Abstract
Lecithin-cholesterol acyltransferase (LCAT) is an enzyme involved in maintaining cholesterol homeostasis. In familial LCAT deficiency (FLD), abnormal lipid deposition causes renal injury and nephrotic syndrome, frequently progressing to ESRD. Here, we describe a 63-year-old Japanese woman with no family history of renal disease who presented with nephrotic syndrome. The laboratory data revealed an extremely low level of serum HDL and undetectable serum LCAT activity. Renal biopsy showed glomerular lipid deposition with prominent accumulation of foam cells, similar to the histologic findings of FLD. In addition, she had subepithelial electron-dense deposits compatible with membranous nephropathy, which are not typical of FLD. A mixing test and coimmunoprecipitation study demonstrated the presence of an inhibitory anti-LCAT antibody in the patient’s serum. Immunohistochemistry and immunofluorescence detected LCAT along parts of the glomerular capillary walls, suggesting that LCAT was an antigen responsible for the membranous nephropathy. Treatment with steroids resulted in complete remission of the nephrotic syndrome, normalization of serum LCAT activity and HDL level, and disappearance of foam cell accumulation in renal tissue. In summary, inhibitory anti-LCAT antibody can lead to glomerular lesions similar to those observed in FLD.
Lecithin-cholesterol acyltransferase (LCAT) is a key enzyme involved in the maintenance of cholesterol homeostasis and regulation of cholesterol transport in the blood.1 It binds to HDL and converts cholesterol to cholesterol esters, causing transformation of nascent discoidally shaped HDL (pre-β-HDL) into mature spherically shaped HDL (α-HDL).2 Through its mediation of HDL metabolism, LCAT is considered to be responsible for reverse cholesterol transport (RCT), by which excess cholesterol in the peripheral cells is delivered to the liver for excretion.3
Two types of inherited LCAT deficiency are known: familial LCAT deficiency (FLD) and fish-eye disease.4,5 Both are autosomal recessive diseases caused by mutation of the LCAT gene and manifest as corneal opacity and low plasma HDL levels.6 In FLD, plasma LCAT is either absent or completely lacks catalytic activity, whereas in fish-eye disease, the mutant LCAT exerts partial LCAT activity (i.e., it fails to exert activity on the HDL lipids, but esterifies cholesterol bound to apoB-containing lipoproteins).7 Therefore, homozygous patients for FLD gene mutation exhibit more severe clinical manifestations, such as normochromic anemia and renal failure.3
Renal disease is the major cause of morbidity and mortality in patients with FLD.8 The kidney disease manifests as proteinuria in the early stage, and increases in severity by the fourth and fifth decades of life, frequently progressing to nephrotic syndrome and ESRD.9 Light microscopy reveals thickening of the glomerular capillary walls, with an irregular bubble appearance of the basement membrane. The mesangium is expanded, often with a bubble appearance, with variable foam cells infiltrating the capillaries and mesangial areas.9
Here, we report a case of a woman in her sixties who presented with nephrotic syndrome caused by acquired severe LCAT deficiency. The patient exhibited glomerular lesions similar to those of FLD, together with changes of membranous nephropathy, which improved with steroid therapy. We performed a detailed examination of the mechanism of LCAT deficiency in this patient.
Case Report
A 63-year-old Japanese woman was admitted to our hospital in the spring of 2010, with edema of the lower extremities. She had been detected to have mild proteinuria in routine medical examinations for 15 years. She had begun to notice bilateral pretibial edema about 3 months before her current hospitalization. She visited her primary care physician, who made the diagnosis, based on her clinical symptoms/signs and laboratory data, of nephrotic syndrome. She was referred to our hospital for further evaluation and treatment of the nephrotic syndrome. Her previous medical history revealed that she had been diagnosed with Sjögren’s syndrome about 20 years ago, and that she had undergone surgery for breast cancer 7 years ago. There was no family history of renal disorders or dyslipidemia.
At admission, she was 153.5 cm tall and weighed 53.3 kg. Her BP was 152/65 mmHg. Mild pitting edema was noted in the lower extremities. There were no ocular abnormalities, including corneal opacity. Her laboratory data were as follows: serum total protein, 5.4 g/dl; serum albumin, 2.5 g/dl; serum creatinine, 0.58 mg/dl; BUN, 24 mg/dl; serum uric acid, 7.0 mg/dl; white blood cell count, 6300/mm3; red blood cell count, 3,240,000/mm3; and hemoglobin, 9.4 g/dl. Urinalysis revealed 1+ occult blood and sediment containing 20 red blood cells per high-power field. The urinary protein excretion was 4.1 g/d. The serum lipid profile is summarized in Table 1.
Table 1.
Laboratory data for serum lipid or lipoprotein levels
| Variable | At First Renal Biopsy | At Second Renal Biopsy | Normal Range |
|---|---|---|---|
| Total cholesterol (mg/dl) | 179 | 201 | 128–219 |
| Cholesteryl esters (mg/dl) | 19 | 141 | 80–200 |
| Free cholesterol (mg/dl) | 138 | 60 | 34–66 |
| LDL cholesterol (mg/dl) | 78 | 134 | 59–139 |
| HDL cholesterol (mg/dl) | 3 | 42 | 45–67 |
| Triglycerides (mg/dl) | 444 | 131 | 30–149 |
| ApoA-I (mg/dl) | 47 | 116 | 126–165 |
| ApoA-II (mg/dl) | 5.8 | 18.6 | 24.6–33.3 |
| ApoB (mg/dl) | 64 | 111 | 66–101 |
| ApoC2 (mg/dl) | 5.6 | 3.4 | 1.5–3.8 |
| ApoE (mg/dl) | 7.4 | 2.8 | 2.8–4.6 |
| Lipoprotein fraction (%) | |||
| α | 3.5 | ND | 31.5–51.5 |
| Pre-β | 33.9 | ND | 2.6–24.6 |
| β | 32.1 | ND | 36.5–53.3 |
| CETP (μg/ml) | 2.1 | ND | 1.8–3.0 |
| LPL activity (ng/ml) | 146 | ND | 140–353 |
| LCAT (U) | 0 | 283 | 235–550 |
CETP, cholesteryl ester transfer protein; LPL, lipoprotein lipase; LCAT, lecithin-cholesterol acyltransferase, ND, not done.
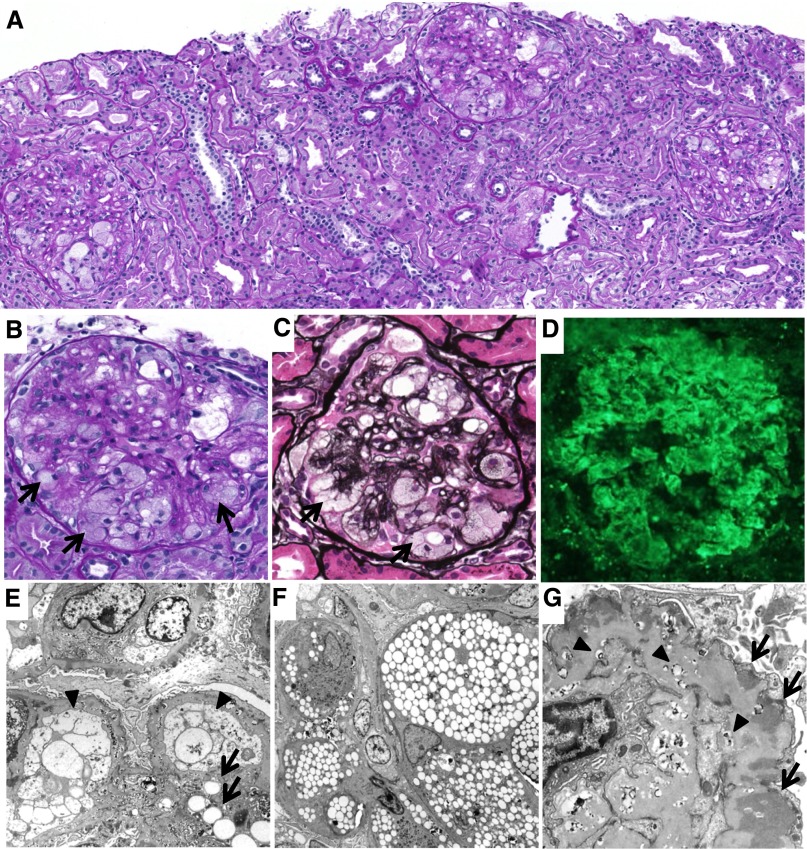
A renal biopsy was performed (Figure 1). The specimen for light microscopy contained 13 glomeruli. Almost all of the glomeruli showed mild mesangial proliferation and mesangial matrix expansion. In addition, marked accumulation of foam cells was observed in most of the glomeruli (Figure 1, B and C). In some of the glomeruli, the glomerular basement membrane (GBM) showed a double contour and spike formation. Tubular atrophy and tubulointerstitial fibrosis were observed in some areas. There were no obvious abnormalities in the blood vessels. Immunofluorescence revealed strong, coarse granular deposition of IgG, mainly along the capillary walls (Figure 1D). Moderate deposition of C3c and weak deposition of IgA, IgM, C3d, C1q, and fibrinogen were also observed in a similar pattern. Electron microscopy revealed extensive lipid deposits in the mesangial area and along the glomerular capillary walls, together with marked accumulation of foam cells. Both mesangial cells and endothelial cells showed foamy changes (Figure 1E). Marked foam cell accumulation was observed in the capillary lumina (Figure 1F). These foam cells were considered to be derived from macrophages, because some macrophages showed foamy changes. In addition, irregular thickening of the GBM with subepithelial electron-dense deposits and osmiophilic lipid deposits was also observed, together with widespread foot process effacement (Figure 1G).
Figure 1.
Histologic findings at the first renal biopsy. (A–C) Light microscopy findings. Glomeruli show marked foam cell accumulation (arrows), together with mild mesangial proliferation and expansion (periodic acid–Schiff staining in A and B, periodic acid silver-methenamine staining in C). (D) Immunofluorescence findings. Coarse granular depositions of IgG are observed mainly along the capillary walls. (E–G) Electron microscopy findings. (E) Lipid deposits are observed in the mesangial area (arrows). The endothelial cells become enlarged and occlude the capillary lumina (arrowheads). (F) The foam cells are accumulated in the capillary lumina. (G) Irregular thickening of the GBM with subepithelial electron-dense deposits (arrows) and osmiophilic lipid deposits (arrowheads) are also observed. Original magnification, ×100 in A and D; ×400 in B and C; ×1200 in E; ×1000 in F; ×2500 in G.
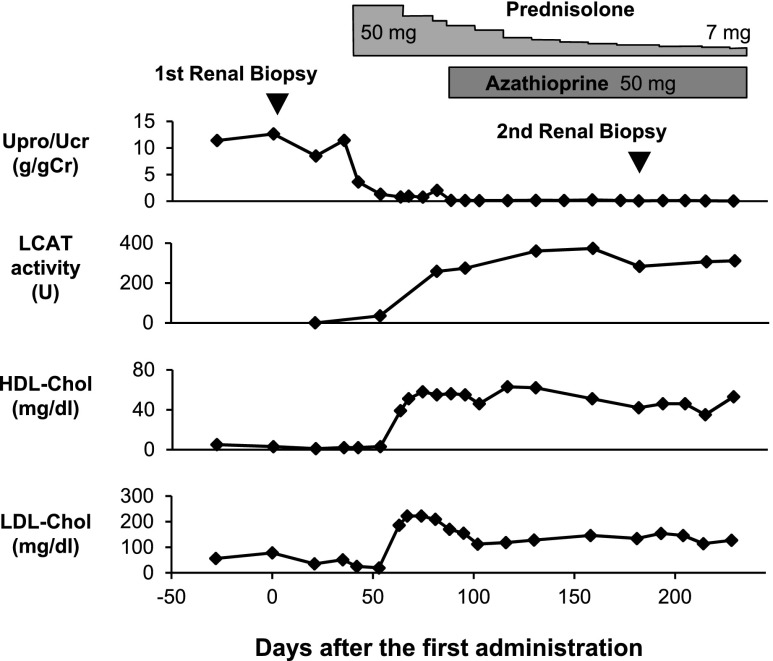
The patient was initiated on treatment for nephrotic syndrome with oral prednisolone at a 50 mg/d dose. As shown in Figure 2, marked reduction of proteinuria was observed soon after the initiation of this treatment. In addition, serum LCAT activity and HDL cholesterol level also increased to within their normal ranges. One month after the initiation of steroid treatment, the patient developed steroid psychosis. Antipsychotropic agents were prescribed and the steroid dose was tapered. Azathioprine was added at a 50 mg/d dose as maintenance therapy.
Figure 2.
Clinical course of the patient. Soon after the initiation of prednisolone therapy, the urinary protein/urinary creatinine ratio decreases and complete remission of the nephrotic syndrome is achieved. The severely deficient LCAT activity and markedly decreased HDL cholesterol level increase to within normal range after the initiation of the prednisolone therapy. Upro/Ucr, urinary protein/urinary creatinine ratio; HDL-Chol, HDL cholesterol; LDL-Chol, LDL cholesterol.
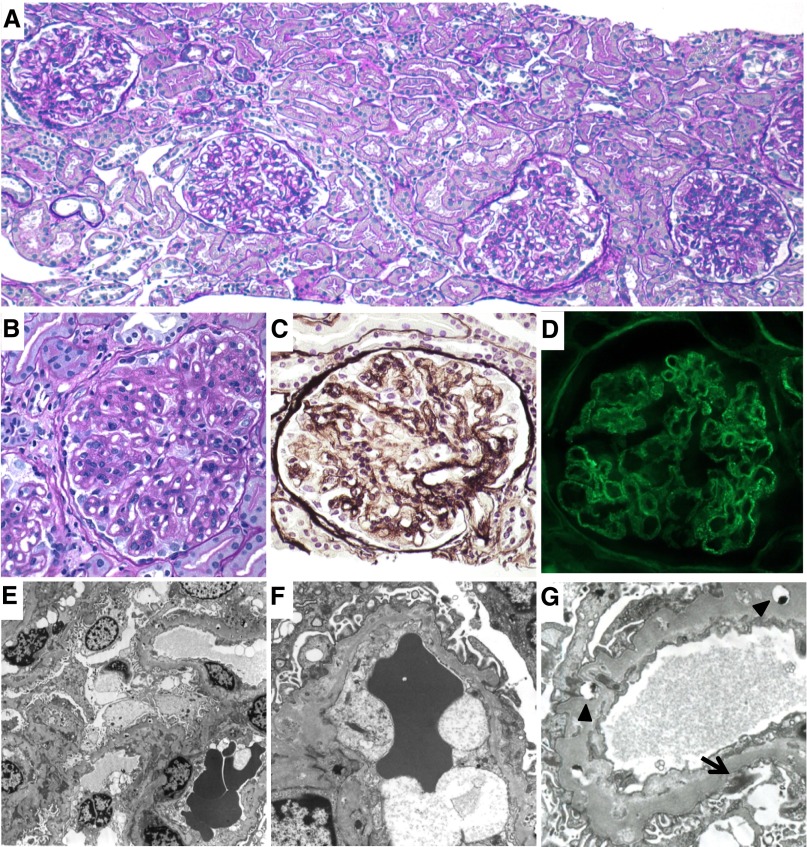
Five months after the initiation of the steroid therapy, a repeat renal biopsy was performed. Light microscopy revealed a marked reduction in the number of foam cells and improvement of the mesangial lesions (Figure 3, A–C). Immunofluorescence showed weak fine granular deposition of IgG along the capillary walls (Figure 3D). Electron microscopy also revealed the disappearance of the foam cells (Figure 3, E and F). No obvious foamy changes of the mesangial cells or endothelial cells were observed. Although the GBM still showed diffuse thickening with fine osmiophilic lipid deposition, the amount of subepithelial electron-dense deposits was apparently reduced (Figure 3G).
Figure 3.
Histologic findings at the second renal biopsy. (A–C) Light microscopy findings. Marked reduction of the foam cells and improvement of the mesangial lesions (periodic acid-Schiff staining in A and B; periodic acid silver-methenamine staining in C). (D) Immunofluorescence findings. Weak fine granular depositions of IgG are observed along the capillary walls. (E–G) Electron microscopy findings. (E and F) The foam cells disappear from the capillary lumina. (G) The apparent decrease in the amount of subepithelial electron-dense deposits (arrow), although the GBM show diffuse thickening with fine osmiophilic lipid depositions (arrowheads). Original magnification, ×100 in A; ×400 in B–D; ×1200 in E; ×4000 in F and G.
Results
Mixing Tests
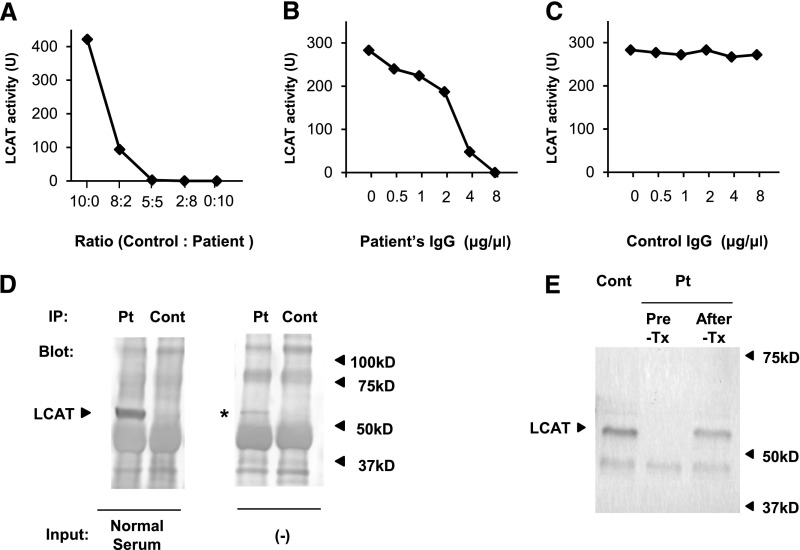
Because the patient’s medical records confirmed that her serum HDL level had mostly been between 20 and 40 mg/dl until 3 years ago, we hypothesized that the severe LCAT deficiency in this patient was acquired. To test this hypothesis, we performed a mixing test using the patient’s serum and healthy control serum. As shown in Figure 4A, the LCAT activity was almost completely suppressed at the mixing ratio (control serum/patient serum) of 5:5, demonstrating the existence of a factor inhibitory for LCAT activity in the patient’s serum. We next purified an IgG fraction from the patient’s serum and mixed it with healthy control serum at different concentrations. The patient’s IgG suppressed LCAT activity at higher concentrations, whereas control IgG from control serum had no effect on LCAT activity (Figure 4, B and C).
Figure 4.
Inhibitory autoantibody against LCAT. (A) Mixing test conducted using the patient’s serum and healthy control serum shows the existence of a factor inhibitory for LCAT activity in the patient’s serum. (B) Addition of the purified patient’s IgG to healthy control serum decreases the LCAT activity in a dose-dependent manner. (C) Addition of purified IgG from healthy control serum to a different control serum has no effect on the LCAT activity. (D) Coimmunoprecipitation study. Patient’s serum (Pt) or healthy control serum (Cont) is preincubated with protein G beads, and then incubated with a different control serum (normal serum). LCAT of normal serum is immunoprecipitated with the patient’s serum (arrowhead), but not with control serum. In addition, protein G beads preincubated with the patient’s serum alone capture LCAT (asterisk), demonstrating that the patient’s serum contained immune complexes composed of IgG and LCAT. (E) Western blot analysis for LCAT. Healthy control serum and the patient’s serum before steroid therapy (Pre-Tx) and after steroid therapy (After-Tx) are pretreated with albumin and IgG removal kits. The treated sera are then subjected to Western blotting for LCAT using rabbit monoclonal anti-LCAT antibody. LCAT is detected in both healthy control serum and the patient’s serum after, but not before, the steroid treatment (arrowhead).
Western Blotting and Coimmunoprecipitation
The results of the mixing tests strongly suggested that the patient’s serum contained an antibody against LCAT. We then performed Western blot analysis of the patient’s serum against recombinant LCAT protein or healthy adult serum. However, the patient’s serum did not react with either the recombinant LCAT or the LCAT in the healthy control serum, under either reducing or nonreducing conditions. We speculated that the antibody might recognize the higher-order structure of LCAT, and performed a coimmunoprecipitation study. The protein G beads preincubated with the patient’s serum captured the LCAT in the healthy control serum, demonstrating that the patient’s serum contained an antibody that bound to LCAT (Figure 4D, left panel). In addition, LCAT was detected in the supernatant of boiled protein G beads preincubated with the patient’s serum alone (Figure 4D, right panel), demonstrating the existence of immune complexes composed of LCAT and anti-LCAT autoantibody in the patient’s serum. We then examined the serum levels of LCAT protein by Western blotting. Each serum was pretreated with an albumin and IgG removal kit, because a preliminary experiment showed that LCAT could be scarcely detected in the healthy control serum, presumably due to interference by the abundant albumin and IgG in the serum. The patient’s serum before steroid treatment exhibited no band, whereas the patient’s serum after steroid treatment or healthy control serum showed a detectable band for LCAT protein (Figure 4E), demonstrating that there was no detectable free LCAT in the patient’s serum before treatment.
Immunostaining for LCAT
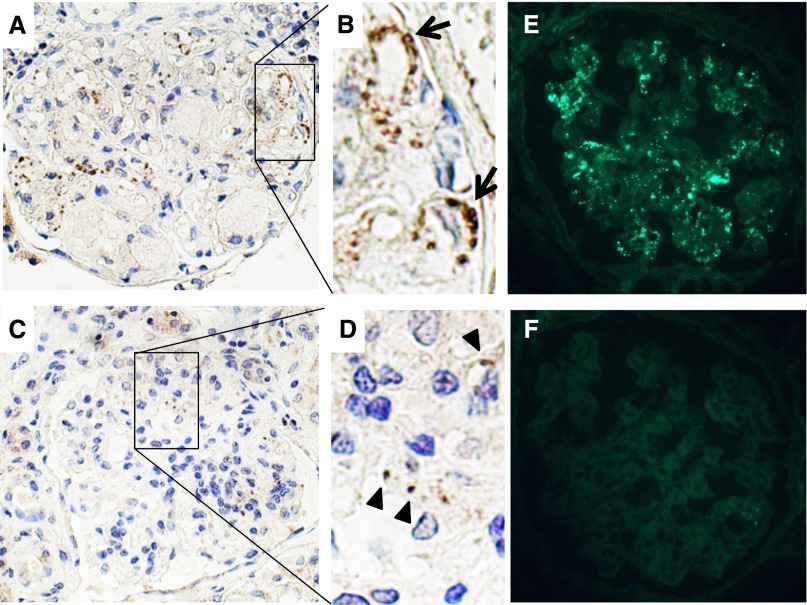
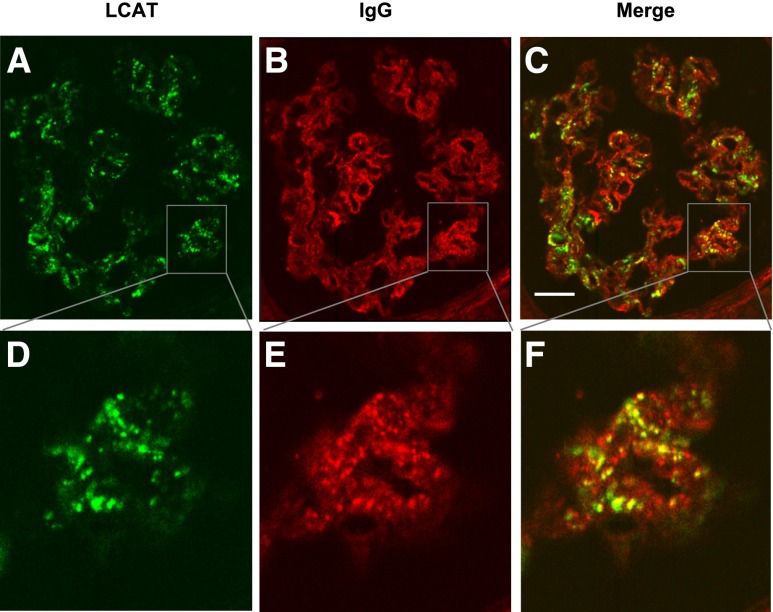
Because of the coexisting membranous nephropathy in our patient, we hypothesized that the glomerular subepithelial deposits might contain LCAT. We performed immunohistochemical staining using formalin-fixed paraffin-embedded tissue sections for both the first and second biopsy specimens and immunofluorescence for the second biopsy specimen, using rabbit monoclonal anti-LCAT antibody. Immunofluorescence of the first biopsy could not be performed because no frozen renal biopsy specimen was left. As shown in Figure 5, A and B, LCAT was detected along parts of the glomerular capillary walls of the first biopsy specimen. Immunohistochemical staining showed that the LCAT deposition was markedly reduced in the second biopsy specimen (Figure 5, C and D). However, immunofluorescence still exhibited granular deposits of LCAT, mainly along glomerular capillary walls (Figure 5E), possibly due to the preservation of antigenicity of LCAT on the frozen section without formalin fixation. The specificity of the rabbit monoclonal anti-LCAT antibody was tested using renal biopsy specimens of different types of GN, including minimal change nephrotic syndrome (n=1), IgA nephropathy (n=1), idiopathic membranous nephropathy (n=2), and lupus membranous nephropathy (n=2). No specific staining was observed in the glomeruli of these samples (data not shown). Finally, double immunofluorescence for LCAT and IgG was performed on frozen sections of the second biopsy specimen using confocal microscopy. Partial colocalization of LCAT and IgG was observed along the glomerular capillary walls (Figure 6).
Figure 5.
Immunostaining for LCAT. (A–D) Immunohistochemical staining on formalin-fixed, paraffin-embedded renal biopsy specimens with rabbit monoclonal anti-LCAT antibody. (A and B) Coarse granular staining is detected mainly along the glomerular capillary walls (arrows) in the first renal biopsy sample. (C and D) Faint granular staining (arrowheads) is observed in the second renal biopsy sample 5 months after the treatment. (E and F) Immunofluorescence on frozen sections of the second renal biopsy. (E) Coarse granular staining is mainly observed along the glomerular capillary walls by rabbit monoclonal anti-LCAT antibody. (F) Specific staining is not observed after elimination of the first antibody.
Figure 6.
Double immunostaining for LCAT and IgG on frozen sections of the second renal biopsy by confocal microscopy. (A and D) LCAT is detected along the glomerular capillary walls by rabbit monoclonal anti-LCAT antibody (green). (B and E) IgG deposition is also detected along the capillary walls by anti-human IgG (red). (C and F) Partial colocalization of LCAT and IgG is observed along the capillary walls (yellow). Scale bar, 20 μm.
Discussion
In this article, we describe the case of a patient with nephrotic syndrome that was associated with severe HDL and LCAT deficiency caused by an inhibitory autoantibody directed against LCAT. The renal biopsy revealed glomerular lipid deposition with prominent foam cell accumulation, similar to the findings in FLD. Very recently, an elderly woman with malignant non-Hodgkin’s lymphoma was first reported to have severe HDL and LCAT deficiency.10 An inhibitory autoantibody against LCAT was detected in the patient’s serum and the HDL levels returned to normal after successful treatment of the lymphoma. However, there was no description of the urinalysis or renal findings in that patient. To the best of our knowledge, our case report is the first in the English literature of a patient with both an inhibitory anti-LCAT antibody in the serum and glomerular lesions on the renal biopsy comparable with those in FLD.
In addition to the aforementioned report of the lymphoma patient with an inhibitory LCAT antibody, we found another report of a patient in whom the presence of anti-LCAT antibody in the serum was suspected from the clinical course.11 According to that report, a 62-year-old male patient with nephrotic syndrome had low serum LCAT activity (<5 nmol/L; normal range, 55–124) and a low serum HDL cholesterol level (11 mg/dl). Renal biopsy revealed lipid deposition similar to that in patients with FLD. After the initiation of steroid therapy, the serum LCAT activity normalized by 2 weeks, followed some time later by partial remission of the nephrotic syndrome. The serum LCAT inhibitory activity was not measured in this patient. However, the renal biopsy showing lipid deposition, reversal of the LCAT activity, and decrease of the urinary protein excretion after the initiation of steroid therapy suggest that this patient may also have had an autoantibody against LCAT in the serum, similar to our patient.
The mechanisms of development of the renal lesions in FLD are not yet precisely understood. Accumulation of lipid components is a characteristic feature of the renal lesions in FLD, and it occurs in both intracellular and extracellular sites.12 The GBM is considered to be injured by these lipids, resulting in proteinuria.12 In addition, a variable form cell infiltration was frequently observed in the glomerular capillary lumina.9 Foam cells are considered to be derived from monocytes or macrophages, which take up LDL and undergo phenotypic transformation into foam cells.13 In our patient, acquired LCAT deficiency led to abnormal lipid accumulation in the glomeruli and induced glomerular injury, similar to that in cases of congenital LCAT deficiency.
LCAT has long been believed to play a crucial and critical role in RCT by contributing to cell cholesterol efflux, the first and rate-limiting step in RCT, by maintaining the unesterified cholesterol gradient between the cell membrane and extracellular acceptors.3 By the RCT theory, HDL transports cholesterol to the liver, and HDL and its major apolipoprotein, apoA-I, remove cholesterol from foam cells.14 However, the role of LCAT in RCT has become controversial, because of opposite findings between animal models and humans.3,15 Neither LCAT-deficient humans nor mice appear to be at a markedly increased risk of atherosclerosis, which might be expected if there were a major defect in RCT.15 The disappearance of the numerous foam cells within the glomeruli after normalization of the LCAT activity in our patient strongly suggests that LCAT plays a critical role in RCT, at least in the removal of foam cells from the glomeruli.
Although the renal histology in our patient resembled that in cases of congenital LCAT deficiency, our patient also had membranous nephropathy, which is not generally found in cases of congenital LCAT deficiency. Phospholipase A2 receptor was recently identified as the antigen responsible for idiopathic membranous nephropathy.16 Subsequently, aldose reductase and SOD2 were reported to be other renal antigens involved in the development of human membranous nephropathy.17 In our patient, we detected LCAT along the glomerular capillary walls by immunohistochemical staining and immunofluorescence, suggesting LCAT as an antigen responsible for the membranous nephropathy. Interestingly, the other Japanese patient with nephrotic syndrome mentioned above who was found to have acquired LCAT deficiency was also detected by electron microscopy to show membranous lesions, although the deposition of LCAT was not examined in that patient. We suppose that LCAT is a rare but possible membranous antigen in idiopathic or secondary membranous nephropathy.
There is no specific treatment established yet for FLD. Transplantation of the cornea or kidney was performed in cases with severe disease, although the disease has been reported to recur in the transplanted kidney.18 Plasma infusions improved the lipid profiles in a short-term study; however, their long-term benefits are yet to be demonstrated.12 Recently, enzyme replacement therapy was shown to be effective for certain lysosomal diseases.19 FLD could be an attractive candidate for enzyme replacement therapy, because LCAT acts in the plasma compartment, without any organ-specific distribution.20 In mouse models of LCAT deficiency, human recombinant LCAT treatment rapidly restored the normal lipoprotein phenotype in LCAT-knockout mice, and increased the cholesterol efflux.20 Enzyme replacement therapy for human congenital LCAT efficiency is still under development.21 Our case report provides encouraging evidence to suggest that restoration of LCAT activity in patients with LCAT deficiency could induce resolution of the abnormal lipid deposition.
Concise Methods
Mixing Tests and Measurements of LCAT Activity
To investigate the presence of inhibitory activity against LCAT in the serum, the patient’s serum was mixed with healthy control serum at different mixing ratios. LCAT activity was measured by an exogenous substrate method with liposomes composed of cholesterol and lecithin (Anasorb LCAT; Daiichi Pure Chemicals, Tokyo, Japan). To determine whether the patient had an inhibitory antibody in the serum directed against LCAT, purified IgG from the patient’s serum or healthy control serum was added in various amounts to a different control serum and the LCAT activity was measured.
IgG Purification
IgG was purified from the patient’s serum and healthy control serum using IgG Purification Kit-G (Dojindo Laboratories, Kumamoto, Japan), in accordance with the manufacturer’s instructions. Briefly, a sample was added to the protein G cartridge tube and centrifuged at 8000×g for 30 seconds. The filtrate was added to the tube and centrifuged again. After washing the tube with washing buffer, IgG was recovered with an elution buffer.
Western Blot Analyses
For the Western blot analysis, 0.05 μg of recombinant human LCAT protein or 3–5 μl of pretreated healthy control serum was diluted in reduced or nonreduced sample buffer and applied to SDS gel, and then transferred to a polyvinyl difluoride membrane (Immobilon-P; Millipore, Bedford, MA). After blocking with nonfat dry milk (Cell Signaling, Danvers, MA), the membrane was incubated with the patient’s serum (×500) or rabbit monoclonal anti-LCAT antibody (×10,000; Epitomics, Burlingame, CA). IgG binding was detected using alkaline phosphatase-conjugated goat polyclonal anti-human IgG (Promega, Madison, WI) or goat polyclonal anti-rabbit IgG (Promega) and chromogen 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Sigma, St Louis, MO).
To examine the amount of LCAT in the serum, the patient’s serum before and after treatment or healthy control serum was pretreated in a separate experiment with the ProteoSeek Antibody-Based Albumin/IgG Removal Kit (Pierce, Rockford. IL), and then applied for electrophoresis under reducing conditions. Rabbit monoclonal anti-LCAT antibody (Clone EPR1384Y; Epitomics) was used to detect LCAT.
Coimmunoprecipitation
For the coimmunoprecipitation analysis, 100 μl of protein G beads (GE Healthcare, Amersham, UK) preincubated with 100 μl of patient’s serum or healthy control serum were incubated overnight at 4°C with or without a different control serum. The protein G beads were collected by centrifugation, washed with Tris-Glycine buffer, and resuspended in 2× SDS-PAGE loading buffer. Samples were heated at 95°C for 5 minutes. The supernatant was examined by Western blotting using rabbit monoclonal anti-LCAT antibody.
Immunohistochemistry and Immunofluorescence
Paraffin-embedded sections were deparaffinized and rehydrated in a routine manner. After the inactivation of endogenous peroxidase with 3% hydrogen peroxide in distilled water for 30 minutes, sections were pretreated with 10% normal goat serum for 30 minutes, and incubated with rabbit monoclonal anti-LCAT antibody (×100), and then with biotinylated goat anti-rabbit IgG (Histofine; Nichirei, Tokyo, Japan). Signals were detected using peroxidase-conjugated streptavidin (Histofine; Nichirei) and diaminobenzidine tetrahydrochloride (Nichirei). The sections were counterstained with hematoxylin.
In a separate experiment, indirect immunofluorescence for LCAT was performed. Optimal cutting temperature (OCT)-embedded frozen kidney sections were fixed in ice-cold methanol-acetone (1:1) for 10 minutes, incubated with rabbit monoclonal anti-LCAT antibody (×100). Staining was detected by FITC-conjugated goat anti-rabbit IgG absorbed with pooled human sera, purified human paraproteins, pooled mouse sera, and pooled mouse plasmacytoma/hybridoma proteins (Southern Biotech, Birmingham, AL). In addition, double immunofluorescence for LCAT and IgG was performed. To detect human IgG, Alexa Fluor 546-conjugated goat anti-human IgG absorbed with pooled mouse, rabbit, and bovine sera (Invitrogen, Carlsbad, CA) was used. The double-stained sections were examined with a Zeiss Axiophot 2 microscope (Zeiss, Oberkochen, Germany) equipped with Bio-Rad MRC1024ES confocal system (Bio-Rad, Hemel Hempstead, UK).
Disclosures
None.
Acknowledgments
The patient in this report gave informed consent to publication of the data.
This research was funded, in part, by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to K.H. and Y.N.) and a grant for progressive renal diseases research, research on rare and intractable disease, from the Japanese Ministry of Health, Labour, and Welfare.
Parts of this article were presented at the American Society of Nephrology 44th Annual Meeting, November 8–13, 2011, in Philadelphia, Pennsylvania.
Footnotes
S.T. and K.H. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Jonas A: Lecithin cholesterol acyltransferase. Biochim Biophys Acta 1529: 245–256, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Zannis VI, Chroni A, Krieger M: Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl) 84: 276–294, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Calabresi L, Franceschini G: Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc Med 20: 50–53, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Gjone E, Norum KR: Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 183: 107–112, 1968 [PubMed] [Google Scholar]
- 5.Carlson LA, Philipson B: Fish-eye disease. A new familial condition with massive corneal opacities and dyslipoproteinaemia. Lancet 2: 922–924, 1979 [PubMed] [Google Scholar]
- 6.Dalrymple LS, Kaysen GA: The effect of lipoproteins on the development and progression of renal disease. Am J Nephrol 28: 723–731, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Calabresi L, Pisciotta L, Costantin A, Frigerio I, Eberini I, Alessandrini P, Arca M, Bon GB, Boscutti G, Busnach G, Frascà G, Gesualdo L, Gigante M, Lupattelli G, Montali A, Pizzolitto S, Rabbone I, Rolleri M, Ruotolo G, Sampietro T, Sessa A, Vaudo G, Cantafora A, Veglia F, Calandra S, Bertolini S, Franceschini G: The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: A comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arterioscler Thromb Vasc Biol 25: 1972–1978, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT: Lecithin: cholesterol acyltransferase—from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 16: 163–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogo A, Kashgarian M: Lecithin-cholesterol acyltransferase deficiency. In: Diagnostic Atlas of Renal Pathology, Philadelphia, PA, Elsevier Saunders, 2005, pp 171–174 [Google Scholar]
- 10.Simonelli S, Gianazza E, Mombelli G, Bondioli A, Ferraro G, Penco S, Sirtori CR, Franceschini G, Calabresi L: Severe high-density lipoprotein deficiency associated with autoantibodies against lecithin:cholesterol acyltransferase in non-Hodgkin lymphoma. Arch Intern Med 172: 179–181, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Sato H, Ito S, Akiu N: A case of nephrotic syndrome with lecithin cholesterol acyltransferase deficiency that was improved by steroid therapy (in Japanese). Ther Res 23: 1329–1330, 2002 [Google Scholar]
- 12.Appel GB, Radhakrishnan J, D'Agati V: Secondary glomerular disease. In: The Kidney, edited by Brenner BM, 6th Ed., Philadelphia, WB Saunders, 2000, pp 1350–1448 [Google Scholar]
- 13.Steinberg D: Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat Med 8: 1211–1217, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Tall AR, Wang N: Tangier disease as a test of the reverse cholesterol transport hypothesis. J Clin Invest 106: 1205–1207, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanigawa H, Billheimer JT, Tohyama J, Fuki IV, Ng DS, Rothblat GH, Rader DJ: Lecithin: cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation 120: 160–169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prunotto M, Carnevali ML, Candiano G, Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto A, Santucci L, Mattei S, Gatti R, Scolari F, Kador P, Allegri L, Ghiggeri GM: Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 21: 507–519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panescu V, Grignon Y, Hestin D, Rostoker G, Frimat L, Renoult E, Gamberoni J, Grignon G, Kessler M: Recurrence of lecithin cholesterol acyltransferase deficiency after kidney transplantation. Nephrol Dial Transplant 12: 2430–2432, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Brady RO: Enzyme replacement for lysosomal diseases. Annu Rev Med 57: 283–296, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rousset X, Vaisman B, Auerbach B, Krause BR, Homan R, Stonik J, Csako G, Shamburek R, Remaley AT: Effect of recombinant human lecithin cholesterol acyltransferase infusion on lipoprotein metabolism in mice. J Pharmacol Exp Ther 335: 140–148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroda M, Bujo H, Aso M, Saito Y: Adipocytes as a vehicle for ex vivo gene therapy: Novel replacement therapy for diabetes and other metabolic diseases. J Diabetes Investig 2: 333–340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]