Abstract
Patterning of embryonic blood vessels occurs in association with nerves. In this issue of Developmental Cell, Li et al. (2013) report that nerve-derived chemokine Cxcl12 (also known as SDF-1), acting through its receptor Cxcr4, initiates blood vessel remodeling along cutaneous nerve trajectories to establish the proper pattern of cutaneous arteries.
An anatomical and functional relationship that has been known for centuries, yet not well described in a molecular and developmental context, is the close association of certain peripheral nerves and blood vessels. Termed “neurovascular congruence,” the mechanistic basis of this relationship is notably established for sympathetic nerves, in which the growing nerve follows specific guidance cues produced by the vasculature to ultimately reach its appropriate targets (Glebova and Ginty, 2005). In contrast, previous work by Mukouyama and colleagues demonstrated that the arterial pattern of the limb skin is based on the prior growth of the cutaneous nerves (Mukouyama et al., 2002, 2005). In this issue of Developmental Cell, Li et al. (2013) describe the molecular components that underlie this latter relationship.
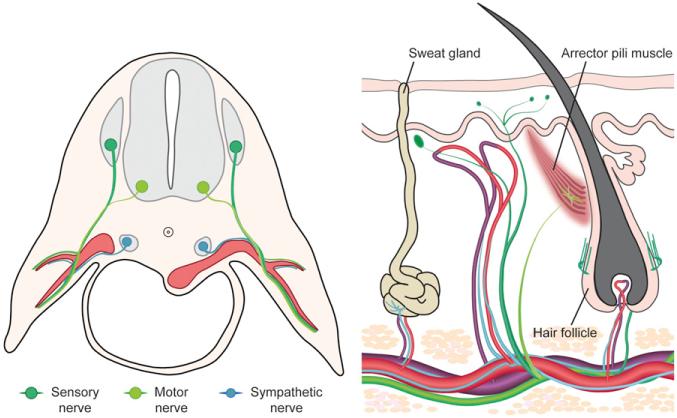
Skin is the largest and the most distant target organ of systemic circulation. Cutaneous arteries are supplied by branches of major arteries of the body and infiltrate the glands, hair follicles, and dermis of the skin. Similarly, venous drainage of the skin is facilitated by cutaneous perforating veins that collect into major veins that ultimately return to the heart. The cutaneous vascular profile generally coincides with cutaneous nerves, which include sensory nerves that sense touch, pain, and temperature, and motor nerves that excite the arrector pili muscles of the hair follicles. Dorsal root sensory nerves and spinal motor nerves together travel long distances along blood vessels that supply the skin. In the skin, these cutaneous nerves arborize and innervate appropriate targets to establish functional neural circuits (Figure 1).
Figure 1. Developmental and Mature Organization of Cutaneous Blood Vessels and Nerves.
Schematic illustrations of the sources of cutaneous (DRG sensory and spinal motor) and sympathetic nerves during development at the time of their initial association with major blood vessels that supply the limb skin (left) and the mature organization of cutaneous blood vessels and nerve endings of the skin (right).
In development, vascular endothelial cells assemble into tubes and then couple into a primitive vascular plexus. This initial vascular pattern becomes remodeled into the mature pattern of larger and smaller arteries and veins by the selective retention and growth of some segments and the loss of others. Mukouyama and colleagues previously used a neurogenin mutant mouse that lacks cutaneous nerves to show that the presence of the nerve is required for cutaneous vascular remodeling (Mukouyama et al., 2002). Subsequent to physical association with cutaneous nerve branches, these vessels acquire an arterial phenotype. These in vivo and in vitro studies identified vascular endothelial growth factor (VEGF-A) as a crucial molecule secreted from the cutaneous nerves that triggers arterial differentiation of nerve-associated vessels, although nerve-derived VEGF is dispensable for the initial recruitment of blood vessels along the nerve (Mukouyama et al., 2005). In the absence of cutaneous innervation, neither blood vessel patterning nor arterial differentiation occurs. Although these studies elucidated the fundamental principle of nerve-vessel alignment in cutaneous arteriogenesis, the molecular mechanisms that establish the initial physical association of blood vessels with nerves remained unknown.
In this recent study, Li et al. (2013) identify Cxcl12 as the critical factor secreted by the nerve that initiates the process of cutaneous vascular remodeling. The authors hypothesized the involvement of chemokine signaling in this process and screened candidate ligands and receptors for expression in dorsal root ganglia(DRG) and skin vascular endothelial cells, respectively. They found that Cxcl12 is expressed in Schwann cells of the cutaneous nerve prior to the reorganization of the cutaneous vascular endothelial plexus. Using an in vitro assay, the authors showed that DRG neurons secrete a soluble activity that induces endothelial cell migration and confirmed using selective inhibitors that Cxcl12 was responsible for this activity. In Cxcl12 mutant embryos or in embryos lacking its receptor Cxcr4, nerve-vessel alignment failed to occur and, for lack of proximity, VEGF-A-driven arterial differentiation also failed to occur. Cxcl12 is well documented to induce endothelial cell migration and vascular assembly (Salcedo and Oppenheim, 2003) but had not previously been described as a factor made by the nervous system to influence vascular biology. The present results regarding Cxcl12, coupled with the authors’ previous observation that VEGF promotes arterial differentiation, account for the two sequential events that result in formation of the cutaneous vascular system and its congruency with the cutaneous nervous system.
In Cxcl12 and Cxcr4 mutant embryos, mesenteric blood vessels that supply the gastrointestinal tract fail to undergo vascular remodeling and arteriogenesis (Ara et al., 2005). This phenotype is similar to the observations now reported by Li et al. (2013) in skin. In principle, the same mechanism of nerve-mediated vascular organization might be involved in arteriogenesis in both organs (and perhaps elsewhere as well). It is important to note, however, that the major arteries and veins supplying the skin, gut, and the rest of the embryo are unaffected by the absence of Cxcl12 (and of nerve-derived VEGF), implying that this mechanism might be limited to smaller peripheral arteries that infiltrate some end-organs.
A vital role of cutaneous circulation and innervation is to regulate body temperature. Excess body heat is removed passively through the skin by the dilation of cutaneous blood vessels and through sweat gland secretory activity. When the body is cold, cutaneous vessels constrict to prevent heat loss, sweat gland activity diminishes, and skin hairs erect (piloerection, or “goose bumps”) to provide an enhanced layer of insulation. The sensation of temperature is relayed by cutaneous sensory nerves to the central nervous system, which then regulates cutaneous vascular tone and sweat gland activity via sympathetic nerves and piloerection via cutaneous motor nerves. The Li et al. (2013) study provides a more comprehensive view of how skin neural circuitry and physiological homeostasis are established. As the cutaneous nerves innervate the skin, their secretion of Cxcl12 and VEGF induces cutaneous vascular organization, which then guides sympathetic axons to reach their appropriate targets (vascular smooth muscle and sweat glands). Functional physiological integration is achieved via these sequential steps of neurovascular interaction during development.
REFERENCES
- Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. Blood. 2005;105:3155–3161. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Annu. Rev. Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou Y, Nagasawa T, Mukouyama YS. Dev. Cell. 2013;24(this issue):359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Oppenheim JJ. Microcirculation. 2003;10:359–370. doi: 10.1038/sj.mn.7800200. [DOI] [PubMed] [Google Scholar]