Abstract
Risk alleles at genome loci containing phospholipase A2 receptor 1 (PLA2R1) and HLA-DQA1 closely associate with idiopathic membranous nephropathy (IMN) in the European population, but it is unknown whether a similar association exists in the Chinese population and whether high-risk alleles promote the development of anti-PLA2R antibodies. Here, we genotyped 2132 Chinese individuals, including 1112 patients with IMN and 1020 healthy controls, for three single nucleotide polymorphisms (SNPs) within PLA2R1 and three SNPs within HLA genes. We also selected 71 patients, with varying genotypes, to assess for circulating anti-PLA2R antibody and for PLA2R expression in glomeruli. Three SNPs within PLA2R1 and one SNP within HLA-DQA1 strongly associated with IMN, and we noted gene–gene interactions involving these SNPs. Furthermore, these risk alleles strongly associated with the presence of anti-PLA2R antibodies and glomerular PLA2R expression. Among individuals who carried risk alleles for both genes, 73% had anti-PLA2R antibodies and 75% expressed PLA2R in glomeruli. In contrast, among individuals who carried protective genotypes of both genes, none had anti-PLA2R antibodies and glomerular expression of PLA2R was weak or absent. In conclusion, the interaction between PLA2R1 and HLA-DQA1 risk alleles associates with the development of IMN in the Chinese population. Individuals carrying risk alleles are predisposed to the generation of circulating anti-PLA2R autoantibodies, which may contribute to the development of IMN.
Idiopathic membranous nephropathy (IMN), characterized by subepithelial glomerular immune deposits and glomerular membrane thickening, is one of the most common reasons for adult nephrotic syndrome.1–3 It is now recognized that IMN is an organ-specific autoimmune disease. To date, two major antigens have been identified in human membranous nephropathy. The first is neutral endopeptidase, the alloantigen involved in neonatal cases of membranous nephropathy that occur in newborn infants from neutral endopeptidase-deficient mothers.4 The second is the M-type phospholipase A2 receptor (PLA2R), the first autoantigen identified in adult IMN patients.5 PLA2R is a type I transmembrane protein expressed on glomerular podocytes, forming subepithelial deposits in situ through binding of circulating anti-PLA2R autoantibodies. Another key finding in membranous nephropathy comes from a genome-wide association study (GWAS) using European white ancestry. Stanescu et al. identified risk alleles at two genome loci containing PLA2R1 and HLA-DQA1, which both contribute to the risk of membranous nephropathy.6 These results strongly support an interaction between HLA-DQ and PLA2R in the pathogenesis of membranous nephropathy. It has been postulated that certain genetic variants of PLA2R1 yield peptides with strong affinity for specific HLA-DQA1 variants that subsequently confer a predisposition to anti-PLA2R autoantibody generation. Although there are studies reporting an association between PLA2R1 gene polymorphisms and IMN in Asian populations from Korea and Taiwan,7,8 no study thus far has evaluated whether the gene interaction between PLA2R1 and HLA-DQA1 contributes to production of anti-PLA2R autoantibodies and development of IMN in an independent cohort.
In China, IMN accounts for >25% of nephrotic syndrome and 6.7% of all biopsy glomerular disease,9 which is not as prevalent as that reported in Western countries.9–14 In this study, we aim to evaluate the association between these risk alleles and the development of IMN in a large Chinese cohort with >2000 participants and to further explore their roles in the generation of anti-PLA2R antibodies and expression of PLA2R in glomeruli.
Results
Study Participants
The characteristics of IMN patients and controls are listed in Supplemental Table 1. Overall, IMN was predominant in men (men/women = 1.26:1) with a mean age of 49±13 years. Healthy controls comprised 527 men and 493 women (men/women = 1.07:1) with a mean age of 35±10 years. There was no significant difference in sex distribution (P=0.06), but the healthy controls were much younger than the IMN patients (P<0.001). Deviation from the Hardy–Weinberg equilibrium was not observed for any of the SNPs in the patients or controls.
Association of Gene Polymorphisms with IMN
Table 1 summarized the allele frequency in IMN patients and controls. Three SNPs (rs35771982, rs3749117, and rs4664308) within PLA2R1 were strongly associated with IMN (P value for association between 1.14×10−29 and 1.34×10−28 with odds ratios [OR] of 2.32 and 2.36). As for the HLA gene, one SNP (rs2187668) in HLA-DQA1 showed a strong association with IMN (OR=2.42; P=6.66×10−14, whereas the other two SNPs, rs11244 in HLA-DOB and rs2301271 in HLA-DQB2, which were identified in the Caucasian population, were not associated with IMN. The genotype distribution was shown in Supplemental Table 2.
Table 1.
PLA2R1 and HLA-DQA1 polymorphisms in patients with IMN and healthy controls in a Chinese population
| Variant | Gene | Position | Allele | Controls (n=1020) | IMN Patients (n=1112) | OR (95% CI)a | P Value | Adjusted P Valueb |
| rs35771982 | PLA2R1 | Exon 5 | C | 615 (30.1) | 344 (15.5) | 2.36 | 1.90×10−30 | 1.14×10−29 |
| G | 1425 (69.9) | 1880 (74.5) | (2.03 to 2.74) | |||||
| rs3749117 | PLA2R1 | Exon 5 | C | 611 (30.0) | 346 (15.6) | 2.32 | 2.23×10−29 | 1.34×10−28 |
| T | 1429 (70.0) | 1878 (84.4) | (2.00 to 2.69) | |||||
| rs4664308 | PLA2R1 | Intron | A | 1427 (70.0) | 1880 (84.5) | 2.35 | 4.17×10−30 | 2.50×10−29 |
| G | 613 (30.0) | 344 (15.5) | (2.02 to 2.73) | |||||
| rs2187668 | HLA-DQA1 | Intron | A | 110 (5.4) | 270 (12.1) | 2.42 | 1.11×10−14 | 6.66×10−14 |
| G | 1930 (94.6) | 1954 (87.8) | (1.93 to 3.05) | |||||
| rs11244 | HLA-DOB | 3′ untranslated region variant | C | 1506 (73.9) | 1641 (73.8) | 1.001 | 0.98 | — |
| T | 534 (26.1) | 583 (26.2) | (0.87 to 1.15) | |||||
| rs2301271 | HLA-DQB2 | Intron | C | 1719 (84.3) | 1851 (83.2) | 1.08 | 0.36 | — |
| T | 321(15.7) | 373 (16.8) | (0.92 to 1.27) |
OR modeled for minor allele.
Bonferroni correction was used when the adjusted P value was reported.
The linkage disequilibrium (LD) analysis revealed that rs35771982, rs3749117, and rs4664308 are in the tight region of LD block in healthy control individuals. The frequency of haplotype GTA was significantly higher in patients (81.9% versus 66.3%; P=1.46×10−16; OR, 2.54; 95% confidence interval [95% CI], 2.03 to 3.19) than that in controls.
Risk genotypes of PLA2R1 were strongly associated with pathologic phenotype (P<0.001) even after adjusting for sex, age, duration of disease, BP, and treatment (Table 2 and Supplemental Table 3). No association was observed between HLA-DQA1 and IMN pathologic phenotype. No association between clinical characteristics and risk alleles of PLA2R1 and HLA-DQA1 was observed regarding baseline proteinuria, kidney function, and BP.
Table 2.
Clinical and pathologic characteristics of IMN in subgroup by genotype of PLA2R1 and HLA-DQA1
| Clinical Parameter | rs4664308 (PLA2R1) | rs2187668 (HLA-DQA1) | |||||
| AA | GA | GG | P Value | AA+GA | GG | P Value | |
| Patients (n) | 803 | 274 | 35 | — | 259 | 853 | — |
| Sex (men/women) | 480/323 | 128/146 | 14/21 | <0.001 | 147/112 | 474/379 | 0.74 |
| Age (yr) | 50±13 | 48±13 | 49±13 | 0.15 | 49±13 | 50±13 | 0.75 |
| Systolic BP (mmHg) | 129±18 | 127±19 | 127±20 | 0.15 | 130±18 | 128±18 | 0.25 |
| Diastolic BP (mmHg) | 81±11 | 80±12 | 81±12 | 0.76 | 82±11 | 80±12 | 0.05 |
| Proteinuria (g/24 h) | 4.50 (2.79–7.07) | 4.60 (3.11–6.76) | 4.56 (2.15–7.94) | 0.82 | 4.62 (3.06–7.06) | 4.57 (2.81–6.99) | 0.97 |
| Serum albumin (g/L) | 26.32±6.85 | 25.79±7.48 | 25.93±8.57 | 0.57 | 26.16±7.06 | 26.19±7.07 | 0.67 |
| Serum creatinine (μmol/L) | 72.00 (61.00–87.00) | 68.00 (57.50–82.00) | 69.10 (57.20–84.00) | 0.04 | 72.00 (60.00–88.00) | 71.09 (60.00–85.00) | 0.59 |
| Total triglyceride (mmol/L) | 2.93±2.84 | 2.66±1.96 | 2.90±2.25 | 0.37 | 2.82±2.38 | 2.88±2.25 | 0.90 |
| Total cholesterol (mmol/L) | 7.79±2.63 | 8.14±3.13 | 8.57±4.26 | 0.11 | 7.81±2.80 | 7.93±2.83 | 0.85 |
| Estimated GFR (ml/min per 1.73 m2) | 105.01±38.37 | 108.93±38.05 | 103.18±42.98 | 0.34 | 104.59±37.59 | 106.30±38.72 | 0.13 |
| Pathology (%)a | <0.001 | 0.54 | |||||
| I-MN | 338 (42.1) | 145 (52.9) | 28 (80) | 111 (42.8) | 400 (46.9) | ||
| II-MN | 352 (43.8) | 99 (36.1) | 7 (20) | 111 (42.8) | 347 (40.7) | ||
| III-MN | 113 (14.1) | 30 (10.9) | 0 (0) | 37 (14.2) | 106 (12.4) | ||
Data are presented as mean ± SEM or median (interquartile range) unless otherwise indicated. GFR was estimated using the Modified Diet in Renal Disease equation for the Chinese population.19 Neither proteinuria nor serum creatinine conforms to normal distribution, so we performed a nonparametric test. Other parameters used one-way ANOVA or t tests for continuous variables or the chi-squared test for dichotomous variables.
A multivariable analysis adjusted for age, sex, duration of disease, BP (systolic BP and diastolic BP), and treatment was used to evaluate the association of PLA2R and HLA-DQA1 risk alleles with the pathology stage.
Genetic Interaction Analyses between PLA2R1 and HLA-DQA1
Logistic regression showed rs4664308 best explaining the signal in this highly linked region (Supplemental Table 4). Thus, the following gene–gene analyses were performed between rs4664308 (PLA2R1) and rs2187668 (HLA-DQA1). Multiplicative interaction analysis indicated that HLA-DQA1 had an interactive effect with PLA2R1 in the Chinese population with IMN (P=5.85×10−3). For additive interaction, as shown in Figure 1, the ORs of risk genotype were 1.54 (95% CI, 0.47 to 5.09) in HLA-DQA1 and 3.40 (95% CI, 2.19 to 5.29) in PLA2R1. The combination of risk genotypes of two genes (rs4664308/rs2187668: AA/GA+AA) conferred an 11.13-fold higher risk (95% CI, 6.47 to 19.15) for the development of IMN compared with either protective genotype (GG/GG) at both loci (Supplemental Table 6). Gene–gene interaction analyzed by the chi-squared test and logistic regression, as well as additive and multiplicative interaction, could be observed between PLA2R1 and HLA-DQA1 (Supplemental Tables 4–8).
Figure 1.
Analysis of gene–gene interaction: ORs for IMN according to SNP and genotype combinations. The combination of the AA or GA (rs2187668 in HLA-DQA1)/AA (rs4664308 in PLA2R1) genotype confers an 11.13-fold risk of IMN reference to GG/GG genotype (P=6.03×10−21). The AA or GA/GA genotype combination confers a 2.89-fold risk of IMN reference to GG/GG (P=1.24×10−4). The GG/AA genotype combination confers a 3.40-fold risk of IMN reference to GG/GG (P=1.17×10−8). Other ORs for genotype combination are not significant.
Association between PLA2R1/HLADQA1 Genotype and Anti-PLA2R Antibody
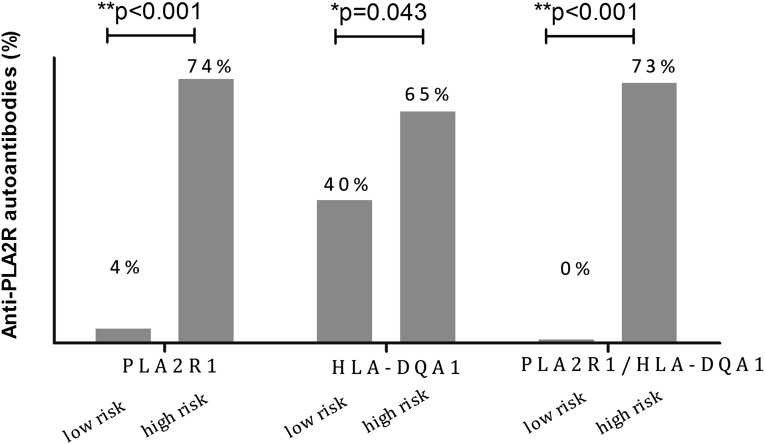
Seventy-one patients were selected according to genotype for the detection of circulating anti-PLA2R antibodies (Table 3). We divided them into two groups based on genotypes of PLA2R1, including 24 patients with protective genotypes of CC in rs35771982, CC in rs3749117, and GG in rs4664308 (PLA2R1 low-risk genotype; n=24) and 47 with high-risk genotypes of GG in rs35771982, TT in rs3749117, and AA in rs4664308 (PLA2R1 high-risk genotype; n=47), or based on the HLA-DQA1 genotype, including the HLA-DQA1 low-risk genotype (GG in rs2187668 of HLA-DQA1; n=40) and the HLA-DQA1 high-risk genotype (AA or GA in rs2187668; n=31). As shown in Figure 2, we detected anti-PLA2R antibodies in serum of only 1 of 24 patients (4%) with the PLA2R1 low-risk genotype and in 35 of 47 patients (74%) in the high-risk genotype with anti-PLA2R autoantibodies (P<0.001). In addition, 16 of 40 patients (40%) with the HLA-DQA1 low-risk genotype and 20 of 31 patients (65%) with the HLA-DQA1 high-risk genotype also had positive results (P=0.04).
Table 3.
Baseline characteristics of 71 IMN patients for detecting circulating anti-PLA2R antibody and PLA2R expression in different genotypes
| Characteristic | PLA2R Low Risk/HLA Low Risk | PLA2R Low Risk/HLA High Risk | PLA2R High Risk/HLA Low Risk | PLA2R High Risk/HLA High Risk | P Value |
| Patients (n) | 19 | 5 | 21 | 26 | |
| Age (yr) | 44±11 | 54±7 | 52±14 | 48±9 | 0.12 |
| Sex (men/women) | 7/12 | 3/2 | 9/12 | 8/18 | 0.62 |
| Systolic BP (mmHg) | 126±23 | 129±11 | 127±14 | 127±18 | 0.99 |
| Diastolic BP (mmHg) | 79±12 | 80±13 | 80±9 | 81±14 | 0.97 |
| Proteinuria (g/24 h) | 4.04 (0.58, 13.15) | 11.07 (2.81, 19.33) | 4.56 (0.69, 16.49) | 4.09 (0.68, 8.12) | 0.85 |
| Serum albumin (g/L) | 28.07±9.05 | 25.22±8.20 | 25.47±5.33 | 26.27±6.74 | 0.70 |
| Serum creatinine (μmol/L) | 76.46±26.99 | 87.04±28.13 | 66.86±19.02 | 66.82±18.83 | 0.16 |
| Total cholesterol (mmol/L) | 8.65±4.98 | 8.74±2.81 | 7.93±2.36 | 7.12±2.08 | 0.49 |
| Total triglycerides (mmol/L) | 3.34±2.78 | 2.52±1.06 | 3.08±1.62 | 2.20±1.03 | 0.23 |
| Estimated GFR (ml/min per 1.73 m2) | 104.32±37.08 | 89.10±35.95 | 118.81±39.30 | 115.94±26.75 | 0.25 |
| Pathology stage (I/II,III) | 16/3 | 5/0 | 10/11 | 10/16 | <0.001 |
| No immunosuppressive treatment (%) | 14 (74) | 2 (40) | 15 (71) | 19 (73) | 0.86 |
| Anti-PLA2R (%) | 0 (0) | 1 (20) | 16 (76) | 19 (73) | <0.001 |
| Anti-PLA2R in patients without immunosuppressive therapy (%) | 0 (0, 0/14) | 0 (0, 0/2) | 11 (73, 11/15) | 17 (89, 17/19) | <0.001 |
| PLA2R staining in glomeruli (%) | 0/19 (0) | 2/5 (40) | 16/21 (76) | 18/24 (75) | <0.001 |
Data are presented as mean ± SEM or median (interquartile range) unless otherwise indicated. PLA2R1 low risk, individuals with CC in rs35771982, CC in rs3749117, GG in rs4664308 within PLA2R1; PLA2R1 high risk, individuals with GG in rs35771982, TT in rs3749117, AA in rs4664308 within PLA2R1; HLA-DQA1 low risk, individuals with GG in rs2187668 within HLA-DQA1; HLA-DQA1 high risk, individuals with AA or GA in rs2187668 within HLA-DQA1.
Figure 2.
Distribution of circulating anti-PLA2R antibody in different genotypes. PLA2R1 low-risk indicates individuals with CC in rs35771982, CC in rs3749117, and GG in rs4664308 within PLA2R1; 4% (1 of 24) of patients in this group are positive for anti-PLA2R antibody. PLA2R1 high-risk indicates individuals with GG in rs35771982, TT in rs3749117, and AA in rs4664308 within PLA2R1; 76% of patients in this group are positive for antibody in this group. HLA-DQA1 low-risk indicates individuals with GG in rs2187668 within HLA-DQA1; 40% of patients in this group are positive for antibodies. HLA-DQA1 high-risk indicates individuals with AA or GA in rs2187668 within HLA-DQA1; 65% of patients in this group are positive for anti-PLA2R antibodies. No patients in the PLA2R1/HLA-DQA1 low-risk group carrying double protective genotypes are positive for anti-PLA2R antibodies; 73% of patients in the PLA2R1/HLA-DQA1 high-risk group carrying double risk genotypes are positive for anti-PLA2R antibodies.
Importantly, we did not detect anti-PLA2R antibodies in any of the 19 patients (0%) with both PLA2R1 and HLA-DQA1 low-risk genotypes. We detected anti-PLA2R antibodies in 1 of the 5 patients (20%) with the HLA-DQA1 high-risk genotype and the PLA2R1 low-risk genotype. In addition, we detected autoantibodies in 16 of the 21 patients (76%) with the PLA2R1 high-risk genotype and the HLA-DQA1 low-risk genotype and 19 of the 26 (73%) patients with both high-risk genotypes (P<0.001). This trend was even more obvious among those not receiving immunosuppressive therapy (n=50), with proportions of 0%, 0%, 73%, and 89% respectively (P<0.001) (Table 3).
Association between PLA2R1 Genotype and PLA2R Expression
We also evaluated PLA2R expression in glomeruli among patients with different genotypes of PLA2R1 and HLA-DQA1 (Figure 3). Immunohistochemical analysis for PLA2R had low expression (faint positive) in kidneys of normal individuals and patients with other glomerular disease, including minimal change disease, IgA nephropathy, or secondary membranous nephropathy (hepatitis virus type B associated nephropathy). Overall, the expression of PLA2R on podocytes highly correlated with circulating anti-PLA2R antibodies in IMN.
Figure 3.
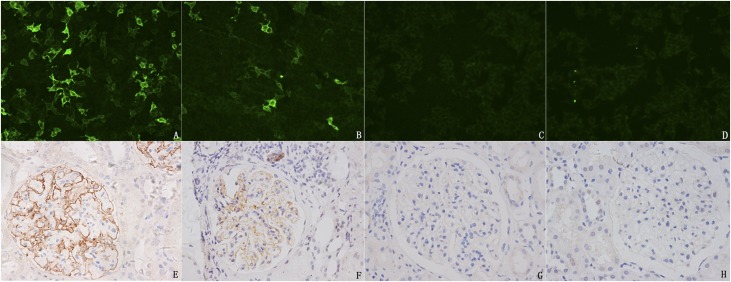
Specification of the diagnostic antibody used for PLA2R detection and PLA2R expression in glomeruli of different kidney tissues. Detection of anti-PLA2R antibodies with indirect immunofluoresent assay (IIFA) Mosaic immunofluorescence. Circulating anti-PLA2R antibody positivity in IMN patients (A and B) and two healthy individuals (C and D). For PLA2R staining in the glomeruli, 36 patients present linear or granular, diffuse PLA2R expression on glomeruli (E and F), and 33 patients present traces of an extremely faint positive staining (G). (H) Secondary membranous nephropathy (hepatitis B virus–associated glomerular nephritis) also shows faint positive staining of PLA2R.
Only 2 of the 24 individuals (8%) with the PLA2R1 low-risk genotype presented with enhanced PLA2R expression in the glomeruli, whereas 34 of the 45 patients (76%) with the high-risk genotype presented enhanced PLA2R expression (P<0.001). Similarly, 16 of the 40 patients (40%) with the HLA-DQA1 low-risk genotype showed enhanced PLA2R expression, and 20 of 29 patients (69%) with the high-risk genotype had enhanced PLA2R expression (P=0.02). None of the 19 patients with both protective genotypes of the PLA2R1/HLA-DQA1 genotype had enhanced expression, whereas 18 of 24 patients (75%) with both risk genotypes showed high expression in glomeruli (P<0.001) (Table 3).
Discussion
In this large Chinese population with >2000 participants, we confirmed that the risk alleles of PLA2R1 and HLA-DQA1 are closely associated with the susceptibility of IMN, and patients with both risk alleles confer >11 times the risk of developing membranous nephropathy. Importantly, our results for the first time shows that patients with these risk alleles, especially with PLA2R1 risk alleles, are closely associated with circulating anti-PLA2R antibodies in serum as well as the expression of PLA2R in glomeruli. None of the patients with protective alleles in both PLA2R1 and HLA genes circulating anti-PLA2R antibodies or enhanced expression of PLA2R were found in podocytes; however, we could detect autoantibodies and enhanced PLA2R expression in the kidney for >70% of persons homozygous for the risk alleles of both variants. Our study supports the hypothesis that coexistence of risk alleles in PLA2R1 and HLA in the same person may circumvent the tightly regulated adaptive immune system and allow for the development of membranous nephropathy. These data expand our understanding of the pathogenesis of IMN.
Stanescu and colleagues reported that sequence variance within the HLA locus and PLA2R1 were associated with the susceptibility of IMN in the Caucasian population. A stronger association with HLA than with PLA2R1 was observed in persons of white ancestry.6 In this study, we found that the frequency of risk alleles in HLA-DQA1 was much less in the Chinese population than in the Caucasian population (12.1% versus 39.2%). It was reported that membranous nephropathy was much less common in the Chinese population than in the white population.9–14 Although both HLA-DQA1 and PLA2R1 are associated with the development of membranous nephropathy in the Chinese population, PLA2R1 gene variance has shown a much stronger association with the development of IMN as well as the production of anti-PLA2R autoantibodies. In this study, we explored other SNPs in HLA-DOB and HLA-DQB2, which showed a relatively strong signal in European GWAS data; however, only HLA-DQA1 was identified as being associated with membranous nephropathy in the Chinese Han population. These data suggest a racial difference in the genetic contribution of membranous nephropathy. Interestingly, we note that the risk alleles of PLA2R1 are so commonly found in the general population that this suggests that other risk factors should jointly contribute to the development of membranous nephropathy. Indeed, as a complex trait determined by genetic and environmental factors, these risk alleles themselves cannot determine the disease development. We also speculate that these risk alleles might have other roles in human evolution.
Seminal studies have identified several autologous antigens that are targets of an autoantibody response in membranous nephropathy. The leading candidate autoantigen is M-type PLA2R in podocytes. Autoantibodies to PLA2R, usually of the IgG4 subclass, are found in 70%–80% of patients with primary membranous nephropathy.5,15 The level of autoantibody to PLA2R correlates with the severity of clinical manifestation and progression of this disease.16 In this study, we found that the production of autoantibodies is closely associated with sequence variance of PLA2R1 and HLA-DQA1, especially the identified SNPs in PLA2R molecules. We could detect anti-PLA2R autoantibodies in >70% patients with risk alleles of PLA2R1 and HLA-DQA1, whereas none of the patients with protective alleles of both genes could be detected with anti-PLA2R antibodies. How would this occur? It is postulated that certain sequence variation in PLA2R1 could control the pattern of antigen-peptide processing through conformational change that is recognized by HLA-DQA1 and facilitate the anti-PLA2R production. In this study, we also found that the PLA2R antigen was detected strongly in glomeruli in patients carrying risk alleles, whereas it was weakly detected in participants with the protective genotype. This finding is consistent with a greater tendency to detect circulating anti-PLA2R antibodies in the serum of patients with high-risk alleles.
To our knowledge, this study represents the largest genetic study in membranous nephropathy that confirms the association between PLA2R1/HLA-DQA1 and disease development. Furthermore, we also identified the association between PLA2R expression, anti-PLA2R antibody generation, and these risk alleles. These findings provide the functional clues, at least the identified SNPs in PLA2R1, for further investigation of the pathogenic contribution in IMN. The major limitation was that this was a validation study based on the reported candidate SNPs of the PLA2R1/HLA-DQA1 gene, which might have different predominance in different populations. More studies on PLA2R1/HLA-DQA1 gene variation and its association with the generation of anti-PLA2R antibodies are needed.
In conclusion, this study has firmly confirmed that PLA2R1/HLA-DQA1 is strongly associated with the susceptibility of IMN in the Chinese population. Furthermore, individuals carrying risk alleles confer a predisposition to anti-PLA2R autoantibody generation and PLA2R expression, which may contribute to the development of membranous nephropathy.
Concise Methods
Patients
A total of 2132 participants, comprising 1112 patients with biopsy-diagnosed IMN and 1020 healthy controls, were enrolled in our study. All of the participants were of Han ethnicity. IMN diagnosis was established by kidney biopsy in patients who lacked features suggestive of secondary causes, such as hepatitis B virus infection, SLE, cancer, or drug.
This study was approved by the Ethics Committee of Peking University and informed written consent was obtained from all participants.
SNP Selection and Genotyping
A total of six SNPs—including rs35771982 and rs3749117 located in exon 5, rs4664308 located in intron 1 of PLA2R1, and rs2187668 in HLA-DQA1, rs11244 in HLA-DOB, and rs2301271 in HLA-DQB2—were selected to validate their association with susceptibility to IMN. Among them, rs4664308 and rs2187668 are reported to be strongly associated with IMN in the Caucasian population of a GWAS study.6 Two SNPs correlated with rs4664308 (r2>0.80), encoding nonsynonymous amino acid (rs3749117, M292V and rs35771982, H300D) in the extracellular C-type lectin domain 1 of PLA2R1, were also selected in this study. A replication study also included two additional HLA SNPs that showed an association with IMN in Caucasians.
Genomic DNA was isolated from whole blood using a modified salt extraction technique. TaqMan allele discrimination assays (Applied Biosystems, Foster City, CA) were used to determine the genotypes according to the manufacturer’s instructions. The variants were detected using an ABI Prism 7500 Sequence Detection System (Applied Biosystems).
PLA2R Antibody and Renal Biopsy Staining Detection
Serum samples from 71 patients with different genotypes were collected 1 day before renal biopsy. Circulating PLA2R autoantibody in serum was assessed by direct immunofluorescence assay with the use of HEK293 cells that were transiently transfected with full-length complementary DNA encoding a PLA2R1 isoform (FA1254-1005-50; EUROIMMUN AG, Lübeck, Germany). The detection was performed on an immunofluoresent assay (IFA) Mosaic slide following the standard instructions as previously reported.17 Antibody positivity was defined as positive staining at serum dilutions ≥1/10. Negativity of anti-PLA2R1 was defined as absence of detectable antibodies at 1/10 dilution.
Renal specimens of the 71 patients were immunohistochemically stained with a rabbit polyclonal anti-PLA2R-AB (Atlas Antibodies AB, Stockholm, Sweden). Staining was performed on formalin-fixed paraffin-embedded renal biopsies. For PLA2R staining, 5-μm-thick sections were deparaffinized, hydrated, and subjected to microwave thermal treatment for 11 minutes for antigen retrieval. The detection system (Dako EnVision HRP; Dako A/S, Copenhagen, Denmark) was an avidin-free two-step indirect method with anti-rabbit (as well as anti-mouse) antibodies produced in goat conjugated with horseradish peroxidase as secondary antibodies. Sections were examined by light microscopy. As negative controls, primary antibodies were replaced by PBS. Enhanced positivity of glomerular PLA2R expression was defined as linear or granular, diffuse PLA2R staining on glomeruli as previously described.18
Statistical Analyses
The genotype frequencies of SNP were tested separately for Hardy–Weinberg equilibrium in patients and controls. Associations between disease and SNP were analyzed by chi-squared tests or by logistic regression analysis. LD was tested using Haploview (Broad Institute, Cambridge, MA). Statistical power was estimated using PS Power and Sample Size Calculation software (Vanderbilt University, Nashville, TN). Gene interaction analyses were conducted by logistic regression and the chi-squared test. Statistical analysis was performed using the t test, nonparametric Mann–Whitney U test, and one-way ANOVA for comparison of continuous variables, as well as the chi-squared test or logistic analysis for categorical variables. Statistical analysis was performed with the SPSS 16.0 software package (SPSS Inc, Chicago, IL). A two-tailed P value <0.05 was considered statistically significant. The Bonferroni correction was applied to adjust P values for multiple comparisons.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the Major State Basic Research Development Program of China (973 program; 2012CB517700 to H.Z.), the National Natural Science Foundation of China (81270795 to J.L.), and the Natural Science Fund of China to the Innovation Research Group (81021004 to M.Z.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Can Genetics Risk-Stratify Patients with Membranous Nephropathy?,” on pages 1190–1192.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012080771/-/DCSupplemental.
References
- 1.Ronco P, Debiec H: Advances in membranous nephropathy: Success stories of a long journey. Clin Exp Pharmacol Physiol 38: 460–466, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ: The pathogenesis of membranous nephropathy: Evolution and revolution. Curr Opin Nephrol Hypertens 21: 235–242, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Couser WG, Steinmuller DR, Stilmant MM, Salant DJ, Lowenstein LM: Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest 62: 1275–1287, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Chin HJ, Na KY, Kim S, Oh J, Chung W, Noh JW, Lee YK, Cho JT, Lee EK, Chae DW; Progressive Renal Disease and Medical Informatics and Genomics Research (PREMIER) members: Single nucleotide polymorphisms in the phospholipase A2 receptor gene are associated with genetic susceptibility to idiopathic membranous nephropathy. Nephron Clin Pract 117: c253–c258, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Liu YH, Chen CH, Chen SY, Lin YJ, Liao WL, Tsai CH, Wan L, Tsai FJ: Association of phospholipase A2 receptor 1 polymorphisms with idiopathic membranous nephropathy in Chinese patients in Taiwan. J Biomed Sci 17: 81, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou FD, Chen M: The renal histopathological spectrum of patients with nephrotic syndrome: An analysis of 1523 patients in a single Chinese center [published online ahead of print August 3, 2011]. Nephrol Dial Transplant doi:10.1093/ndt/gfr401 [DOI] [PubMed] [Google Scholar]
- 10.Zeng CH, Chen HM, Wang RS, Chen Y, Zhang SH, Liu L, Li LS, Liu ZH: Etiology and clinical characteristics of membranous nephropathy in Chinese patients. Am J Kidney Dis 52: 691–698, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Cahen R, Francois B, Trolliet P, Gilly J, Parchoux B: Aetiology of membranous glomerulonephritis: a prospective study of 82 adult patients. Nephrol Dial Transplant 4: 172–180, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Gluck MC, Gallo G, Lowenstein J, Baldwin DS: Membranous glomerulonephritis. Evolution of clinical and pathologic features. Ann Intern Med 78: 1–12, 1973 [DOI] [PubMed] [Google Scholar]
- 13.Honkanen E: Survival in idiopathic membranous glomerulonephritis. Clin Nephrol 25: 122–128, 1986 [PubMed] [Google Scholar]
- 14. Hanko JB, Mullan RN, O'Rourke DM, McNamee PT, Maxwell AP, Courtney AE: The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant 24: 3050–3054, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Qin W, Beck LH, Jr, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z: Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22: 1137–1143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA: Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 82: 797–804, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.