Abstract
Acute lung injury represents the result of multiple pathways initiated by local or systemic insults and is characterized by profound vascular permeability, pulmonary edema, and life-threatening respiratory failure. Permeability-reducing therapies are of potential clinical utility but are currently unavailable. We hypothesized that polyethylene glycol (PEG) compounds, inert and non-toxic polymers that serve as a surrogate mucin lining in intestinal epithelium, may attenuate agonist-mediated lung endothelial cell (EC) barrier dysfunction. High molecular weight PEG (PEG15-20) produced rapid, dose-dependent increases in transendothelial electrical resistance (TER) in human lung endothelium cultured on gold microelectrodes, reflecting increased paracellular integrity. The maximal effective concentration of 8% PEG induced a sustained 125% increase in TER (40 hrs), results similar to barrier-enhancing agonists such as sphingosine 1-phosphate (40% increase in TER). Maximal PEG barrier enhancement was achieved at 45–60 min and PEG effectively reversed both thrombin- and LPS-induced EC barrier dysfunction. Consistent with the increase in TER, immunofluorescent studies demonstrated that PEG produced significant cytoskeletal rearrangement with formation of well-defined cortical actin rings and lamellipodia containing the actin-binding proteins, cortactin and MLCK, known participants in cell-matrix and cell-cell junctional adhesion. Finally, PEG challenge induced rapid alterations in levels of MAP kinase and MLC phosphorylation. In summary, PEG joins a number of EC barrier-regulatory agents which rapidly activate barrier-enhancing signal transduction pathways which target the cytoskeleton and provides a potential therapeutic strategy in inflammatory lung injury.
Keywords: PEG, LPS, thrombin, endothelium, barrier function
INTRODUCTION
The pulmonary endothelium is a functionally dynamic tissue that serves as a semi-permeable barrier between blood and the interstitium and airspaces of the lung. Under normal conditions, the endothelial cell (EC) monolayer provides a non-thrombogenic barrier limiting the access of luminal cells to the underlying tissues. In response to pro-inflammatory stimuli (thrombin, TNF-α, reactive oxygen species, and interleukin-1β), EC actively contract to form paracellular gaps which increase vascular permeability and promotes fluid and leukocytes to access the tissue interstitium to produce edema. Oxygen radicals and digestive enzymes released by PMNs serve to eliminate the source of inflammation and initiate resolution of the inflammatory process with PMN apoptosis and phagocytosis of damaged cells by macrophages. Timely release and removal of chemical mediators orchestrating cellular responses are of extreme importance for the onset and resolution of the inflammatory process.
Pathophysiological perturbation of lung vascular integrity, commonly observed in the inflammatory syndrome evoked by sepsis, leads to massive pulmonary edema that hinders gas exchange and results in respiratory failure. Sepsis is the leading cause of acute lung injury and death in ICU patients despite supportive care (antibiotic treatment, surgical drainage of fluids, mechanical ventilation). Therefore, therapeutic strategies that target EC integrity and lead to reversal of increased vascular permeability would have obvious clinical impact utility.
The regulatory mechanisms involved in maintenance of the EC barrier are poorly understood, however, we previously reported that sphingosine 1-phosphate (S1P), a potent phospholipid angiogenic factor released from activated platelets (Schaphorst et al., 2003), produces significant endothelial barrier enhancement (Garcia et al., 2001). Through ligation of Gi-coupled S1P1 receptor (previously named endothelial differentiation gene-1 or EDG-1), S1P initiates a series of downstream events including Rac activation, cortactin translocation, peripheral myosin light chain (MLC) phosphorylation, focal adhesion and VE-cadherin rearrangement, that result in enhancement of the EC cortical actin ring, improved cell-cell and cell-matrix interactions, and increased endothelial barrier function in vitro (Dudek et al., 2002; Dudek and Garcia, 2001; Dudek et al., 2004; Garcia et al., 2001; Shikata et al., 2003a; Shikata et al., 2003b). In addition, we have recently demonstrated the in vivo capacity for S1P to attenuate endotoxin-induced murine and canine models of sepsis and ALI (McVerry and Garcia, 2005; McVerry et al., 2004; Peng et al., 2004; Peng et al., 2003), supporting the potential therapeutic utility of S1P in edema states. We have also identified additional novel potential permeability-reducing agents including hepatocyte growth factor (Liu et al., 2002; Singleton et al., 2007), adenosine triphosphate (Jacobson et al., 2006; Kolosova et al., 2005), hylauronan (Singleton et al., 2006), simvastatin (Jacobson et al., 2005; Jacobson et al., 2004), and activated protein C (Finigan et al., 2005), many which share common signaling pathways with S1P in the evolution of EC barrier protection.
Polyethylene glycol (PEG) compounds are water-soluble, non-toxic polymers of differing molecular weight, different physical properties (e.g. viscosity) and application use. For example, PEG with added electrolytes is the basis of laxatives, sold under the brand name GoLYTELY, used for bowel preparation and drug overdoses. The non-toxic nature of PEG allows drugs to be anchored to PEG to reduce toxicity and provide longer dosing intervals through a process called PEGylation. A recent application utilized conjugation of nitric oxide molecule to PEG in order to anchor the compound to the cell membrane and controlling the level of NO which reduces ischemia/reperfusion injury in endothelial cells (Bertuglia et al., 2006). Utilization of PEG alone as a drug is less frequent, however, PEG potentially exhibits clinically beneficial properties aside from membrane anchoring of molecules and drugs or providing molecular barrier. For example, PEG has been used to provide a molecular barrier on the glycocalyx of the endothelium to preventing acute platelet deposition to the surface of damaged arteries (Deible et al., 1998a; Deible et al., 1998b). Furthermore, we recently utilized PEG to act as a surrogate mucin lining by providing protection against bacterial infections on intestinal epithelial cells in a murine model of lethal gut-derived sepsis (Wu et al., 2004). However, the most compelling use of PEG has been in the repair of severed neurons with rapid fusion of severed spinal cord nerve fibers following PEG treatment (Borgens and Shi, 2000; Shi and Borgens, 1999; Shi and Borgens, 2000; Shi et al., 1999) and for successful repair of guinea pig (Borgens and Bohnert, 2001) and canine (Laverty et al., 2004) spinal cord injuries. The mechanism of PEG-induced neuroprotection involves direct interaction of PEG with the mitochondria (Luo et al., 2004) and inhibiting free radical production after acute spinal cord injury (Luo et al., 2002). Recently, we screened various PEG compounds in a murine model of lethal gut-derived sepsis and identified a high molecular weight PEG (PEG15-20) that provides protection against bacterial infections on intestinal epithelial cells (Wu et al., 2004).
In the current study, we hypothesized that unconjugated PEG15-20 may provide protection against agonist-induced human lung vascular endothelial barrier dysfunction and sought to characterize its mechanism of action. Our results indicate that PEG15-20 produced rapid and sustained dose-dependent increases in transendothelial electrical resistance (TER), reflecting increased paracellular integrity similar to barrier-enhancing agonists such as sphingosine 1-phosphate (S1P), and effectively reversed both thrombin- and LPS-induced EC barrier dysfunction. PEG-mediated barrier protection was linked to significant cytoskeletal rearrangement and formation of a well-defined cortical actin and spatially localized MLC phosphorylation, events previously shown to strengthen cell-matrix and cell-cell junctional adhesion. Thus, PEG joins a number of EC barrier-regulatory agents which rapidly activate actin-associated barrier-enhancing signal transduction pathways with potential therapeutic benefit in inflammatory lung injury.
Materials and Methods
Reagents
Polyethylene glycol (P-2263 HMW PEG 15,000–20,000 Da), sphingosine 1-phosphate, human thrombin (cell culture grade), fetal bovine serum (FBS), phosphate buffer saline, telostein gelatin, heparinase III, and bovine serum albumin were purchased from Sigma-Aldrich (St. Louis, MO). All primary antibodies were generated against human antigens in which anti-ERK, anti-phospho-ERK (Thr202/Tyr204), anti-MLC, and anti-diphospho-MLC (Thr18/Ser19) were from Cell Signaling Technology (Beverly, MA). Anti-mouse and anti-rabbit secondary antibodies conjugated to horse radish peroxidase, enhanced chemiluminescence (ECL), and ECL-Plus are purchased from Amersham Biosciences, Inc /GE Health Sciences (Piscataway, NJ). Texas Redphalloidin, anti-mouse and rabbit Alexa 488 secondary antibodies, and Prolong mounting solution are from Molecular Probes (Eugene, OR). Pharmacological inhibitors Ro-32-0432, Calphostin C, phorbol-12-myristate-13-acetate (PMA), pertussis toxin (PTX), ML-7, Rho-kinase Inhibitor, U0126, LY294002, PP2, and genistein were purchased from EMD Biosciences (San Diego, CA).
Cell culture
Human lung microvessel endothelial cells (HLMVEC) and human pulmonary artery endothelial cells (HPAEC) were purchased from Lonza/Cambrex, Inc. (Allendale, NJ) and grown in manufacture's recommended Endothelial Growth Medium-2-Microvessel (EGM-2MV) or Endothelial Growth Medium-2 (EGM-2) consisting of defined growth factors and supplemented with additional FBS up to 10% final concentration. Cells were grown at 37°C in 5% CO2 incubator and used from passage 6–9. For experiments, ECs were plated at appropriate density (875,000 cells/D60; 300,000 cells/D35; 100,000 cells/CultureSlide well; 75,000 cells/ECIS well) and used 3 days after plating unless otherwise noted with medium changed 1 day prior to experimentation.
Immunofluorescence
ECs were seeded onto 8 chambers, collagen-coated Culture Slides (BD Biosciences, Lexington, KY). After agonist stimulation, cells were washed with phosphate buffered saline once and fixed with 3.7% formaldehyde for 5 min, permeabilized with 0.25% Triton X-100 in PBS for 3 min, washed and probed with primary antibodies at 1:100–1:200 dilution for 45 min. F-actin is probed with Texas Red-phalloidin at 1:200 dilution. Secondary antibodies are diluted at 1:200 dilution and incubated for 30 min. Slides are mounted with Prolong™ anti-fade reagent with DAPI. Stained cells were visualized using a Nikon Eclipse TE2000 inverted microscope (Nikon Inc., Melville, NY) and images acquired using SPOT software (Diagnostic Instruments, Sterling Heights, MI). Integrated densities of fluorescence staining are measured and quantified using Adobe Photoshop CS3 as described by Luke Miller (http://www.lukemiller.org/journal/2007/08/quantifying-western-blots-without.html).
Fluorescence video-microscopy
EC were grown to ~80% confluency and transfected using the amaxa HCAEC Nucleofection kit (amaxa Inc, Gaithersburg, MD). Basically, cells were trypsinized and counted with a hemacytometer; 500,000 cells were used in each transfection. Cells were pelleted @ 200g and all of the supernatant was removed. Cells were resuspended in 100 μl of amaxa nucleofection solution and 1 μg each of pEGFP-C1/MLCK2EGFP and pDsRed-N1/cortactin plasmids were added to the transfection mixture. Immediately the transfection mixture was transferred to an amaxa cuvette and the cells were electroporated with program S-5 in the amaxa Nucleofector™ system. Immediately 500 μl of prewarmed culture medium were added to the cuvette and the mixture was transferred to a 35-mm dish containing equilibrated warm culture medium and a gelatin-coated 25 mm coverslip. Cells were imaged within 24–48 hrs of transfection. EC were bathed in 2 ml EGM-2 and maintained at 37°C with a heating stage for the entire assay. Transfected cells were illuminated with Ar 488 nm and He/Ne 561nm lasers and movies were recorded with a Leica TCS SP5 confocal microscope with an active resonance scanner set to 8000 Hz and a 63X oil objective lens (NA 1.4). Twelve-bit 512 × 512 images were acquired sequentially scan line-by-scan line every six seconds and with a line averaging setting of eight. Cells were first imaged in a prestimulation movie for six minutes, then bath medium was aspirated and 8% PEG was added to the cells and digital recording was continued for another forty minutes. All raw image data were processed with ImageJ 1.39c software (Wayne Rasband, NIH, http://rsb.info.nih.gov/ij). Images were background subtracted then smoothed with a Kalman stack filter. Fluorescence signal was normalized to sixteen bits using the enhance contrast plug-in with pixel saturation of 0.01%.
Electrical resistance measurements
An electrical cell-substrate impedance sensing (ECIS) system (Applied Biophysics, Troy, NY) was used to measure transendothelial electrical resistance (TER) with ECs grown on gold microelectrodes (Tiruppathi et al., 1992). ECs were plated directly onto an ECIS plate (8W10E) and cultured for 2–3 days. Confluency was assessed as minimum basal resistance of 2000 ohms for HLMVEC and 1000 ohms for HPAEC. Due to its low solubility, PEG is dissolved in EGM2 at the desired final concentration, and stimulation of cells is achieved by replacement of media with vehicle EGM2 or PEG dissolved in EGM2. Data pooling and analysis were performed using Epool software created in-house. Pooled data are expressed either as non-normalized resistance or normalized resistance relative to the point of agonist challenge (t = 0 hr).
Permeability Assay
Measurements of FITC-dextran clearance to assess changes in endothelial permeability were performed with the In Vitro Vascular Permeability Assay Kit (ECM640, Millipore, Billerica, MA), and the methods were modified to optimized for detecting barrier-enhancement. EC were plated on collagen-coated inserts at 100,000 cells/insert and grown to confluency. Then, cells were challenged with the addition of the agonist in the luminal chamber only. After 1–2 hr stimulation, FITC-dextran (1:20 dilution, 40kDa, Sigma, St. Louis) was added to the luminal chambers. After 2–24hr of incubation, the inserts were removed and the abluminal chamber media was collected. Fluorescence detection was determined on the CytoFluor Multi-well Plate Reader Series 4000 (Applied Biosystems, Foster City, CA) by adjusting the gain for the unstimulated control sample to read approximately 1000 relative fluorescence unit (RFU) for normalization. The readings were calculated with the use of a standard curve to FITC-dextran concentration, which were then used in the following equation to determine the permeability coefficient of FITC-dextran (Pf):
where [A] is abluminal concentration in mg/ml; t is time in seconds; A is area of membrane (0.3 cm2); V is volume of abluminal chamber (0.5 cm3); [L] is luminal concentration in mg/ml; and therefore Pf is expressed in cm/s (Tinsley et al., 2000).
Western blots
After agonist stimulation, cells were washed with cold Endothelial Basal Medium (EBM) once and extracted with 0.3% SDS lysis buffer (300 μl/D60) containing protease inhibitors (1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 0.2 TIU/ml aprotinin, 10 μM leupeptin, 5 μM pepstatin A). DNA is sheared with a 26-gauge syringe. Each sample was boiled for 5 min, and diluted with 5X sample buffer (0.56 M Tris pH7.0, 10% SDS, 25% β-ME, 25% sucrose, 0.025% bromophenol blue). Sample proteins were separated with a 10% or 15% homogenous SDS-PAGE gel using the Mini-Protean II (Bio-Rad, Hercules, CA). Proteins were transferred onto Immobilion-P PVDF membrane (Millipore, Bedford, MA), and immunoblotted with primary antibodies (1:1000, 4°C, overnight) followed by secondary antibodies conjugated to HRP (1:5000, room temperature, 30 min) and detected with enhanced chemiluminescence (Pierce ECL or SuperSignal West Dura, Pierce Biotechnology, Rockford, IL) on Biomax MR film (Kodak, Rochester, NY).
Calcium measurements
ECs are grown to confluency on 20 × 40 mm coverslips and preloaded with 5 μM Fura-2-AM (Molecular Probes, Eugene, OR) for 15 min. Fluorescence was measure with an Aminco-Bowman Series 2 luminescence spectrometer at excitation wavelength of 340 and 380 nm and emission wavelength of 510 nm. Upon agonist challenge, intracellular calcium in nM is calculated from 340/380 ratio using calibration curves and software as previously described (Usatyuk et al., 2003).
RESULTS
PEG induces potent endothelial barrier enhancement
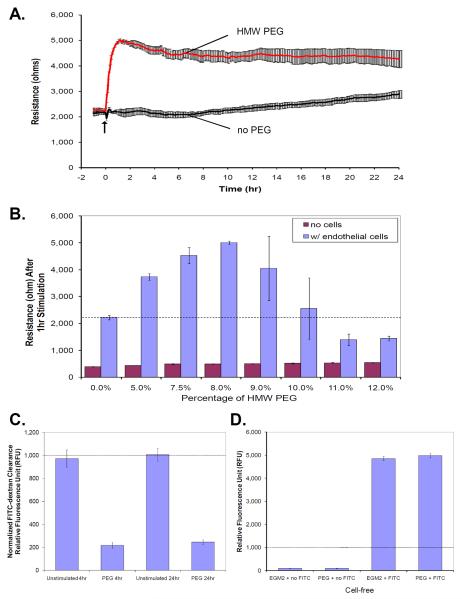
To examine the effect of PEG on EC barrier function, human lung EC were grown to confluency on gold microelectrodes and changes in TER in response to agonist were monitored. Compared to vehicle control EGM2, PEG15-20 (8% dissolved in EGM2) induces a rapid and potent barrier enhancement as detected by increased TER from 2221.9 ± 81.1 ohms to 5001.9 ± 62.8 ohms (Figure 1A), a 125% increase in EC TER. The response is immediate but the potency of the response resulted in a plateau response that occurs 1hr post-stimulation with barrier enhancement sustained for more than 40 hrs (data not shown). Dose response studies demonstrate that 8% PEG is the optimal concentration to induce EC barrier enhancement (Figure 1B) with increasing concentrations of PEG above 10% resulting in TER below baseline values, i.e. barrier dysfunction. To assess that PEG does not cause an artifact by directly increasing TER independent of a cell monolayer, we measured the dose response of PEG in the absence of cells overlying the gold microelectrodes (Figure 1B). Resistance values increased from baseline values of 394 ± 9.2 ohm (0% PEG in media) to a range of 429.3 ± 2.9 ohms to 537.3 ± 16.4 ohms, for 5% and 12% PEG respectively, which demonstrate that PEG alone causes minimal change in resistance measurements and that cellular responses to PEG challenge underlie the dramatic TER enhancement.
Figure 1. HMW PEG15-20 induces sustained and dose-dependent endothelial barrier enhancement.
Human lung EC were cultured on gold microelectrodes and subjected to transendothelial electrical resistance (TER) measurements to detect changes in barrier permeability. Increases in resistance correspond to tightening of junctional adhesion resulting in enhanced barrier function, while decreases in resistance correspond to barrier dysfunction and increased paracellular permeability. A) The addition of PEG15-20 to an endothelial monolayer at time = 0 hr results in a rapid increase in barrier enhancement that is sustained for over 24hr. B) To verify that PEG does not have an artifactual effect on TER measurements, TER values for various concentration of PEG with and without cells were taken. In the absence of cells, increasing concentration of PEG does not alter the TER measurements while the addition of endothelial monolayer provides a basal resistance of 2160 ± 64 ohm (dotted line). Furthermore, PEG induces a biphasic response in which increasing concentration of PEG up to 8% enhances the TER and >10% PEG decreases TER. Thus, changes in resistances observed in experiments with cells are a result of cellular responses to PEG. Permeability assay, measuring FITC-dextran clearance through endothelial cells grown on co-culture inserts, were also performed to validate the TER data. C) Confluent cells were challenged with 8% PEG for 2hr and then FITC-dextran tracking dyes were added to the media. Inserts were removed to stop the experiment after 4hr and 24hr post-PEG challenge and media from the lower well were sample for fluorescence with a luminometer. Normalized data to the unstimulated time points demonstrate that PEG significantly decreased FITC-dextran clearance at 4hr and 24hr post-challenge which correlates with the TER data. To verify that PEG did not quench the fluorescence of the tracking dye, a cell-free system with either EGM2 or 8%PEG in EGM2 with or without FITC-dextran were mixed and the fluorescence was measured. D) Fluorescence were not detected in either EGM2 nor PEG itself and the addition of FITC-dextran significantly enhanced fluorescence measurement which were not affected by either EGM2 or PEG suggesting PEG does not have an artifactual effect on fluorescence measurement in the endothelial permeability assay.
To complement and validate the TER results, we performed comparable in vitro vascular permeability experiments measuring the effect of PEG on FITC-dextran clearance. EC grown on co-culture inserts were pretreated with control EGM2 or 8% PEG in EGM2 for 2hr followed by the addition of FITC-dextran tracking dye for additional 2hr and 22hr incubation, for 4hr- and 24hr-post PEG challenge time points. Data were normalized by adjusting the gain of the fluorimeter such that the control time point is approximately 1000 relative fluorescence unit (RFU). Consistent with TER data, PEG reduced FITC-dextran clearance from 971.9 ± 74.5 RFU of control to 216.5 ± 23.0 RFU at 4hr post-challenge and from 1005.5 ± 53.9 RFU of control to 244.8 ± 18.9 RFU at 24hr post-challenge (Figure 1C), suggesting that PEG enhances endothelial barrier function by reducing permeability and the effect is sustained for at least 24hr. The permeability coefficient of FITC-dextran clearance for PEG stimulated cells is (Pf = 0.315 cm/s), which is drastically reduced in comparison to baseline unstimulated cell (Pf = 1.745 cm/s). To exclude PEG-mediated chelation of the tracking dye fluorescence (a potential artifact) we utilized a cell-free system with measurement of the fluorescence of control EGM2 and 8% PEG in EGM2 with and without FITC-dextran. Without the addition of FITC-dextran, the fluorescence reading for control EGM2 was 91.8 ± 7.7 RFU and PEG was 95.6 ± 15.4 RFU. In the presence of FITC-dextran, the fluorescence reading for control EGM2 was 4852.3 ± 82.7 RFU and PEG was 4979.5 ± 86.8 RFU (Figure 1D), indicating that PEG does not chelate the fluorescence of the tracking dye. Both TER and permeability data support that PEG enhances endothelial barrier function and reduces endothelial permeability.
Physical presence of PEG is required for EC signal transduction and barrier protection
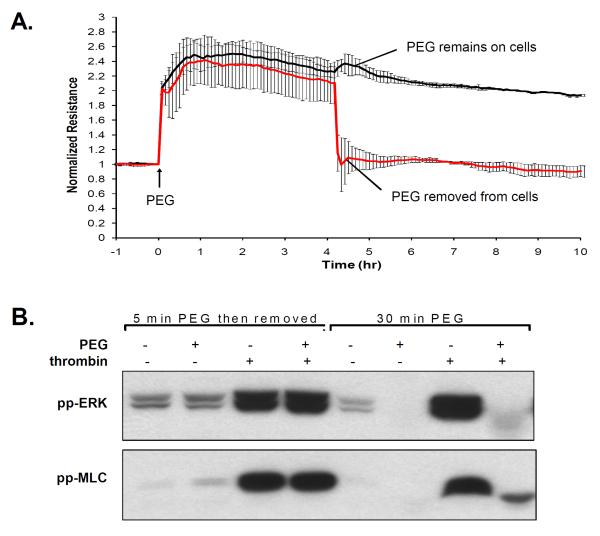
We previously demonstrated that PEG can activate intestinal epithelial cells within minutes and the polymer does not wash off from the surface of the cells, suggesting that PEG may coat the cell surface and induce a prolonged response (Wu et al., 2004). We examined the adhesive properties of PEG challenge by removal of PEG during TER measurement (Figure 2A). EC were stimulated with 8% PEG for 4hr and then PEG was removed by replacing the media. Upon PEG stimulation, the resistance increased 2.5-fold, but then immediately dropped to basal level upon PEG removal, suggesting that unlike intestinal epithelial cells, the presence of PEG is necessary to induce the dramatic barrier enhancement on vascular EC. Furthermore, we examined the effect of PEG removal on PEG-mediated alterations in signal transduction evoked by thrombin, an edemagenic agonist which induces dramatic increases in ERK and MLC phosphorylation (Figure 2B). Pretreatment with PEG (8%, 30 min) attenuated thrombin-induced ERK and MLC phosphorylation, however, pretreatment and removal of PEG (8% PEG, 5 min, then removal with EGM and incubation for another 25 min) results in no inhibition of thrombin-induced ERK and MLC phosphorylation. The ability of PEG to enhance TER and inhibit thrombin-induced ERK and MLC phosphorylation is highly dependent on the presence of PEG on vascular EC.
Figure 2. PEG is required to be physically present to induce barrier enhancement and to block thrombin-induced ERK and MLC phosphorylation.
Prior data on epithelial cells suggest that PEG actives cells even upon removal of PEG. The response of PEG removal is examined in human lung EC. A) The effect of PEG on TER is assessed by pretreating cells with PEG (8%, 4hrs) and then removal of PEG upon replacement with EGM2 as compared with cells in which PEG was left on the cells. B) To examine the effect of PEG on signal transduction, cells were either pretreated with vehicle or PEG (8%, 30 min), and then stimulated with thrombin (1U/ml, 5 min). In comparison, cells were also pretreated with PEG for 5 min followed by EGM2 replacement to remove PEG (25 min incubation), and subsequently stimulated with vehicle or thrombin. Cell lysate were processed via Western blots probed with either antibodies specific for phospho-ERK (Thr202/Tyr204) or phospho-MLC (Thr18/Ser19). The presence of PEG abolishes thrombin-induced ERK and MLC phosphorylation, but removal of PEG eliminates the inhibitory effects of PEG.
PEG does not act as a physical barrier
PEG does not directly alter gold microelectrode TER values in the absence of cells suggesting that PEG does not act as a physical barrier to TER determinations. To address whether PEG provides such a physical barrier upon interaction with cell membrane surface, we examined the ability of two agonists to alter PEG-induced endothelial barrier enhancement. In Figure 3A, ECs were pretreated with vehicle EGM2 or PEG (8%, 24 hrs) and then stimulated with the potent calcium ionophore, ionomycin (10 μM) thereby inducing a rapid influx of extracellular calcium and potent endothelial barrier disruption as previously described (Garcia et al., 1997). Ionomycin quickly reduces TER values within minutes of agonist challenge. However, the addition of ionomycin to established PEG-induced TER barrier enhancement resulted in delayed ionomycin-mediated EC barrier disruption that returns TER values back to baseline 7 hrs post-stimulus. Although PEG pretreatment appears to protect against ionomycin-induced barrier disruption by delaying the decreased TER values, the ability of ionomycin to induce gradual reduction in TER value suggests that PEG does not act as a physical barrier. Complementary experiments measuring permeability via FITC-dextran clearance demonstrated that PEG pretreatment blocked ionomycin-induced increase in permeability at 4 hr post-stimulation and significantly attenuated at 24hr post-stimulation (Figure 3B), supporting the ability of PEG to delay ionomycin-induced barrier dysfunction. We similarly utilized ECs pretreated with vehicle EGM2 or PEG (8%, 1hr) and then stimulated with the edemagenic agonist, thrombin, and observed a thrombin-induced transient drop in TER which was unaffected by PEG pretreatment suggesting adequate access of thrombin to PAR receptors at the cell surface (Figure 3C). The maximum drop in thrombin-induced TER in the presence of PEG remains significantly higher than baseline resistance, again supporting the notion that PEG does not act as a physical EC barrier. Despite the inability of PEG to block thrombin-induced changes in TER, the elevated TER induced by PEG is sufficient to protect against both thrombin- and LPS-induced increase in FITC-dextran clearance (Figure 4D). Comparison of the Pf demonstrates that thrombin and LPS increased the rate of FITC-dextran clearance from baseline 1.744 cm/s to 11.391 cm/s and 8.868 cm/s, respectively. However, PEG pretreatment reduced the Pf of thrombin and LPS challenge to 0.427 cm/s and 2.139 cm/s, respectively, as compared to PEG alone at 0.315 cm/s.
Figure 3. PEG does not act simply as a physical barrier.
EC cultured on gold microelectrodes were pretreated with vehicle EGM2 or PEG (8%, 24 hrs) and then stimulated with the A) calcium ionophore, ionomycin (10 μM). In the control, the influx of extracellular calcium causes a potent endothelial barrier disruption, but PEG pretreatment delays the barrier disruption. The ability of ionomycin to gradually bring the resistance down to baseline suggests that even in the presence of cells, PEG does not act as a physical barrier. B) To validate the TER data, confluent cells grown in co-culture inserts were pretreated with either EGM2 or 8% PEG for 2hr and then challenged with 10 μM ionomycin. FITC-dextran tracking dyes were added to the media. Inserts were removed to stop the experiment after 4hr and 24hr post-PEG challenge and media from the lower well were sample for fluorescence with a luminometer. Normalized data to the unstimulated time points demonstrate that PEG significantly decreased FITC-dextran clearance at 4hr and 24hr post-challenge which correlates with the TER data. Using a physiological relevant agonist, similar experiment was performed measuring TER in which ECs were pretreated with vehicle EGM2 or PEG (8%, 1hr) and then stimulated with C) 1 U/ml thrombin. In the control, thrombin causes a transient drop in TER. PEG pretreatment did not block this transient drop in TER, suggesting thrombin was able to access cell surface PAR receptors further supporting the absence of a physical barrier produced by PEG. Note, however, that the maximum drop in thrombin-induced TER in the presence of PEG is still significantly higher than baseline resistance and thereby offering endothelial barrier protection by enhancing overall barrier resistance. D) We confirm that the elevated baseline resistance provided protection by performing in vitro permeability assay measuring FITC-dextran clearance. EC were pretreated with 8% PEG for 2hr, followed by addition of either thrombin (1U/ml, 2hr) or LPS (1μg/ml, 4hr) with FITC-dextran tracking dye. Pretreatment with PEG significantly attenuated both thrombin and LPS-induced increase in FITC-dextran clearance.
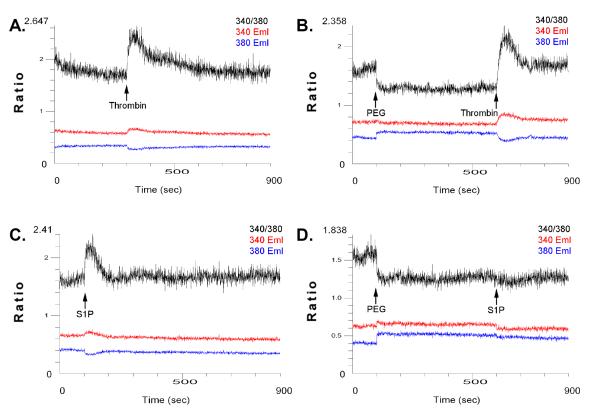
Figure 4. PEG does not induce a transient intracellular calcium spike but has differential effects on thrombin- and S1P-induced calcium signaling.
ECs are grown on glass coverslips and preloaded with 5 μM Fura2-AM for 15 min. Fluorescence was measured with an Aminco-Bowman Series 2 luminescence spectrometer at excitation wavelengths of 340 and 380 nm and emission wavelength of 510 nm. Cells were stimulated with either A) 1U/ml thrombin, C) 1μM S1P as compared with 8% PEG pretreatment with subsequent addition of B) thrombin or D) S1P. Intracellular calcium in nM was calculated from the 340/380 ratio using calibration curves and software.
PEG does not induce intracellular calcium spike
Calcium is a common signaling intermediate that regulates endothelial barrier function. Using ratiometric fluorescent calcium indicator dyes, we explored the role of PEG in modulating calcium signaling using EC grown on glass coverslips and preloaded with Fura-2-AM (5 μM, 15 min) with fluorescence measured using luminescence spectrometer at excitation wavelengths of 340 and 380 nm and emission wavelength of 510 nm. The addition of 8% PEG does not trigger an intracellular calcium signaling (Figure 4B, D) whereas, both the endothelial barrier-disrupting agonist, thrombin (Figure 4A), and the barrier-enhancing agonist, S1P (Figure 4B), known triggers of intracellular calcium signaling, each produce a strong spike in cytosolic calcium. Interestingly, pretreatment with 8% PEG followed by sequential stimulation with thrombin or S1P produced differential responses with PEG pretreatment attenuating S1P-induced calcium signaling but not thrombin-induced calcium spikes (Figure 4B, D). The inability of PEG to block thrombin-induced calcium spike correlates with the inability of PEG to block thrombin-induced declines in TER values (Figure 3C).
PEG induces adherens junction and cytoskeletal reorganization
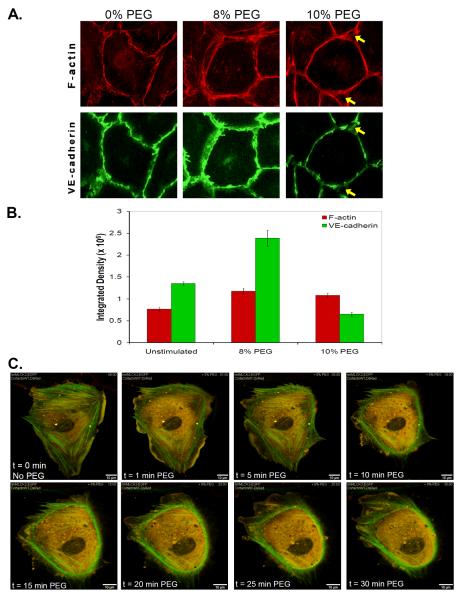
Endothelial barrier integrity is maintained by cell-cell contacts regulated in part by dimerization of adherens junction components such as VE-cadherins which are anchored to the actin cytoskeleton for structural stability. Typically, endothelial barrier disruption is characterized by the dissociation of junctional cadherin associations and the formation of central stress fibers of actin filaments that induce cellular contraction and mediate paracellular gap formation. In contrast, endothelial barrier enhancement is characterized by increased VE-cadherin association at the cell-cell junctions along with increased F-actin bundling at the cortical or periphery of the cells which reinforces the junctional protein stability with the cytoskeleton. We examined the effect of PEG on cytoskeletal reorganization via immunofluorescence staining of ECs stimulated with vehicle, 8% or 10% PEG (1hr), fixed and stained to visualize F-actin and VE-cadherin (Figure 5A). Changes in PEG-stimulated staining are quantified and expressed as integrated densities (Figure 5B). In comparison to controls, 8% PEG induces enhanced cortical actin (integrated density from 764,005 + 47,409 to 1,171,854 + 70,148.7) and VE-cadherin (from 1,344,476 + 48,985 to 2,385,016 + 182,518) staining similar to other barrier-enhancing agents such as S1P. The bundling of F-actin and increased ruffling of lamellipodia structures at the periphery of each cell correlates with the dramatic barrier enhancement. Increasing above the 8% optimal concentration, 10% PEG induces an exaggeration of F-actin staining at the junctions with a thinner but more intense staining of the cortical band (integrated density from 764,005 + 47,409 to 1,080,869 + 47,849) but a decrease in junctional VE-cadherin staining (from 1,344,476 + 48,985 to 651,271 + 43,186). The dissociation of the junctional adhesion and the exaggerated bundling of F-actin cause individual cells to round up and thereby forming junctional pores (yellow arrows) between three cells, which may contribute to the decreased barrier enhancement compared to 8% PEG.
Figure 5. PEG induces cytoskeleton redistribution.
Endothelial barrier function has been shown to be dynamically regulated by alterations in the actin cytoskeleton and junctional proteins. To visualize the effects of PEG on cytoskeletal and junctional remodeling, lung EC are stimulated with PEG (8% or 10%, 1hr) and immunostained for A) F-actin with Texas Red-Phalloidin and VE-cadherin with anti-VE-cadherin antibodies conjugated to Alexa 488. Higher concentration of PEG (10%) which induces lower barrier-enhancing properties reveals some junctional gap formation at tri-laminar region between multiple cells (yellow arrows). B) The integrated densities of the fluorescence stainings are measured and quantified. To visualize cytoskeleton motility in real-time, EC were sparsely plated on gelatin-coated glass coverslips and transfected with DS Red-cortactin (Red) and EGFP-nmMLCK2 (Green). After 48hr post-transfection, cells were imaged using time-lapse fluorescence video-microscopy C) in which the media was replaced with 8% PEG, which induces MLCK reorganization from central stress fibers to the periphery and also stimulates cortactin ruffling and lamellae formation.
Cortical actin formation and barrier-enhancing properties in response to several barrier-enhancing agonists have been linked to dynamic rearrangement of two key cytoskeletal proteins, cortactin and the non muscle isoform, nmMLCK. We previously demonstrated that cortactin binds nmMLCK and both proteins are recruited to the cortical region of the cell where nmMLCK catalyzes phosphorylation of peripheral MLC resulting in enhanced cell-cell adhesion and endothelial barrier function (Dudek et al., 2004). Since PEG induces cortical cytoskeleton redistribution seen with other barrier-enhancing agents, we examined the role of cortactin and nmMLCK in response to PEG. Constructs encoding Ds-Red cortactin and EGFP-nmMLCK2 were transfected into EC, and single cell cytoskeletal responses to PEG challenge was observed in real time using fluorescence video-microscopy (Figure 5C). Prior to PEG challenge, nmMLCK is organized prominently along stress fibers with minor co-localization of cortactin and MLCK diffusely throughout the cell but concentrated colocalization in lamellepodia of unstimulated cells. Immediately (<1 min) after PEG challenge, nmMLCK redistributes from central stress fibers to the periphery in a prominent nmMLCK-containing cortical ring with cortactin and MLCK also co-localized at the ruffling lamellipodia. After 30 min of PEG challenge, ruffling lamellipodia are observed with nmMLCK and cortactin participating in formation of a cortical ring suggesting a potential mechanism for structural stability for the extending lamellipodia. Similar lamellipodial formation is often observed in barrier protective agonist-challenged confluent EC monolayers, where ruffling lamellipodia are thought to contribute to enhanced endothelial barrier function by increasing junctional interaction with adjacent cell. Therefore, PEG appears to induce cortical actin, VE-cadherin, cortactin, and nmMLCK reorganization to the cell periphery thereby stablilizing cell-cell junctions.
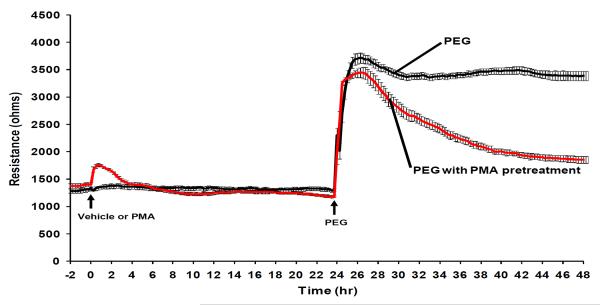
Prolonged PMA pretreatment attenuates barrier-sustaining effect of PEG
As PEG induces cytoskeleton remodeling (i.e. cortical actin ring formation and lamellipodia formation) similar to other barrier-enhancing agents, we next explored whether PEG activates common barrier-protective signaling pathways. Ligation of S1P with the Gi-coupled receptor, S1P1 receptors activates signaling pathways resulting in potent EC barrier-enhancing response. Transactivation of S1P1 receptors has been reported for various non-S1P barrier protective agonists such as activated protein C (Finigan et al., 2005), hyaluronan (Singleton et al., 2006), insulin-like growth factor (El-Shewy HM, Johnson KR,), nerve growth factor (Toman et al., 2004), FcepsilonRI (Jolly et al., 2004), and edaravone (Omori et al., 2007). Furthermore, the S1P analogue FTY720, downregulates S1P1 receptors via receptor internalization (Graler and Goetzl, 2004). Using prolonged FTY720 exposure (10 μM, 24hr) and pertussis toxin (PTX, 100 ng/ml, 4hr) pretreatment, we examined if S1P1 receptors play a role in PEG-induced endothelial barrier enhancement (Table 1). Neither inhibition of Gi-coupled receptors with PTX, or S1P1 receptor downregulation with prolonged FTY720 exposure, attenuated the PEG-induced increases in TER suggesting PEG does not activate Gi-coupled receptors and does not transactivate S1P1 receptors to enhance endothelial barrier function. As positive control experiments, both PTX and FTY720 pretreatments attenuated S1P-induced endothelial barrier enhancement. Next, we explore a spectrum of downstream signaling targets using a variety of pharmacological inhibitors: ML-7 (MLCK), Y-27632 (Rho kinase), U0126 (ERK), LY294002 (PI3K), PP2 (Src kinases), and genistein (tyrosine kinases). Inhibition of kinase activities by 30 min pretreatment with each inhibitor failed to alter PEG-induced barrier enhancement (Table 1).
Table 1. Effects of pharmacological inhibitor pretreatments on PEG-induced endothelial barrier enhancement.
EC were pretreated with various agents (1μM S1P, 10μM FTY720, 100ng/ml PTX, Heparinase III for 4hr, or 10μM ML-7, 100nM Rho-kinase Inhibitor, 10μM U0126, 10μM LY294002, 10μM PP2, or 200μM Genistein for 30 min) and subsequently challenged with either 1μM S1P or 8% PEG to induced endothelial barrier enhancement. None of the agonists or inhibitors blocked PEG-induced barrier enhancement.
| ECIS | S1P 24hr | FTY720 24hr | Heparinase III 4hr | PTX 4hr | ML-7/RhoK inhibitor/U0126/LY294002/PP2/Gen | PMA 24hr |
|---|---|---|---|---|---|---|
| S1P barrier enhancement | No effect | Blunted | - | Blocked | - | Blocked |
| PEG barrier enhancement | No effect | No effect | No effect | No effect | No effect | Affect only latter phase |
As PKC isotypes are additional known barrier-regulatory molecules, we next downregulated specific PMA-sensitive PKC isoforms via prolonged PMA pretreatment. We previously demonstrated that in human lung EC, the addition of 100nM PMA induces a modest increase in TER that returns to baseline (Bogatcheva et al., 2003). However, pretreatment with prolonged PMA (100 nM, 24 hr) attenuated the barrier-sustaining effect of PEG without affecting the initial increase in TER (Figure 6). In comparison, S1P-induced barrier enhancement is completely blocked by prolonged PMA pretreatment (Figure 6). As prolonged PMA pretreatment lacks specificity for non-PKC protein expressions, we examined the effects of general pharmacological PKC inhibitors, Ro-32-0432 and calphostin C, on PEG-induced EC barrier enhancement. These studies indicate that PKCs do not appear to be involved in PEG-induced barrier enhancement (data not shown) and are consistent with unique PEG-mediated barrier-protective signaling pathways.
Figure 6. Effects of prolong PMA pretreatments on PEG-induced endothelial barrier enhancement.
EC were pretreated with 100nM PMA for 24hr and subsequently challenged with either 1μM S1P or 8% PEG to induced endothelial barrier enhancement. Prolonged phorbol ester pretreatment attenuated barrier-sustaining effects of PEG but not the initial barrier-enhancement.
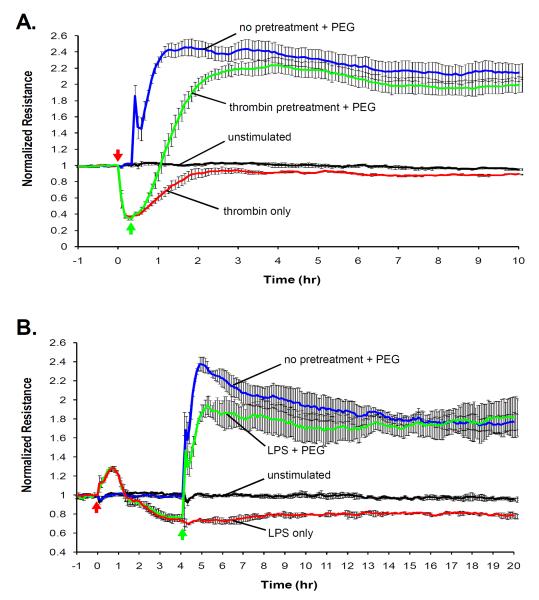
PEG restores agonist-mediated barrier dysfunction
Pretreatment with PEG followed by thrombin challenge suggested that PEG may serve to provide barrier protection despite the absence of an inhibitory effect on thrombin-induced calcium spikes (Figure 4B) or the magnitude of thrombin-induced TER changes (Figure 3C). PEG increases the level of normalized TER to such a substantially elevated level that the maximum and temporal thrombin-induced barrier disruption remains above level of unstimulated baseline barrier integrity, and thus, provides relative barrier protection. To assess the maximal utility of PEG pretreatment in a clinical setting, we examined the use of PEG (8% PEG) as a post-agonist treatment during established EC barrier dysfunction produced by challenged with either 1 U/ml thrombin for 30 min (Figure 7A) or 1 ng/ml LPS for 4 hr (Figure 7B). Thrombin induces a transient barrier disruption, and the addition of PEG at the maximum point of barrier dysfunction accelerated the barrier-restoration process resulting in the enhancement of barrier function comparable to that at the level of PEG with no pretreatment. Alternatively, LPS induces barrier dysfunction through upregulation of various cytokines resulting in a more gradual and prolonged barrier dysfunction. Addition of PEG 4hr after LPS challenge also restored barrier function above and beyond the level of baseline barrier integrity. Therefore, PEG can effectively reverse and restore clinically relevant agonist-induced barrier dysfunction.
Figure 7. PEG protects against agonist-induced endothelial barrier dysfunction.
The ability of PEG to reverse barrier dysfunction induced by thrombin or LPS, is assessed with TER measurements. Cells are pretreated (red arrows) with either 1 U/ml thrombin (0.5 hr) or 1 ng/ml LPS (4 hr) followed by 8% PEG (green arrows). PEG is a potent barrier-enhancing agent that can effectively reverse A) thrombin- and B) LPS-induced increases in permeability.
DISCUSSION
Novel therapies are desperately needed to address the profound physiologic derangements produced by dysregulated vascular permeability, particularly during inflammatory states. We have determined that PEG15-20 induces an immediate, dose-dependent augmentation of endothelial cell barrier function with full effect evolving at 45–60 min post-stimulation. Relative to other well-characterized barrier-enhancing agents that induce ~40% increases in TER values and which remains sustained for several hours, PEG induces a remarkable 125% increase in TER values which are elevated for over 40 hours (Figure 1A). This barrier enhancement represents a cellular response since the polymer alone has little effect on resistance measurements in the absence of cells (Figure 1B). The TER data are validated by in vitro permeability assays in which PEG reduces the FITC-dextran clearance to 22.3% of the basal permeability level, or 4.49-fold enhancement of endothelial barrier function over basal level (Figure 1C). Preliminary experiments using other high molecular weight polymers (20 kDa and 35 kDa) also induced enhanced TER (data not shown) suggesting that the intrinsic physical property of increased molecular size may contribute to the barrier enhancing effect. Hypertonic solution has been hypothesized to cause endothelial shrinkage and destabilize barrier properties by increasing junctional width and possibly weakening cell-matrix interaction via induction of endothelial protein tyrosine phosphorylation (Ragette et al., 1997). This concept of PEG-induced cell shrinkage was observed in our time lapse video (Figure 5C), which is also supported by several papers citing that PEG reduces swelling of anoxia-induced epithelial cells (Kreisberg et al., 1980) and prevents osmotic swelling in transplant organ preservation (Faure et al., 2002). However, cell shrinkage would mimic cellular contraction and likely cause barrier dysfunction unless the magnitude of the cell shrinkage is negligible in a monolayer of cells and the cells respond to reinforce the cell-cell junctions. Interestingly, this has been demonstrated in which hypertonic solution that cause “intermediate” increases (from 31 mOsmg/kg to 37–50 mOsmg/kg) in glucose osmolarity may enhance endothelial barrier function (Safdar et al., 2003) and protect against acid-induced lung injury in rats (Safdar et al., 2005). The mechanism is hypothesized to involve increased endothelial adherens junction assembly and cortical actin formation. However, doses higher than 50 mOsm reduced the barrier enhancement (Safdar et al., 2005) suggesting that relatively high hypertonic solution may ultimately lead to cell shrinkage and therefore increased vascular permeability. We also observed that PEG induced similar increases in VE-cadherin at the adherens junctions as well as cortical actin formation (Figure 5) which we have previously described with other barrier enhancing agents such as S1P (Garcia et al., 2001) and HGF (Liu et al., 2002; Singleton et al., 2007). While we have not yet precisely identified the mechanism by which PEG exerts EC barrier protection, however, PEG remains one of the most potent barrier-enhancing agent tested to date and confirmed by both measurement of TER and FITC-dextran clearance. The possibility exists that PEG-mediated membrane fusion is involved in EC barrier protection, however, as removal of PEG eliminated the barrier-enhancing effect, membrane fusion is much less likely a cause for barrier protection compared to interaction of higher molecular weight polymers with distant cell surface receptors. Similarly, PEG does not appear to serve as a physical molecular barrier precluding interactions between cell membrane surface and the media as suggested on studies of platelet deposition in damaged endothelium (Deible et al., 1998a). PEG induced a potent increase in TER only when added to intact endothelium, but not when added to bare microelectrodes (Figure 1B). Furthermore, our finding demonstrates that in PEG treated endothelium that LPS, thrombin and ionomycin retain the capacity to access cell membrane surface to invoke barrier disrupting signals such as calcium influx (Figure 4B), declines in TER values (Figure 3C). These studies indicate that it is unlikely that PEG effects on EC barrier regulation occur as a consequence of a physical barrier but rather via interaction with cell surface components which alter signal transduction pathways resulting in cytoskeletal rearrangement. In this regard, while preliminary, we have also explored the interface of PEG and plasma membrane contact as PEG has been reported to form a molecular barrier on the glycocalyx (Deible et al., 1998a; Deible et al., 1998b), and we previously demonstrated the role of glycosaminoglycans (GAGs) in mediating polyarginine-induced endothelial barrier dysfunction (Dull et al., 2003). The hypothesis that PEG induces endothelial barrier enhancement through cross-linking of endothelial glycocalyx and cell surface GAGs was not supported by experiments involving heparinase III to digest GAGs, an intervention which failed to alter PEG-induced barrier enhancement (data not shown). We are currently exploring the use of siRNA that target syndecans in the glycocalyx.
An abundant body of evidence now supports the concept that cytoskeletal rearrangement is central to the regulation of EC barrier properties i.e. barrier disruption, barrier maintenance, and barrier enhancement. We have previously demonstrated that specific spatial reorganization of actin cytoskeleton is critical in modulating the functional regulation of paracellular permeability. For example, the edemagenic agent, thrombin, cleaves protease-activated receptors and signals downstream Rho-dependent pathways to induce increases in central stress fiber formation and MLC phosphorylation which in concert result in cellular contraction and increased paracellular gap formation and permeability (Birukova et al., 2004; Garcia et al., 1986; Garcia et al., 1996). In contrast, the barrier-enhancing agent, S1P, ligates the S1P1 receptor and activates Rac-dependent pathways to induce cortical actin formation at the cell periphery (Garcia et al., 2001) and both cortactin and nmMLCK localization in lamellipodia (Dudek et al., 2004; Lee et al., 2006). Direct binding of cortactin with nmMLCK results in MLC phosphorylation at the cell periphery in which the increased acto-myosin interaction stabilizes cell-cell junctional adhesion and results in enhanced endothelial barrier function (Dudek et al., 2002; Dudek et al., 2004). We demonstrated that PEG markedly increased cortical actin ring formation and VE-cadherin redistribution at the peripheral region of the cells (Figure 5A and 5B), a feature observed with multiple barrier-enhancing stimuli, including S1P (Garcia et al., 2001), shear stress (Birukov et al., 2003), HGF (Liu et al., 2002; Singleton et al., 2007), simvastatin (Jacobson et al., 2004). Furthermore, we demonstrated that PEG rapidly (1 min) induces nmMLCK and cortactin colocalization in the ruffling lamellipodia and redistributes nmMLCK away from central stress fibers to form a prominent cortical ring with cortactin-staining lamellipodia (Figure 5C). These findings suggest that acto-myosin interaction at the cortical ring provides cellular stability while the lamellipodia extends to neighboring cells to interact and tightening cell-cell adhesion. It is also noteworthy that the impressive cytoskeleton reorganization observed in a solitary cell implies that PEG induces barrier enhancement through cellular motility rather than membrane fusion to adjacent cells. Therefore, the dramatic PEG-induced cytoskeleton reorganization of actin, cortactin, VE-cadherin, and nmMLCK provides evidence of cellular motility correlating with the remarkable enhanced barrier function.
We also explored whether PEG-mediated functional and morphological changes utilized signal transduction pathways common to other barrier-enhancing agonists. For example, S1P enhances endothelial barrier function through the ligation of S1P1 receptor, activation of Gi, which stimulates ERK and PI-3 kinase and serves to recruit Akt to the membrane. Upon activation, Akt phosphorylates S1P1 receptor at threonine 236 (Lee et al., 2001), which induces Rac1 to promote cortical actin assembly (Dudek et al., 2004; Garcia et al., 2001). Furthermore, S1P1 receptor activation induces nmMLCK to the periphery where localized MLC phosphorylation occurs (Dudek et al., 2004; Garcia et al., 2001). Our results suggest a fundamentally distinct signaling paradigm as PEG, unlike S1P, downregulated ERK activity and reduced levels of MLC phosphorylation (Figure 2B) and attenuated thrombin-induced MLC phosphorylation. Furthermore, inhibition of Gi-coupled receptors, S1P1 receptors, Rho kinase, PI-3-kinase, Src kinase, or tyrosine kinases did not attenuate PEG-induced barrier enhancement (Table 1) suggesting an alternative, convergence pathway. We also evaluated the commonly involved protein kinase C (PKC) signaling pathway where prolonged phorbol ester (PMA) exposure and downregulation of PMA-sensitive PKC isoforms completely block S1P-induced endothelial barrier enhancement and noted attenuation of the sustaining effect of PEG-induced barrier enhancement (Figure 6). However, as PMA pretreatment did not block the initial PEG-mediated barrier enhancement, we interpret this information as suggesting the early phase is PKC- independent, a finding supported by studies with general PKC inhibitors, Ro-32-0432 and calphostin C (data not shown). Furthermore, the initial PEG response appears involve calcium-independent signaling since PEG did not invoke a transient calcium spike (Figure 4B, D). Interestingly, PEG has differential effect on the calcium signaling of other calcium-dependent agonists, S1P and thrombin. Pretreatment with PEG blocks S1P- but not thrombin-induced calcium signaling (Figure 4). This is consistent with the ability of PEG pretreatment to block further increase in S1P-mediated barrier enhancement but not thrombin-induced decrease in TER (Figure 3C). Both the barrier-enhancing agonist, S1P, and the edemagenic agonist, thrombin, activate calcium signaling, but result in divergent effector responses due to differences in spatial localization of the calcium transients. Nevertheless, the pro-barrier integrity effects of PEG appear to affect upstream signaling prior to calcium activation for S1P.
Although PEG-induced endothelial barrier enhancement does not appear to activate conventional known barrier protective signaling pathways, we have conducted studies which address the utility of PEG as a therapeutic option in treatment vascular permeability in the context of lung injury. Upon thrombin and LPS challenge, PEG effectively reversed endothelial barrier disruption within minutes and ultimately enhanced the barrier function to the augmented level (Figure 7). In comparison to the PEG-NO used to protect from ischemia/reperfusion injury (Bertuglia et al., 2006), we clearly demonstrate that high molecular weight PEG15-20 alone, unconjugated to any bioactive molecules, has the ability to protect against endothelial barrier dysfunction. We explored various optimization methodologies and have identified the concentration of 8% PEG15–20 as the effective, in vitro dose in enhancing lung endothelial barrier function, as well as to reverse thrombin- and LPS-induced barrier dysfunction. Surprisingly, higher concentration of PEG causes decreased barrier function despite the non-toxic nature of PEG in general. In comparison to the gut-sepsis model of injury, it is likely that the vascular endothelium is less tolerant than the intestinal epithelium by nature and thus the use of 10% PEG15–20 is beneficial as a treatment on epithelial but not endothelial cells.
In summary, our studies now add PEG to a list of potent human lung endothelial barrier-enhancing agents with prominent effects on the architecture of the endothelial cytoskeleton. The concentration of 8% PEG is an effective, in vitro dose in enhancing lung endothelial barrier function, able to reverse thrombin- and LPS-induced barrier dysfunction. We have also characterized morphological changes of cortical actin and MLCK formation along with cortactin recruitment to ruffling lamellipodia and VE-cadherin recruitment to the junctions. In addition we demonstrated the ability of PEG to inhibit ERK and MLC signaling pathways. Lastly, we've verified in vitro that PEG provides effective protection against agonist-induced barrier dysfunction as well as reverse agonist-induced barrier dysfunction. In short, the efficacy and rapidity of PEG to restore barrier dysfunction and sustain elevated endothelial barrier integrity makes it an ideal therapeutic agent to reverse pulmonary dysfunction. These preliminary studies are extremely promising, but require additional studies in animal models in order to view PEG as a viable treatment in the ICU to combat acute lung injury.
ACKNOWLEDGMENTS
We thank Lakshmi Natarajan for continued maintenance and supply of high quality endothelial cell culture stocks. This work was supported by grants from the National Heart, Lung, and Blood Institute (Garcia, HL058064; Dudek, HL088144) and the National Institute of General Medical Sciences (Alverdy, GM062344).
REFERENCES
- Bertuglia S, Veronese FM, Pasut G. Polyethylene glycol and a novel developed polyethylene glycol-nitric oxide normalize arteriolar response and oxidative stress in ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H1536–1544. doi: 10.1152/ajpheart.01114.2005. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285:L785–797. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Verin AD, Wang P, Birukova AA, Birukov KG, Mirzopoyazova T, Adyshev DM, Chiang ET, Crow MT, Garcia JG. Phorbol esters increase MLC phosphorylation and actin remodeling in bovine lung endothelium without increased contraction. Am J Physiol Lung Cell Mol Physiol. 2003;285:L415–426. doi: 10.1152/ajplung.00364.2001. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Bohnert D. Rapid recovery from spinal cord injury after subcutaneously administered polyethylene glycol. J Neurosci Res. 2001;66:1179–1186. doi: 10.1002/jnr.1254. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. Faseb J. 2000;14:27–35. doi: 10.1096/fasebj.14.1.27. [DOI] [PubMed] [Google Scholar]
- Deible CR, Beckman EJ, Russell AJ, Wagner WR. Creating molecular barriers to acute platelet deposition on damaged arteries with reactive polyethylene glycol. J Biomed Mater Res. 1998a;41:251–256. doi: 10.1002/(sici)1097-4636(199808)41:2<251::aid-jbm10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Deible CR, Petrosko P, Johnson PC, Beckman EJ, Russell AJ, Wagner WR. Molecular barriers to biomaterial thrombosis by modification of surface proteins with polyethylene glycol. Biomaterials. 1998b;19:1885–1893. doi: 10.1016/s0142-9612(98)00098-2. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Birukov KG, Zhan X, Garcia JG. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem Biophys Res Commun. 2002;298:511–519. doi: 10.1016/s0006-291x(02)02492-0. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, Garcia JG. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285:L986–995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- Faure JP, Hauet T, Han Z, Goujon JM, Petit I, Mauco G, Eugene M, Carretier M, Papadopoulos V. Polyethylene glycol reduces early and long-term cold ischemia-reperfusion and renal medulla injury. J Pharmacol Exp Ther. 2002;302:861–870. doi: 10.1124/jpet.102.033688. [DOI] [PubMed] [Google Scholar]
- Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamburg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Schaphorst KL, Shi S, Verin AD, Hart CM, Callahan KS, Patterson CE. Mechanisms of ionomycin-induced endothelial cell barrier dysfunction. Am J Physiol. 1997;273:L172–184. doi: 10.1152/ajplung.1997.273.1.L172. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, 2nd, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986;128:96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Verin AD, Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost. 1996;22:309–315. doi: 10.1055/s-2007-999025. [DOI] [PubMed] [Google Scholar]
- Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. Faseb J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol. 2004;30:662–670. doi: 10.1165/rcmb.2003-0267OC. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol. 2006;291:L289–295. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, Adyshev D, Usatyuk P, Romer LH, Jacobson JR, Natarajan V, Pearse DB, Garcia JG, Verin AD. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res. 2005;97:115–124. doi: 10.1161/01.RES.0000175561.55761.69. [DOI] [PubMed] [Google Scholar]
- Kreisberg JI, Mills JW, Jarrell JA, Rabito CA, Leaf A. Protection of cultured renal tubular epithelial cells from anoxic cell swelling and cell death. Proc Natl Acad Sci U S A. 1980;77:5445–5447. doi: 10.1073/pnas.77.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty PH, Leskovar A, Breur GJ, Coates JR, Bergman RL, Widmer WR, Toombs JP, Shapiro S, Borgens RB. A preliminary study of intravenous surfactants in paraplegic dogs: polymer therapy in canine clinical SCI. J Neurotrauma. 2004;21:1767–1777. doi: 10.1089/neu.2004.21.1767. [DOI] [PubMed] [Google Scholar]
- Lee JF, Ozaki H, Zhan X, Wang E, Hla T, Lee MJ. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem Cell Biol. 2006;126:297–304. doi: 10.1007/s00418-006-0143-z. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. Faseb J. 2002;16:950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- Luo J, Borgens R, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. 2002;83:471–480. doi: 10.1046/j.1471-4159.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Borgens R, Shi R. Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury. J Neurotrauma. 2004;21:994–1007. doi: 10.1089/0897715041651097. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170:987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- Omori K, Shikata Y, Sarai K, Watanabe N, Wada J, Goda N, Kataoka N, Shikata K, Makino H. Edaravone mimics sphingosine 1-phosphate-induced endothelial barrier enhancement in human microvascular endothelial cells. Am J Physiol Cell Physiol. 2007 doi: 10.1152/ajpcell.00524.2006. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- Peng X, Sammani S, McVerry BJ, Hassoun PM, Garcia JG. Sphingosine 1-phosphate reduces lung vascular permeability in a murine model of LPS-mediated acute lung injury. Am J Resp Crit Care Med. 2003;167:A778. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- Ragette R, Fu C, Bhattacharya J. Barrier effects of hyperosmolar signaling in microvascular endothelium of rat lung. J Clin Invest. 1997;100:685–692. doi: 10.1172/JCI119581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar Z, Wang P, Ichimura H, Issekutz AC, Quadri S, Bhattacharya J. Hyperosmolarity enhances the lung capillary barrier. J Clin Invest. 2003;112:1541–1549. doi: 10.1172/JCI18370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar Z, Yiming M, Grunig G, Bhattacharya J. Inhibition of acid-induced lung injury by hyperosmolar sucrose in rats. Am J Respir Crit Care Med. 2005;172:1002–1007. doi: 10.1164/rccm.200501-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F, Garcia JG. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- Shi R, Borgens RB. Acute repair of crushed guinea pig spinal cord by polyethylene glycol. J Neurophysiol. 1999;81:2406–2414. doi: 10.1152/jn.1999.81.5.2406. [DOI] [PubMed] [Google Scholar]
- Shi R, Borgens RB. Anatomical repair of nerve membranes in crushed mammalian spinal cord with polyethylene glycol. J Neurocytol. 2000;29:633–643. doi: 10.1023/a:1010879219775. [DOI] [PubMed] [Google Scholar]
- Shi R, Borgens RB, Blight AR. Functional reconnection of severed mammalian spinal cord axons with polyethylene glycol. J Neurotrauma. 1999;16:727–738. doi: 10.1089/neu.1999.16.727. [DOI] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. Faseb J. 2003a;17:2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, Garcia JG. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK and paxillin. J Appl Physiol. 2003b;94:1193–1203. doi: 10.1152/japplphysiol.00690.2002. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity: Role of c-Met, Tiam1/Rac1, dynamin 2 and cortactin. J Biol Chem. 2007 doi: 10.1074/jbc.M702573200. [DOI] [PubMed] [Google Scholar]
- Tinsley JH, De Lanerolle P, Wilson E, Ma W, Yuan SY. Myosin light chain kinase transference induces myosin light chain activation and endothelial hyperpermeability. Am J Physiol Cell Physiol. 2000;279:C1285–1289. doi: 10.1152/ajpcell.2000.279.4.C1285. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S, Bigbee JW, Spiegel S. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol. 2004;166:381–392. doi: 10.1083/jcb.200402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- Wu L, Zaborina O, Zaborin A, Chang EB, Musch M, Holbrook C, Shapiro J, Turner JR, Wu G, Lee KY, Alverdy JC. High-molecular-weight polyethylene glycol prevents lethal sepsis due to intestinal Pseudomonas aeruginosa. Gastroenterology. 2004;126:488–498. doi: 10.1053/j.gastro.2003.11.011. [DOI] [PubMed] [Google Scholar]