Abstract
BACKGROUND
Venous thromboembolic (VTE) disease has a high incidence following trauma, but debate remains regarding optimal prophylaxis. Thrombelastography (TEG) has been suggested to be optimal in guiding prophylaxis. Thus, we designed a phase II randomized controlled trial to test the hypothesis that TEG-guided prophylaxis with escalating low–molecular weight heparin (LMWH), followed by antiplatelet therapy would reduce VTE incidence.
METHODS
Surgical intensive care unit trauma patients (n = 50) were randomized to receive 5,000 IU of LMWH daily (control) or to TEG-guided prophylaxis, up to 5,000 IU twice daily with the addition of aspirin, and were followed up for 5 days. In vitro studies were also conducted in which apheresis platelets were added to blood from healthy volunteers (n = 10).
RESULTS
Control (n = 25) and TEG-guided prophylaxis (n = 25) groups were similar in age, body mass index, Injury Severity Score, and male sex. Fibrinogen levels and platelet counts did not differ, and increased LMWH did not affect clot strength between the control and study groups. The correlation of clot strength (G value) with fibrinogen was stronger on Days 1 and 2 but was superseded by platelet count on Days 3, 4, and 5. There was also a trend in increased platelet contribution to clot strength in patients receiving increased LMWH. In vitro studies demonstrated apheresis platelets significantly increased clot strength (7.19 ± 0.35 to 10.34 ± 0.29), as well as thrombus generation (713.86 ± 12.19 to 814.42 ± 7.97) and fibrin production (274.03 ± 15.82 to 427.95 ± 16.58).
CONCLUSION
Increased LMWH seemed to increase platelet contribution to clot strength early in the study but failed to affect the overall rise clot strength. Over time, platelet count had the strongest correlation with clot strength, and in vitro studies demonstrated that increased platelet counts increase fibrin production and thrombus generation. In sum, these data suggest an important role for antiplatelet therapy in VTE prophylaxis following trauma, particularly after 48 hours.
Keywords: Postinjury hypercoagulability, platelets, functional fibrinogen, thrombelastography (TEG), venous thromboembolism (VTE)
Deep vein thrombosis (DVT) and pulmonary embolism (PE), collectively known as venous thromboembolisms (VTEs), affect an estimated 900,000 people in the United States yearly, resulting in nearly 300,000 deaths.1 The estimated 600,000 nonfatal cases result in several hundred thousand hospitalizations or extended hospital stays, costing an estimated $5.8 to $7.8 billion.1,2 The incidence of symptomatic VTEs are also common following trauma and are reported to be approximately 6% to 7%, despite receiving recommended chemical and/or mechanical prophylaxis.3,4 However, the true incidence of both symptomatic and asymptomatic VTEs in the trauma population may be substantially higher.3,5,6
Although the difference in the incidence of VTEs observed in retrospective studies between patients on prophylaxis compared with those who are not is compelling, the effect of prophylaxis on severely injured patients remains unclear. Several retrospective, prospective, and randomized controlled studies have failed to show differences.7–13 Moreover, a recent review of the trauma literature concluded that there is no evidence that any existing method of VTE prophylaxis is superior to other methods or even to no prophylaxis.14,15 In addition, standard heparin-based VTE prophylaxis often leads to inadequate antifactor Xa (anti-Xa) levels and increased VTE rates in this population.16 Failure to detect differences in the trauma population may be caused by inadequate timing or dosing of heparin or to neglecting the contribution of platelets to thrombus formation.
Currently, there are no optimal plasma-based coagulation assays to assess hypercoagulability. Thrombelastography (TEG) has been proposed to be superior to plasma-based coagulation assays in identifying hypercoagulable states, which can diagnose and quantify hypercoagulability.17 Moreover, a recent study suggested that TEG is more sensitive than anti-Xa levels and may help guide chemical prophylaxis in the prevention of VTEs.18 Therefore, we designed a phase II randomized, controlled trial to test the hypothesis that TEG-guided VTE prophylaxis is safe and would reduced the incidence of both symptomatic and asymptomatic VTE compared with standard heparin-based prophylaxis.
PATIENTS AND METHODS
This randomized, controlled phase II study was conducted at the Denver Health Medical Center, the academic Level 1 trauma center for the University of Colorado Denver, and was approved by the Colorado Multiple Institutional Review Board. This study was registered with the NIH (#NCT01050153). All patients considered for inclusion were trauma patients admitted to the surgical intensive care unit (SICU) in which VTE prophylaxis with low–molecular weight heparin (LMWH) was indicated. Inclusion criteria were patients 18 years or older, who experienced blunt or penetrating trauma requiring admission to the SICU, who would normally receive LMWH therapy for VTE prophylaxis as standard of care, and for whom informed consent by the patient, legally authorized representative, or proxy decision maker could be obtained. Exclusion criteria included the presence of any absolute contraindication to LMWH (heparin hypersensitivity, heparin-induced thrombocytopenia, Model for End-Stage Liver Disease [MELD] > 12, ongoing resuscitation for hemorrhagic shock, known bleeding disorder or ongoing coagulopathy after injury, and subdural or epidural hematoma), any relative contraindication to LMWH (new intracranial lesions, neoplasm or monitoring devices, extravascular thrombolytic therapy, severe uncontrolled hypertension, arterial dissection, recent intraocular surgery, recent intracranial or spine surgery, or conditions associated with increased risk of hemorrhage), presence or removal within the last 12 hours of an epidural or spinal catheter, or recent neuroaxial anesthesia or spinal puncture, or patient history with known use of drugs affecting hemostasis such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors or other anticoagulants within 1 week before hospitalization.
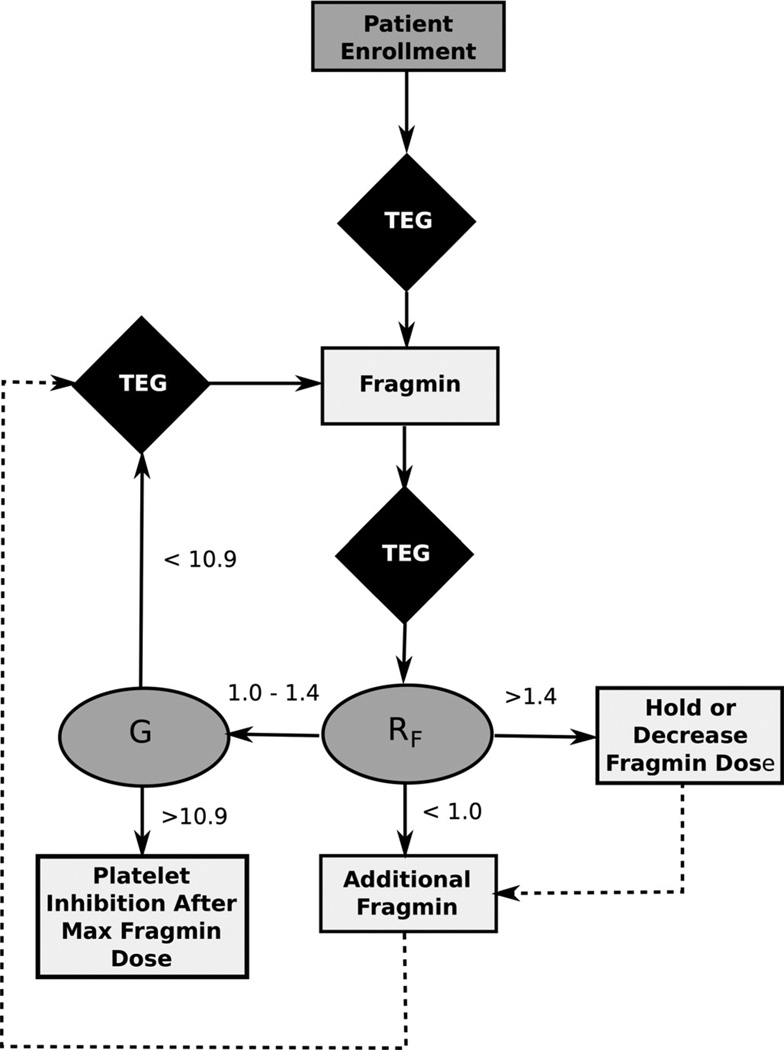
Patients were randomized into a control group or a TEG-guided treatment group, based on a predesigned randomization table. For patients in the control group, dalteparin 5,000 IU was administered subcutaneously once daily. For patients in the TEG-guided group, VTE prophylaxis was guided by the difference in R times obtained from simultaneously running citrated Kaolin TEGs with (RKaolin) and without heparinase (RHeparinase). Dalteparin was initiated if the TEG RF (RKaolin − RHeparinase) was less than 1 minute (RF < 1.0) with a starting dose of 5,000 IU subcutaneously once daily (Fig. 1). The dalteparin dose was then adjusted based on the TEG results four hours postdose, with the goal treatment being an RF value at or between 1.0 and 1.4 minutes. For RF values less than 1 minute, dalteparin was increased by 2,500 IU, to a maximum dose of 10,000 IU daily, divided and given every 12 hours. The dalteparin dose could not be increased more than once in a 24-hour period. For RF values greater than 1.4 minutes, the dalteparin dose was decreased by 2,500 IU if receiving greater than 5,000 IU daily or held if receiving only 5,000 IU once daily. Antiplatelet therapy was initiated once the maximal dose of LMWH was reached and for a G value of greater than 10.9. TEG Platelet Mapping was also performed to ensure the percent inhibition of the arachidonic acid and adenosine diphosphate (ADP) pathways did not exceed 50% with anti-platelet therapy. Aspirin was initiated at a dose of 81 mg and was increased daily to a dose of 325 mg or until inhibition exceeded 50%. Patients were followed up for 5 days in the SICU.
Figure 1.
VTE prophylaxis algorithm for patients randomized to the TEG-guided prophylaxis group.
Conventional plasma-based coagulation tests were also measured including activated partial thromboplastin time (aPTT), international normalized ratio (INR), platelets, antifactor Xa, antithrombin III, and functional fibrinogen levels. The TEG-based Functional Fibrinogen assay assesses the fibrinogen component to clot strength and strongly correlates to the clinical standard von Clauss fibrinogen levels.19 Fibrinogen contribution to clot strength was calculated by MAFibrinogen / MAKaolin. Since clot strength is defined by fibrinogen and platelet contributions, the platelet contribution to clot strength was calculated by 1 – (MAFibrinogen / MAKaolin). Furthermore, the incidence, timing, and management of clinically diagnosed, symptomatic DVT and PE, occurring at any time during the hospitalization, were recorded and confirmed by appropriate diagnostic tests. Patients who exhibited symptoms of a DVT underwent a duplex ultrasound, and patients with symptoms of a PE underwent a CT angiogram of the chest. Regardless of symptoms, all patients received a duplex ultrasound of the lower and upper extremities on study Day 5.
To assess the effect of platelets on TEG parameters, in vitro studies were conducted. Studies were performed on citrated whole-blood samples obtained from healthy volunteers (n = 10). Venipuncture was performed with a 21-gauge needle in an antecubital vein, and blood was collected into two separate 3.5-mL plastic Vacutainers containing 3.2% citrate. In one sample, 700 µL of apheresis platelets, obtained from Bonfils Blood Center, was added to roughly triple the platelet concentration and was gently mixed. Both Kaolin and Functional Fibrinogen TEG assays were performed within 30 minutes of collection, and all TEG parameters were recorded.
Statistical Analysis
All analyses were conducted using SAS version 9.3 for Windows (SAS Institute Inc., Cary, NC). Patient data were analyzed on an “intent-to-treat” basis. Randomization effectiveness was assessed by comparing demographic and injury severity variables between groups. Continuous variables were reported as mean and SEM when normally distributed and as median and interquartile range (IQR) when nonnormally distributed. In comparing the two study groups, we used χ2 tests or Fisher’s exact tests for proportions, t test for normally distributed continuous variables, and the Wilcoxon test for continuous nonnormally distributed variables. Variables measured over time were compared using repeated-measures analysis of variance (ANOVA), and post hoc pairwise comparisons at individual times adjusted using Tukey’s method. In vitro studies were analyzed using a paired t test. All tests were two tailed, and overall experiment error significance set at p < 0.05. The Pearson correlation statistics were used to evaluate the association of fibrinogen level and platelet count to clot strength (as measured by the G value) and the correlation coefficient with correspondent 95% confidence intervals (calculated using Fisher’s z transformation and bias adjustment).
RESULTS
A total of 61 patients were eligible to participate in the study, of whom 11 declined, resulting in 50 patients being enrolled. The control group (n = 25) and TEG-guided prophylaxis group (n = 25) were similar in demographics, including age, body mass index, and sex. Patients had similar injury severity including Injury Severity Score (ISS), base deficit, and Acute Physiology and Chronic Health Evaluation scores (Table 1). Initial coagulation parameters (aPTT, INR, fibrinogen levels, antithrombin III levels, anti-Xa levels, platelet count, and hemoglobin) did not differ between groups. Furthermore, initial citrated Kaolin TEG parameters were similar between groups (Table 1). The median time from injury to study enrollment was 3.0 days (IQR, 2–3) for the control group and 2.0 days (IQR, 2–3) for the TEG-guided group (Wilcoxon p = 0.98). The doses of LMWH and aspirin are shown in Table 2.
TABLE 1.
Baseline Demographics and Coagulation Parameters
| Control (n = 25) |
TEG (n = 25) |
p | |
|---|---|---|---|
| Demographic | |||
| Male, n (%) | 19 (76) | 17 (68) | 0.40* |
| Age, mean (SEM), y | 40 (2.44) | 38 (2.87) | 0.67 |
| Body mass index, mean (SEM), kg/m2 | 29.23 (1.30) | 28.37 (1.39) | 0.65 |
| Injury Severity | |||
| ISS, median (IQR) | 27 (16–36) | 27 (19–33) | 0.92† |
| APACHE, median (IQR) | 20 (17–26) | 23 (17–26) | 0.99† |
| Lactate, mean (SEM), mmol/L | 3.59 (0.43) | 3.97 (0.32) | 0.47 |
| Base deficit, mean (SEM), mEq/L | 7.336 (0.77) | 8.04 (0.76) | 0.52 |
| Hemoglobin, mean (SEM), g/dL | 9.00 (0.33) | 8.53 (0.25) | 0.26 |
| Operations/interventions median (IQR) | 1 (0–2) | 2 (1–2) | 0.12† |
| Coagulation | |||
| Platelet count, mean (SEM), 1,000/µL | 139.16 (9.78) | 120.32 (8.20) | 0.15 |
| Fibrinogen, mean (SEM), mg/dL | 636.00 (39.60) | 562.64 (27.18) | 0.13 |
| Antithrombin III, mean (SEM), % | 72.45 (3.53) | 62.60 (3.64) | 0.06 |
| aPTT, mean (SEM), s | 34.17 (1.65) | 35.53 (1.09) | 0.5 |
| INR, mean (SEM) | 1.35 (0.07) | 1.34 (0.03) | 0.91 |
| Anti-Xa, mean (SEM) | 0.09 (0.00) | 0.09 (0.01) | 0.33 |
| Kaolin TEG | |||
| SP, mean (SEM), min | 4.63 (0.35) | 4.13 (0.44) | 0.38 |
| R, mean (SEM), min | 5.31 (0.32) | 5.26 (0.31) | 0.9 |
| K, mean (SEM), min | 1.43 (0.06) | 1.51 (0.08) | 0.41 |
| Angle, mean (SEM), degrees | 68.54 (1.06) | 66.40 (1.82) | 0.31 |
| MA, mean (SEM), mm | 65.84 (1.35) | 64.37(1.10) | 0.40 |
| G, mean (SEM), dynes/cm2 | 10.26 (0.76) | 9.36 (0.49) | 0.32 |
| LY30, mean (SEM), (%) | 0.01 (0.00) | 0.01 (0.00) | 0.76 |
χ2 test.
Wilcoxon nonparametric test.
All other p values were from t test for continuous variables.
APACHE Acute Physiology and Chronic Health Evaluation; G, clot strength; K, kinetic time; LY30, lysis 30 minutes following MA; MA, maximum amplitude; R, reaction time; SP, split point.
TABLE 2.
Number of Patients in Each Group Receiving LMWH and Aspirin (ASA), as well as the Dose Given
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| Control (n = 25) | |||||
| LMWH dose 1 | |||||
| 5,000 U | 25 (100%) | 23 (92%) | 21 (84%) | 21 (84%) | 23 (92%) |
| TEG-guided (n = 25) | |||||
| LMWH dose 1 | |||||
| 5,000 U | 25 (100%) | 24 (96%) | 22 (88%) | 19 (76%) | 17 (68%) |
| LMWH dose 2 | |||||
| 2,500 U | 2 (8%) | 12 (48%) | 4 (16%) | 2 (8%) | 1 (4%) |
| 5,000 U | 0 (0%) | 0 (0%) | 0 (0%) | 15 (60%) | 13 (52%) |
| ASA | |||||
| 81 mg | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 5 (20%) |
| 162 mg | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) |
| 325 mg | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
The proposed TEG-guided VTE prophylaxis algorithm did not seem to affect standard TEG parameters including R time, K time, α angle, MA, G, and LY30 compared with the control group through Day 5. Trends in R time and G over the 5-day study period are shown in Table 3. In addition, there was no difference in aPTT, INR, fibrinogen levels, platelet count, anti-Xa, and antithrombin III levels between the control and TEG-guided prophylaxis groups during the 5-day study period (Table 3).
TABLE 3.
Trends in R Time (p = 0.10), Clot Strength (G) (p = 0.64), Fibrinogen (p = 0.58), and Platelet Count (p = 0.39), Expressed as Mean ± SEM, as well the Pearson Correlation Statistics of Fibrinogen and Platelet Count With Clot Strength During the 5-day Study Period
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| Control (n) | |||||
| R time, min | 5.32 ± 0.32 | 6.08 ± 0.52 | 4.96 ± 0.21 | 4.70 ± 0.29 | 5.03 ± 0.26 |
| G, dynes/cm2 | 10.26 ± 0.76 | 11.29 ± 0.76 | 12.25 ± 0.91 | 13.83 ± 0.85 | 14.72 ± 0.87 |
| Fibrinogen, mg/dL | 636 ± 137 | 671 ± 117 | 715 ± 120 | 716 ± 159 | 691 ± 147 |
| Platelets, 1,000/µL | 139 ± 49 | 154 ± 83 | 183 ± 135 | 216 ± 185 | 262 ± 172 |
| Correlation coefficients (R, 95% CI) | |||||
| Fibrinogen and G R (95% CI) | 0.68 (0.14–0.91) | 0.63 (0.28–0.84) | 0.25 (−0.24 to 0.64) | 0.55 (0.11–0.81) | 0.45 (0.01 to 0.75) |
| p | 0.0142 | 0.0012 | 0.3004 | 0.0144 | 0.0395 |
| Platelets and G R (95% CI) | 0.56 (0.20–0.79) | 0.44 (0.04–0.71) | 0.76 (0.50 to 0.89) | 0.69 (0.33–0.88) | 0.57 (0.19 to 0.81) |
| p | 0.0037 | 0.0282 | <0.0001 | 0.0007 | 0.0045 |
| TEG-guided (n = 25) | |||||
| R time, min | 5.26 ± 0.31 | 5.75 ± 0.34 | 4.98 ± 0.23 | 5.31 ± 0.43 | 5.68 ± 0.55 |
| G, dynes/cm2 | 9.36 ± 0.49 | 10.47 ± 0.48 | 12.35 ± 0.64 | 14.16 ± 0.85 | 14.17 ± 0.74 |
| Fibrinogen, mg/dL | 563 ± 102 | 610 ± 136 | 624 ± 154 | 652 ± 161 | 687 ± 158 |
| Platelets, 1,000/µL | 120 ± 41 | 128 ± 35 | 148 ± 54 | 199 ± 75 | 268 ± 83 |
| Correlation coefficients (R, 95% CI) | |||||
| Fibrinogen and G R (95% CI) | 0.56 (0.04–0.84) | 0.73 (0.44–0.88) | 0.66 (0.34 to 0.84) | 0.50 (0.07–0.77) | 0.36 (−0.13 to 0.71) |
| p | 0.0306 | <0.0001 | 0.0003 | 0.0202 | 0.1385 |
| Platelets and G R (95% CI) | 0.55(0.19–0.78) | 0.45 (0.05–0.73) | 0.67 (0.35 to 0.85) | 0.56 (0.14–0.81) | 0.55(0.11 to 0.81) |
| p | 0.0036 | 0.025 | 0.0003 | 0.0098 | 0.0147 |
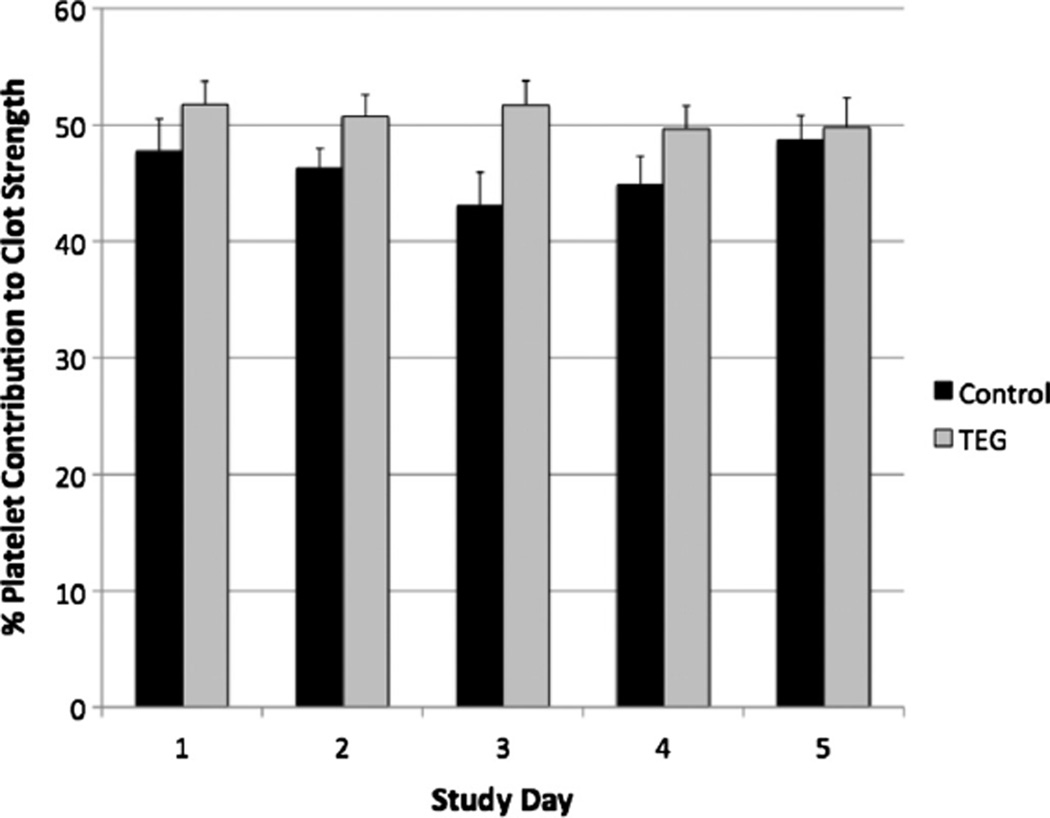
Although fibrinogen levels and platelet count did not differ between groups, the TEG-guided prophylaxis patients had higher platelet contribution to clot strength on Days 2 (46.26% ± 1.68% vs. 50.72% ± 1.87%) and 3 (43.08% ± 2.85% vs. 51.7% ± 2.09%) compared with the control group, suggesting an early effect on platelet function owing to increased LMWH administration (Fig. 2). However, these trends were not significant by repeated-measures ANOVA. Moreover, the correlation of both fibrinogen level and platelet count to clot strength for each day was assessed and is reported in Table 3. For Days 1 and 2, fibrinogen levels had the strongest association to clot strength compared with platelet count. However, platelet count had a stronger correlation to clot strength compared with fibrinogen levels on study Day 3, which persisted through study Day 5 (p < 0.05) (Table 3).
Figure 2.
Platelet contribution to clot strength measured during the 5-day study period. There was no significant difference in platelet contribution to clot strength using repeated measures ANOVA (p = 0.8183). However, there was a trend toward a higher platelet contribution to clot strength in the TEG-guided prophylaxis group on study Days 2 (46.26% ± 1.68% vs. 50.72% ± 1.87%) and 3 (43.08% ± 2.85% vs. 51.7% ± 2.09%) compared with the control group.
Most patients received at least one dose of LMWH daily during the 5-day study period (Table 2). However, by Day 5, fewer patients in the TEG-guided prophylaxis group received at least one dose of heparin compared with those in the control group (68% vs. 92%). Two patients (8%) in the TEG-guided prophylaxis group had their dose of heparin held owing to an elevated RF value greater than 1.4 minutes on Day 5, which was not statistically different from that of the control group (0 patient). The remainder of patients who had their LMWH held in the TEG-guided prophylaxis group had their dose held for additional procedures and/or operations. By study Day 4, 60% of patients in the TEG-guided prophylaxis group were requiring an additional 5,000 IU of LMWH, and by study Day 5, 52% required an additional 5,000 IU of LMWH (Table 2). However, 24% and 32% of patients in the TEG-guided group on Days 4 and 5, respectively, did not receive any LMWH for the reasons stated previously.
Despite increased doses of LMWH, few patients achieved an RF value between 1.0 and 1.4 minutes. By Day 5, only 12% of patients (n = 3) reached a goal RF, but this was not statistically different from the control group, which had 8% of patients (n = 2) with a goal RF value. Within the 5-day study period, no patients developed a VTE. After the 5-day study period and upon transfer out of the SICU, patients were given the standard of care, which consisted of 5,000 IU LMWH once daily until discharge. One patient from the control group developed a PE during this period. In addition, one patient in the control group inadvertently received an additional dose of 5,000 IU of dalteparin on study Day 4.
For the in vitro studies, blood from 10 healthy volunteers was obtained. These volunteers had a mean age of 35 ± 6 years, and 50% were male. With the addition of apheresis platelets, the platelet count increased from a baseline of 96.44 ± 9.82 to 324.22 ± 35.82 1,000/µL (p < 0.0001) (Table 4). As a result, clot strength (G) significantly increased (7.19 ± 0.35 to 10.34 ± 0.29 dynes/cm2) (p < 0.0001). Interestingly, R time (10.22 ± 0.52 minutes to 7.12 ± 0.25 minutes) and K time (2.99 ± 0.14 minutes to 1.66 ± 0.08 minutes) shortened (p < 0.0001), and the angle (51.43 ± 1.48 degrees to 66.63 ± 1.05 degrees) and functional fibrinogen level (274.03 ± 15.82 mg/dL to 427.95 ± 16.58 mg/dL) increased (p < 0.0001) with a paradoxical decrease in the percent platelet contribution to clot strength (74.21% ± 1.55% to 65.10% ± 1.06%) (p = 0.0006).
TABLE 4.
TEG Parameters on Blood From Healthy Volunteers at Baseline and After the Addition of Apheresis Platelets
| Baseline (n = 10) |
Platelets (n = 10) |
p | |
|---|---|---|---|
| R, mean (SEM), min | 10.22 ± 0.52 | 7.12 ± 0.25 | <0.0001 |
| K, mean (SEM), min | 2.99 ± 0.14 | 1.66 ± 0.08 | <0.0001 |
| Angle, mean (SEM), degrees | 51.43 ± 1.48 | 66.63 ± 1.05 | <0.0001 |
| MA, mean (SEM), mm | 58.90 ± 1.10 | 67.40 ± 0.62 | <0.0001 |
| G, mean (SEM), dynes/cm2 | 7.19 ± 0.35 | 10.34 ± 0.29 | <0.0001 |
| TG, mean (SEM), mm/min | 713.86 ± 12.19 | 814.42 ± 7.97 | <0.0001 |
| Platelet count, mean (SEM), 1,000/µL | 96.44 ± 9.82 | 324.22 ± 35.82 | <0.0001 |
| Fibrinogen, mean (SEM), mg/dL | 274.03 ± 15.82 | 427.95 ± 16.58 | <0.0001 |
| % Fib, mean (SEM) | 25.79% ± 1.55% | 34.90% ± 1.06% | 0.0006 |
| % Plt, mean (SEM) | 74.21% ± 1.55% | 65.10% ± 1.06% | 0.0006 |
All TEG parameters significantly changed to promote a hypercoagulable state. Interestingly, thrombin generation (TG), fibrinogen, and the percent fibrinogen contribution to clot strength (% Fib) significantly increased, while the percent platelet contribution to clot strength (% Plt) decreased.
All p values were from a paired t test for continuous variables.
G, clot strength; K, kinetic time; MA, maximum amplitude; R, reaction time; TG, thrombus generation.
DISCUSSION
These data suggest that current standards of VTE prophylaxis may be inadequate based on both conventional and TEG-based standards in severely injured trauma patients. TEG has confirmed that most postinjury patients become hyper-coagulable, as evidenced by clot strength (G) and by the time VTE prophylaxis is started, and continue to increase in hypercoagulability despite VTE prophylaxis. Although TEGhas been proposed as a more sensitive assay to guide prophylaxis, RF values rarely exceeded 1.0 minute despite giving 10,000 IU of LMWH. Furthermore, no more than 8% of patients had an RF value greater than 1.4 minutes, requiring doses of LMWH to be held. Increasing the LMWH dose twofold seemed to have no effect on R times and overall clot strength, confirming that postinjury patients are extremely hypercoagulable and resistant to standard heparin-based prophylaxis.
Although there was no overall difference in clot strength between groups, it still remained unclear if additional heparin had any effect on the individual components of clot strength. Therefore, we evaluated the functional contributions of both fibrinogen and platelets to clot strength. In healthy individuals, fibrinogen functionally contributes approximately 20% to clot strength, while platelets contribute 80%. However, immediately following trauma, there is a shift in these contributions to clot strength in which both fibrinogen and platelets contribute approximately 50% each functionally. This early decrease in platelet contribution can be explained by increased platelet inhibition following trauma despite normal platelet counts.20,21 However, on postinjury Day 5 or 6 (study Day 3), platelets become the dominant contributors to clot strength.
Although platelet contributions to clot strength increased in both groups, patients in the TEG-guided prophylaxis group seemed to have a higher increase in the platelet contribution to clot strength on Days 2 and 3 compared with the control group. This trend was no longer seen beyond Day 3 since the platelet contribution to clot strength subsequently increased in the control group. Therefore, additional doses of LMWH may increase the initial platelet contribution to clot strength early in the hospital course. One explanation is that heparin may in fact activate platelets. Basic studies have consistently demonstrated that the addition of heparin to platelets induces aggregation and enhances their responsiveness to weak stimuli, such as ADP.22 In addition, heparin has been reported to associate directly with the platelet surface,23 produce changes in platelet morphology, 24 promote P-selectin expression,22 and may bind fibrinogen to αIIbβ3, ultimately leading to downstream activation.25,26 Although this increase in platelet contribution to clot strength was not significant by repeated-measures ANOVA, there seems to be a relationship, which should be further explored. However, it remains unclear if an approximate 10% increase in platelet function would substantially add to a clinically significant change in clot strength in this population, but it does further support the role of the platelet in hypercoagulability after injury.
More interestingly, as new platelets are formed and platelet function increases, the synergistic environment with increasing fibrinogen levels further enhances the hypercoagulable state. This is evidenced by the linear regression analysis of comparing clot strength to fibrinogen levels and platelet count, which demonstrates an initial high association with fibrinogen followed by a higher association with platelets on study Day 3. Furthermore, our in vitro studies show that with increased number of platelets, the fluid phase of coagulation is enhanced through the promotion of fibrin formation, thrombus generation, and an increased percent fibrinogen contribution to clot strength. This paradoxically observed phenomenon can be explained through the cell-based model of hemostasis.27 This model proposes that hemostasis occurs in a stepwise process but is highly regulated by tissue factor-bearing cells and platelets. Once coagulation is initiated on tissue factor-bearing cells, platelets become activated, and cofactors Va and VIIIa as well as IXa and XIa rapidly localize on the platelet surface converting factor X to Xa.27,28 Consequently, thrombin generation is propagated on the platelet surface and is the principle site of the majority of thrombin generation. Therefore, increasing the platelet number and surface area may ultimately promote thrombin generation and fibrin formation.
For these reasons, we believe our data indicate platelets as a potential target for VTE prophylaxis, especially in the setting of heparin-based VTE prophylaxis. Although recognized to effectively reduce the risks of myocardial infarctions, ischemic strokes, and other major occlusive arterial events,29,30 antiplatelet therapy has not been recommended for VTE prophylaxis.30–32 However, adequately powered studies have been lacking. Recent meta-analyses concluded that antiplatelet therapy significantly reduced VTE events, and the randomized, placebo-controlled PE prevention (PEP) trial of low-dose aspirin showed that low-dose aspirin resulted in a 43% proportional reduction in PEs and a 29% proportional reduction in symptomatic DVT in orthopedic surgery patients.33–35 Moreover, another recent randomized trail has shown that aspirin alone is effective in preventing recurrent VTE events.36 Furthermore, basic and clinical research have implicated platelets as key mediators in acute lung injury, adult respiratory distress syndrome, and multiple-organ failure.37–40
Since this study was designed as a phase II clinical trial to evaluate our study design, to ensure safety, and to detect differences between standard VTE prophylaxis and a TEG-based algorithm, there are several limitations to this study. A lower than expected incidence of VTE events occurred, and therefore, our study could not determine if TEG-guided VTE prophylaxis was superior to the standard of care. Our study period was also limited to 5 days while patients remained in the SICU. A TEG-guided prophylaxis algorithm may need to be extended since clot strength continued to rise beyond study Day 5. In addition, adherence to VTE prophylaxis in the TEG-guided prophylaxis group was significantly less than the control group on Day 5, which was caused by increased procedures and/or operations. The control group also had suboptimal noncompliance, as high as 16%. Frequent interruptions in VTE prophylaxis may have contributed to the lack of differences observed between groups and highlights a potential clinical problem in managing trauma patients requiring multiple procedures.
In addition, platelet function, as derived from the Functional Fibrinogen assay, also has limitations. TEG does have a Platelet Mapping assay, which has been used to assess platelet function in postinjury patients, but it is not the optimal tool since it is limited to detecting inhibition in only the ADP and arachidonic acid pathways.20 Thrombin is the most potent activator of platelets and, therefore, may be the ideal platelet activator to assess platelet function after injury. The Functional Fibrinogen assay uses a GPIIbIIIa antagonist and does not inhibit thrombin, unlike the Platelet Mapping assay. The other limitation of this assay is that factor XIII levels can affect the results of the assay through fibrin cross-linking, but factor XIII levels were not measured in this study. However, the effect of in vitro addition of factor XIII has been published, which demonstrated that supraphysiologic levels of factor XIII increases both overall clot strength as well as the clot strength of the fibrinogen assay.41 These increases did not correspond to the concentration of factor XIII added, only increased the amplitude by 6 mm to 8 mm, and increased both the fibrinogen and standard assays similarly. Therefore, determining the fibrinogen contribution to clot strength should not be affected by factor XIII levels since it would affect both the Kaolin TEG and Functional Fibrinogen assay equally. It is also important to understand that both the Functional Fibrinogen assay and the clinical standard of measuring fibrinogen (Clauss method) are functional assays and do not measure fibrinogen levels directly. Instead, they measure fibrin formation and cross-linking, and the fibrinogen levels are then subsequently extrapolated based on a standard curve. Therefore, there is likely a presence of nonfunctional fibrinogen, which may be recruited with increased platelets, and thus explaining the rise in fibrinogen levels in the in vitro data after the addition of platelets.
Although this study was also designed to evaluate the role of platelet function in hypercoagulability after injury, the study design precluded a systematic analysis. The initiation of antiplatelet therapy occurred late in the study algorithm. Therefore, we were unable to test the role of antiplatelet therapy owing to the study design, but presumably more patients would have required aspirin if study continued beyond 5 days. We also did not observe statistical differences in RF values between groups as expected, which further highlights the importance of randomized trials. Although an observational study revealed an association between increased changes in R time (RF) to a lower incidence of VTE, suggesting a resistance to LMWH, it was not shown that this could be modified with increased doses of LMWH.18 This observational study included a mixed SICU population, with likely different mechanisms of hyper-coagulability. Therefore, increased doses of LMWH may achieve goal RF values in some patients; however, this was not shown in our randomized trial with a specific trauma population. Therefore, specific factors in trauma patients may further affect the bioavailabilty or pharmacokinetics of LMWH in this population.42
Although an emphasis in VTE prophylaxis is usually placed on preventing thrombin generation, platelets also have a vital role in clot integrity and should be considered as a second therapeutic target in VTE prophylaxis. Basic research has consistently demonstrated the activating effect of heparin on platelets, and now with the use of TEG, we provide evidence that thrombocytosis, which is commonly seen following severe injury, augments the fluid phase of coagulation, and increases fibrin production and thrombus generation. Although the safety of early aspirin therapy following injury has not been thoroughly evaluated, preinjury aspirin therapy has not been associated with increased mortality and may in fact improve outcomes in some patients.40,43 Moreover, in our randomized study, we had no adverse bleeding events, even with a twofold increase in the dose of LMWH and a relatively early administration of aspirin. Furthermore, surgeons are operating more through aspirin therapy in coronary artery bypass and vascular surgeries, as well as urgent and emergent surgeries, with few, if any, adverse outcomes. Therefore, TEG as well as the TEG-based functional fibrinogen assay may be useful in guiding future therapies and aid in understanding the mechanism of hypercoagulability after injury. However, future studies are needed and should evaluate a longer course of TEG-guided VTE prophylaxis, a more aggressive escalation in LMWH prophylaxis, as well as initiating aspirin therapy earlier to prevent VTE in this patient population.
Acknowledgments
DISCLOSURE
This study was supported by the National Institutes of Health (P50 GM049222 T32 GM008315 grants).
DISCUSSION
Dr. Mitchell Jay Cohen (San Francisco, California): This is a well-written paper by a group with an outstanding track record of solid investigation in the area of coagulation after injury.
In this study the group led by Harr and colleagues performed a small randomized controlled study to test the effectiveness of TEG-based dosing on low molecular weight heparin for venous thromboembolism treatment.
Here they report the results of this trial and, more specifically, present data suggesting that platelet function contributes to hypercoagulability after injury.
While the aim was to show the ability to achieve differences in clot strength and other viscoelastic measures after low molecular weight heparin in the TEG-based dosing group, they were unable to achieve those changes due to multiple biological and clinical reasons, essentially rendering this a negative study in the primary endpoint.
They did, however, glean extremely intriguing data regarding the contributors to clot strength after injury. I have several comments and questions, the first is about trial design and the ultimate clinical trial.
There was essentially no difference between the groups in terms of what actual heparin dosing they received. And I think we need some more specific insight as to why this happened. Were these clinical problems? Were they biological problems? Were they dosing problems?
The second is only three patients in the experimental group and only two in the control group ultimately achieved any goal change in the R value which was the target via TEG.
Why was this? Can you speculate why this was, whether this was an issue with overall dosing of the low molecular weight heparin, some biologic availability problem? And have you ever been able to achieve targeted Delta-R subsequently with altered or more aggressive dosing?
From here the authors seek to examine the relative contributions of platelet strength versus fibrinogen on clot strength.
While the authors report an increase in platelet contribution to clot strength, the effect is very, very small. Do you think that this is clinically or biologically significant, these changes in percentages that you saw on Day 2 and Day 3?
Second, while there is intuitive evidence regarding the effect of heparin on platelet function-and I do say the discussion is very, very well written and a very good review of this very complicated topic-it remains uncertain to me and many others whether the conclusion that clot strength is entirely a product of fibrinogen as measured by TEG with all the residual effects being associated with platelets. I think that that’s very biologically unclear.
As you know, our group has published prolonged platelet dysfunction despite adequate platelet numbers. Have you done anything to confirm this additive or this product that you base your analysis on?
Have you done any platelet mapping or aggregometry on match samples? I would extend this to ask if you have measured factor levels, including Factor XIII which, as you know, is associated with fibrin cross-linking and strength.
Ultimately, while I very much like your analysis and it, indeed, fits my bias, I don’t think that there is enough data yet to conclusively attribute these relative drivers of clot strength.
Lastly, I have a question about the ex-vivo experiments. In these you spiked ex-vivo blood from healthy volunteers with apheresis platelets.
In these samples the fibrinogen level increased significantly. Can you explain this? I’m not clear where this fibrinogen came from. Are these platelets activated?
Are you activating coagulation via tissue factor that’s either on the platelet or associated with microparticles? Is there contact activation in your system somehow playing a role? I remain very unclear on these seemingly paradoxical results. They are very interesting but any insight you have would be helpful.
Lastly, I very much share the suggestion that veno-thromboembolism prophylaxis should be a combination of anticoagulant and antiplatelet therapies. Can you comment on the risk of bleeding in these patients and what you might see as the ideal timing of these therapies in our patients?
Overall this is a very intriguing study by a group that is a leader in the study of coagulation after injury. I applaud Dr. Harr and the group for a very well written manuscript and examination of this complicated but clinically crucial issue. I look forward to many years of good work from this group.
Dr. Marc A. de Moya (Boston, Massachusetts): Great talk. Great presentation. I wanted to just ask you if you could elaborate a little bit more-because you probably didn’t talk about it too much for lack of time during your presentation-in regard to what is the gold standard in terms of activated Factor X levels versus TEG levels for prophylaxis.
Maybe you are shooting for the wrong level so please shed some light on that question.
Dr. Jeff Ustin (Cleveland, Ohio): My question concerns the mechanism of increased coagulability and hypercoagulability associated with increased clot strength or platelet strength. Is there a correlation or is there something mechanistic you can point to that would produce a higher coagulability state?
Dr. Jeffrey Harr (Denver, Colorado): Thank you very much, Dr. Cohen. Due to the trial design, there was a slow escalation of LMWH therapy to ensure the safety of increasing the standard LMWH dose by 100%. Consequently, there was no initial difference between groups in the dose of LMWH given. However, by study day 2, 48% of patients in the TEG-guided group received at least 7500 U of LMWH, and by study days 4 and 5, greater than 50% were receiving 10,000 U of LMWH. Therefore, the biggest differences were seen near the end of the study period.
However, there were also issues with compliance. In multiple patients within both the control and study groups, the dose of LMWH needed to be held due to further procedures or operations. This is usually a common practice to decrease bleeding complications from procedures. However, we may be holding doses of LMWH unnecessarily for the fact that patients remained hypercoagulable despite increased doses of LMWH. After completing this phase II clinical trial, we feel that 10,000 U of LMWH is safe to give for VTE prophylaxis, and should be given earlier post-injury. Therefore, a new study should be designed with earlier administration of high-dose LMWH and aspirin therapy to detect greater differences between the control and study groups.
Surprisingly, very few patients achieved a goal RF value. This goal was established based on retrospective studies, which observed that patients who had a significant difference in their delta R values had a decreased association with VTE events. Being more aggressive with increasing the LMWH dosing, may have increased the RF values, but both the timing and dosing remain unclear. There is one study which speculates that the bioavailability of LMWH is decreased in post-injury patients due to peripheral edema, but a cause and effect relationship has not been established, and some of the data presented suggest the pharmacokinetics of LMWH instead. Therefore, we believe there are other post-injury factors which contribute to decreased LMWH efficacy in post-injury patients. However, we believe these factors may be overcome with additional LMWH.
This may include a three times a day dosing regimen to increase trough levels. Unfortunately, we are unclear of the doses needed, or even if achieving an RF value between 1 and 1.4 minutes by heparin is safe and efficacious. Therefore, additional studies are needed to determine if 5000 Units three times daily is an alternative dosing regimen to obtain appropriate VTE prophylaxis.
The effect was small and was maximal on study day 3 with a difference of approximately 8%. Although the trend over the 5-day study period was not significant using repeated measures ANOVA, increased doses of LMWH appears to have an effect. It is difficult to ascertain how significant this effect is due to the multiple limitations of the study. It is possible that this effect may have been greater if increased LMWH doses were escalated more aggressively. However, it remains unclear if an approximate 10% increase in platelet function would substantially add to clot strength. Regardless, it does continue to support the role for anti-platelet therapy in post-injury VTE prophylaxis.
Unfortunately, we did not perform Platelet Mapping on all patients, and only employed it on patients who received anti-platelet therapy. Although platelet mapping helps understand post-injury platelet function, it is not the optimal tool since it is limited to detecting inhibition in only the ADP and AA pathways. Thrombin is the most potent activator of platelets, and therefore may be the ideal platelet activator to assess post-injury platelet function. This is exactly what the Functional Fibrinogen assay observes. It is true that Factor XIII plays a significant role in clot stability through fibrin cross-linking and could affect the functional fibrinogen assay. Although we have not studied this, or measured Factor XIII levels in our study, the effect of in vitro addition of Factor XIII has been published. Supraphysiologic levels of Factor XIII increases both overall clot strength as well as the clot strength of the fibrinogen assay. These increases did not correspond to the concentration of Factor XIII added, and only increase the amplitude by a few millmeters. Therefore, determining the fibrinogen contribution to clot strength should not be affected by Factor XIII levels since it would affect both the Kaolin TEG and FF assay equally.
The finding of increased fibrinogen levels was also unexpected for our group. However, it can be explained by the cell-based model of hemostasis. I think it is important to understand that the functional fibrinogen assay, as well as the clinical standard, the Clauss method, are functional assays, and are limited by substrates in an in vitro setting. They are not actually measuring fibrinogen, but fibrin formation and cross-linking, and the fibrinogen level is then extrapolated based on a standard curve. Now, the cell-based model of hemostasis emphasizes the role of platelets in thrombin generation, since the platelet surface is where many of the cofactors needed for thrombin generation assemble. Therefore, if more substrate, or platelet surface area, is added to the equation, more fibrinogen will be converted to fibrin, reflecting higher levels of fibrinogen. In addition, it is the platelet, as well as other myeloid cells, which supplies the active subunit of Factor XIII. Technically, these platelets are not active since they are inhibited with a GPIIbIIIa receptor antagonist; however, they still contribute their surface area for cofactor complexes to assemble. The functional fibrinogen assay employs tissue factor as an activator, and for this experiment, tissue factor was added after the addition of platelets, and just prior to running the TEG.
As a phase II clinical trial, one aspect was to evaluate the safety of increasing the dose of LMWH by 100%. Subsequently, we found no adverse bleeding events. Moreover, this study demonstrates that by post-injury day 2 or 3, when the decision is made to start VTE prophylaxis, patients have a range from normal to hypercoagulable TEG parameters. However, by post-injury day 3 or 4, which is our study day 2, almost all patients were hypercoagulable based on clot strength. Therefore, a more aggressive and earlier LMWH administration is likely to be safe without any adverse events in bleeding. Greater care may need to be implemented in patients with closed-head injuries. Regarding the use of anti-platelet therapy, a recent retrospective study found no increased risk with pre-injury anti-platelet therapy use and mortality. We found similar results in our review of the Glue Grant database, but also found that pre-injury anti-platelet therapy may reduce pulmonary dysfunction, multiple organ failure, and mortality. More importantly, many surgeons are now operating through aspirin therapy in coronary artery bypass surgeries, as well as for urgent and emergent cases, with few, if any, adverse outcomes. Therefore, once the decision is made to start heparin-based VTE prophylaxis, anti-platelet therapy should also be started that same day, or shortly after, once no adverse bleeding is confirmed.
With regard to Dr. de Moya’s questions, currently anti-XA levels are considered to be the gold standard. TEG is still evolving and has not been fully validated. However, TEG actually allows us to physically see the effect heparin has on coagulation, and further helps us quantify this effect. Furthermore, TEG is the best measure of global coagulation that we have and I think it eventually might become a key to better understand post-injury hypercoagulability and provide insight on how to tailor management of DVT prophylaxis to each individual.
Finally, in regard to Dr. Ustin’s questions, following severe injury, there are multiple mechanisms responsible for hypercoagulability leading to VTE. Due to disruption of tissue and significant vascular injury, there is an increase in tissue factor and collagen exposure, as well as the activation of platelets through thrombin, collagen, and epinephrine. Decreased venous blood flow and oxygen tension from shock, also results in an upregulation of multiple stress-response genes, including hypoxia inducible factor and P-selectin, as well as other genes responsible for pro-coagulant proteins. As the patient is resuscitated, reperfusion injuries lead to increases in inflammation, resulting in increased release of acute phase reactant proteins, which include fibrinogen, prothrombin, factor VIII, and von Willebrand factor. Moreover, platelets also increase due to increased inflammation, and are released in a milieu optimal for activation. Furthermore, severely injured trauma patients are immobilized, which further contributes to the fulfillment of Virchow’s triad: venous stasis, endothelial injury, and hypercoagulability.
Footnotes
This study was presented at the 71st annual meeting of the American Association for the Surgery of Trauma, September 12–15, 2012, in Kauai, Hawaii.
AUTHORSHIP
J.H., E.E.M., T.L.C., E.G., M.V.W., and C.C.S. performed the literature search. E.E.M., A.B., C.C.S., A.S., E.G., M.V.W., and A.G. contributed in the study design. A.G., E.G., and M.V.W. performed the data collection. A.S., J.H., T.L.C., and A.G. perfumed the data analysis. J.H., T.L.C., E.E.M., A.B., C.C.S., and A.S. performed the data interpretation. J.H., E.E.M., T.L.C., C.C.S., and A.S. wrote the article. J.H., E.E.M., A.B., C.C.S., and A.S. provided the critical revision.
REFERENCES
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475–486. doi: 10.18553/jmcp.2007.13.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toker S, Hak DJ, Morgan SJ. Deep vein thrombosis prophylaxis in trauma patients. Thrombosis. 2011;2011:505373. doi: 10.1155/2011/505373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shackford SR, Davis JW, Hollingsworth-Fridlund P, Brewer NS, Hoyt DB, Mackersie RC. Venous thromboembolism in patients with major trauma. Am J Surg. 1990;159:365–369. doi: 10.1016/s0002-9610(05)81272-3. [DOI] [PubMed] [Google Scholar]
- 5.Schultz DJ, Brasel KJ,Washington L, Goodman LR, Quickel RR, Lipchik RJ, Clever T, Weigelt J. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J Trauma. 2004;56:727–731. doi: 10.1097/01.ta.0000119687.23542.ec. [DOI] [PubMed] [Google Scholar]
- 6.Pierce CA, Haut ER, Kardooni S, Chang DC, Efron DT, Haider A, Pronovost PJ, Cornwll EE., 3rd Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–937. doi: 10.1097/TA.0b013e318166b808. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield LJ, Proctor MC, Rodriquez JL, Luchette FA, Cipolle MD, Cho J. Posttrauma thromboembolism prophylaxis. J Trauma. 1997;42:100–103. doi: 10.1097/00005373-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Edwards M, Felder S, Ley E, Srour M, Mirocha J, Margulies DR, Salim A. Venous thromboembolism in coagulopathic surgical intensive care unit patients: is there a benefit from chemical prophylaxis? J Trauma. 2011;70:1398–1400. doi: 10.1097/TA.0b013e318217868d. [DOI] [PubMed] [Google Scholar]
- 9.Knudson MM, Collins JA, Goodman SB, McCrory DW. Thromboembolism following multiple trauma. J Trauma. 1992;32:2–11. doi: 10.1097/00005373-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Knudson MM, Lewis FR, Clinton A, Atkinson K, Megerman J. Prevention of venous thromboembolism in trauma patients. J Trauma. 1994;37:480–487. doi: 10.1097/00005373-199409000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Upchurch GR, Demling RH, Davies J, Gates JD, Knox JB. Efficacy of subcutaneous heparin in prevention of venous thromboembolic events in trauma patients. Am Surg. 1995;61:749–755. [PubMed] [Google Scholar]
- 12.Velmahos GC, Nigro J, Tatevossian R, Murray JA, Cornwell EE, 3rd, Belzberg H, Asensio JA, Berne TV, Demetriades D. Inability of an aggressive policy of thromboprophylaxis to prevent deep venous thrombosis (DVT) in critically injured patients: are current methods of DVT prophylaxis insufficient? J Am Coll Surg. 1998;187:529–533. doi: 10.1016/s1072-7515(98)00223-3. [DOI] [PubMed] [Google Scholar]
- 13.Meissner MH, Chandler WL, Elliott JS. Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability? J Trauma. 2003;54:224–231. doi: 10.1097/01.TA.0000046253.33495.70. [DOI] [PubMed] [Google Scholar]
- 14.Velmahos GC, Kern J, Chan LS, Oder D, Murray JA, Shekelle P. Prevention of venous thromboembolism after injury: an evidence-based reportVpart I: analysis of risk factors and evaluation of the role of vena caval filters. J Trauma. 2000;49:132–139. doi: 10.1097/00005373-200007000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Velmahos GC, Toutouzas KG, Brown C, Vassiliu P, Gkiokas G, Rhee P. Thromboprophylaxis does not protect severely injured patients against pulmonary embolism. Am Surg. 2004;70:893–896. [PubMed] [Google Scholar]
- 16.Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M, Kong A, Lekawa ME, Dolich MO, Cinat ME, et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma. 2010;68:874–880. doi: 10.1097/TA.0b013e3181d32271. [DOI] [PubMed] [Google Scholar]
- 17.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–275. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van P, Cho SD, Underwood SJ, Morris MS, Watters JM, Schreiber MA. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009;66:1509–1515. doi: 10.1097/TA.0b013e3181a51e33. [DOI] [PubMed] [Google Scholar]
- 19.Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, Silliman CC. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39:45–49. doi: 10.1097/SHK.0b013e3182787122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma. 2012;73:13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Z, Theroux P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low-molecular-weight heparin and with a direct thrombin inhibitor. Circulation. 1998;97:251–256. doi: 10.1161/01.cir.97.3.251. [DOI] [PubMed] [Google Scholar]
- 23.Sobel M, Adelman B. Characterization of platelet binding of heparins and other glycosaminoglycans. Thromb Res. 1988;50:815–826. doi: 10.1016/0049-3848(88)90341-6. [DOI] [PubMed] [Google Scholar]
- 24.Anand SX, Kim MC, Kamran M, Sharma SK, Kini AS, Fareed J, Hoppensteadt DA, Carbon F, Cavusoglu E, Varon D, et al. Comparison of platelet function and morphology in patients undergoing percutaneous coronary intervention receiving bivalirudin versus unfractionated heparin versus clopidogrel pretreatment and bivalirudin. Am J Cardiol. 2007;100:417–424. doi: 10.1016/j.amjcard.2007.02.106. [DOI] [PubMed] [Google Scholar]
- 25.Sobel M, Fish WR, Toma N, Luo S, Bird K, Mori K, Kusumoto S, Blystone SD, Suda Y. Heparin modulates integrin function in human platelets. J Vasc Surg. 2001;33:587–594. doi: 10.1067/mva.2001.112696. [DOI] [PubMed] [Google Scholar]
- 26.Gao C, Boylan B, Fang J, Wilcox DA, Newman DK, Newman PJ. Heparin promotes platelet responsiveness by potentiating αIIbβ3-mediated outside-in signaling. Blood. 2011;117:4946–4952. doi: 10.1182/blood-2010-09-307751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–965. [PubMed] [Google Scholar]
- 28.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 29.Collaborative overview of randomized trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patient. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 30.Collaborative overview of randomized trials of antiplatelet therapy—II: maintenance of vascular graft of arterial patency by antiplatelet therapy. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:159–168. [PMC free article] [PubMed] [Google Scholar]
- 31.Consensus Conference. Prevention of venous thrombosis and pulmonary embolism. JAMA. 1986;256:744–749. [PubMed] [Google Scholar]
- 32.Cohen AT, Skinner JA, Kakkar VV. Antiplatelet treatment for thromboprophylaxis: a step forward or backwards? BMJ. 1994;309:1213–1215. doi: 10.1136/bmj.309.6963.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clagett GP, Anderson FA, Geerts W, et al. Prevention of venous thromboembolism. Chest. 1998;114(suppl 5):531S–560S. doi: 10.1378/chest.114.5_supplement.531s. [DOI] [PubMed] [Google Scholar]
- 34.Collaborative overview of randomized trials of antiplatelet therapy—III: reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:235–246. [PMC free article] [PubMed] [Google Scholar]
- 35.Collins R, Baigent C, Sandercock P, Peto R. Antiplatelet therapy for thromboprophylaxis: the need for careful consideration of the evidence from randomized trials. Antiplatelet Trialists’ Collaboration. BMJ. 1994;309:1215–1217. doi: 10.1136/bmj.309.6963.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355:1295–1302. [PubMed] [Google Scholar]
- 37.Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–1967. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 38.Harr JN, Moore EE, Wohlauer MV, Fragoso M, Gamboni F, Liang X, Banerjee A, Silliman CC. Activated platelets in heparinized shed blood: the “second hit” of acute lung injury in trauma/hemorrhagic shock models. Shock. 2011;36:595–603. doi: 10.1097/SHK.0b013e318231ee76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harr JN, Moore EE, Johnson JL, Chin TL, Wohlauer MV, Maier R, Cuschieri J, Sperry J, Banerjee A, Silliman CC, et al. Anti-platelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit Care Med. 2012 doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thuesinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increase clot firmness in rotation thromboelastometry (ROTEM) Thromb Haemost. 2010;104:385–391. doi: 10.1160/TH09-12-0858. [DOI] [PubMed] [Google Scholar]
- 42.Haas CE, Nelsen JL, Raghavendran K, Mihalko W, Beres J, Ma Q, Forrest A. Pharmacokinetics and pharmacodynamics of enoxaparin in multiple trauma patients. J Trauma. 2005;59:1336–1343. doi: 10.1097/01.ta.0000197354.69796.bd. [DOI] [PubMed] [Google Scholar]
- 43.Bonville DJ, Ata A, Jahraus CB, Arnold-Lloyd T, Salem L, Rosati C, Stain SC. Impact of preinjury warfarin and antiplatelet agents on outcomes of trauma patients. Surgery. 2011;150:861–868. doi: 10.1016/j.surg.2011.07.070. [DOI] [PubMed] [Google Scholar]