Abstract
Double-stranded probes are homogeneous biosensors for rapid detection of specific nucleotide sequences. These double-stranded probes have been applied in various molecular sensing applications, such as real-time polymerase chain reaction and detection of bacterial 16S rRNA. In this study, we present the design and optimization of double-stranded probes for single-cell gene expression analysis in living cells. With alternating DNA/LNA monomers for optimizing the stability and specificity, we show that the probe is stable in living cells for over 72 hours post-transfection and is capable of detecting changes in gene expression induced by external stimuli. The probes can be delivered to a large number of cells simultaneously by cationic liposomal transfection or to individual cells selectively by photothermal delivery. We also demonstrate that the probe quantifies intracellular mRNA in living cells through the use of an equilibrium analysis. With its effectiveness and performance, the double-stranded probe represents a broadly applicable approach for large-scale single-cell gene expression analysis toward numerous biomedical applications, such as systems biology, cancer, and drug screening.
Introduction
The investigation of complex biological processes, such as tissue morphogenesis, collective cell migration, and gene regulatory networks, can be benefited tremendously by novel biosensing techniques with high spatiotemporal resolution.1 In particular, molecular probes with qualities including high stability, sensitivity, and specificity are highly sought-after for long-term monitoring of gene expression in individual cells. Investigating the dynamic regulation of gene expression, for instance, requires single-cell analysis techniques to monitor the dynamics of cellular responses. While single-cell polymerase chain reaction (PCR) and high-throughput microfluidic analysis are available to measure gene expression down to the single-cell level,2, 3 the requirement of cell lysis prior to measurement introduces challenges in analyzing the dynamics and spatial distribution of genes for a large number of cells. Thus, innovative biosensing techniques are required to address these challenges in investigating complex biological systems.
Molecular beacon, as an example, has been developed for gene expression analyses in living cells and has been broadly utilized in various biological applications.4–10 A molecular beacon is a short hairpin oligonucleotide that binds to a specific target oligonucleotide sequence and produces a fluorescence signal. For intracellular detection, the stability of molecular beacons is a major consideration particularly when they are employed in long-term gene expression monitoring. DNA sequences encompassing molecular beacon probes are vulnerable to nuclease digestion and nonspecific binding by DNA binding proteins. These processes can in turn generate false-positive signals. To overcome the stability issue, nuclease-resistant nucleic acid monomer such as 2’-O-methyl RNA,11, 12 peptide nucleic acids (PNA),13, 14 and locked nucleic acids (LNA)15, 16 have been incorporated into the molecular beacon design. For instance, LNA and 2’-O-methyl bases have been shown to improve the stability of molecular beacons inside living cells, while retaining similar or better solubility and molecular recognition features to natural DNA. In the case of LNA, alternating DNA/LNA bases has been demonstrated to balance between the binding affinity and the stability of the probes, resulting in enhanced performance for intracellular measurement.17
Another homogeneous scheme for sensing specific nucleic acid sequences is the double-stranded probe, which consists of two complementary oligonucleotide sequences labeled with a fluorophore and a quencher at the 5' and 3' ends.18–21 When the probes enter a cell lacking the probe's specific target mRNA, the donor and quencher sequences remain in close proximity, diminishing the fluorescence signal. In the presence of the target mRNA, the quencher sequence is displaced from the donor sequence due to the thermodynamically driven binding event between the donor and the target sequences (Figure 1a–b). This separation between quencher and donor sequences enables the fluorophore to fluoresce. When compared with molecular beacons, the double-stranded probe has several advantages including the possibility of adjusting the quencher-to-fluorophore ratio for noise minimization as well as the flexibility of modifying the length of the quencher sequence to improve the specificity and kinetics of the assay.18 The double-stranded probe has been successfully demonstrated for single base mismatch discrimination,22 transcription factor detection,23, 24 quantification of bacterial 16S rRNA,20 and real-time PCR.25 Nevertheless, several key aspects of the double-stranded probe for intracellular gene expression measurement have not been explored and optimized systematically. In particular, the feasibility of adopting nuclease-resistant building blocks in the double-stranded probe should be evaluated to improve the long-term stability and specificity of the probe in living cells. Furthermore, methods for high-throughput delivery to multiple cells simultaneously and selective delivery to individual cells rapidly should be developed for elucidating complex biological processes. The ability and specificity of using double-stranded probes for measuring inducible genes should also be evaluated. More importantly, the applicability of the double-stranded probe for the quantification of intracellular mRNA needs to be explored toward systems investigation of biological processes.
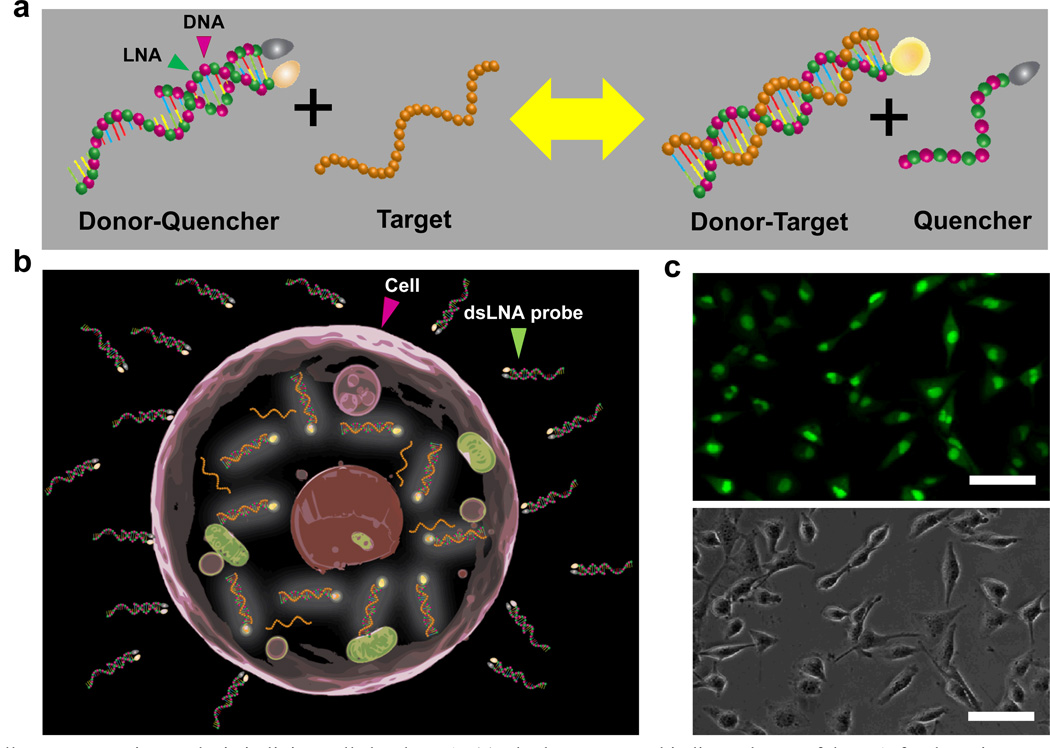
Fig. 1.
Single-cell gene expression analysis in living cells by dsLNA. (a) The homogenous binding scheme of dsLNA for detecting a nucleic acid target. (b) Detection of intracellular mRNA by dsLNA probes. (c) Fluorescence (top) and bright-field (bottom) images of MDA-MB-231 cells transfected with dsLNA probes targeting β-actin mRNA. Scale bars, 50 µm.
In this study, we adopt LNA monomers in the design of double-stranded probes (dsLNA) for monitoring intracellular gene expression at the single-cell level. In particular, we design dsLNA probes targeting β-actin, heme oxygenase-1 (HO-1), heat shock protein 70 (HSP70), and a random sequence control by incorporating alternating DNA/LNA bases with a goal of optimizing their stabilities and specificity. The stability of the dsLNA probes are compared with other nucleotide modifications. We also optimize the procedures for transfecting dsLNA with cationic liposomal transfection reagents for detecting the gene expression in a large number of cells concurrently, which can be used to study how cells respond to their environment, interact with each other, and undergo other complex biological processes. We also explore a photothermal nanoblade technique for delivery to individual cells selectively. To evaluate the ability of dsLNA probes to detect inducible genes, we carry out tert-butylhydroquinone (tBHQ) treatment and temperature elevation studies to induce HO-1 and HSP70 gene expression, respectively. The behaviors of the probe inside living cells are studied systematically by transfecting donor-quencher (DQ) duplex and donor-target (DT) duplex into the cells. By considering an equilibrium analysis, we evaluate the feasibility of quantifying the mRNA concentration in living cells using dsLNA probes.
Materials and methods
Probe Design and preparation
A dsLNA probe consists of two oligonucleotide sequences for rapid detection of specific nucleic acid sequences. A donor sequence complementary to a target mRNA is designed and is synthesized with alternating DNA/LNA monomers. The donor sequence is labeled with a fluorophore (6-FAM) located at the 5’ end. For homogeneous detection of the target gene, a shorter, alternating DNA/LNA sequence complementary to the donor sequence is designed and labeled with a quencher (Iowa black) at its 3' end. To compare its stability, two other double-stranded probes were designed consisting of DNA only or 2'-O-methyl RNA. All nucleic acid probes and corresponding target sequences in this study were synthesized by Integrated DNA Technologies Inc. Three different probes targeting β-actin, HO-1 and HSP70 mRNAs were designed based on sequences from the NCBI GenBank database (Table 1). A random probe sequence not significantly complementary to any known intracellular mRNA was designed as a negative control. The specificity of all probes sequences were evaluated by the NCBI Basic Local Alignment Search Tool (BLAST) database. Before introducing the probe to the cells, the dsLNA probes were prepared by mixing donor and quencher sequences in a buffer of 10 mM Tris-EDTA and 0.2 M NaCl. After 5 minutes of incubation in a water bath at 95°C, the probes were allowed to slowly cool down to room temperature over the course of 3 hours before they were ready to transfect into cells and detect the gene of interest. A low background noise was achieved at a ratio of 1 donor to 2.5 quencher sequences and the ratio was used in this study.
Table 1.
Sequence of probes with alternating DNA/LNA monomer modification in this study. Bold italic letters indicates LNA monomers.
| Target Gene | Probe Type | Length (base) | ΔGDQ (kcal/mol) | ΔGDT (kcal/mol) | |
|---|---|---|---|---|---|
| β-actin | Donor (D) | 20 | −10.9 | −25.8 | |
| Quencher (Q) | 10 | ||||
| Target (T) | 5’-/CTCTTCCAGCCTTCCTTCCT/-3’ | 20 | |||
| HO-1 | Donor (D) | 20 | −11.8 | −26.6 | |
| Quencher (Q) | 10 | ||||
| Target (T) | 5’/AACAAGGAGAGCCCAGTCTT/3’ | 20 | |||
| HSP70 | Donor (D) | 20 | −10.2 | −24.9 | |
| Quencher (Q) | 10 | ||||
| Target (T) | 5’-/ATCACCATCACCAACGACAA/-3’ | 20 | |||
| Random | Donor (D) | 20 | −11.1 | −26.2 | |
| Quencher (Q) | 10 | ||||
| Target (T) | 5’/TATCGGTGCGCTTGTCGCGT/3’ | 20 |
Cell Culture and transfection of the probe
Human mammary gland adenocarcenoma, MDA-MB-231, was obtained from ATCC (Manassas, VA). Cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO), 2 mM HEPES buffer, and antibiotics (0.1% Gentamycin, Sigma). Cells were stored at 37°C in a humidified incubator containing 5% CO2. Cells were plated overnight in 24-well plates at an initial concentration of 105 cells/ml before transfection. Once reaching 90–95% confluency, cells were transfected with 1.0 µg probe at different transfection reagent ratios. Transfection reagents, including Lipofectamine, FuGENE HD, and Lipofectamine 2000 in opti-MEM (Invitrogen, Grand Island, NY) were tested to assess the probe transfection efficiency. In other experiments, cells were transfected with Lipofectamine 2000 to measure intracellular gene expression (Figure 1c).
Delivery with the photothermal nanoblade was performed as described.26, 27 Briefly, a Nd:YAG laser (Minilite I; Continuum) operated at 532 nm wavelength and 6 ns pulsewidth was applied to induce a ultrafast cavitation bubble near a titanium-coated micropipette. The cavitation bubble locally ruptured the cell membrane to allow delivery of the dsLNA probe to the cells with high efficiency and viability. With a liquid delivery system synchronized with the pulsed laser, a volume of ~1 pL of the dsLNA probe solution was delivered to the cells.
Inducible gene detection
To examine the ability of dsLNA probe to detect gene expression changes in cells, experiments were performed utilizing either chemical or physical stimuli to upregulate the target gene of interest. In the chemical stimulation experiment, cells transfected with dsLNA probes were treated with 50 µM tBHQ, a known HO-1 activator, for 16 hours prior to taking measurements.28 To induce changes of HSP70 mRNA, a heat-responsive gene,29 the temperature was increased from 37°C to 42°C for 1.5 hours. The cells were then incubated at 37°C for 5 hours before the intensity measurement.
Intracellular probe concentration calibration
Two different experiments were performed using random donor probes hybridized with the synthetic targets, i.e., random donor-target (DT) duplex, to estimate the probe concentration transfected into a cell. We first plated cells on a cover slip overnight. Cells were transfected with a random DT duplex and intensity measurements were performed after 12 hours. Since the random probe sequence has no complementary target gene inside the cell, intracellular signals were primarily due to the DT duplex transfected. In the second experiment, we placed different concentrations of a pre-hybridized random DT duplex between two cover slips. It is known that the height of breast cancer cells is typically ~5 µm.30 We introduced the random DT duplex to create a 5 µm-thick sample of solution between two cover slips. We measured the intensity levels of the random DT duplex at different concentrations to obtain a linear calibration curve to quantify the probe concentration inside a cell. The donor-quencher (DQ) probe concentration is normalized for the differences in intensity and transfection efficiency of DQ and DT probes. By measuring the cell spreading area, we estimated the average cell volume to be ~4600 µm3. The average concentration of dsLNA probes in the cells can then be estimated and applied for obtaining the standard curve to quantify intracellular mRNA in living cells.
Equilibrium Analysis
An equilibrium analysis has been previously demonstrated to describe the probe-target binding dynamics without any fitting parameters.22 The dsLNA probe can be represented by two coupled reversible binding reactions (equations (1) and (2)).
| (1) |
| (2) |
where D is the donor probe, Q is the quencher probe, and T is the target. DQ and DT are the fluorophore–quencher probe and the fluorophore–target duplex. These binding reactions lead to equilibrium constant equations (3) and (4), where KDQ and KDT are the equilibrium constants of donor–quencher and donor–target hybridizations respectively. The equilibrium constants depend on the Gibbs free energy change, ΔG, associated with the hybridization of the complementary probe sequences. The equilibrium constant can be estimated by K = e−ΔG/RT where R and T are the gas constant and the temperature respectively. Also, the mass conservation law results in equations (5) and (7), where D0, Q0, and T0 represent the initial concentrations of donor, quencher and target sequences.
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
In our experiment, the concentration of the donor is smaller than the concentration of the quencher, i.e., D << Q; therefore, we assume that Q = Qo. By solving equations (3)–(7), the equilibrium concentrations of the free donor [D], the donor–quencher [DQ], and the donor–target [FT] are given by equations (8)–(10).
| (8) |
| (9) |
| (10) |
where M = 1 + QKDQ + KDTTo - DoKDT, and N = KDT + QKDQ KDT. At a low background level, the fluorescence signal can be determined by equation (10) according to the target concentrations. The free energy change (and the equilibrium constant) is directly estimated by Mfold based on the probe sequence and experimental conditions.31 In this study, all three dsLNA probe designs have the same length of donor and quencher sequences (Table 1). In the calculation, we ignored the slight differences in free energy among the probes and apply one standard curve for the quantitative estimation of the concentration of intracellular mRNA.
Imaging and data analysis
Probe intensities were monitored with an inverted fluorescence microscope (TE2000-U, Nikon) and fluorescence images were acquired using a CCD camera (SensiCamQE, Cook corp.) at different time points. All images were taken with a 1.0 second exposure time under the same conditions in order to compare the relative fluorescence intensity. In the photothermal nanoblade experiment, bright field and fluorescence images were captured using a Zeiss Axiovert 40CFL inverted fluorescence microscope with a Canon digital camera. Data collection and imaging analysis were performed using the NIH ImageJ software. Approximately 100 cells were measured for each set of experiments and the data are expressed as mean ± SEM.
Results
Nucleic acid modification of double-stranded probes
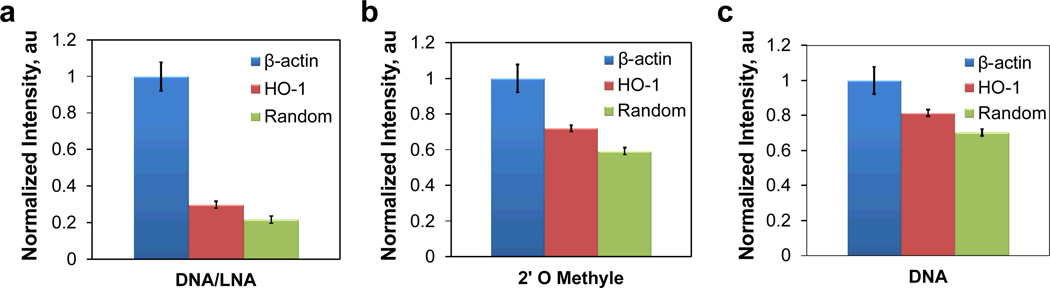
In order to optimize the probe for intracellular RNA detection in living cells, we first studied the signal-to-noise ratio of double-stranded probes with alternating DNA/LNA, 2’-O-methyl RNA, and DNA bases targeting β-actin, HO-1, and random sequences. β-actin mRNA, which is a housekeeping gene, has a large number of copies in the cell, and therefore is anticipated to show a high intensity. In contrast, the random probe, with no complementary intracellular mRNA target in the cell, represents the background noise inherent to the assay. The intensities of the probes in the cytoplasm are presented in Figure 2. Figure 3 shows images of cells transfected with dsLNA probes for intracellular mRNA detection in the cytoplasm. The dsLNA probe with alternating DNA/LNA monomers displayed signal-to-noise ratios over 8:1 and 4:1 for detecting β-actin and HO-1 mRNA relative to the control (Figure 2a). By comparison, the probe with 2’O methyl RNA modification showed a lower signal-to-noise ratio (less than 2), while the DNA probe had the lowest signal-to-noise ratio of ~1.4 (Figure 2b–c). The low signal-to-noise ratios of 2’O methyl RNA and DNA probes are possibly due to the non-specific binding of probes and nuclease degradation.17, 32 The data show that the probe with alternating DNA/LNA monomer has the highest signal-to-noise ratio. The result is consistent with previous data showing the exceptionally high endurance of LNA modified probes16 and demonstrates the applicability of LNA in double-stranded probes for intracellular detection.
Fig. 2.
Normalized fluorescence intensities of double-stranded probes in the cytoplasm with different modifications: (a) alternating DNA/LNA, (b) 2’-O-methyl RNA, and (c) DNA. The probes are designed to specifically detect β-actin and HO-1 mRNAs. A random sequence is designed as the control.
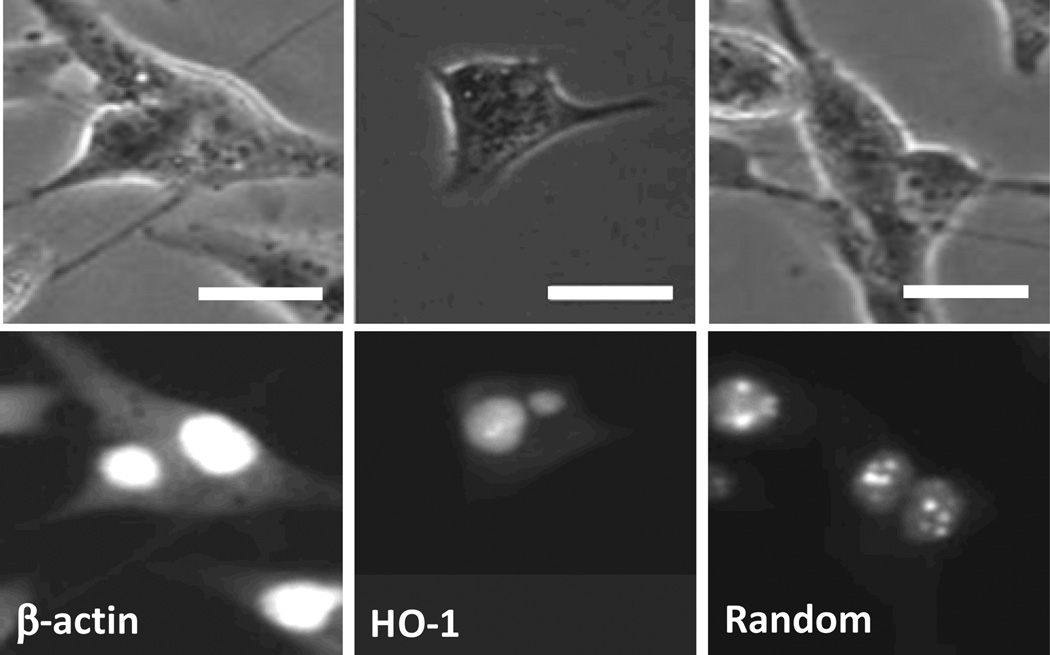
Fig. 3.
Bright-field (top) and fluorescence (bottom) images of cells transfected with dsLNA probes targeting β-actin mRNA, HO-1 mRNA, and random sequence. Scale bars, 20 µm.
Intracellular delivery of dsLNA probes
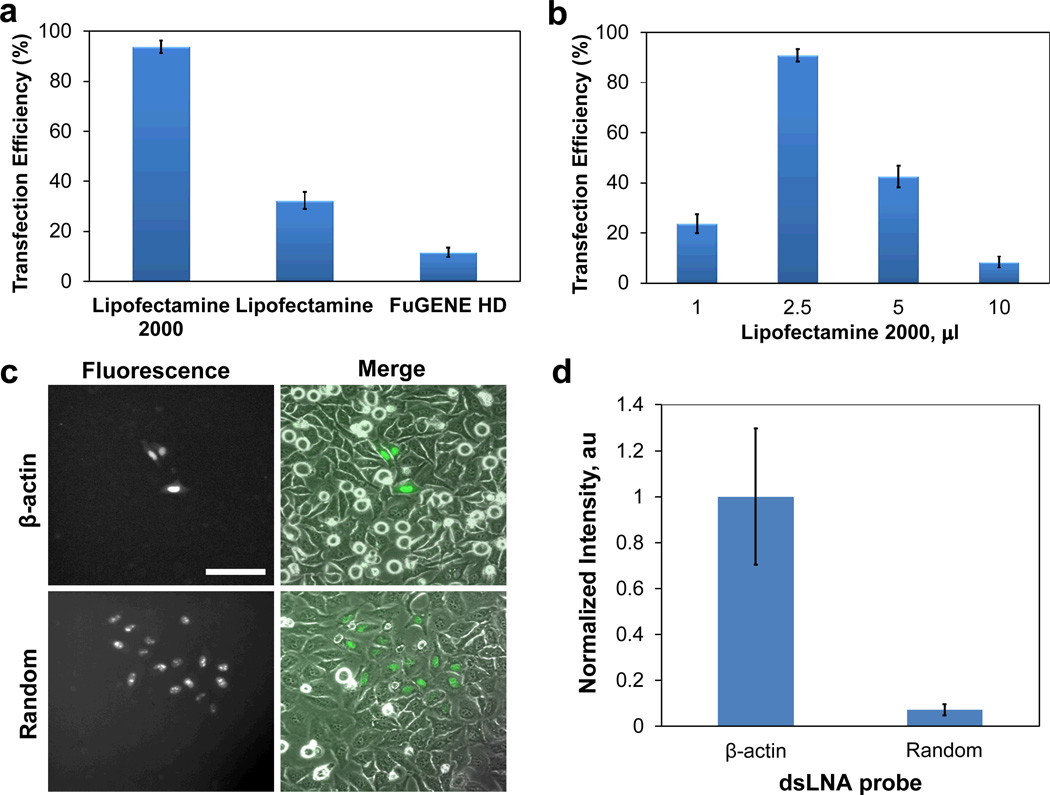
To allow simultaneous detection of a large number of cells, different transfection reagents were evaluated to maximize the transfection efficiency. Figure 4a illustrates the transfection efficiencies achieved using different transfection reagents for delivering the dsLNA probes. Transfection efficiency was measured as the percentage of cells transfected. Lipofectamine gave 35% transfection efficiency while FuGENE HD resulted in 10% transfection efficiency in our experimental condition. Lipofectamine 2000 achieved over 95% transfection efficiency, by far the best of the transfection reagents tested for transfecting dsLNA. In addition to choosing the proper transfection reagent for the study, the optimal reagent-to-probe ratio was also established to maximize the transfection efficiency.33 We fixed the probe to 1.0 µg and applied various amounts of Lipofectamine 2000. An optimal value of 2.5 µl Lipofectamine 2000 was observed to achieve over 95% transfection efficiency (Figure 4b). Increasing or decreasing this value resulted in a reduction of the transfection efficiency.
Fig. 4.
Delivery of dsLNA probes to cells. Transfection efficiency with (a) different transfection reagents and (b) different amounts of Lipofectamine 2000 per 1.0 µg dsLNA probe. (c) Photothermal delivery of dsLNA probes targeting β-actin mRNA and random sequence to HeLa cells. Scale bars, 100 µm. (d) Normalized fluorescence intensities of dsLNA probes in the cytoplasm delivered by photothermal nanoblade.
Investigation of complex biological systems, such as cell tracking and probing intracellular transportation, can be benefited by selective delivery of molecular probes. For instance, molecular beacons are often delivered to living cells by microinjection. Cells can function normally in the experiments and the results are comparable to reverse transcription PCR.34, 35 However, the microinjection technique could be invasive, slow, and labor intensive. To address the requirement of single-cell delivery, we explored delivery of dsLNA probes by photothermal nanoblade, which has shown to have high efficiency and cell viability.26, 27 The dsLNA probe can be delivered to the cells with high efficiency. Figure 4c shows photothermal delivery of dsLNA probes to three individual cells selectively. The result is in agreement with previous photothermal nanoblade studies that the efficiency for delivering small molecules can be close to 100%.26, 27 Furthermore, β-actin probe displayed a high intensity in the cytoplasm compared to the random probe, suggesting specific detection of intracellular mRNA (Figure 4d). These results show the robustness of dsLNA using different delivery approaches and demonstrate the applicability of photothermal nanoblade for delivering dsLNA.
Inducible Gene Detection
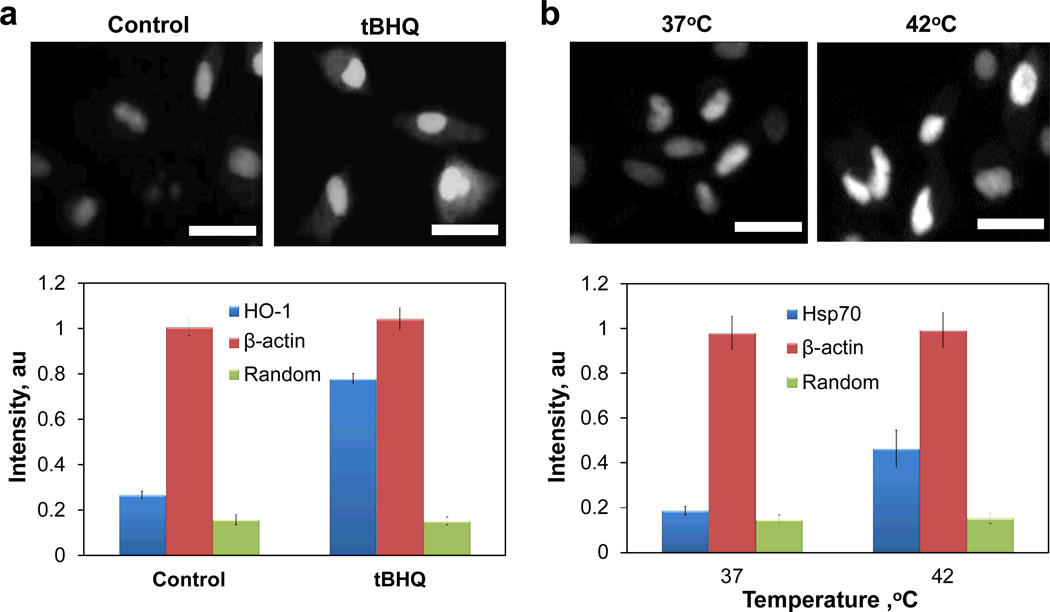
Experiments were performed to evaluate the dsLNA probe’s ability to detect inducible genes in living cells specifically. Previous reports have shown that treating MDA-MB-231 cells with tBHQ can upregulate heme oxygenase-1 (HO-1) mRNA through the Nrf2 signaling pathway.28 Figure 5a shows the intensity level of probes inside the cell after 50 µM tBHQ treatment. Data revealed that the β-actin and random probe intensity levels in the cytoplasm remained constant, while the value for the HO-1 probe increased significantly with the addition of tBHQ. An experiment was also designed to detect HSP70 mRNA induced by elevated temperature (Figure 5b). The HSP70 probe intensity increased when the cells were heated at 42°C for 1.5 hours followed by cultured at 37°C for 5 hours post-heating. The intensities for other probes tested remained stable throughout the duration of experiment. These data demonstrate the specificity of the dsLNA probe and verify its ability to detect the expression of inducible genes in living cells.
Fig. 5.
Detection of inducible genes with dsLNA probes. (a) Fluorescence intensities of dsLNA probes in cells treated with or without 50 µM tBHQ. (b) Fluorescence intensity of dsLNA probes in cells cultured at 37 or 42°C. Scale bars, 20 µm.
Intracellular stability and dynamics of the dsLNA probe
To evaluate the stability and the dynamics of the dsLNA probes in cells, experiments were performed using the donor-quencher (DQ) probe as well as the donor-target (DT) duplex, in which the donor probe was pre-hybridized with the complementary synthetic target prior to cell transfection. Figure 6 shows the normalized fluorescence intensity of the β-actin and random probes over 4 days. The random DQ probe expressed a low fluorescence intensity throughout the duration of the experiment in the cytoplasm (Figure 6a). In contrast, the β-actin DQ probe showed a high signal in the cytoplasm that endured over a long period of time. Specifically, the β-actin DQ probe intensity reached a stable value at 12 hours after transfection and maintained a constant value for 60 hours before the intensity gradually decreased. The drop off in the intensity level after 72 hours is possibly due to probe degradation and the dilution effect of cell proliferation. In the nucleus, however, both the β-actin and random DQ probes behaved differently (Figure 6b). For the β-actin probe, the intensity level was maximized at 54 hours while the random probe signal peaked at 12 hours after transfection, suggesting different intracellular dynamics of the specific and non-specific probes. The observed fluorescence of the random DQ probe in the nucleus was significantly elevated compared to the fluorescence in the cytoplasm. This observation suggests that there could be degradation and other molecular interactions of the probe in the nucleus. The data also revealed that the β-actin probe signal reached levels more than double that of the random probe in the nucleus, suggesting that there could be target gene binding in the nucleus. This is consistent with the observation that the β-actin probe has a high intensity throughout the nucleus whereas the random probe is rapidly sequestered and localized in the nucleoli (Figure 3).36 Figure 6c–d shows the DT duplex in the cytoplasm and nucleus. As expected, the pre-hybridized β-actin and random DT duplexes fluoresce at comparable levels, regardless of their presence in the nucleus or cytoplasm. While the relative fluorescence of the DT duplex in the nucleus is 3 times higher than the value in the cytoplasm, the fluorescence intensity of the duplexes increases at similar speeds in both β-actin and random probes. Furthermore, the maximum intensity of both DT duplexes occurred at 12 hours post-transfection in the cytoplasm whereas this peak was observed in the nucleus 24 hours after transfection. The results further support a kinetic transition of double-stranded probes from the cytoplasm to the nucleus.
Fig. 6.
Kinetics of dsLNA probes in living cells. (a–b) Fluorescence intensities of donor-quencher (DQ) probes in the cytoplasm (a) and nucleus (b). (c–d) Fluorescence intensities of of the donor probe pre-hybridized with synthetic target (DT) in the cytoplasm (c) and nucleus (d).
Quantification of intracellular mRNA
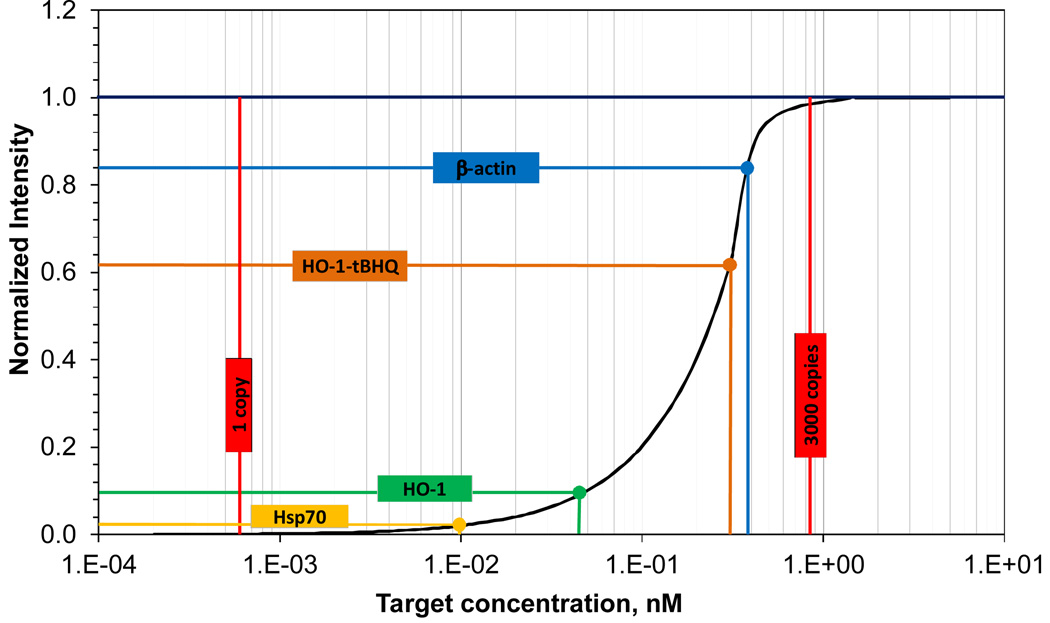
The intracellular probe concentration is required to estimate the target mRNA concentration using the equilibrium analysis. To determine the intracellular probe concentration, a serial dilution experiment was performed on cover slips for calibrating the probe intensity. A linear intensity calibration curve was then determined to correlate the fluorescence intensity and the probe concentration. Based on the calibration curve normalized for DQ, we estimated the average DQ probe concentration in the cytoplasm was 0.49 nM. Applying this value to the equilibrium analysis, we obtained a standard curve for quantitative measurements of mRNAs in living cells (Figure 7). The dsLNA probe has a dynamic range of 2–3 orders of magnitude, which is sensitive between 1 pM and 1 nM (equivalent ~3 to ~3000 copies of mRNA per cell). Using this standard curve, the average concentration and copy number of mRNA inside the cells were estimated based on the fluorescence intensity. In particular, 1,080 copies of β-actin mRNA (~0.38 nM) were estimated to be inside a cell. This value is in good agreement with a previous report that there are ~1,400 copies of β-actin mRNA in a cell, despite the differences in the experimental conditions.37 For cells treated with and without tBHQ, the normalized intensities of the HO-1 probes were 0.63 and 0.10 respectively, which represented a 7-fold difference in the concentration of HO-1 mRNA from 0.32 nM to 0.045 nM. This value is also in reasonable agreement with quantitative real-time PCR data, which showed an 11-fold concentration increase in HO-1 after tBHQ treatment, with the same cell type and experimental conditions.28 Furthermore, approximately 27 copies (~0.0095 nM) of HSP70 mRNA molecules are inside a cell based on the standard curve. This value is consistent with gel electrophoresis measurement that ~20 copies of HSP70 mRNA are expressed in each HeLa cell.38 These results collectively demonstrate the potential of the dsLNA probes for quantitative measurements in living cells.
Fig. 7.
Standard curve of the dsLNA probe for quantitative measurements of intracellular nRNA. The standard curve is estimated based on the equilibrium analysis with 0.49 nM probe transfected into the cytoplasm. The normalized intensities of β-actin, HO-1 and Hsp70 probes measured in the experiment are indicated in the figure.
Discussion
Developing efficient biosensing approaches for measuring gene expression in individual living cells will have profound impacts on various biomedical applications. This study focuses on the optimization of the double-stranded probe to address several key issues, including specificity, stability, and probe delivery, toward intracellular gene expression detection in living cells. Our data suggest that the dsLNA probes were not only able to detect specific genes inside a cell over time, but also sense intracellular changes in inducible genes due to applied physical or chemical stimuli. In concert with molecular beacon studies,16, 17, 39 our results suggest the effectiveness of alternating DNA/LNA monomers in double-stranded probes for providing a high resistance to nuclease digestion as well as protection against non-specific binding. By incorporating LNA monomers, the signal-to-noise ratio of the probe is significantly improved compared to DNA and 2’O methyl RNA. In general, the signal-to-noise ratio of the dsLNA probe for intracellular detection is similar to molecular beacons. Under the same condition, the dsLNA probe can often display better sensitivity and kinetics due to the ability to adjust the quencher-to-fluorophore ratio as well as the quencher length. Furthermore, the stability of LNA allows intracellular detection with the dsLNA probe for over three days. The long term stability is a critical aspect for studying many complex biological processes, which often last for days. In addition to the stability, probe delivery is another important factor to be considered. Using the optimized conditions for liposomal transfection, over 95% of cells can be transfected simultaneously allowing large scale single-cell analysis. Furthermore, we demonstrate rapid delivery of dsLNA probe to individual cells by photothermal nanoblade. Compared to microinjection, photothermal nanoblade allows rapid delivery with high efficiency and cell viability. These capabilities will enable the dsLNA probe to be applied in a diverse set of applications.
Our results have also revealed two fundamental aspects of the dsLNA probe. We observed a size dependence on the kinetics of nuclear transportation of the dsLNA probe. The random probe translocated into the nucleus significantly faster than the β-actin probe. This observation can be explained by the lack of an intracellular complementary target sequence with the random probe, allowing the probe to freely pass into the nucleus through nuclear pore complexes.40 In comparison, the large β-actin mRNA hybridized with the β-actin probe and kept it in the cytoplasm for an extended period of time before entering into the nucleus. The size dependence on nuclear transportation was further supported by the experiment where a pre-hybridized DT duplex was transfected into the cells. The DT probes entered the nucleus at the same rate for the β–actin and random probes. The size dependence provides a possible explanation of the stability of the dsLNA probes over molecular beacons, which is generally smaller than the dsLNA probes. In fact, it has been reported that molecular beacons in MDA-MB-231 has a finite life span of ~30 min and conventional molecular beacons are typically stable in cells for less than 24 hours.41 Secondly, our results demonstrate quantitative detection of intracellular mRNA in living cells. We have shown comparable results with quantitative real-time PCR under the same experimental conditions. Our results are also in reasonable agreement with other cell types and techniques, such as gel electrophoresis and real-time PCR. Quantitative detection in living cells has not been demonstrated in either molecular beacons or double-stranded probes. The ability for quantitative measurement in living cells will enable a new paradigm in quantitative systems biology and dynamic investigation of complex biological systems.
Conclusion
In summary, this study establishes the double-stranded probes for intercellular gene expression analysis. Our results demonstrate that dsLNA probes can continuously monitor and quantify mRNA in living cells for days. We have also demonstrated quantification of the number of mRNA in viable cells. Overall, the double-stranded probe represents a powerful approach toward the long-term monitoring of intracellular RNA molecules and carries the potential for use in a wide spectrum of biomedical applications in the future.
Acknowledgements
This work is supported by NIH Director's New Innovator Award (1DP2OD007161-01) and NSF (0900899).
Notes and references
- 1.Ottino JM. Nature. 2004;427:399. doi: 10.1038/427399a. [DOI] [PubMed] [Google Scholar]
- 2.Lecault V, White AK, Singhal A, Hansen CL. Current Opinion in Chemical Biology. 2012;16:381–390. doi: 10.1016/j.cbpa.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi K, Kajiyama T, Kambara H. Nature methods. 2009;6:503–506. doi: 10.1038/nmeth.1338. [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Tang Z, Yang CJ, Kim Y, Fang X, Li W, Wu Y, Medley CD, Cao Z, Li J, Colon P, Lin H, Tan W. Angew Chem Int Ed Engl. 2009;48:856–870. doi: 10.1002/anie.200800370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TH, Peng YH, Zhang CY, Wong PK, Ho CM. Journal of the American Chemical Society. 2005;127:5354–5359. doi: 10.1021/ja042642i. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, Sohn D, Tan W. International journal of clinical and experimental pathology. 2008;1:105–116. [PMC free article] [PubMed] [Google Scholar]
- 7.Santangelo PJ, Nitin N, Bao G. Journal of Biomedical Optics. 2005 doi: 10.1117/1.2011402. 10, - [DOI] [PubMed] [Google Scholar]
- 8.Tyagi S, Kramer FR. Nature Biotechnology. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 9.Marras SAE, Tyagi S, Kramer FR. Clinica Chimica Acta. 2006;363:48–60. doi: 10.1016/j.cccn.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Wong PK. Bioanalysis. 2010;2:1689–1699. doi: 10.4155/bio.10.116. [DOI] [PubMed] [Google Scholar]
- 11.Chen AK, Behlke MA, Tsourkas A. Nucleic Acids Research. 2008:36. doi: 10.1093/nar/gkn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majlessi M, Nelson NC, Becker MM. Nucleic Acids Research. 1998;26:2224–2229. doi: 10.1093/nar/26.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen K, Vogel U, Rockenbauer E, Nielsen KV, Kolvraa S, Bolund L, Nexo B. Molecular and Cellular Probes. 2004;18:117–122. doi: 10.1016/j.mcp.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Seitz O. Angewandte Chemie-International Edition. 2000;39 3249-+ [Google Scholar]
- 15.Campbell MA, Wengel J. Chemical Society Reviews. 2011;40:5680–5689. doi: 10.1039/c1cs15048k. [DOI] [PubMed] [Google Scholar]
- 16.Wu YR, Yang CJ, Moroz LL, Tan WH. Analytical Chemistry. 2008;80:3025–3028. doi: 10.1021/ac702637w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CJ, Wang L, Wu YR, Kim YM, Medley CD, Lin H, Tan WH. Nucleic Acids Research. 2007;35:4030–4041. doi: 10.1093/nar/gkm358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gidwani V, Riahi R, Zhang DD, Wong PK. Analyst. 2009;134:1675–1681. doi: 10.1039/b906077d. [DOI] [PubMed] [Google Scholar]
- 19.Li QQ, Luan GY, Guo QP, Liang JX. Nucleic Acids Research. 2002:30. doi: 10.1093/nar/30.2.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riahi R, Mach KE, Mohan R, Liao JC, Wong PK. Analytical Chemistry. 2011;83:6349–6354. doi: 10.1021/ac2012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison LE, Halder TC, Stols LM. Anal Biochem. 1989;183:231–244. doi: 10.1016/0003-2697(89)90473-9. [DOI] [PubMed] [Google Scholar]
- 22.Meserve D, Wang ZH, Zhang DD, Wong PK. Analyst. 2008;133:1013–1019. doi: 10.1039/b804853c. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Gidwani V, Zhang DD, Wong PK. Analyst. 2008;133:998–1000. doi: 10.1039/b809113g. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Gidwani V, Sun Z, Zhang DD, Wong PK. Journal of Association for Laboratory Automation. 2008;13:243–248. [Google Scholar]
- 25.Kennedy B, Arar K, Reja V, Henry RJ. Anal Biochem. 2006;348:294–299. doi: 10.1016/j.ab.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Wu TH, Teslaa T, Teitell MA, Chiou PY. Optics Express. 2010;18:23153–23160. doi: 10.1364/OE.18.023153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu TH, Teslaa T, Kalim S, French CT, Moghadam S, Wall R, Miller JF, Witte ON, Teitell MA, Chiou PY. Analytical Chemistry. 2011;83:1321–1327. doi: 10.1021/ac102532w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XJ, Zhang DD. Plos One. 2009:4. doi: 10.1371/journal.pone.0005492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Diller KR, Aggarwal SJ. J Biomech Eng. 2003;125:794–797. doi: 10.1115/1.1632522. [DOI] [PubMed] [Google Scholar]
- 30.Mendez MG, Kojima S, Goldman RD. FASEB J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuker M. Nucleic Acids Research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molenaar C, Marras SA, Slats JCM, Truffert JC, Lemaitre M, Raap AK, Dirks RW, Tanke HJ. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.17.e89. art. no.-e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, Ciccarone VC. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Tang HX, Yang XH, Wang KM, Tan WH, Liu B, He LF, Wang W. Chinese Sci Bull. 2008;53:357–361. [Google Scholar]
- 35.Perlette J, Tan WH. Analytical Chemistry. 2001;73:5544–5550. doi: 10.1021/ac010633b. [DOI] [PubMed] [Google Scholar]
- 36.Nitin N, Bao G. Bioconjugate chemistry. 2008;19:2205–2211. doi: 10.1021/bc800322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjarnadottir H, Jonsson JJ. J Biotechnol. 2005;117:173–182. doi: 10.1016/j.jbiotec.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Wu BJ, Hurst HC, Jones NC, Morimoto RI. Molecular and Cellular Biology. 1986;6:2994–2999. doi: 10.1128/mcb.6.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Yang CYJ, Medley CD, Benner SA, Tan WH. Journal of the American Chemical Society. 2005;127:15664–15665. doi: 10.1021/ja052498g. [DOI] [PubMed] [Google Scholar]
- 40.Terry LJ, Shows EB, Wente SR. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 41.Medley CD, Drake TJ, Tomasini JM, Rogers RJ, Tan W. Anal Chem. 2005;77:4713–4718. doi: 10.1021/ac050881y. [DOI] [PubMed] [Google Scholar]