Abstract
New adjuvants and delivery strategies are needed to optimize the ability of protein-based vaccines to elicit CD8+ T cell responses. We have developed a model vaccine formulation containing ovalbumin (OVA) and the double-stranded RNA analog poly(inosinic acid)–poly(cytidylic acid) (poly(I:C)), a TLR3 agonist. OVA and poly(I:C) were each ion-paired to cetyltrimethylammonium bromide (CTAB) to produce hydrophobic complexes, which were co-encapsulated in pH-sensitive polyketal (PK3) microparticles (1–3 μm) using a single emulsion method. Loading levels ranged from 13.6 to 18.8 μg/mg OVA and 4.8 to 10.3 μg/mg poly(I:C). Murine splenic dendritic cells (DCs) pulsed with PK3-OVA–poly(I:C) microparticles, at antigen doses of 0.01 and 0.1 μg/mL, induced a higher percentage of IFNγ-producing CD8+ T cells than DCs treated with PK3-OVA particles or soluble OVA/poly(I:C). A higher antigen dose (1 μg/mL) was less effective, which can be attributed to CTAB toxicity. At the lowest antigen dose (0.01 μg/mL), PK3-OVA–poly(I:C) microparticles also enhanced TNF-α and IL-2 production in CD8+ T cells. These data demonstrate the potential of polyketal microparticles in formulating effective CD8+ T cell-inducing vaccines comprising protein antigens and dsRNA adjuvants.
Keywords: Drug delivery, Microsphere, Co-polymer, Immunostimulation, Vaccine
1. Introduction
The development of delivery systems for recombinant protein-based vaccines remains a major challenge in the field of drug delivery. Protein subunit vaccines have great potential for treating cancer and chronic viral infections, such as HIV and hepatitis C, by activating dendritic cells (DCs) to induce a CD8+ cytotoxic T lymphocyte (CTL) response [1,2]. Because proteins alone are weakly immunogenic, protein-based vaccines are typically adjuvanted with immunostimulatory molecules such as agonists for Toll-like receptors (TLRs) [3,4]. TLR agonists are strong stimulators of the innate immune system and can selectively bias the T-helper cell polarization toward a TH1 phenotype, and cytotoxic CD8+ T cell responses, as well as TH2 responses [5–7]. The simultaneous processing of protein antigen and engagement of TLRs leads to activation of DCs and subsequent cross-priming of CTLs [8,9]. Thus, a promising strategy for TH1-inducing vaccines is to target endosomal TLRs (3, 7, and 9) using delivery systems which can simultaneously release antigen and adjuvants in the phagolysosomes of DCs.
Poly(inosinic acid)–poly(cytidylic acid) (poly(I:C)), a synthetic dsRNA analog, is an agonist for TLR3 and is a potent stimulator of innate immune responses [9]. Treatment of antigen-presenting cells (APCs) with poly(I:C) leads to secretion of inflammatory cytokines such as Type I interferons, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-12, and enhances the cross-priming of CD8+ cytotoxic T lymphocytes [9–11]. Recently, poly(I:C) has shown promise as a vaccine adjuvant administered with anthrax and HIV antigens in mice [12,13]. In clinical applications, TLR3 offers a potential advantage over TLR9 as an adjuvant target due to the TLR expression patterns in human DCs. TLR9 is selectively expressed in human plasmacytoid DCs [14,15], which produce high levels of Type I interferons and stimulate humoral responses but do not generate significant levels of IL-12 [16,17]. On the other hand, TLR3 is expressed in human myeloid DCs, which are capable of IL-12 production, and consequently TLR3 engagement can stimulate TH1-biased cross-priming of CTLs [14,15,17].
The TH1-inducing property of poly(I:C) makes it a potentially effective vaccine adjuvant; however, the systemic inflammation resulting from high doses of soluble poly(I:C) may limit its acceptable dosage in a vaccine formulation. For example, mice injected intraperitoneally (i.p.) with poly(I:C) at doses of 2–12 mg/ kg exhibit sickness behavior, adverse effects on body weight and temperature, and increased levels of IFN-α and IFN-β in the bloodstream [18,19]. Similarly, i.p. injection of 3 mg/kg of poly(I:C) in rats resulted in reduced running wheel activity and increased expression of IFN-α in the central nervous system [20]. There is thus a need to minimize systemic levels of poly(I:C) by targeting poly(I:C) to DCs.
Polymeric microparticles have been widely investigated as delivery systems for protein-based vaccines due to their enhanced uptake by phagocytic cells, which facilitates antigen presentation in DCs [21–24]. Another benefit is that antigen and adjuvant molecules can be co-encapsulated in microparticles, resulting in simultaneous delivery of antigen and TLR agonists to Phagolysosomes and enhanced cross-priming of T cells [23,25–27]. While polymeric microparticles are advantageous as vaccine delivery vehicles, there is a need for improvements over existing carrier polymers, of which the most widely studied is poly(lactic-co-glycolic acid) (PLGA). One area of concern with PLGA is that the hydrolysis products, lactic acid and glycolic acid, create an acidic microclimate within degrading particles, which may lead to deterioration of nucleic acid adjuvants [28–31]. Another limitation of PLGA is the absence of a stimulus-responsive release mechanism to promote accelerated release of the cargo molecules within the phagolysosome.
To address these concerns, we have developed a new family of biodegradable polymers, termed polyketals, which contain pH-sensitive ketal linkages in the polymer backbone and have non-acidic degradation products [32–34]. We have previously shown that PCADK, a polyketal synthesized from 1,4-cyclohexanedimethanol, is effective in encapsulating superoxide dismutase [33], and we have recently developed polyketal co-polymers based on PCADK which exhibit tunable hydrolysis kinetics and degrade into biocompatible small molecules [34]. In this study, the co-polyketal PK3 (Fig. 1), which is synthesized from 1,4-cyclohexanedimethanol and 1,5-pentanediol, was used to encapsulate ovalbumin (OVA) and poly(I:C). PK3 has a hydrolysis half-life of 39 days at pH 7.4 and 1.8 days at pH 4.5 [34]. The rapid degradation kinetics of PK3 at lysosomal pH enables PK3 microparticles to release poly(I:C) in the phagolysosome, where poly(I:C) can bind TLR3 and initiate co-stimulatory signaling (Fig. 2). The objective of this study was to investigate the efficacy of PK3 microparticles containing OVA and poly(I:C), using an in vitro model measuring CD8+ T cell stimulation by vaccine-pulsed DCs.
Fig. 1.
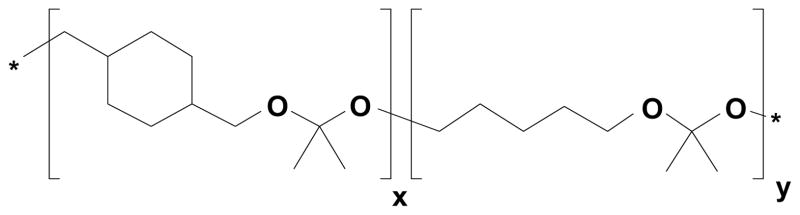
Structure of co-polyketal PK3, which is synthesized from 1,4-cyclohexanedimethanol, 1,5-pentanediol, and 2,2-dimethoxypropane via the acetal exchange polymerization [32,34].
Fig. 2.
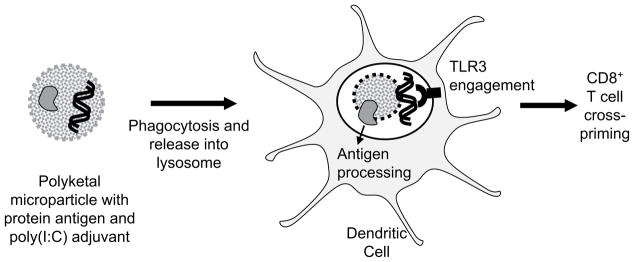
Biodegradable polyketal microparticles containing protein antigen and poly(I:C) adjuvant are phagocytosed by dendritic cells (DCs). pH-Sensitive polyketal degrades and releases protein antigen and dsRNA analog poly(I:C) in phagolysosome. Poly(I:C) engages TLR3, thereby activating DC and cross-priming CD8+ cytotoxic T lymphocytes.
2. Materials and methods
2.1. Materials
1,4-Cyclohexanedimethanol, 1,5-pentanediol, p-toluenesulfonic acid, 2,2-di-methoxypropane poly(vinyl alcohol) (PVA, average Mw 31,000–50,000, 98–99% hydrolyzed), albumin from chicken egg white (ovalbumin, A5503), fluorescamine, brefeldin A, and cetyltrimethylammonium bromide (CTAB) were purchased from Sigma-Aldrich. Poly(riboinosinic acid)–poly(ribocytidylic acid) (poly(I:C)) was obtained as a lyophilized salt from Amersham Biosciences. SYBR Green I and Oli-Green were purchased from Invitrogen. CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS reagent) was obtained from Promega. IDTE pH 7.5 buffer (10 mM Tris, 0.1 mM EDTA) was obtained from Integrated DNA Technologies, Inc., OmniPur nuclease-free water from EMD Biosciences, RPMI-1640 medium from Lonza, Cytofix/Cytoperm from BD Biosciences, and fetal bovine serum (FBS) from Hyclone. OVA-specific MHC Class I H-2Kb-restricted peptide (SIINFEKL) was prepared by the Microchemical Peptide Core Facility at Emory University.
2.2. Synthesis of a co-polyketal based on 1,4-cyclohexanedimethanol and 1,5-pentanediol (PK3)
Synthesis of the co-polyketal PK3 is described previously [34]. A typical synthesis of PK3 is carried out in a 25 mL two-necked flask connected to a short-path distilling head. 1,4-Cyclohexanedimethanol (5.0 g, 34.7 mmol) and 1,5-pentanediol (0.903 g, 8.67 mmol) were dissolved in 30 mL of distilled benzene at 100 °C. Re-crystallized p-toluenesulfonic acid (3.5 mg, 0.0197 mmol) dissolved in 3.5 mL of ethyl acetate was then added. Distilled 2,2-dimethoxypropane (DMP, 5.33 mL, 43.3 mmol) was added to initiate the reaction. Additional doses of DMP (2.5 mL, 20.3 mmol) and benzene (5 mL) were subsequently added to the reaction every hour for 6 h via a metering funnel at slow drip rate to compensate for DMP and benzene that had distilled off. After 20 h, the reaction was stopped with the addition of 2 mL of triethylamine. The polymer was isolated by precipitation in cold hexanes (−20 °C) followed by vacuum filtration. The recovered polymer was analyzed by gel permeation chromatography (GPC) using a Shimadzu LC-10AD/SPD-10A liquid chromatography system (Shimadzu Scientific Instruments, Columbia, MD) with a Shodex KF-803 GPC column. The GPC trace indicated a weight-average molecular weight of Mw = 4770, with a polydispersity index of 1.65, based on polystyrene standards (Polymer Laboratories, Amherst, MA).
2.3. Hydrophobic ion-pairing of poly(I:C) with CTAB
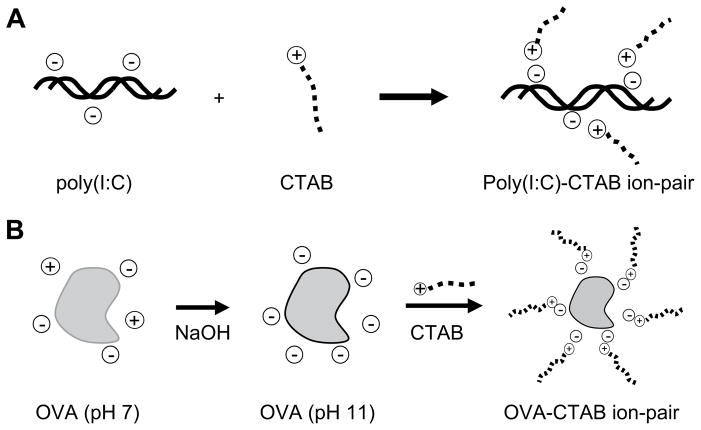
Poly(I:C) was initially dissolved in IDTE pH 7.5 buffer at a concentration of 2.26 mg/mL, as determined by the UV absorbance at 260 nm (50 μg/mL/A.U., per supplier’s literature). The poly(I:C) solution was diluted to 0.1 mg/mL in OmniPur nuclease-free water and kept at 4 °C. Next, 10 mL of poly(I:C) solution (1 mg, 3.15 μmol anion) was combined with 1 mL of CTAB solution (1.52 mg, 4.17 μmol), resulting in a precipitate which was collected by centrifugation at 20,000 × g for 20 min at 5 °C (Fig. 3A). The lyophilized poly(I:C):CTAB complex was dissolved in 4:1 chloroform–methanol at an approximate concentration of 4.0–6.2 mg/mL as determined by absorbance at 260 nm. The yield was 73–93% of the starting mass of poly(I:C). The molar ratio of CTAB cations to poly(I:C) anions was 1.32:1.
Fig. 3.
Hydrophobic ion-pairing procedure. (A) Poly(I:C) solution or (B) OVA solution at pH 11 is paired with CTAB in equimolar ratio of opposite charges to form a hydrophobic complex.
2.4. Hydrophobic ion-pairing of OVA with CTAB
OVA was first converted to the stabilized form by heating a 10 mg/mL OVA solution to 55 °C for 20 h in 100 mM, pH 10 sodium phosphate buffer, followed by desalting in a PD-10 column [35]. The stabilized OVA solution was diluted to 1 mg/ mL in OmniPur water and adjusted to pH 11 by addition of NaOH. Next, 10 mL (10 mg, 0.233 μmol) of OVA pH 11 solution (4 °C) was added to 364 μL of cetyltrimethylammonium bromide (CTAB) solution (3.64 mg, 10.0 μmol), resulting in a precipitate which was collected by centrifugation at 20,000 × g for 20 min at 5 °C (Fig. 3B). The lyophilized OVA:CTAB ion-pair complex was dissolved in 1:1 chloroform–DMSO, at an approximate concentration of 1.3–2.0 mg/mL (7–11% mass yield) as determined by UV absorbance at 280 nm (UV-1700 Spectrophotometer, Shimadzu Scientific Instruments, Columbia, MD). The molar ratio of CTAB cations to OVA anions was 0.7:1. The conversion to stabilized OVA was performed to minimize OVA aggregation during the ion-pairing step; an increase in the ion-pairing yield was observed when the stabilization step was included.
2.5. Preparation of PK3 microparticles containing OVA:CTAB and poly(I:C):CTAB complexes
Microparticles were fabricated using an oil-in-water emulsion, solvent evaporation method. The oil phase contained 50 mg of PK3 dissolved in 400 μL of chloroform, plus 0.8 mg of OVA:CTAB in 400 μL of 1:1 chloroform–DMSO and 0.93 mg of poly(I:C):CTAB in 150 μL of 4:1 chloroform–methanol. Control batches included OVA:CTAB particles, poly(I:C):CTAB particles, or empty particles. The total volume of the organic phase was made up to 1.2 mL with chloroform and homogenized in 15 mL of 5% PVA solution for 2 min at 24,000 rpm (PowerGen 500 Homogenizer, Fisher Scientific, Pittsburgh, PA). The resulting emulsion was poured into 85 mL of 1% PVA solution and stirred for 4 h to evaporate the chloroform. The PVA solutions were buffered with 10 mM sodium phosphate (pH 8) to prevent hydrolysis of the acid-sensitive polyketal. The microparticles were collected by centrifugation at 10,000 × g, washed once with OmniPur water, re-suspended in 5 mL OmniPur water, and lyophilized overnight. Dynamic light scattering (DLS) measurements were obtained with a 90 Plus Particle Size Analyzer (Brookhaven Instruments Corp., Holtsville, NY). Microparticle images were generated by a Hitachi X800 field emission scanning electron microscope at the Center for Nanostructure Characterization and Fabrication at Georgia Tech.
2.6. Determination of OVA and poly(I:C) loading
Microparticles were digested by adding 200 μL of 0.01 M HCl (with 0.25% SDS) to approximately 5 mg of particles. After 5–10 min, with occasional vortexing and bath sonication, the slightly hazy solution was neutralized with 400 μL of 0.05 M pH 9.5 sodium bicarbonate buffer. OVA standard dilutions were prepared in the same neutralized solution as the particles. The samples were analyzed for OVA content using a fluorescamine assay in a 96-well plate using a Synergy™ HT microplate reader (BioTek Instruments, Inc., Winooski, VT). Poly(I:C) content was measured by diluting the samples in IDTE pH 7.5 buffer and staining with SYBR Green I or Oli-Green nucleic acid dye. Poly(I:C) standard dilutions were prepared in IDTE pH 7.5 buffer, and samples were analyzed using an RF-5301PC spectrofluorophotometer (Shimadzu Scientific Instruments, Columbia, MD).
2.7. MTS cell viability assay
RAW264.7 macrophage cells (ATCC, Manassas, VA) were cultured per ATCC’s protocol, in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS and Pen/ Strep/Fungizone. Cells were seeded overnight in 96-well plates at 15,000 cells/ 150 μL/well. Empty polyketal microparticles and particles containing OVA, poly(I:C), or OVA + poly(I:C) were re-suspended in complete medium with vortexing and bath sonication. Microparticle suspensions and solutions of OVA, poly(I:C), and CTAB were diluted in complete medium, and 50 μL of each dilution was added to triplicate wells. After 5 h incubation, Cell Titer 96 MTS reagent was added at 30 μL per well, and after 1 h incubation the difference in absorbance at 490 and 650 nm was recorded (Bio-Tek Synergy™ HT). Measurements were corrected for background (DMEM + MTS reagent) and scaled to positive control wells (untreated cells + MTS).
2.8. Isolation of splenic DCs and OT-1 CD8+ T cells from mice
C57BL/6 mice (Charles River Labs, Wilmington, MA) were injected i.p. with Flt-3 ligand, at 20 μg/mouse, for 8 days. On day 9, mice were sacrificed and the spleens harvested, and CD11c+ DCs isolated by positive selection with anti-CD11c magnetic beads (Miltenyi Biotech, Auburn, CA). C57BL/6-Tg (OT-I)-RAG1〈tm1Mom〉 mice were obtained from Taconic Farms Inc (Hudson, NY) through the NIAID exchange program [36,37]. OT-I CD8+ T cells were obtained by harvesting splenocytes followed by MACS purification with anti-CD8a magnetic beads (Miltenyi Biotech, Auburn, CA) according to manufacturer’s instructions. All studies were conducted following the guidelines of the Institutional Animal Care and Use Committee at Emory University.
2.9. In vitro stimulation of OT-1 cells by PK3 microparticle-pulsed DCs
CD11c+ DCs (1.5 × 105 cells/0.2 mL/well) isolated from the spleens of mice injected with Flt-3 ligand were pulsed with soluble or microparticle-encapsulated OVA protein and poly(I:C) at concentrations of 1.0, 0.1, and 0.01 μg/mL of OVA and 0.5, 0.05, and 0.005 μg/mL of poly(I:C), for 5 h at 37 °C. Cells were washed twice with PBS and co-cultured with ~7.5 × 105 OT-I CD8+ T cells (1:5 ratio of DCs to CD8+ T cells) for 4 days at 37 °C. For the last 5 h of culture, fresh culture media containing OVA-specific MHC class I-restricted SIINFEKL peptide (1 μg/mL) was added with 5 μg/mL brefeldin A, prior to intracellular staining as described below.
2.10. Intracellular cytokine staining and flow cytometry
SIINFEKL peptide pulsed cells were stained for surface CD8α as well as the intracellular cytokines IFNγ, TNFα, and IL-2 (BD Biosciences, San Diego, CA) following manufacturer’s protocols and instructions. Briefly, peptide-restimulated cells were cell surface stained for CD8α in FACS staining buffer (PBS +5% FBS) in the presence of Fc Block (Anti CD16/32, 2.4G2, BD Biosciences) for 30 min at 4 °C. Cells were washed twice with staining buffer, fixed and permeabilized using BD Cytofix/Cytoperm (BD Biosciences) for 15 min at 4 °C. Cells were washed twice with Perm Wash (BD Biosciences) and stained for intracellular cytokines (IFNγ, TNFα and IL-2) for 30 min at 4 °C, then washed twice with Perm Wash and once with FACS staining buffer. FACS data was acquired using a FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA) and data was analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
3. Results and discussion
We have developed a new vaccine formulation, based on microparticles composed of the co-polyketal PK3, which contain OVA and poly(I:C), a TLR3-inducing adjuvant. We chose PK3 for these studies because of its fast hydrolysis kinetics, having a hydrolysis half-life of 39 days at pH 7.4 and 1.8 days at pH 4.5, and neutral degradation products [34]. PK3 microparticles should degrade rapidly after phagocytosis at lysosomal pH, releasing antigen and poly(I:C) in the phagolysosome, and allow for poly(I:C) to engage TLR3. Rapid release of antigen and immunostimulatory adjuvant is also advantageous to achieve CD8+ T cell responses, because in vivo programming of CD8+ effector and memory T cell responses occurs within the first 1–2 days following an acute infection [38]. Another important property of PK3 is that it does not have acidic degradation products, in contrast to polyesters such as poly(lactic-co-glycolic acid) (PLGA), polyanhydrides, and poly (ortho ester)s. A potential concern with polyester-based particles is that the acidic microenvironment within degrading particles may be deleterious to nucleic acids being delivered [30]. We therefore investigated PK3 microparticles for delivery of antigen and poly(I:C) to DCs.
3.1. Encapsulation of OVA and poly(I:C) in PK3 microparticles using ion-pairing with CTAB
In order to encapsulate OVA and poly(I:C) in polyketal micro-particles via a single emulsion method, we used hydrophobic ion-pairing to convert the water-soluble OVA and poly(I:C) into hydrophobic complexes that could be dissolved in organic solvent mixtures (Fig. 3). The ion-pairing/single emulsion method resulted in loading levels ranging from 4.8 to 10.3 μg poly(I:C) per mg particle and 13.6 to 18.8 μg OVA per mg particle. The overall efficiency of the ion-pairing and encapsulation steps ranged from 22.1 to 51.3% for poly(I:C) and 8.4 to 12.9% for OVA. The PK3 microparticle batches yielded 40.0–72.8% of the starting polymer weight. The microparticle size range was 1 to 3 μm effective diameter as determined by DLS; this particle size range was confirmed by the SEM image shown in Fig. 4.
Fig. 4.

Scanning electron microscope image of polyketal microparticles containing ion-paired OVA and poly(I:C).
Hydrophobic ion-pairing has been reported as a means of altering the solubility of proteins and nucleic acids, by combining detergents (cationic or anionic lipids) with biomolecules at the proper charge stoichiometry [39,40]. Hydrophobic ion-paired complexes of nucleic acids and various proteins such as lysozyme, insulin, and growth factors, have been encapsulated in PLGA or PLA microparticles, and this method has resulted in improved loading levels or release profiles [41–43]. The ion-pairing/single emulsion method was chosen for this study in order to improve the encapsulation efficiency of water-soluble components in comparison to the double (water–oil–water) emulsion method. The ion-pairing/single emulsion process resulted in a better encapsulation efficiency for poly(I:C) than previous attempts using a double emulsion method, whereas the encapsulation efficiency for OVA was similar for the two methods. Because the objective was to encapsulate the protein antigen and TLR3 adjuvant in the same particle, the ion-pairing/single emulsion method was the preferred process for fabricating polyketal microparticles with OVA and poly(I:C). In this study, we have achieved the first hydrophobic ion-pairing of poly(I:C) and the first co-encapsulation of ion-paired OVA and poly(I:C) in a microparticle.
3.2. Cell toxicity of polyketal microparticles containing ion-paired OVA and poly(I:C)
An MTS cell viability assay was performed to measure the cytotoxicity of polyketal microparticles containing ion-paired complexes of OVA and/or poly(I:C). RAW264.7 cells treated with microparticles containing OVA:CTAB and/or poly(I:C):CTAB complexes were 50% viable at a concentration range of 0.05–0.25 mg/mL. Plain polyketal microparticles were less toxic, with 70% cell viability at 1.25 mg/mL (Fig. 5A). Soluble OVA and poly(I:C) did not show appreciable toxicity to RAW264.7 cells over the range tested, however the ion-pairing surfactant CTAB showed significant toxicity at 0.8 μg/mL (Fig. 5B). This value corresponds roughly to the amount of CTAB (1.2 μg/mL) present in ion-paired OVA/poly(I:C) microparticles at 0.05 mg/mL. This data for CTAB toxicity also falls within the range reported for other cell lines treated with CTAB-containing liposomes, micellar solutions, cationic vesicles, and nanoparticles [44–46]. Based on these data, the greater toxicity of the OVA/poly(I:C)-containing polyketal microparticles, compared to plain polyketal microparticles, can be attributed to CTAB and not OVA or poly(I:C).
Fig. 5.
Cell viability of RAW264.7 macrophages treated for 5 h with (A) PK3 micro-particles containing OVA and/or poly(I:C) or empty microparticles or (B) soluble OVA, poly(I:C), or CTAB.
3.3. Cross-priming of CD8+ T cells by DCs pulsed with OVA/poly(I:C)-containing microparticles
The polyketal microparticle-based vaccine is designed to deliver protein antigen and TLR3 stimuli to the phagosomes of DCs, where they will induce activation of DCs and cross-priming of CD8+ T cells. To determine the efficiency with which the TLR3 and antigen encapsulated in polyketal microparticles induced cross-presentation, we used an in vitro system in which murine splenic DCs were pulsed with the microparticles, and then co-cultured with splenocytes from an OT-1 mouse. The OT-1 mouse strain expresses a transgenic T cell receptor that recognizes the OVA peptide SIIN-FEKL presented by major histocompatibility complex (MHC) class I molecules. Following the co-culture, the activated T cells were re-stimulated with the SIINFEKL peptide, in the presence a Golgi block, to facilitate the accumulation of cytokines within the cells. We used an anti-CD8+ surface stain to identify cytotoxic T lymphocytes (CTLs) and intracellular cytokine staining for IFNγ, TNFα, and IL-2 to measure T cell activation and differentiation. A representative set of flow cytometry scatter plots for the 0.01 μg/mL antigen dose is shown in Fig. 6, and the dose-dependent results for the three individual cytokines are presented in Figs. 7–9.
Fig. 6.
Representative flow cytometry plots showing percentage of IFNγ-, TNFα- and IL-2-producing CD8+ T cells stimulated by DCs pulsed with PK3-encapsulated OVA and poly(I:C) (0.01 μg/mL antigen dose).
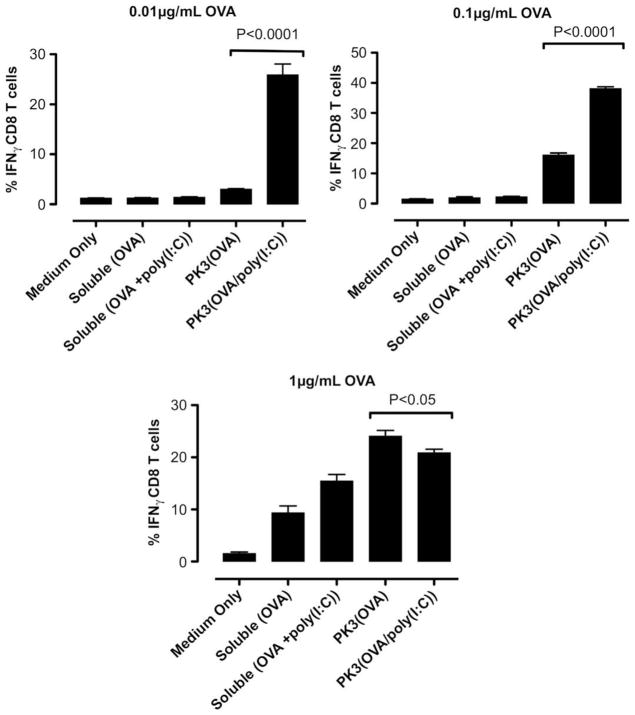
Fig. 7.
In vitro DC-OT-1 cross-priming by soluble and PK3 microparticle vaccine formulations containing OVA or (OVA + poly(I:C)); percentage of IFNγ-producing CD8+ T cells (n = 4 wells per group). Data shown above is one representative experiment out of two independent experiments with similar trends.
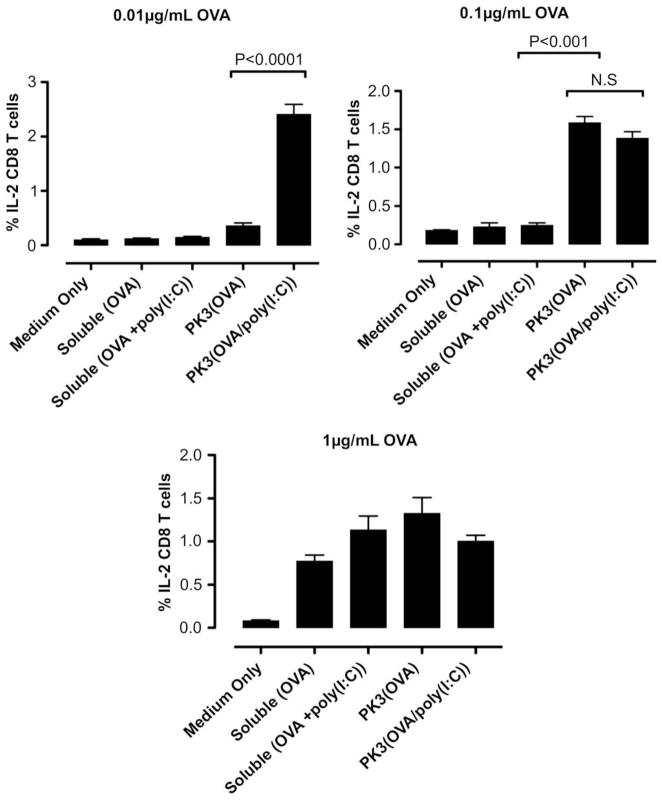
Fig. 9.
In vitro DC-OT-1 cross-priming by soluble and PK3 microparticle vaccine formulations containing OVA or (OVA + poly(I:C)); percentage of IL-2-producing CD8+ T cells (n = 4 wells per group). Data shown above is one representative experiment out of two independent experiments with similar trends.
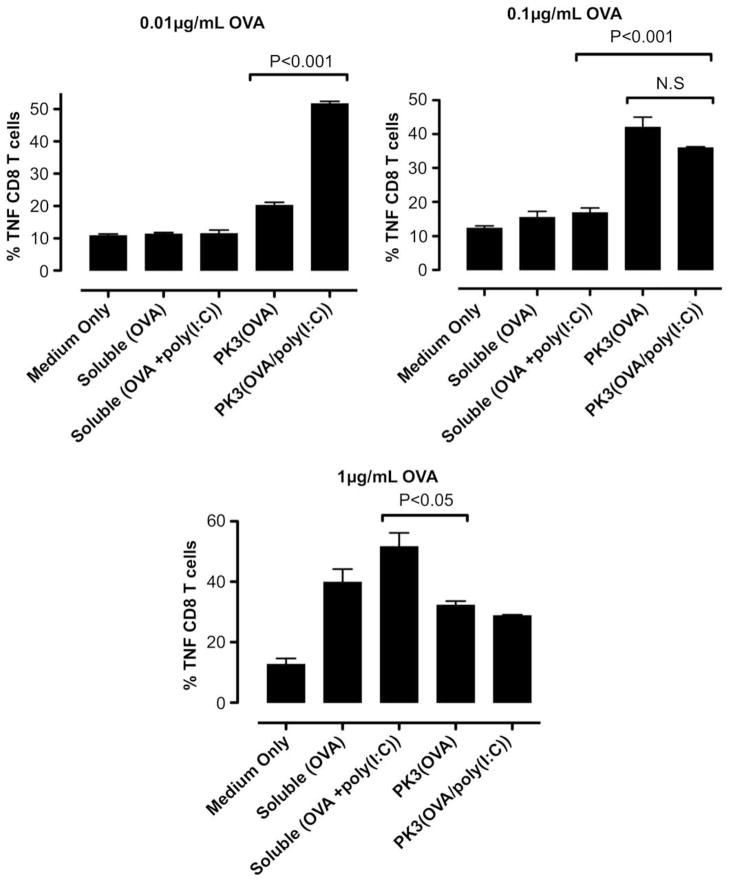
The results of the intracellular cytokine staining indicate that at doses of 0.01 and 0.1 μg/mL OVA, the polyketal microparticles containing OVA and poly(I:C) induced significantly higher levels of IFNγ+CD8+ T cells than OVA-containing microparticles or soluble OVA/poly(I:C) (Fig. 7). Notably, the 0.01 μg/mL dosage of OVA/poly(I:C)-containing microparticles induced stronger T cell activation than a 10-fold higher dose (0.1 μg/mL) of OVA microparticles. At the lowest dose (0.01 μg/mL), the co-delivery of antigen and TLR3 agonist also significantly enhanced the generation of TNFα+ CD8+ and IL-2+CD8+ cells when compared to the control formulations (Figs. 8 and 9). At the highest dosage (1 μg/mL OVA, 50 μg/mL microparticles), however, the IFNγ+CD8+ T cell response for the OVA/poly(I:C)-containing microparticles was attenuated (Fig. 7). This can be attributed to the toxicity of CTAB ion-paired microparticles at this dose, based on the 45% cell viability observed with RAW 264.7 cells treated for 5 h with the same formulation and dosage (Fig. 5). Nonetheless, these results demonstrate that at low antigen/adjuvant doses, the polyketal microparticles containing OVA and poly(I:C) are highly effective at cross-priming CD8+ T cells. Also, comparison of the PK3(OVA + poly(I:C)) and PK3(OVA) formulations indicates that the inclusion of poly(I:C) in the vaccine formulation enhances the cross-priming of CTLs. This is consistent with reported studies that show a strong TH1-biased adaptive immune response and antigen-specific cross-priming of CTLs through activation of TLR3 [9,47,48]. In the present study, the TH1-inducing property of poly(I:C) is utilized by the polyketal-based OVA/poly(I:C) vaccine formulation to stimulate cross-priming of CTLs as shown by high levels of IFNγ, TNFα, and IL-2 production in CD8+ T cells.
Fig. 8.
In vitro DC-OT-1 cross-priming by soluble and PK3 microparticle vaccine formulations containing OVA or (OVA + poly(I:C)); percentage of TNFα-producing CD8+ T cells (n = 4 wells per group). Data shown above is one representative experiment out of two independent experiments with similar trends.
4. Conclusions
We have demonstrated the development of a vaccine delivery system for a protein antigen and TLR-inducing nucleic acid adjuvant. Ovalbumin and the double-stranded RNA analog poly(I:C) were each ion-paired to the surfactant CTAB to produce hydrophobic complexes which were encapsulated in biodegradable polyketal microparticles. The co-polyketal PK3 exhibits pH-sensitivity, and thus the microparticles are designed to be stable at physiological pH yet release the vaccine components within the acidic phagolysosomes of DCs. We have demonstrated that the PK3-based vaccine formulation enhances the ability of DCs to cross-prime cytotoxic T lymphocytes in vitro, as evidenced by production of IFN-γ, TNF-α, and IL-2. Very low doses of antigen and adjuvant are required, which can be attributed to the rapid intracellular release from the PK3 particles. The ion-paired polyketal formulation represents a significant contribution to the field of microparticle vaccine delivery systems, as it is the first hydrophobic ion-pairing of the double-stranded RNA analog poly(I:C) and the first co-encapsulation of ion-paired protein antigen and poly(I:C) in a microparticle. The methods developed here can potentially be used for other protein antigens and TLR ligands for vaccines and can be applied to intracellular delivery of nucleic acids such as plasmid DNA, mRNA, and interfering RNA.
Acknowledgments
This work was funded in part by the Georgia Tech/Emory Center for the Engineering of Living Tissues (funded by NSFEEC-9731643), NSF-BES-0546962 CAREER award, NIH U01 HL80711-01, NIH R21 EB006418, J&J/GT Health Care Innovation Seed Grant Proposal; and by NIH R01 DK 57665-01, R01 AI48638-01, R01 AI056499-01, N01 A150025, N01 A1-50019, R01 AI064463 and U19 AI057266 (to B.P.). M.J.H. was funded by an NSF Graduate Research Fellowship and a GAANN Fellowship through the Center for Drug Design, Development, and Delivery at Georgia Tech.
We thank Chen-Yu Kao and Sydney M. Shaffer of the Murthy laboratory (at Georgia Tech) for synthesizing a batch of PK3 polymer used in this study. We also thank Dr. Marcin Kwissa of the Pulendran laboratory (at the Emory Vaccine Center) for his assistance with the immunological protocols.
References
- 1.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 3.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11(4 Suppl):S63–8. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 4.Heit A, Busch DH, Wagner H, Schmitz F. Vaccine protocols for enhanced immunogenicity of exogenous antigens. Int J Med Microbiol. 2008;298(1–2):27–32. doi: 10.1016/j.ijmm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 6.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199(1):227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 7.Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol. 2005;174(5):2457–65. doi: 10.4049/jimmunol.174.5.2457. [DOI] [PubMed] [Google Scholar]
- 8.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, et al. A subset of toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol. 2003;170(8):4102–10. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 9.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433(7028):887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 10.Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJM, Brand A, et al. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163(1):57–61. [PubMed] [Google Scholar]
- 11.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappa B by Toll-like receptor 3. Nature. 2001;413(6857):732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 12.Sloat BR, Shaker DS, Le UM, Cui Z. Nasal immunization with the mixture of PA63, LF, and a PGA conjugate induced strong antibody responses against all three antigens. FEMS Immunol Med Microbiol. 2008;52(2):169–79. doi: 10.1111/j.1574-695X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 13.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105(7):2574–9. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadowaki N, Ho S, Antonenko S, Malefyt RD, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31(11):3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103(8):3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Kanzler H, Duramad O, Cao W, Liu Y-J. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107(6):2423–31. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 18.Davis J, Weaver J, Kohut M, Colbert L, Ghaffar A, Mayer E. Immune system activation and fatigue during treadmill running: role of interferon. Med Sci Sports Exerc. 1998;30(6):863–8. doi: 10.1097/00005768-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav Immun. 2007;21(4):490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Katafuchi T, Kondo T, Yasaka T, Kubo K, Take S, Yoshimura M. Prolonged effects of polyriboinosinic:polyribocytidylic acid on spontaneous running wheel activity and brain interferon-alpha mRNA in rats: a model for immunologically induced fatigue. Neuroscience. 2003;120(3):837–45. doi: 10.1016/s0306-4522(03)00365-8. [DOI] [PubMed] [Google Scholar]
- 21.Newman KD, Elamanchili P, Kwon GS, Samuel J. Uptake of poly(D,L-lactic-co-glycolic acid) microspheres by antigen-presenting cells in vivo. J Biomed Mater Res. 2002;60(3):480–6. doi: 10.1002/jbm.10019. [DOI] [PubMed] [Google Scholar]
- 22.Jiang WL, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly (lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57(3):391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Heit A, Schmitz F, Haas T, Busch DH, Wagner H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur J Immunol. 2007;37(8):2063–74. doi: 10.1002/eji.200737169. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Pollock KGJ, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;21(9–10):849–55. doi: 10.1016/s0264-410x(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 25.Hamdy S, Elamanchili P, Alshamsan A, Molavi O, Satou T, Samuel J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly(D,L-lactic-co-glycolic acid) nanoparticles. J Biomed Mater Res A. 2007;81A(3):652–62. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- 26.Standley SM, Mende I, Goh SL, Kwon YJ, Beaudette TT, Engleman EG, et al. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem. 2007;18(1):77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 27.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 28.Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nat Biotechnol. 2000;18(1):24–5. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- 29.Shenderova A, Burke TG, Schwendeman SP. The acidic microclimate in poly(lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm Res. 1999;16(2):241–8. doi: 10.1023/a:1018876308346. [DOI] [PubMed] [Google Scholar]
- 30.Walter E, Moelling K, Pavlovic J, Merkle HP. Microencapsulation of DNA using poly(DL-lactide-co-glycolide): stability issues and release characteristics. J Control Release. 1999;61(3):361–74. doi: 10.1016/s0168-3659(99)00151-0. [DOI] [PubMed] [Google Scholar]
- 31.Taluja A, Youn YS, Bae YH. Novel approaches in microparticulate PLGA delivery systems encapsulating proteins. J Mater Chem. 2007;17(38):4002–14. [Google Scholar]
- 32.Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem. 2005;16(6):1340–2. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Yang SC, Heffernan MJ, Taylor WR, Murthy N. Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjug Chem. 2007;18(1):4–7. doi: 10.1021/bc060259s. [DOI] [PubMed] [Google Scholar]
- 34.Yang SC, Bhide M, Crispe IN, Pierce RH, Murthy N. Polyketal copolymers: a new acid-sensitive delivery vehicle for treating acute inflammatory diseases. Bioconjug Chem. 2008;19(6):1164–9. doi: 10.1021/bc700442g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatta H, Nomura M, Takahashi N, Hirose M. Thermostabilization of ovalbumin in a developing egg by an alkalinity-regulated, two-step process. Biosci Biotechnol Biochem. 2001;65(9):2021–7. doi: 10.1271/bbb.65.2021. [DOI] [PubMed] [Google Scholar]
- 36.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68(5):869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 37.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 38.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25(1):171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 39.Powers ME, Matsuura J, Brassell J, Manning MC, Shefter E. Enhanced solubility of proteins and peptides in nonpolar solvents through hydrophobic ion-pairing. Biopolymers. 1993;33(6):927–32. [Google Scholar]
- 40.Meyer JD, Manning MC. Hydrophobic ion pairing: altering the solubility properties of biomolecules. Pharm Res. 1998;15(2):188–93. doi: 10.1023/a:1011998014474. [DOI] [PubMed] [Google Scholar]
- 41.Yoo HS, Park TG. Biodegradable nanoparticles containing protein-fatty acid complexes for oral delivery of salmon calcitonin. J Pharm Sci. 2004;93(2):488–95. doi: 10.1002/jps.10573. [DOI] [PubMed] [Google Scholar]
- 42.Fu K, Harrell R, Zinski K, Um C, Jaklenec A, Frazier J, et al. A potential approach for decreasing the burst effect of protein from PLGA microspheres. J Pharm Sci. 2003;92(8):1582–91. doi: 10.1002/jps.10414. [DOI] [PubMed] [Google Scholar]
- 43.Patel MM, Zeles MG, Manning MC, Randolph TW, Anchordoquy TJ. Degradation kinetics of high molecular weight poly(L-lactide) microspheres and release mechanism of lipid:DNA complexes. J Pharm Sci. 2004;93(10):2573–84. doi: 10.1002/jps.20176. [DOI] [PubMed] [Google Scholar]
- 44.Cortesi R, Esposito E, Menegatti E, Gambari R, Nastruzzi C. Effect of cationic liposome composition on in vitro cytotoxicity and protective effect on carried DNA. Int J Pharm. 1996;139(1–2):69–78. [Google Scholar]
- 45.Kuo J-HS, Jan M-S, Chang C-H, Chiu H-W, Li C-T. Cytotoxicity characterization of catanionic vesicles in RAW 264. 7 murine macrophage-like cells. Colloids Surf B Biointerfaces. 2005;41(2–3):189–96. doi: 10.1016/j.colsurfb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Delie F, Berton M, Allemann E, Gurny R. Comparison of two methods of encapsulation of an oligonucleotide into poly(D,L-lactic acid) particles. Int J Pharm. 2001;214(1–2):25–30. doi: 10.1016/s0378-5173(00)00627-x. [DOI] [PubMed] [Google Scholar]
- 47.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, et al. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus-and HIV-1-specific T cell responses. J Immunol. 2003;171(8):4320–8. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 48.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]