Abstract
Growth factor-induced activation of Akt occursin the majority of human breast cancer cell lines resulting in a variety of cellular outcomes, including suppression of apoptosis and enhanced survival. We demonstrate that epidermal growth factor (EGF)-initiated activation of Akt is mediated by the ubiquitous calcium sensing molecule, calmodulin, in the majority of human breast cancer cell lines. Specifically, in estrogen receptor (ER)-negative, but not ER-positive, breast cancer cells, Akt activation is abolished by treatment with the calmodulin antagonist, W-7. Suppression of calmodulin expression by siRNAs against all three calmodulin genes in c-Myc-overexpressing mouse mammary carcinoma cells results in significant inhibition of EGF-induced Akt activation. Additionally, transient expression of constitutively active Akt (Myr-Akt) can overcome W-7-mediated suppression of Akt activation. These results confirm the involvement of calmodulin in the Akt pathway. The calmodulin independence of EGF-initiated Akt signaling in some cells was not explained by calmodulin expression level. Additionally, it was not explained by ER status or activation, since removal of estrogen and ablation of the ER did not convert the ERpositive, W-7 insensitive, MCF-7 cell line to calmodulin dependent signaling. However, forced overexpression of either epidermal growth factor receptor (EGFR) or ErbB2 did partially restore calmodulin dependent EGF-stimulated Akt activation. This is consistent with observation that W-7 sensitive cells tend to be estrogen independent and express high levels of EGFR family members. In an attempt to address how calmodulin is regulating Akt activity, we looked at localization of fluorescently tagged Akt and calmodulin in MCF-7 and SK-BR-3 cells. We found that both Akt and calmodulin translocate to the membrane after EGFstimulation, and this translocation to the same sub-cellular compartment is inhibited by the calmodulin inhibitor W-7. Thus, calmodulin may be regulating Akt activity by modulating its sub-cellular location and is a novel target in the poor prognosis, ER-negative subset of breast cancers.
Keywords: Akt, Calmodulin, Estrogen receptor, EGFR, ErbB2
Introduction
Growth and survival signals initiated at the cell surface by ligand–receptor interactions often impinge on the serine/threonine kinase, Akt, a central regulator of survival, apoptosis, and oncogenesis. Akt interferes with programmed cell death by phosphorylating and thereby inactivating a number of proteins involved in apoptosis including BAD, Forkhead transcription factors, GSK-3b, and MDM-2 [1–6]. Akt has been shown to be activated in a wide variety of cancers where it contributes to inappropriate cell survival and oncogenesis. Constitutive activation of Akt (produced by overexpression of growth factor receptors, chromosomal amplification of Akt or phosphatidylinositol 3-kinase [PI-3 kinase], or by deletion of the lipid phosphatase PTEN) is a common tumorigenic event in a number of cancers [7]. In breast cancer cell lines, Akt activation has been shown to contribute to survival and cellular resistance to chemotherapeutic drugs such as doxorubicin and tamoxifen, and to predict for poor outcome in patients [8, 9].
Akt activation, resulting from growth factor stimuli, occurs through both PI-3 kinase-dependent and PI-3 kinase-independent interactions, which recruit Akt to the plasma membrane (PM) where it is phosphorylated and activated by PDK1 and PDK2 on Thr308 and Ser473, respectively. The 30phosphorylated phosphoinositides (PIP3) generated by PI-3 kinase act as a lipid second messenger to recruit Akt to the PM via its pleckstrin homology (PH) domain, a specific PIP3-binding motif, and serves as the PI-3 kinase-dependent initiating event in Akt activation. The ubiquitous calcium sensing molecule calmodulin seems to mediate a PI-3 kinase-independent mechanism of Akt activation through calmodulin kinase kinase [10]. In mammalian cells, calmodulin regulates a variety of crucial biological functions including neurotransmission, proliferation, cell metabolism and development [11–13]. Calmodulin classically acts on specific calmodulin dependent protein kinases to exert its function. One specific kinase which directly phosphorylates Akt in a calcium-dependent manner is calmodulin kinase kinase [10]. Interestingly, calmodulin has also been shown to regulate PI-3 kinase-dependent activation of Akt in neurons. Akt activation and the survival of neuronal cells was shown to depend on calcium and calmodulin in a PI-3 kinase-dependent manner, as calmodulin antagonism prevented Akt activation by inhibiting in vivo generation of PIP2 and PIP3 phosphoinositides [14].
Our previous work highlighted the PI-3 kinase/Akt pathway as a key regulator of EGF-mediated survival of c-Myc-overexpressing carcinoma cells derived from MMTV-c-Myc mouse mammary tumors (henceforth called Myc83 cells) [15]. Since mammary epithelial cells release abundant calcium from intracellular stores upon ligand stimulation [16, 17], and since it has been shown that calmodulin is overexpressed in breast tumors, breast cancer cell lines and transgenic mouse models of breast cancer [18, 19], we previously investigated the role of calcium and calmodulin in the survival of these mammary carcinoma cells [20]. We reported that calmodulin was a novel modulator of Akt activity and additionally showed that the survival of c-Myc-overexpressing mammary carcinoma cells was dependent on calmodulin-mediated Akt activation.
Studying the mechanism of calmodulin-mediated Akt activation, we showed that Akt could be co-immunoprecipitated with calmodulin in an EGF-dependent manner, and yet, PI-3 kinase activity remained intact in the presence of a calmodulin inhibitor. This suggests that calmodulin interacts with Akt and mediates its activation downstream of PLCc and PI-3 kinase [20]. We therefore hypothesized that calmodulin was a direct and fundamental regulator of Akt activation and a potential therapeutic target in human breast cancer.
In the present study, we demonstrate that siRNAmediated suppression of cellular calmodulin levels inhibits EGF-induced Akt activation, and that the constitutive activation of membrane-targeted Myr-Akt is unaffected by a pharmacological calmodulin inhibitor. These findings provide evidence of a direct role for calmodulin in the Akt pathway and suggest that calmodulin may modulate Akt activation by regulating the sub-cellular localization of Akt, and indeed we were able to show that the calmodulin inhibitor W-7 blocks EGF-induced re-localization of Akt to cell membranes.
In addition, we report that the calmodulin-dependence of Akt activation can be demonstrated in the majority of human breast cancer cell lines, but that interestingly, the few cell lines where this is not the case are almost all estrogen receptor (ER) positive. This suggests that distinct signal transduction pathways are active in the different classes of breast cancer cells, though the apparent reduced dependence on calmodulin for Akt activation in ER-positive cells does not appear to be directly related to the ER activity. We examine the role of the ER and epidermal growth factor (EGF) family receptors in determining a breast cancer cell’s dependence on calmodulin to activate Akt.
This newly appreciated role for calmodulin in mediating Akt activation in ER-negative breast cancers may have important therapeutic implications, since patients diagnosed with ER-negative breast cancers tend to have a worse clinical outcome, and the unique calmodulin–Akt interaction, selectively operant in these cells, presents a new survival mechanism which might be exploited therapeutically.
Methods
Cell lines
Human breast cancer cell lines, with the exception of MCE5, MB7 and MB8, were obtained from the Lombardi Comprehensive Cancer Center Tissue Culture Shared Resource, Georgetown University. MCF-7 cells were grown in improved modified Eagle’s minimum essential medium (IMEM—Biofluids, Frederick, MD) supplemented with 10% fetal bovine serum (FBS). Hs578T, MDA-MB-231(NIH), MDA-MB-453, MDA-MB-468, SK-BR-3, and T47D cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) with sodium pyruvate (Biofluids, MD) and 10% FBS. BT474 and BT549 cells were grown in RPMI (Biofluids) with 10% FBS; and MDA-MB-361 in Leibowitz media (Biofluids) with 10% FBS. Myc83 cells, a mouse mammary carcinoma cell line derived from c-Myc overexpressing mammary tumors resulting in MMTV-cmyc transgenic mice, were grown as previously reported [20]. Primary human mammary epithelial cells (HMEC) obtained from Cambrex (Walkersville, MD) were cultured for no more than eight passages in mammary epithelial basal medium (Cambrex), supplemented with bovine pituitary extract (BPE), hydrocortisone, hEGF, insulin, gentamicin/amphotericin. MCE5 cells, stable epidermal growth factor receptor-expressing (EGFR) MCF-7 cells (also named MCF-7-EGFR) were previously described and obtained from Dr. Dorraya El-Ashry, (Miami Miller School of Medicine, Miami, FL) [21]. MCE5 cells were grown in phenol red free IMEM with 10% charcoal stripped serum from Gemini Bio-Products (West Sacramento, CA). Stable ErbB2 expressing MCF-7 cells (clones MB7 and MB8) were previously described [22] and obtained from Dr. Francis G. Kern, (Adelson Medical Research Foundation, Los Angeles, CA) and grown in phenol red free IMEM with 10% charcoal stripped serum from Gemini (West Sacramento, CA).
Antibodies and reagents
Rabbit polyclonal antibodies phospho-Akt (S473), phospho-Akt (T308), total Akt, phospho-p44/42 MAPK (T202/Y204), and phospho-CREB (S133) were obtained from Cell Signaling Technology (Danvers, MA). Mouse monoclonal antibodies against EGFR, ErbB2, phospho-ErbB2 (Y1248) were obtained from NeoMarkers (Fremont, CA). Anti-b-actin was obtained from Sigma (St. Louis, MO). Mouse monoclonal antibody against calmodulin was obtained from Upstate (Lake Placid, NY). Cathepsin D antibody was obtained from BD Transduction Lab (San Diego, CA). The calmodulin inhibitor, W-7 and the internal calcium chelator, BAPTA-AM were obtained from Calbiochem (San Diego, CA). ICI 182,780 was obtained from Tocris Biosciences (Ellisville, MO). 17-beta-estradiol (E2), obtained from Sigma (St. Louis, MO). Treatment with pharmacological inhibitors and preparation of whole cell lysates Semiconfluent cell monolayers were serum-starved overnight or as indicated and then incubated with 30 lM of W-7 for 30 min, or 10 lM BAPTA-AM for 90 min. Where indicated, cells were stimulated with 10 nM EGF for 5 min, 50 ng/ml IGF for 5 min, 100 nM insulin for 5 min, 20% FBS for 15 min, and 100 lM pervanadate for 5 min, at 37° C. Cells were washed once in 19 PBS and lysed on ice with a 19 Lysis buffer from cell signaling supplemented with 1 mM PMSF. Lysates were processed and resolved on Tris–Glycine SDS-PAGE, and transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore) as previously described [20]. Membranes were incubated in primary antibody for 2 h, then biotinylated secondary antibody for 1 h, and detected with Vectastain ABC elite kit (Vector Laboratories) or HRPconjugated secondary antibody and enhanced chemiluminescence (PerkinElmer Life and Analytical Sciences).
Estrogen depletion and ER ablation
MCF-7 cells were grown to 70% confluence in IMEM plus 10% FBS and then stripped of estrogen by washing the cell monolayer three times with phenol red free IMEM and replacing the medium with phenol red free IMEM supplemented with 10% charcoal stripped serum (Gemini). This procedure was repeated four more times during the course of one day after which the cells were incubated overnight. The following morning the cells were trypsinized using phenol red free trypsin (Biofluids), plated in phenol red free IMEM plus 10% charcoal stripped serum, and where indicated, supplemented with 1 nM estrogen or 100 nM ICI 182,780 for 48 h. Cells were then serumstarved overnight in phenol red free IMEM plus HEPES, supplemented with 1 nM estrogen or 100 nM ICI 182,780 where indicated. Cells were pretreated with 30 lM W-7 for 30 min in serum free media and then stimulated with 10 nM EGF for 5 min. Cell lysates were prepared as described above and processed for determination of Akt activity by Western blotting, also described above.
Amaxa nucelofection
Myc83, SK-BR-3 orMCF-7 cells were passaged 2 days prior to nucleofection, and grown to no more than 80% confluence on the day of transfection. 1 9 106 cells were resuspended in 100 ll T solution (Amaxa, Inc. Gaithersburg, MD) plus indicated amount of DNA. Cells were then pulsed in the Amaxa Nucleofector II system (Cologne, Germany) using program T-20, or A-23 for MCF-7. Cells were plated in six well plates containing complete media, incubated for 24 h, and then used as described in figure legends.
RNA preparation and PCR-based detection of calmodulin gene expression
Myc83 cells were cultured as described and total RNA was prepared using Tri Reagent (Sigma), following the manufacturer’s instructions. RNA quality and concentration was determined by absorbance measurements and a 5 lg sample was used to prepare cDNA using the SuperScript First-Strand Synthesis System following the manufactures instructions (Invitrogen). The sequences for PCR primers specific for the three mouse calmodulin genes (CALM-1 -2 and -3) were obtained from the Primer Bank resource [23] and were used in PCR reactions with the Myc83 cDNA using the conditions recommended. A total of 5 ll samples of each reaction was analyzed by electrophoresis on a 3% agarose gel which was stained with ethidium bromide and photographed. The Primer Bank ID’s for the primers used are as follows: CALM1—6753244a1, CALM2—6680832a1, CALM3—6680834a1. The same primers were used in real time PCR evaluation of siRNA mediated knock down of the calmodulin genes as described below using the Primer Bank PCR protocol.
siRNA transfection
A total of 1 × 106 Myc83 cells were nucleofected as described above with 5 υg siRNAs generated against calmodulin 1, calmodulin 2, and calmodulin 3 from Dharmacon (Lafayette, CO). Control cells were nucleofected with equal amounts of control siRNA consisting of a pool of siRNA against Luciferase (Dharmacon, Lafayette, CO). After nucleofection, cells were transferred to Myc83 complete media and incubated for 48 h in complete media. On Day 2 after nucleofection, cells were washed and serum starved for 6 h in serum free media and then stimulated with or without 60 ng/ml (10 nM) EGF for 5 min. After EGF stimulation, the cells were lysed and processed as described above.
Plasmids
Calmodulin-red fluorescent protein (RFP) was made by ligating calmodulin from CaM-YFP construct (a gift from Dr. Thomas Michel, Brigham and Woman’s Hospital, Boston, MA) in mRFP-N vector. Akt1-GFP (green fluorescent protein) was a gift from Dr. Julian Downward, London Research Institute, UK.
Fluorescence microscopy
MCF-7 and SK-BR-3 cells were cotransfected with calmodulin-mRFP and Akt-GFP using LipofectAMINE 2000 (Invitrogen) according to manufacturer’s instructions. The cells were reseeded onto 12 mm glass coverslips 6 h after transfection. The cells on coverslips were serum-starved for 24 h in DMEM and then stimulated with 10 nM EGF for 5 min at 37°C. In the case of W-7 pretreatment, cells were incubated with 30 uM W-7 for 30 min prior to EGF stimulation. The cells were then fixed with 2% paraformaldehyded in phosphate buffered saline (PBS) for 15 min at 37°C, washed and mounted in Vectashield (Vector Laboratories). Laser Confocal images were obtained using a Zeiss LSM 510 confocal system.
Results
EGF-induced Akt activation is dependent on calmodulin in the majority of human breast cancer cell lines and primary HMECs
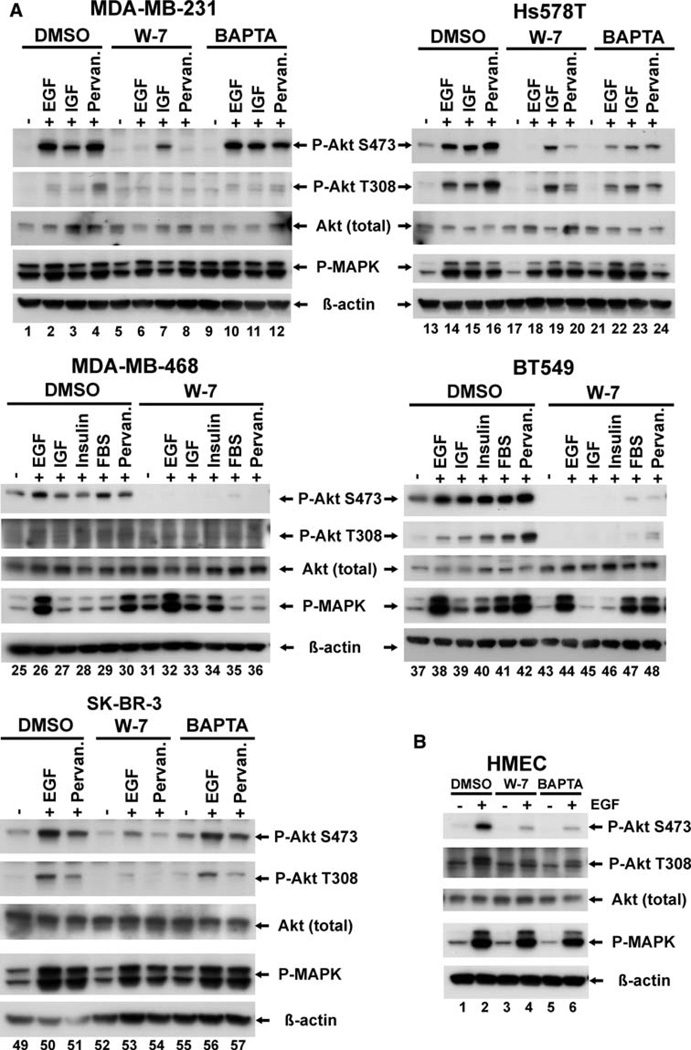
Our previous observations implicated calmodulin as a regulator of EGF-induced Akt activation and the survival of c-Myc-overexpressing, Myc83 mouse mammary tumor cells [20]. In this study, we set out to investigate whether calmodulin is a general mediator of Akt activity in human breast cancer cells. To do this we attempted to select a panel of breast cancer cell lines that would be representative of the diversity of human breast tumors [24] consisting of: (1) ER-negative, invasive breast cancer cell lines exhibiting a more stromal/mesenchymal genetic signature—MDA-MB-231, Hs578T, BT549, MDA-MB-468 and SK-BR-3 [25, 26], and (2) ER-positive, estrogen dependent breast cancer cell lines—MCF-7, T47D and BT474 [27]. In an initial experiment, these cells, along with non-transformed HMEC’s, were screened to identify treatments that would result in the induction of Akt activity. The cells were serum starved and then stimulated by either EGF, insulin-like growth factor 1 (IGF-1), insulin, FBS, or treatment with pervanadate (data not shown). Treatments that resulted in Akt phosphorylation were then selected to evaluate calmodulin-dependent signaling in that particular cell line.
Serum starved cells were pre-incubated for 30 min with 30 lM W-7, a selective calmodulin antagonist [12], for 90 min with 10 lM BAPTA-AM, an internal calcium chelator, or for comparable periods of time, with medium containing the vehicle alone (0.1% DMSO), and then stimulated to induce Akt activation. As shown in Fig. 1, pretreatment with the calmodulin inhibitor, W-7, but not DMSO, completely abolished EGF-induced Akt activation in MDA-MB-231, Hs578T, BT549, MDA-MB-468 and SK-BR-3 cells and also inhibited the basal Akt phosphorylation seen in serum starved Hs578T, MDA-MB-468 and BT549 cell lines (Fig. 1a, lanes 17, 31, 43). EGF induced Akt activation was also blocked in HMEC cells (Fig. 1b), and similar results were seen in MDA-MB-453 breast cancer cells (data not shown). Treatment with W-7 had no effect on MAP kinase (MAPK) activation, suggesting that the suppressive effect of W-7 on Akt activation was not due to a non-specific, or general toxic effect of the drug.
Fig. 1.
Effect of the calmodulin antagonist, W-7, and the internal calcium chelator, BAPTA-AM on EGF-induced Akt activation in breast cancer cell lines. ER negative breast cancer cells (a) and HMEC (ER-negative human basal mammary epithelial cells) (b) were grown to semi-confluence as described in section “Materials and methods”, then serum-starved overnight, and were pre-incubated with 0.1% vehicle control, Me2SO (DMSO), 30 lM W-7 for 30 min, or 10 lM BAPTA-AM for 90 min, as indicated. The cells were stimulated, as indicated, for 5 min with either 10 nM EGF, 50 ng/ml IGF-I, or 100 nM insulin, for 15 min with 20% FBS, or for 5 min with 100 lM pervanadate. After ligand stimulation, cells were washed with 19 PBS and lysed. Cell lysates were processed as described in material and methods. Equal amounts of total protein containing lysates were resolved on SDS-PAGE, transferred onto PVDF membrane and immunoblotted for activated Akt using phospho-specific Akt (S473) and phospho-specific Akt (T308) antibodies. Immunoblotting for activated MAP kinase (P-MAPK) revealed that W-7 inhibitory effects are specific for Akt. Immunoblotting with Akt (total) and β-actin antibodies served as a loading control. Each blot is a representative of at least two separate experiments.
Since it is known that mammary epithelial cells release calcium from intracellular stores upon ligand stimulation [16, 17], and since our previous work demonstrated that Akt activation was calcium dependent in Myc83 mouse cells [20], we also examined the calcium dependence of Akt activation using the cell permeant calcium chelator BAPTA-AM. With the exception of Hs578T and the HMECs, pretreatment with BAPTA-AM was unable to inhibit Akt activation in the cells tested (Fig. 1a, b, and data not shown). In Hs578T, BAPTA-AM pretreatment inhibited, but did not abolish ligand-induced Akt phosphorylation, whereas EGF-induced Akt activation was almost completely abolished in the HMECs. These data suggest that both calcium-activated calmodulin and calcium-free calmodulin may be capable of modulating Akt activity in breast cell lines.
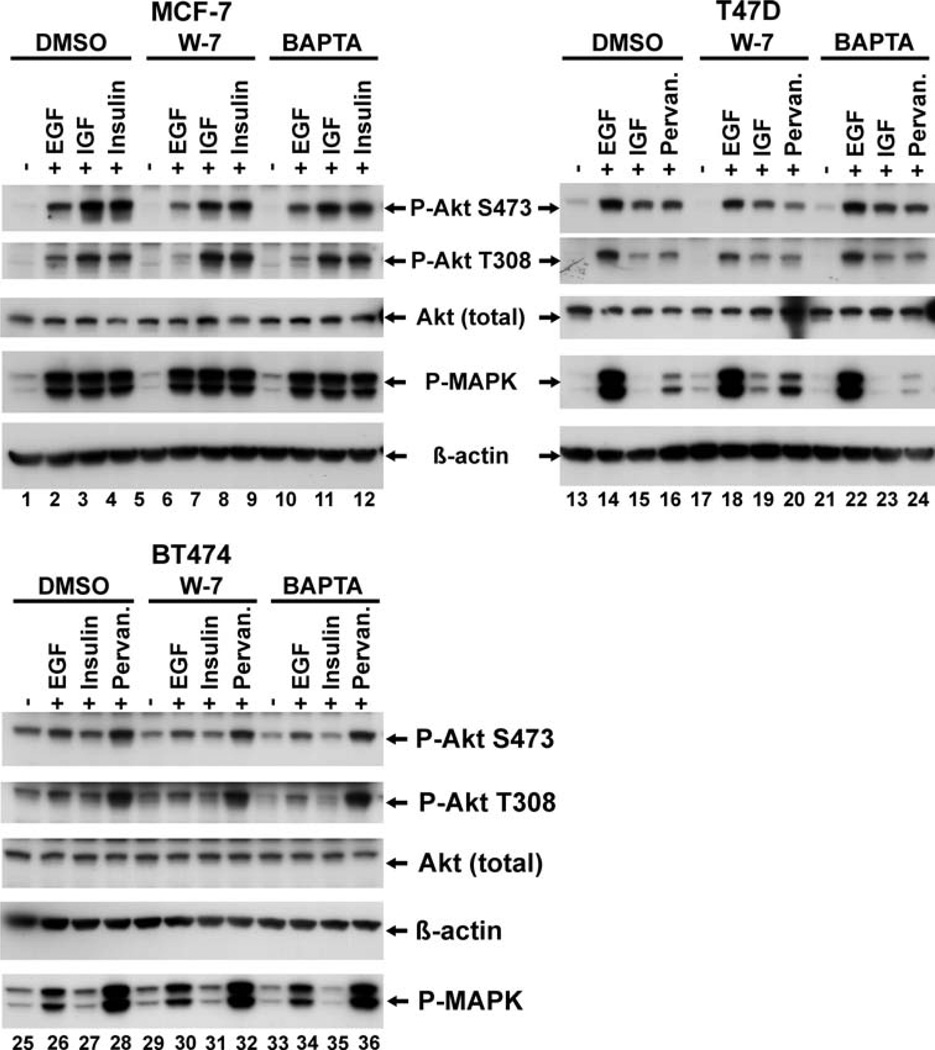
Akt activation is calmodulin-independent in ER positive breast cancer cell lines
In a few of the cell lines tested in the panel, we found that Akt activation was in fact not dependent on calmodulin. Treatment with W-7 had little or no effect in the ability of several stimuli to induce Akt activation (Fig. 2). When we examined the characteristics of these refractory lines, we were interested to note that all were estrogen dependent, ER-positive breast cancer cells.
Fig. 2.
Akt activation in some ER-positive human breast cancer cell lines is calmodulin independent. Semi-confluent cultures of the indicated cell lines were serum-starved and pre-treated where indicated with either 0.1% DMSO, 30 lM W-7 for 30 min, or 10 lM BAPTA for 90 min, and where indicated, stimulated for 5 min with either 10 nM EGF, 50 ng/ml IGF, 100 nM insulin or exposed to 100 lM pervanadate. Cells were lysed and analyzed for Akt activation as described for Fig. 1. Each blot show is a representative of at least two separate experiments.
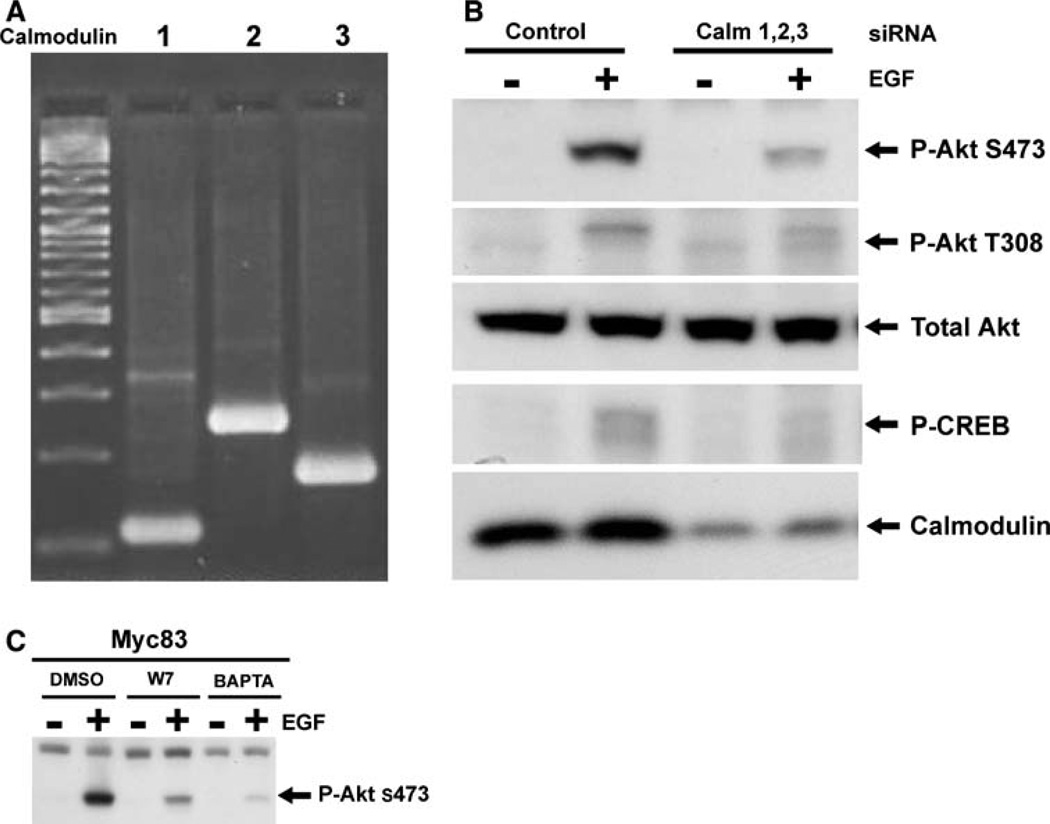
Suppression of calmodulin protein levels using siRNAmediated gene expression knockdown mimics the effect of calmodulin inhibition and substantially diminishes EGF-induced Akt phosphorylation
We wished to further establish the role of calmodulin in the Akt pathway, and also to validate the data generated using the calmodulin inhibitor by demonstrating that the suppression of Akt activation produced by W-7 is the result of its action on calmodulin and not due to some non-specific drug effect. We reasoned that if the effect of W-7 on Akt activation was indeed the result of suppression of calmodulin activity, then suppressing calmodulin protein levels should produce a similar effect on Akt activation. We therefore, set out to use siRNA mediated gene knockdown to suppress calmodulin protein levels and evaluated the impact of this intervention on Akt activation. Unlike most genes in mammalian genomes, three separate calmodulin genes, located on different chromosomes, encode a single calmodulin protein [28]. PCR-based evaluation of cDNA prepared from Myc83 cells revealed that, as expected, all three calmodulin genes are expressed (Fig. 3a). As a result, in order to significantly suppress protein levels, it was necessary to develop an siRNA strategy targeting of all three calmodulin genes simultaneously. Following extensive optimization of this approach, Amaxa-mediated nucleofection of combined siRNAs against calm1, calm2 and calm3 successfully resulted in reduced calmodulin protein expression, compared to cells nucleofected with control luciferase siRNA (Fig. 3b). Suppression of calmodulin protein levels resulted in a marked decrease in EGF-induced Akt phosphorylation. Total Akt expression was unaltered by the calmodulin siRNAs, and the levels of phospho-CREB, a target of calmodulin-dependent protein kinase II, were suppressed, thereby serving as a positive control for the suppression of calmodulin activity. Nucleofection with a luciferasedirected control siRNA had no effect on EGF-induced Akt activation, and a further control for off-target effects of the calmodulin-directed siRNAs is provided by the observation that nucleofection with all three siRNAs was required to produce the effect. Omitting the siRNA against any one of the three genes resulted in no suppression of calmodulin protein levels, and no alteration in EGF-induced AKT activation (data not shown). The results obtained with calmodulin siRNAs confirmed, at the genetic level, the role of calmodulin in the Akt signal pathway, and substantiated the results seen using the calmodulin inhibitor, W-7, as demonstrated in Myc83 cells (Fig. 3c).
Fig. 3.
Suppression of calmodulin expression by siRNA results in significant suppression of EGF-induced Akt-activation. The expression of all three calmodulin genes in Myc83 cells was confirmed by reverse transcription-PCR analysis of RNA from the cells using primers specific for Calm1, Calm2 and Calm3 as described in the “Methods” section (a). Myc83 cells were transfected using amaxa Nucleofector II system as described in section “Materials and methods” with 5 lg of siRNA against each calmodulin gene or equal amounts of control luciferase siRNA. After 48 h, the cells were serum starved for 6 h and then stimulated with or without EGF (10 nM/5 min). Cells lysates were processed as previously described and equal amount of total protein containing lysates were analyzed for calmodulin protein levels using calmodulin monoclonal antibody. Akt activation was assayed by phospho-specific Akt antibodies. Western blot for phospho-CREB served as a positive control for down regulation of calmodulin and calmodulin activity. Total Akt serves as a loading control. Figure shown is a representative of two successful siRNA experiments (b). Myc83 cells were serum starved and pretreated where indicated with either 0.1% DMSO, 30 lM W-7 for 30 min, or 10 lM BAPTA for 90 min and stimulated with or without EGF (10 nM/5 min). Akt activity was assessed by Western blot as described above (c).
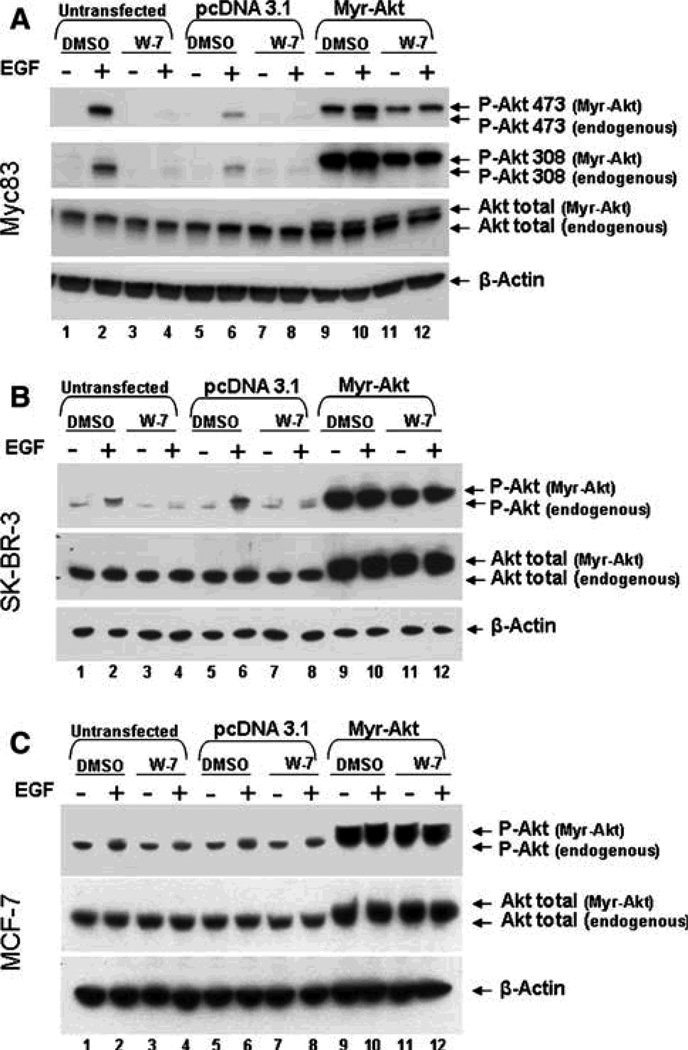
Phosphorylation of a constitutively active Akt construct (Myr-Akt) is not suppressed by calmodulin inhibition by W-7, in breast cancer cell lines
The addition of a myristoylation signal to the Akt coding sequences (Myr-Akt), results in a protein that is constitutively targeted to the plasma membrane and, therefore, bypasses normal regulatory mechanisms controlling Akt activation. We employed this construct to begin to dissect the mechanism underlying the role of calmodulin in Akt activation. Mammary cancer cells (Myc83, MCF-7 and SK-BR-3) were nucleofected with 3 lg of empty pcDNA3.1 vector control, or the Myr-Akt construct using the Amaxa system (Fig. 4). One day after nuclofection, the cells were serum starved overnight, pretreated for 30 min with either DMSO or 30 lM W-7, and then stimulated with EGF or control medium. As seen in Fig. 4a, W-7 abolished Akt activation in untransfected and control, pcDNA3.1-transfected Myc83 cells (Fig. 4, lanes 2, 4, 6, 8). In serum starved Myc83 cells transfected with the Myr-Akt construct, as expected, the myristoylated Akt is phosphorylated even in the absence of EGF stimulation (Fig. 4a, lane 9). Upon EGF stimulation, the endogenous Akt also becomes activated (Fig. 4a, lane 10, P-Akt 473, lower band). In the presence of the calmodulin inhibitor, W-7, the endogenous P-Akt band is abolished, while activation of the transiently expressed Myr-Akt is unaffected (Fig. 4a, lane 12). Thus, myristoylation of the Akt renders the activation of the molecule calmodulin-independent, suggesting a possible role for calmodulin in the regulation of the sub-cellular localization of Akt.
Fig. 4.
Transient expression of Myr-Akt can overcome the inhibitory effects of W-7 in breast cancer cells. Untransfected Myc83 (a), SKBR- 3 (b), or MCF-7 (c) cells (lanes 1–4) were compared to cells transfected by Amaxa nucleofection as described in section “Materials and methods” with either 3 lg of empty vector control pcDNA 3.1 (lanes 5–8) or 3 lg Myr-Akt expression construct (lanes 9–12). Twenty-four hours post-transfection the cells were serum starved overnight, then pretreated with either DMSO or 30 lM W-7 for 30 min, and then stimulated with 10 nM EGF for 5 min. Cell were lysed and analyzed for Akt activity as previously described. The blots shown are a representative of at least three separate experiments.
Similar results were seen when Myr-Akt was exogenously expressed in the ER-negative human breast cancer cell line SK-BR-3 (Fig. 4b). Again, the membrane targeted Myr-Akt was resistant to W-7 treatment (Fig. 4b, lanes 11 and 12, upper band). Not surprisingly, MCF-7 cells again consistently resisted the effects of the calmodulin inhibitor, as Akt activation was not significantly changed in endogenous Akt or Myr-Akt in transfected MCF-7 cells, as demonstrated in Fig. 4c.
The lack of calmodulin dependence for Akt activation seen in some cells does not appear to be explained by calmodulin expression level, or ER activity or status
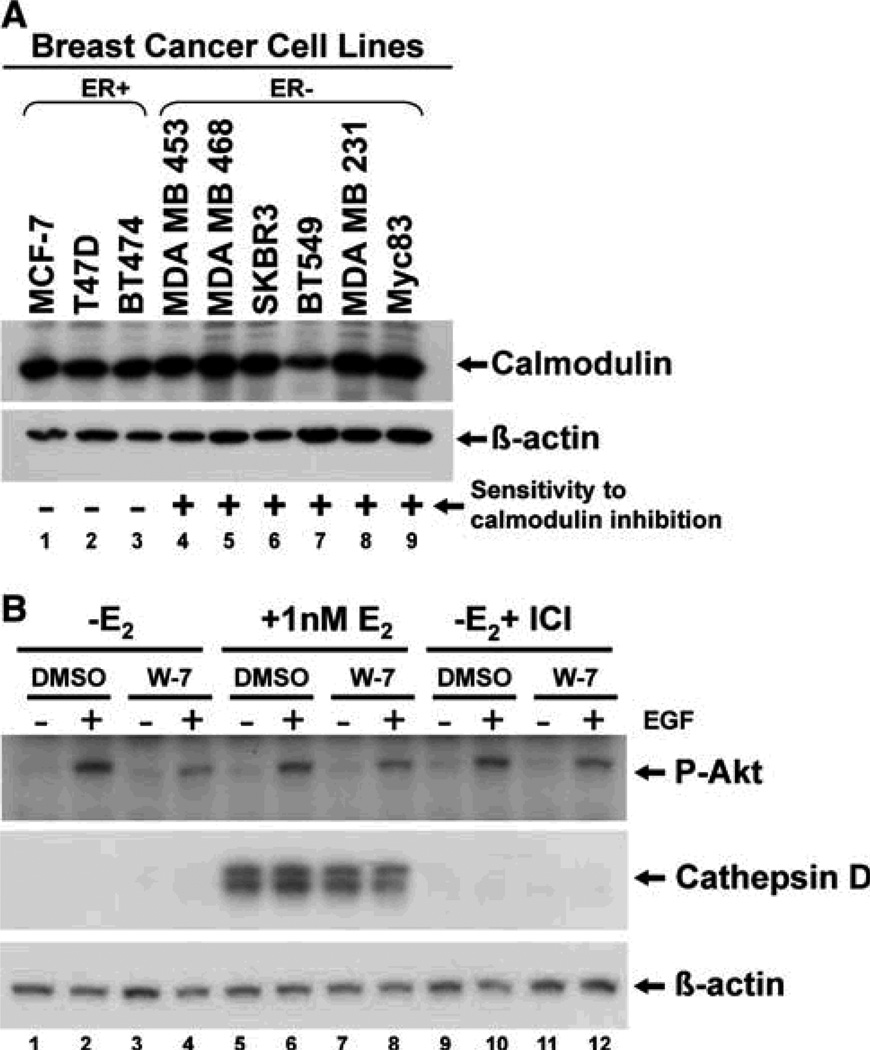
The panel of cell lines screened for W-7 sensitivity exhibit diverse range of genetic and phenotypic features which exemplify many of the common alterations seen in human breast tumors, including cells with mutant p53, activated Ras, loss of PTEN expression, or loss of functional Rb, etc., none of which alterations were apparently correlated with W-7 sensitivity [24, 29]. Based on the calmodulin knockdown data it seemed possible that the level of calmodulin expressed within the cell might influence W-7 sensitivity. We therefore examined the level of calmodulin present in the cells by Western blot analysis. As seen in Fig. 5, the W-7 insensitive cell lines (MCF-7, T47D and BT474; Fig. 5a, lanes 1–3) expressed relatively similar amounts of calmodulin protein to W-7 sensitive (+) cell lines (MDA-MB-453, MDA-MB-468, SK-BR3, BT549, and MDA-MB-231—lanes 4–9).
Fig. 5.
(a) Calmodulin expression can not explain the differential response to calmodulin inhibition seen in ER-positive versus ERnegative breast cancer cell lines. Actively growing breast cancer cells were lysed and processed as previously described, and analyzed for calmodulin expression by Western blot. Equal amounts of total protein containing lysates were resolved on SDS-PAGE, then transferred to PVDF and probed for calmodulin. Cell lines that were sensitive to calmodulin inhibition, observed by inhibition of Akt activation, are indicated by (+). Cell lines that do not demonstrate inhibition of Akt activity in the presence of W-7 are labeled (−). (b) No effect of W-7 treatment on Akt activation by estrogen removal, or ER ablation by treatment with ICI 182,780 in ER-positive MCF-7 cells. MCF-7 cells were grown to semi-confluence and then removed of estrogen as described in materials and methods. Estrogen stripped MCF-7 cells were either untreated or, where indicated, treated with 1 nM bestradiol (E2) (lanes 5–8) or 100 nM ICI 182,780 (lanes 9–12) for 48 h. MCF–7 cells were serum starved overnight in phenol red-free IMEM plus HEPES buffer, then pre-treated, where indicated, with either DMSO or 30 lMW-7 for 30 min, then unstimulated or stimulated with 10 nM EGF for 5 min. Equal amounts of cell lysates were processed for Western blot as previously described and analyzed for Akt activation by phospho-Akt S473 antibody. Cathepsin D expression, an estrogen responsive gene, is used as a control to demonstrate proper estrogen removal. Beta-actin serves as a loading control. This figure is a representative of two independent experiments.
As noted above, the most obvious difference between the W-7 sensitive and in-sensitive cell lines is that all of the insensitive ones are ER-positive and estrogen dependent. In light of this and since there is strong evidence in the literature demonstrating that ER activity can regulate the PI3K/Akt pathway in breast cancer cells [30, 31], we postulated that the presence or activity of the ER may be bypassing the need for calmodulin in the activation of Akt in ER-positive breast cancer cell lines.
To address this question, we examined the impact on EGF mediated Akt activation of estrogen withdrawal alone, or withdrawal in combination with treatment with an antiestrogen which is known to target the ER for destruction (ICI 182,780), using the estrogen dependent breast cancer cell line MCF-7. The cells were stripped of estrogen and grown for two days under three different conditions: (1) with charcoal stripped serum only, (2) charcoal stripped serum plus 1 nM 17-beta-estradiol (E2), or (3) charcoal stripped serum plus 100 nM ICI 182,780 (to ablate the ER). The cells were then serum starved overnight (still in the presence or absence of E2 and ICI 182,780), exposed to the calmodulin inhibitor, W-7, and stimulated with EGF. As seen in Fig. 5b, MCF-7 cells were still able to activate Akt in the presence of W-7, even in the absence of estrogen (Fig. 5b, lanes 2 and 4), and indeed, under conditions in which the ER is being ablated (Fig. 5b, lanes 10 and 12). Adequacy of the estrogen withdrawal was assessed by evaluating the expression of the highly estrogen responsive gene cathepsin D. Estrogen withdrawal resulted in suppression of cathepsin D expression to undetectable levels, which was not altered by treatment with ICI 182,780, but treatment with 1 nM estradiol resulted in re-expression of cathepsin D protein. These results demonstrate that neither removal of estrogen nor ablation of the ER restored calmodulin-dependent Akt signaling in the ER-positive MCF-7 cells.
Forced overexpression of EGFR and ErbB2 partially restores calmodulin-dependent Akt signaling in the ER-positive, W-7-insensitive MCF-7 cell line
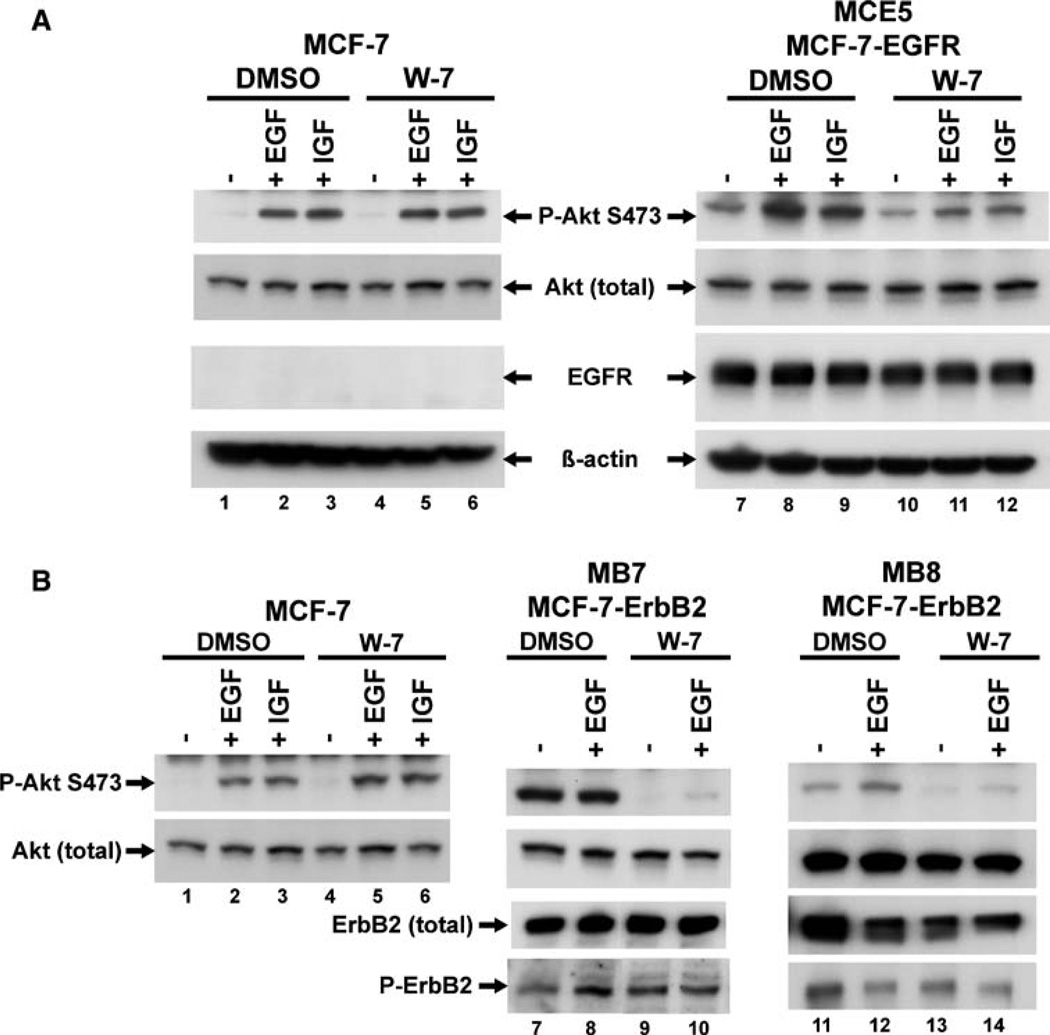
In addition to ER expression status we noticed that many of the W-7 sensitive cells express high levels of epidermal growth factor receptor (EGFR) family members, whereas the ER positive cells do not. We therefore postulated that overexpression of one or more of this family of receptors, a common feature of ER negative breast cancer, might be involved in the calmodulin dependent Akt activation. To examine this possibility we decided to determine if forcing the overexpression of one of these receptors in an ER positive cell would make Akt activation calmodulin dependent. We obtained MCF-7 cells that have been engineered to overexpress either EGFR (MCE5—kindly provided by Dr. Dorraya El-Ashry, University of Michigan) [22] or ErbB2 (MCF-7-ErbB2, clones MB7 and MB8—graciously provided by Dr. Francis G. Kern, Adelson Medical Research Foundation) [21], and tested them to determine if EGF-induced Akt activation was calmodulin dependent.
Serum starved cells were pretreated with W-7 or DMSO, stimulated with EGF and then analyzed, as described above. MCE5 (MCF-7-EGFR) cells express significantly more EGFR than the parental MCF-7 cells, and exhibit high basal Akt activation (Fig. 6a, lanes 1 and 7). As before, exposure to the calmodulin inhibitor did not inhibit EGF-induced Akt activation in parental MCF-7 cells (Fig. 6a, lanes 2 and 5), but did result in a reduction of EGF-induced Akt phosphorylation in MCF-7-EGFR cells (Fig. 6a, lanes 8, and 11). Interestingly, unlike what was seen with the EGFR-amplified breast cancer cell line MDA-MB-468 (Fig. 1a, lane 31), treatment with W-7 did not result in significant inhibition of basal Akt activation in MCE5 cells, (Fig. 6a, lane 10). These results suggest that overexpression of EGFR can make EGF-induced Akt activation partially dependent on calmodulin in cells that were previously W-7 insensitive.
Fig. 6.
Stable expression of EGFR or ErbB2 in MCF-7 cells partially restores calmodulin dependent signaling compared to parental W-7 insensitive MCF-7 cells. MCF-7 cells were grown to semi-confluence in 10% FBS, MCF-7-EGFR cells (MCE5) (a) and MCF-7-ErbB2 cells (Clones MB7 and MB8) (b) were grown to semi-confluence in phenol red-free IMEM plus 10% charcoal stripped serum. Cells were serum starved over night and pre-treated with either DMSO or 30 lM W-7 for 30 min. MCF-7 cells were unstimulated or stimulated with 10 nM EGF, or 50 ng/ml IGF for 5 min, where indicated. MCF-7-EGFR cells, because of their high EGFR expression levels, and MCF-7-ErbB2 cells MB7 and MB8, because of their high ErbB2 expression levels, were unstimulated or stimulated with only 24 ng/ml EGF for 10 min, or 50 ng/ml IGF for 5 min where indicated. Cell lysates were processed as previously described and equal amount of total protein containing lysates were analyzed for Akt activation by phospho-S473 specific Akt antibody. EGFR expression was confirmed in MCF-7-EGFR cells using anti-EGFR antibody. Total ErbB2 and activated phopspho-ErbB2 expression was confirmed in MCF-7-ErbB2 MB7 and MB8 cells using anti-ErbB2 and anti-phospho-ErbB2 monoclonal antibodies. Total Akt levels were unchanged and beta-actin serves as a loading control. This figure is a representative of three independent experiments.
The two independent MCF-7-ErbB2 clones overexpress ErbB2 to slightly different degrees, with the MB7 cells expressing slightly more than the MB8 cells. In both clones, however, the ErbB2 was found to be constitutively phosphorylated (Fig. 6b, P-ErbB2). In these overexpressing clones, but not in the parental MCF-7 cells, pretreatment with W-7 resulted in suppression of both basal and EGF stimulated phosphorylation of Akt (Figs. 6b, lanes 9, 10, 13, 14 and 6b, lanes 4 and 5).
To further address the possible involvement of estrogen signaling as a modulator of the calmodulin dependence of Akt activation, we tested the W-7 sensitivity of MB7 cells when grown in medium containing untreated serum (with estrogen), and when grown in medium supplemented with charcoal stripped serum (estrogen free). Under both conditions, W-7 was able to block EGF-stimulated Akt activation (data not shown), providing further evidence that the presence of ER alone or in combination with its ligand does not inhibit the calmodulin dependence of Akt activation.
These data suggest that calmodulin-dependent signaling to Akt is not affected by the presence of estrogen or ER activity, but rather may be influenced by the over-expression of growth factor receptors.
EGF-induced localization of calmodulin and Akt at the plasma membrane is inhibited by W-7 in both MCF-7 and SK-BR-3 cell lines
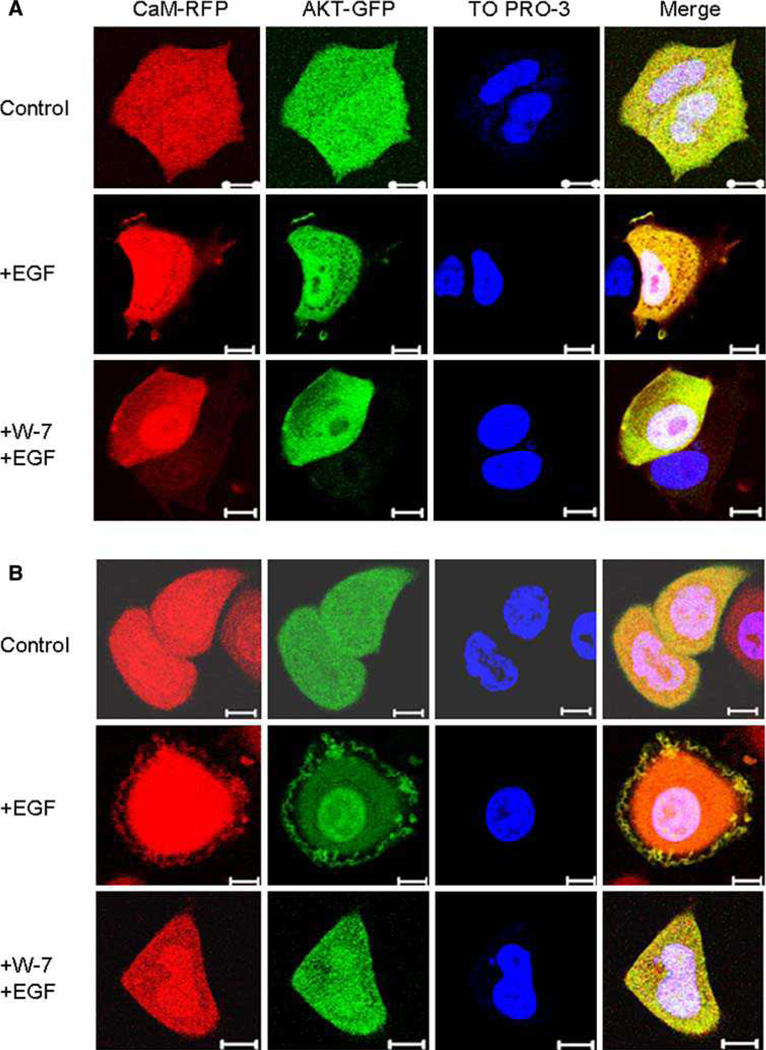
Our data demonstrating that phosphorylation of the constitutively active Akt construct was not suppressed by calmodulin inhibition suggested a possible mechanism by which the activation of wild-type Akt might be occurring. The constitutively active construct has a myristoylation signal that results in the targeting of the protein to the plasma membrane where it is phosphorylated independent of its normal activating signals. This suggested that inhibition of calmodulin function in ER-negative cells might be blocking the EGF stimulated relocalization of Akt to the PM, thereby blocking its activation, and that this blockade does not occur in ER positive cells. To test this possibility we made use of fluorescently tagged Akt and calmodulin constructs in order to follow the sub cellular localization of these proteins before and after EGF stimulation in the presence and absence of calmodulin inhibition, in both ER positive and negative cells.
MCF-7 (ER-positive) and SK-BR-3 (ER-negative) cells were transiently co-transfected with calmodulin-RFP and Akt1-GFP, then serum starved and treated with the indicated materials as described in the “Methods” section, and then examined by fluorescent microscopy (Fig. 7). Calmodulin and Akt were distributed mainly in the cytoplasm and nucleus of cells in serum free medium. Stimulation of the cells with EGF caused translocation of both calmodulin and Akt to the membrane, demonstrating that calmodulin and Akt localize to the same cellular compartment. As predicted, treatment with W-7 blocked the translocation of both calmodulin and Akt to the plasma membrane in SKBR-3 cells. Interestingly, however, this translocation was also blocked by W-7 in MCF-7 cells, which seems to rule out the possibility of differential localization of calmodulin and Akt in ER-positive and ER-negative cell lines as an explanation for the different responses of Akt activation by the calmodulin inhibitor in the two types of cells.
Fig. 6.
Stable expression of EGFR or ErbB2 in MCF-7 cells partially restores calmodulin dependent signaling compared to parental W-7 insensitive MCF-7 cells. MCF-7 cells were grown to semi-confluence in 10% FBS, MCF-7-EGFR cells (MCE5) (a) and MCF-7-ErbB2 cells (Clones MB7 and MB8) (b) were grown to semi-confluence in phenol red-free IMEM plus 10% charcoal stripped serum. Cells were serum starved over night and pre-treated with either DMSO or 30 lM W-7 for 30 min. MCF-7 cells were unstimulated or stimulated with 10 nM EGF, or 50 ng/ml IGF for 5 min, where indicated. MCF-7-EGFR cells, because of their high EGFR expression levels, and MCF-7- ErbB2 cells MB7 and MB8, because of their high ErbB2 expression levels, were unstimulated or stimulated with only 24 ng/ml EGF for 10 min, or 50 ng/ml IGF for 5 min where indicated. Cell lysates were processed as previously described and equal amount of total protein containing lysates were analyzed for Akt activation by phospho-S473 specific Akt antibody. EGFR expression was confirmed in MCF-7-EGFR cells using anti-EGFR antibody. Total ErbB2 and activated phopspho-ErbB2 expression was confirmed in MCF-7-ErbB2 MB7 and MB8 cells using anti-ErbB2 and anti-phospho-ErbB2 monoclonal antibodies. Total Akt levels were unchanged and beta-actin serves as a loading control. This figure is a representative of three independent experiments.
Discussion
Akt is a key regulator of multiple cellular processes including proliferation and cell survival. In a previous study we discovered that survival signaling provided by EGF-induced Akt activation in a c-Myc overexpressing mouse mammary cancer cell line could be inhibited by the pharmacological calmodulin inhibitor W-7 [20]. The goals of the work we present here were: (1) to confirm that the inhibition of Akt activation was indeed the result of the inhibition of calmodulin function and not due to some independent property of the drug, (2) to determine if this intriguing phenomenon was limited to this particular mouse cancer cell line or represented a more general mechanism for Akt activation in human mammary epithelial or cancer cells, and (3) to identify the characteristics of cells that allow this pathway of Akt activation to function.
No pharmacological inhibitor is perfect, and so we first wanted to be sure that the suppression of Akt activation we were studying was indeed the result of inhibition of calmodulin by W-7, and not the result of some non-specific activity of the agent. To address this concern we decided to use an entirely independent, non-pharmacological approach to suppress calmodulin activity; knocking down calmodulin protein levels using siRNA technology. We reasoned that if the suppression of Akt activation produced by W-7 was indeed mediated by its inhibition of calmodulin activity, then reducing the cellular calmodulin concentration should also block Akt activation. This simple idea turned out to be much more challenging to achieve than we had anticipated. In addition to the complication noted above, that calmodulin is unique in the genome in having three independent genes coding for exactly the same amino acid sequence, thereby necessitating the simultaneous knockdown of three independent gene products, we soon discovered that suppressing the levels of one calmodulin gene increases the expression of the others (not shown). Nevertheless, after selecting and optimizing a cocktail of three siRNAs, one against each gene product, we were able to significantly suppress calmodulin protein levels and demonstrate that this indeed resulted in suppression of EGF-induced Akt activation (Fig. 3b). Leaving any one of the three siRNAs out of the cocktail results in loss of knockdown and loss of suppression of Akt activation, thereby controlling for off-target effects of the individual siRNAs (not shown). During this process we determined that the better the knockdown of calmodulin level, the more effective the suppression of Akt activation. In addition to these studies, we examined the ability of several other chemically distinct compounds reported to inhibit calmodulin activity to suppress EGF induced Akt activity. All of these compounds were able to suppress EGF-stimulated Akt activity (not shown), and since it is unlikely that they would all have the same non-specific activities, we take this as further evidence that the inhibition of calmodulin activity is indeed the key target.
Further evidence for the specificity of the inhibitor is provided by the fact that when targeted to the PM by virtue of a myristylation signal, the phosphorylation of Akt occurs as expected, in the presence of W-7 even as the compound is blocking the phosphorylation of the endogenous protein. This demonstrates that W-7 is not non-specifically inhibiting the kinases involved in Akt activation nor grossly perturbing cellular function such that these events cannot occur.
These data, along with the significant experience with W-7 in the literature lead us to conclude that the effects we are studying do indeed relate to inhibition of calmodulin rather than some non-specific effect.
To address the second goal of the study, we assembled a panel of human cell lines, including, breast cancer cell lines designed to represent the spectrum of phenotypes and genotypes typically found in human breast cancer [24, 29], as well as some non-transformed mammary epithelial cell cultures. As described above, treatment with W-7 suppressed Akt activation in the majority of the cell lines tested, including the non-transformed mammary epithelial cells (Fig. 1 and data not shown). This demonstrates that calmodulin dependent Akt activation is a common mechanism in many breast cancer cells, but since EGF-induced Akt-activation was also suppressed by W-7 in non-transformed HMEC (Fig. 1b), it suggests that calmodulin is likely involved in the regulation of Akt activity in normal mammary tissue.
Several cell lines, however, were refractory to the inhibitory effects of W-7 on Akt activation (Fig. 2), allowing us to proceed with the third goal of this study: characterizing the features of cells in which Akt activation is calmodulin dependent, compared to those in which it is not, with the goal of beginning to understand the mechanisms involved. We first set out to address the possibility that simple overexpression of calmodulin might underlie the difference in W-7 sensitivity. We had shown that knocking down calmodulin levels could suppress EGFinduced Akt activation, mimicking the effects of W-7 and so it seemed reasonable that overexpression of calmodulin might make cells resistant to the effects of the compound. Analysis of calmodulin levels in the cell lines by immunoblotting did not reveal dramatic differences in calmodulin levels between the various cell lines and no correlation between the levels and W-7 sensitivity was evident (Fig. 5). We believe, therefore, that calmodulin expression level is not a likely source of the differences in W-7 sensitivity. It is possible, however, that this conclusion is too simplistic. The nucleic acid sequence of the exons in the three genes that code for calmodulin in the human genome are almost as divergent as is possible for DNA coding for the same amino acid sequence [28]. This demonstrates that all three genes have been carefully conserved over evolutionary time and implies that each gene has some individual function that cannot be provided by the other two genes. Indeed the promoters and 30 and 50 UTRs of the three genes are quite different suggesting divergent function. Studies of calmodulin expression in neurons and other systems suggest that indeed the three transcripts serve different functions and may code for different pools of calmodulin within the cell [32–34]. Western blot analysis simply looks at total calmodulin levels within the cells without regard for which transcript is predominant, or the sub-cellular localization of the protein. No large scale immunohistochemical study of calmodulin expression in breast cancer is described in the literature; however, differences in the levels of the three transcripts are evident in published micro-array data sets, comparing normal mammary gland to cancer, and ER-negative to ER-positive disease, suggesting that this is an issue that merits further study [35–38]. However, our finding that altering the level of one calmodulin transcript can alter the expression of the others highlights the complexity of such analysis, particularly since there is no way of distinguishing between the protein products of the three genes. We are developing immunofluorescence based approaches to study calmodulin dynamics in our cell systems and plan to initiate an immunohistochemical study of calmodulin expression in breast cancer.
The cell lines that make up our panel have been studied in great detail and characterized with respect to many of the genetic and epigenetic alterations frequently found in breast cancer. The panel contains lines with mutant p53, some with mutant Ras, some with loss of PTEN expression, and some with loss of functional Rb, etc., none of which alterations was apparently correlated with W-7 sensitivity.
The most obvious difference between cells in which W-7 blocked Akt activation and those in which it did not was ER status. However, acute inhibition of ER mediated signaling, by estrogen deprivation or treatment with an antiestrogen known to lead to ER destruction, did not render the activation of Akt W-7 sensitive in MCF-7 cells. This suggests that the difference is not simply due to the presence of the receptor or the expression of some estrogen responsive gene in ER positive cells.
Long before the gene expression array studies were able to develop elaborate descriptions of the ER-positive phenotype in terms of expression array signatures, it has been appreciated that ER-positive breast cancer is different from ER-negative breast cancer in many more ways than simply the presence or absence of the receptor. It has, for example, frequently been noted that there seems to be an inverse correlation between ER status and the expression of EGFR family members. ER-negative breast cancer cells, tend to overexpress members of the EGFR family, compared to ER positive cells [26, 39–42] and this is certainly true of the cell lines in our panel. For example MDA-MB-231, and MDA-MB-468 both are ER-negative and express over 250,000 and 2,000,000 EGF binding sites per cell respectively [39], compared to MCF-7 cells, which are ER positive, and express only 2,400 EGFR binding sites per cell [39].
Since the calmodulin-dependence of Akt activation seemed to correlate somewhat with high level expression of EGFR, we decided to examine the effect that enforced overexpression of EGFR or ErbB2 has on the W-7 sensitivity of one of the ER positive cell lines—MCF-7. As described above, we found that EGF-stimulated Akt activation became sensitive to W-7 in cells overexpressing either of these receptors and that this effect was seen independent of the presence of estrogen in the culture medium.
At first glance these and the previous data would seem to suggest a simple relationship between the level of EGFR family member expression and calmodulin dependent Akt activation, independent of ER status, however, we believe that the situation is rather more complex. Multiple lines of evidence suggest that overexpression of EGFR family members, or constitutive activation of the signaling molecules down-stream of these receptors, is incompatible with ER mediated signaling. As noted above, there is an inverse relationship between ER expression and the expression of EGFR family members, both in clinical samples and cell lines. During the development of the transfected MCF-7 clones we have used in this study, it was discovered that stable expression of EGFR, or ErbB2 in MCF-7 cells could only be maintained if the clones were grown in estrogen depleted media [21, 22]. If the clones were grown in media containing estrogen, both receptor protein and mRNA levels were suppressed. Culture of the clones once more in estrogen depleted medium resulted in the re-expression of the receptors over several weeks. Similarly, transfection of MCF-7 cells with constitutively active components of the MAPK pathway results in the reversible loss of ER expression in cells grown in estrogen containing medium [43]. In contrast, if estrogen-dependent breast cancer cells are grown in the prolonged absence of estrogen, or are selected for resistance to antiestrogens, the cells that grow out are frequently found to have upregulated expression of one of the EGFR family members or exhibit enhanced down-stream signaling [44–47]. Taken together these data suggest that if deprived of estrogenic stimuli, ER positive breast cancer cells will come to depend on proliferative signals down-stream from EGFR family members in much the same way as ER negative breast cancer cells. Indeed a recent array based study has shown that cells driven down this pathway acquire a pattern of gene expression that is reminiscent of that seen in ER negative breast cancers [43].
We hypothesize the acquisition of the calmodul in dependence of Akt activation is one aspect of this phenotypic shift and as such probably does not depend on the absolute level of the receptors expressed so much as the signaling pathways operant within the cell that are driving proliferation.
The precise mechanisms underlying calmodulin-dependent Akt activation are not completely understood and this area is the focus of our ongoing studies, though the data presented here and in our previous work clearly suggest that direct interaction between calmodulin and Akt is involved, and that inhibition might depend on altered protein trafficking. Our previous work showed by coimmunoprecipitation assays that Akt associates with calmodulin in an EGF-dependent manner, in Myc83 cells [20], and we have shown that the same is true in the human breast cancer cells that we have used here (not shown). Additional evidence for a direct interaction between the two proteins has come from the literature. For example, in a study in which an mRNA-displayed proteome library was screened for previously uncharacterized calmodulin binding proteins [48], Akt was identified as a calmodulin binding partner. This finding was then validated by a CaMSepharose pull-down assay and Akt was shown to be associated with calmodulin in a calcium dependent manner in both mouse brain lysates and in HeLa cell extracts [48], and a recent report by Rihe Liu and colleagues has demonstrated that calmodulin binds directly to the PH domain of Akt [49]. We are in the process of investigating the nature of the association between calmodulin and Akt, and have identified a putative IQ motif, a conserved calmodulin binding motif resembling IQXXXRGXXXR, [13] within Akt (between amino acids 161 and 176).
In the present study we have extended our earlier observations and shown that both calmodulin and Akt are translocated to the PM on EGF-stimulation and that this translocation is blocked by treatment with W-7, suggesting that calmodulin is regulating Akt activity by modulating the sub-cellular localization of Akt. This idea is reinforced by our finding that direct targeting of Akt to the plasma membrane by the addition of a myristoylation signal overcomes the dependence on calmodulin for activation. It would have been satisfying had we found that EGF-stimulated Akt translocation to the plasma membrane was not blocked by treatment with W-7, however, this was not the case and although Akt becomes activated in response to EGF in the ER positive cells when treated with W-7, the translocation appears to be blocked. This is an intriguing finding that suggests that Akt activation is occurring differently in these cells. As noted above, it is not clear if the ER has a direct role in this process or if the differences seen relate to other features in the cell associated with the ER positive phenotype. Our studies conducted in the presence or absence of estrogen suggest that if ER is involved, its action is independent of ligand binding. Furthermore, the insensitivity of the system to treatment with the ICI 182,780, which is known to result in suppression of ER levels, again argues against a direct effect, although some un-liganded receptor might be available for interactions, even under these conditions. The ER is known to interact with calmodulin suggesting the possibility that this interaction might render W-7 treatment ineffective. We consider this possibility unlikely, but plan to study calmodulin-dependent Akt activation in cells transiently transfected with ER-alpha.
A recent finding has provided another possible explanation for why some breast cancer cell lines might lack dependence on calmodulin for Akt activation. A transforming mutation found in the PH domain of AKT1 in some human cancers is located in one of the putative Akt/calmodulin interaction domains and it would be intriguing to determine if the breast cancer cell lines that do not require calmodulin to activate Akt upon EGF stimulation possess this mutation, and whether this mutation disrupts calmodulin Akt binding, since the PH domain of Akt has been shown to harbor the calmodulin binding site [49, 50].
It is interesting to note that contrary to our previous finding in mouse carcinoma cells, the majority of human breast cancer cells do not require calcium to mediate calmodulin dependent signaling. In fact, the majority of cell lines screened for sensitivity to W-7 were able to phosphorylate Akt in the presence of internal calcium chelation by BAPTA-AM, even though Akt activation was dependent on calmodulin (Fig. 1a and data not shown). This suggests that calcium-independent calmodulin, or apocalmodulin, is capable of mediating Akt activation in the human cells utilized in the present study. Apocalmodulin differs from calcium-bound calmodulin in its tertiary structure, and like calcium-bound form, is known to be involved in functions vital to cellular life, including microtubule function, chromosome segregation and endocytosis [13, 51, 52].
We are in the process of studying the biological implications of our finding that Akt activation is calmodulindependent in ER negative breast cancer cells. It is interesting to note that previous work with calmodulin inhibitors has demonstrated that estrogen independent cells show enhanced sensitivity to these agents. MDA-MB-231 cells were shown to be significantly more sensitive to growth inhibition by the calmodulin inhibitor W-13, than ER-positive, estrogen dependent MCF-7 cells [53]. Furthermore, MCF-7 cells selected for resistance to an antiestrogen were shown to be more sensitive to the growth inhibitory effects of a calmodulin antagonist than the parental MCF-7 cells [53]. It is tempting to speculate that this may present an attractive therapeutic opportunity, and that the use of a calmodulin inhibitor at the same time as antihormonal therapy with antiestrogens or aromatase inhibitors might prevent the development of hormone independent disease.
Acknowledgements
This work is dedicated to the memory of our colleague, Dr. Robert B. Dickson, who passed away before submission of this manuscript. We thank Dr. Dorraya El-Ashry and Dr. Francis G. Kern for the use of the MCE5, MB7 and MB8 cell lines. Assistance from LCCC Tissue Culture Share Resource is gratefully acknowledged (NIH 4P30CA51008). This work was supported in part by National Institutes of Health Grant 2RO1AG014963 and United States Department of Defense Grant DAMD 17-01-1-0251 (to R.B.D.). C.M.C. was supported by a predoctoral training grant from the United States Department of Defense (DAMD 17-01-1-0246). T.B.D. was partly supported by an NIH Specialized Program of Research Excellence (SPORE) grant in breast cancer to Lombardi Comprehensive Cancer Center (2P50CA58185) and by United States Department of Defense postdoctoral training grant DAMD 17-00-1-0271.
References
- 1.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 2.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 3.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 4.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Tamaskovic R, Yang Z, Brazil DP, Merlo A, Hess D, et al. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem. 2004;279:35510–35517. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 8.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 9.Perez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–545. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the proteinkinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 11.DeLorenzo RJ. The calmodulin hypothesis of neurotransmission. Cell Calcium. 1981;2:365–385. doi: 10.1016/0143-4160(81)90026-9. [DOI] [PubMed] [Google Scholar]
- 12.Hidaka H, Sasaki Y, Tanaka T, Endo T, Ohno S, Fujii Y, et al. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci USA. 1981;78:4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurado LA, Chockalingam PS, Jarrett HW. Apocalmodulin. Physiol Rev. 1999;79:661–682. doi: 10.1152/physrev.1999.79.3.661. [DOI] [PubMed] [Google Scholar]
- 14.Egea J, Espinet C, Soler RM, Dolcet X, Yuste VJ, Encinas M, et al. Neuronal survival induced by neurotrophins requires calmodulin. J Cell Biol. 2001;154:585–597. doi: 10.1083/jcb.200101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramljak D, Coticchia CM, Nishanian TG, Saji M, Ringel MD, Conzen SD, et al. Epidermal growth factor inhibition of c-Myc-mediated apoptosis through Akt and Erk involves Bcl-xL upregulation in mammary epithelial cells. Exp Cell Res. 2003;287:397–410. doi: 10.1016/s0014-4827(03)00135-6. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa J, Furuya K, Miyata S, Nakashima T, Kiyohara T. EGF enhances Ca(2+) mobilization and capacitative Ca(2+) entry in mouse mammary epithelial cells. Cell Biochem Funct. 2000;18:215–225. doi: 10.1002/1099-0844(200009)18:3<215::AID-CBF875>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa J, Kiyohara T. Suppression of EGF-induced cell proliferation by the blockade of Ca2+ mobilization and capacitative Ca2+ entry in mouse mammary epithelial cells. Cell Biochem Funct. 2001;19:213–219. doi: 10.1002/cbf.914. [DOI] [PubMed] [Google Scholar]
- 18.Krishnaraju K, Murugesan K, Vij U, Kapur BM, Farooq A. Calmodulin levels in oestrogen receptor positive and negative human breast tumours. Br J Cancer. 1991;63:346–347. doi: 10.1038/bjc.1991.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai KV, Xiao N, Wang W, Gangi L, Greene J, Powell JI, et al. Initiating oncogenic event determines gene-expression patterns of human breast cancer models. Proc Natl Acad Sci USA. 2002;99:6967–6972. doi: 10.1073/pnas.102172399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem. 2004;279:38903–38911. doi: 10.1074/jbc.M405314200. [DOI] [PubMed] [Google Scholar]
- 21.Miller DL, El-Ashry D, Cheville AL, Liu Y, McLeskey SW, Kern FG. Emergence of MCF-7 cells overexpressing a transfected epidermal growth factor receptor (EGFR) under estrogen-depleted conditions: evidence for a role of EGFR in breast cancer growth and progression. Cell Growth Differ. 1994;5:1263–1274. [PubMed] [Google Scholar]
- 22.Liu Y, El-Ashry D, Chen D, Ding IY, Kern FG. MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat. 1995;34:97–117. doi: 10.1007/BF00665783. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross DT, Perou CM. A comparison of gene expression signatures from breast tumors and breast tissue derived cell lines. Dis Markers. 2001;17:99–109. doi: 10.1155/2001/850531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 27.Thompson EW, Martin MB, Saceda M, Clarke R, Brunner N, Lippman ME, et al. Regulation of breast cancer cells by hormones and growth factors: effects on proliferation and basement membrane invasiveness. Horm Res. 1989;32(Suppl 1):242–249. doi: 10.1159/000181356. [DOI] [PubMed] [Google Scholar]
- 28.Fischer R, Koller M, Flura M, Mathews S, Strehler-Page MA, Krebs J, et al. Multiple divergent mRNAs code for a single human calmodulin. J Biol Chem. 1988;263:17055–17062. [PubMed] [Google Scholar]
- 29.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5:195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 30.Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH. Upregulation of PI3 K/Akt signaling by 17beta-estradiol through activation of estrogen receptor-alpha, but not estrogen receptorbeta, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun. 2005;336:1221–1226. doi: 10.1016/j.bbrc.2005.08.256. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, et al. Phosphatidylinositol-3-OH Kinase (PI3 K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- 32.Palfi A, Vizi S, Gulya K. Differential distribution and intracellular targeting of mRNAs corresponding to the three calmodulin genes in rat brain. A quantitative in situ hybridization study. J Histochem Cytochem. 1999;47:583–600. doi: 10.1177/002215549904700502. [DOI] [PubMed] [Google Scholar]
- 33.Toutenhoofd SL, Strehler EE. The calmodulin multigene family as a unique case of genetic redundancy: multiple levels of regulation to provide spatial and temporal control of calmodulin pools? Cell Calcium. 2000;28:83–96. doi: 10.1054/ceca.2000.0136. [DOI] [PubMed] [Google Scholar]
- 34.Toutenhoofd SL, Strehler EE. Regulation of calmodulin mRNAs in differentiating human IMR-32 neuroblastoma cells. Biochim Biophys Acta. 2002;1600:95–104. doi: 10.1016/s1570-9639(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 35.Ginestier C, Cervera N, Finetti P, Esteyries S, Esterni B, Adelaide J, et al. Prognosis and gene expression profiling of 20q13-amplified breast cancers. Clin Cancer Res. 2006;12:4533–4544. doi: 10.1158/1078-0432.CCR-05-2339. [DOI] [PubMed] [Google Scholar]
- 36.Gruvberger S, Ringner M, Chen Y, Panavally S, Saal LH, Borg A, et al. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979–5984. [PubMed] [Google Scholar]
- 37.Gruvberger SK, Ringner M, Eden P, Borg A, Ferno M, Peterson C, et al. Expression profiling to predict outcome in breast cancer: the influence of sample selection. Breast Cancer Res. 2003;5:23–26. doi: 10.1186/bcr548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Davidson NE, Gelmann EP, Lippman ME, Dickson RB. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987;1:216–223. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- 40.Filmus J, Pollak MN, Cailleau R, Buick RN. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem Biophys Res Commun. 1985;128:898–905. doi: 10.1016/0006-291x(85)90131-7. [DOI] [PubMed] [Google Scholar]
- 41.Rae JM, Scheys JO, Clark KM, Chadwick RB, Kiefer MC, Lippman ME. EGFR and EGFRvIII expression in primary breast cancer and cell lines. Breast Cancer Res Treat. 2004;87:87–95. doi: 10.1023/B:BREA.0000041585.26734.f9. [DOI] [PubMed] [Google Scholar]
- 42.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, El-Ashry D. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 2006;66:3903–3911. doi: 10.1158/0008-5472.CAN-05-4363. [DOI] [PubMed] [Google Scholar]
- 44.Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol Endocrinol. 2001;15:1344–1359. doi: 10.1210/mend.15.8.0678. [DOI] [PubMed] [Google Scholar]
- 45.Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–3910. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson RI, Hutcheson IR, Britton D, Knowlden JM, Jones HE, Harper ME, et al. Growth factor signalling networks in breast cancer and resistance to endocrine agents: new therapeutic strategies. J Steroid Biochem Mol Biol. 2005;93:257–262. doi: 10.1016/j.jsbmb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Riggins RB, Thomas KS, Ta HQ, Wen J, Davis RJ, Schuh NR, et al. Physical and functional interactions between Cas and c-Src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res. 2006;66:7007–7015. doi: 10.1158/0008-5472.CAN-05-3952. [DOI] [PubMed] [Google Scholar]
- 48.Shen X, Valencia CA, Szostak JW, Dong B, Liu R. Scanning the human proteome for calmodulin-binding proteins. Proc Natl Acad Sci USA. 2005;102:5969–5974. doi: 10.1073/pnas.0407928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong B, Valencia CA, Liu R. Ca2+/calmodulin directly interacts with the pleckstrin homology domain of AKT1. J Biol Chem. 2007;282:25131–25140. doi: 10.1074/jbc.M702123200. [DOI] [PubMed] [Google Scholar]
- 50.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 51.Kakiuchi S, Sobue K, Fujita M. Purification of a 240 000 Mr calmodulin-binding protein from a microsomal fraction of brain. FEBS Lett. 1981;132:144–148. doi: 10.1016/0014-5793(81)80449-8. [DOI] [PubMed] [Google Scholar]
- 52.Kakiuchi S, Yamazaki R, Teshima Y, Uenishi K, Yasuda S, Kashiba A, et al. Membrane-bound protein modulator and phosphodiesterase. Adv Cyclic Nucleotide Res. 1978;9:253–263. [PubMed] [Google Scholar]
- 53.Strobl JS, Peterson VA. Tamoxifen-resistant human breast cancer cell growth: inhibition by thioridazine, pimozide and the calmodulin antagonist, W-13. J Pharmacol Exp Ther. 1992;263:186–193. [PubMed] [Google Scholar]