels, mice in both genotypes were treated with insulin. The increased G6Pase transcript level in kidney cortex in mice lacking IR in the proximal tubule suggests that gluconeogenesis in kidney is relatively higher in the KO mice than in their WT littermates.
Figure 3.
Increased renal glucose-6-phosphatase gene expression and activity in KO mice. mRNA levels (n=6–7/genotype) (A) and specific activity (B) of G6Pase (n=10/genotype). (C) mRNA levels of FOXO-1 (n=6–7/genotype) in the renal cortex of KO mice relative to WT. *P<0.05 between the two genotypes by unpaired t test. Pi, inorganic phosphate.
We also measured G6Pase activity in the mice by estimating inorganic phosphate release in renal cortex slices. We found that the specific activity of G6Pase averaged about 20% higher in the renal cortex from KO mice relative to WT littermates (88.9±5.1 versus 71.2±4.6 μmol inorganic phosphate/mg protein per minute; P=0.02) (Figure 3B). Forkhead box protein O-1 (FOXO-1) mRNA expression in the cortex of these mice (Figure 3C) was not statistically different. FOXO-1 is a transcription factor downregulated by p-Akt (a kinase activated by IR-PI3K signaling). However, this regulation of FOXO-1 activity is classically thought to result from a phosphorylation change rather than changes in its expression. Elevation in FOXO-1 activity has been associated with increased transcriptional regulation of genes involved in gluconeogenesis.16,17
Our results concur with the finding of enhanced gluconeogenesis in renal proximal tubules of insulin-resistant Zucker rats.18 In this regard, we have shown reduced IR in the proximal tubule of insulin-resistant Zucker rats.6 These data also suggest that transcriptional regulation may be the major mechanism of G6Pase modulators that account for variations observed in G6Pase activity.13 Moreover, in different models of acute diabetes in rats, both G6Pase mRNA and activity are increased in the kidney to the same extent as in the liver.12
These studies suggest that the reduced IR expression in the proximal tubule is sufficient in and of itself to result in a mildly diabetic phenotype and indicate enhanced renal gluconeogenesis as a candidate mechanism. Thus, reduced or altered renal epithelial IR signaling may be particularly relevant for abnormal glucose metabolism associated with insulin resistance in the metabolic syndrome. Sechi and colleagues do not support that the kidney develops insulin resistance in the same manner as the muscle or liver.19–21 Their studies were, however, based primarily on mRNA expression of the renal IR and radiolabeled ligand binding.
In contrast, we show decreased renal expression of IR protein and phosphorylated IR, the first step in insulin signaling, in alternative rat models of insulin resistance,6 such as the obese Zucker rats,6 which have been demonstrated by other investigators18 to have elevated renal gluconeogenesis. Some of the differences may be due to model/strain differences. Furthermore, Sechi et al.22 have reported abnormal glucose metabolism in nondiabetic patients with early renal failure. Nevertheless, patients with kidney disease are often susceptible to hyperglycemia,23 and if left untreated this may progress to diabetes. Overall these results support a physiologic role for IR in the proximal tubule to affect systemic glucose levels independent of other metabolic disturbances and suggest that downregulation of IR in renal proximal tubule in the insulin-resistant state may further contribute to hyperglycemia through enhanced gluconeogenesis.
Concise Methods
Mice with the targeted deletion of insulin receptor in the proximal tubule of the kidney were generated by using a Cre-loxP recombination strategy as described previously.2 Briefly, homozygous IRloxP male mice were bred to heterozygous Cre recombinase–carrying females in which Cre-recombinase was driven by the type 1 γGT-Cre in a series of crosses. To generate mice for experiments, female KO mice were crossed with homozygous IRloxP male mice to produce WT (homozygous IRloxP, Cre-negative) and KO (homozygous IRloxP, Cre-positive) littermates at about a 50:50 ratio. All mice studied were male. They were 4–8 months of age, on a mixed 129/Sv/C57Bl6 background, essentially the background in which the floxed IR mice8.9.10 were generated after numerous backcrosses of female KO mice to homozygous floxed males to produce successive generations of experimental KO mice. The original γGT-Cre transgenic mice were primarily on a C57Bl6 genetic background.
To determine regions of the kidney with active Cre-recombinase, LacZ/γGT-Cre mice were perfused transcardially with 1× PBS followed by 20 ml of 2% glutaraldehyde. The kidneys were then removed and bisected transversely, exposing the medulla and the cortex. The bisected sections were immediately stained for the product of β-galactosidase at 37°C for 1 hour using a B-Gal staining kit (Mirus 2600). The sections were then photographed using a Zeiss dissecting scope and a Canon A590 camera with a fitted objective at 1.2× and 5× magnification or processed to slides for higher-power microscopy (200×) with a Photometrics Cool Snap camera (Scanalytics) mounted to a Nikon Eclipse E600 microscope. Genotyping was performed on tail DNA samples using DirectPCR Lysis Reagents (Viagen Biotech). A set of mice (n=8/genotype) were euthanized, and kidneys and other tissues were harvested to perform Western blotting using antibodies against the IR-β subunit as described previously.2,6,24
Blood glucose was measured in tail blood of young male mice after fasting for 8 hours using a glucometer (Lifescan Ultra II glucometer). For the glucose tolerance test, mice were intraperitoneally injected with 20% dextrose, 5 ml/kg of body weight, followed by measurement of blood glucose levels at 15, 30, 60, 90, and 120 minutes. To evaluate insulin sensitivity, a 1.5-hour hyperinsulinemic-euglycemic clamp with a continuous infusion of human insulin (Humulin-R, Eli Lily) at a rate of 0.12 mU/30 g of body weight per minute was carried out as described elsewhere.25 Plasma insulin levels were measured using an ELISA-based assay (Alpco). Mice were periodically housed in metabolic cages to collect urine for the determination of sodium and potassium by ion-selective electrodes (ELISE Electrolyte System; Beckman Instruments).
For G6Pase activity, male mice were fasted for 4 hours before study, followed by intraperitoneal injection of insulin (0.5 U/kg body weight) in 300 μl of saline plus 300 μl of 25% dextrose in saline to amplify the difference between KO and WT mice. After 20 minutes, mice were euthanized and right kidneys were removed. Kidney cortex was used to estimate G6Pase by the method of Baginsky.26 The inorganic phosphate liberated was estimated by the method of Fiske and Subbarow.27 Enzyme activity was expressed as µmoles of inorganic phosphate formed/mg protein per minute at 37°C. Protein was determined by the Bradford method with bovine serum albumin as a standard.
Total RNA prepared from kidney and liver of the KO mice were used for RT-PCR analysis of IR gene expression. Briefly, cDNA was synthesized using a random hexamer, followed by PCR reaction with the following set of primers: a forward primer on exon 3 of IR, 5′-GCTGCACAGCTGAAGGCCTGT-3′, and a reverse primer on exon 5, 5′-CTCCTCGAATCAGATGTAGCT-3′. Total RNA from kidney was used for real-time quantitative RT-PCR to compare G6Pase and FOXO-1 gene expression between the genotypes using SYBR Green Master Mix as per the manufacturer's instructions (Applied Biosytems) as described previously.2,28 The following primer sequences were used: G6Pase: 5′-TTTCCCCACCAGGTCGTGGCT-3′ (F) and 5′-CCCATTCTGGCCGCTCACACC-3′ (R); FOXO-1: 5′-ATGTGTTGCCCAACCAAA-3′ (F) and 5′-ATGTAGCCTGCTCACTAA-3′ (R). β-actin gene was also analyzed as a control (calibrator) by using specific primers [5′-GGCGGACTGTTACTGAGCTGCG-3′ (F) and 5′-GCTGTCGCCTTCACCGTTCCA3′ (R)]. Fold changes in gene expression were calculated using the 2−ΔΔCT method.
Quantitative data are expressed as the mean ± SEM. Differences between WT and KO mice were determined by unpaired t test if variability and distribution of data did not differ. When data were not normally distributed or variability differed, data were compared by the Mann-Whitney rank-sum test.
Disclosures
None.
Acknowledgments
The authors wish to thank Dr. Eric G. Neilson (Northwestern University, Chicago, IL) for his generous gift of the γGT-Cre mice and Allison Yunghans, MS (Georgetown University) and Drirh Khare (Sanjay Gandhi Postgraduate Institute of Medical Sciences) for technical assistance.
This work was supported by the Department of Biotechnology, government of India, Ramalingaswami grant (BT/HRD/35/02/17/2008 to S.T) and the National Institutes of Health (R01 DK082507 to C.E). G.P. and R.S.S. were supported by Junior Research Fellowships from University Grant Commission and Indian Council of Medical Research, government of India, respectively.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Butlen D, Vadrot S, Roseau S, Morel F: Insulin receptors along the rat nephron: [125I] insulin binding in microdissected glomeruli and tubules. Pflugers Arch 412: 604–612, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, Ecelbarger CM: Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci U S A 105: 6469–6474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stumvoll M, Meyer C, Mitrakou A, Nadkarni V, Gerich JE: Renal glucose production and utilization: New aspects in humans. Diabetologia 40: 749–757, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Stumvoll M, Meyer C, Mitrakou A, Gerich JE: Important role of the kidney in human carbohydrate metabolism. Med Hypotheses 52: 363–366, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Cano N: Bench-to-bedside review: Glucose production from the kidney. Crit Care 6: 317–321, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA: Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrol 18: 2661–2671, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR: A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR: Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR: Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96: 329–339, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Wirthensohn G, Guder WG: Renal substrate metabolism. Physiol Rev 66: 469–497, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Mithieux G, Vidal H, Zitoun C, Bruni N, Daniele N, Minassian C: Glucose-6-phosphatase mRNA and activity are increased to the same extent in kidney and liver of diabetic rats. Diabetes 45: 891–896, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Minassian C, Zitoun C, Mithieux G: Differential time course of liver and kidney glucose-6 phosphatase activity during long-term fasting in rat correlates with differential time course of messenger RNA level. Mol Cell Biochem 155: 37–41, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Miyake K, Ogawa W, Matsumoto M, Nakamura T, Sakaue H, Kasuga M: Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J Clin Invest 110: 1483–1491, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR: Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87–97, 2000 [PubMed] [Google Scholar]
- 16.Valenti L, Rametta R, Dongiovanni P, Maggioni M, Fracanzani AL, Zappa M, Lattuada E, Roviaro G, Fargion S: Increased expression and activity of the transcription factor FOXO1 in nonalcoholic steatohepatitis. Diabetes 57: 1355–1362, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, Shulman GI, Veniant MM: Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 55: 2042–2050, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Eid A, Bodin S, Ferrier B, Delage H, Boghossian M, Martin M, Baverel G, Conjard A: Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J Am Soc Nephrol 17: 398–405, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA: Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int 64: 2163–2171, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Sechi LA, Griffin CA, Schambelan M: Effect of dietary sodium chloride on insulin receptor number and mRNA levels in rat kidney. Am J Physiol 266: F31–F38, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA: Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens 16: 973–978, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Sechi LA, Catena C, Zingaro L, Melis A, De Marchi S: Abnormalities of glucose metabolism in patients with early renal failure. Diabetes 51: 1226–1232, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Shehab-Eldin W, Zaki A, Gazareen S, Shoker A: Susceptibility to hyperglycemia in patients with chronic kidney disease. Am J Nephrol 29: 406–413, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Tiwari S, Nordquist L, Halagappa VKM, Ecelbarger CA: Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK: Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53: 1060–1067, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Baginsky ES, Foa PP, Zak B: Glucose-6-phosphatase. In: Methods of Enzymatic Analysis, 2nd Ed., edited by Bergmeyer HU, New York, Academic Press, 1974, pp 788–792 [Google Scholar]
- 27.Fiske CH, Subbarow J: The colorimetric determination of phosphorous. J Biol Chem 66: 375–400, 1925 [Google Scholar]
- 28.Tiwari S, Zhang Y, Heller J, Abernethy DR, Soldatov NM: Atherosclerosis-related molecular alteration of the human CaV1.2 calcium channel alpha1C subunit. Proc Natl Acad Sci U S A 103: 17024–17029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]