Abstract
There are approximately 277 000 new cases of pancreatic cancer and 266 000 deaths from pancreatic cancer annually, indicating a mortality rate of 96% of the cases diagnosed. Because of the ineffectiveness of therapies, a major emphasis needs to be placed on prevention. This paper reviews the epidemiology and risk factors for pancreatic cancer, and uses this information to propose plausible research directions for determining the biological mechanisms mediating the effects of risk factors on the promotion of pancreatic cancer, with a focus on the pancreatic stellate cell.
Keywords: diabetes, obesity, pancreatic cancer, smoking, stellate cell
Epidemiology and risk factors
Epidemiology
Recently, data published by the International Agency for Research on Cancer reported the incidence and mortality from 182 countries in 2008 for 27 different cancers.1 The estimated number of new pancreatic cancer cases was 277 000 worldwide. The number of pancreatic cancer deaths during this time period was estimated at 266 000. These were sobering statistics, suggesting that the mortality rate is 96% of the incidence rate. Comparable statistics for colon cancer were 1 233 000 new cases and 608 000 deaths for a mortality of 49%; breast cancer, with 1 383 000 new cases and 458 000 deaths for a mortality rate of 33%, and prostate cancer, with 913 000 new cases and 258 000 deaths for a mortality rate of 28%.
Pancreatic cancer death is the eighth or ninth most frequent cause of cancer death worldwide, and is the fourth or fifth most common cause of cancer death in developed countries.1,2 Japan has the highest incidence of pancreatic cancer, with a rate of 156 deaths per 1 000 000 inhabitants. Comparable numbers for Italy are 138; for France, 118; for the USA, 102; and for Russia, 86. The rates are three to four times greater in countries significantly north or south of the Equator (i.e. North America, Europe, Australia, and New Zealand) compared to those near the equator (i.e. South Asia). Some of these differences can be accounted for by differences in life expectancy, as the incidence of this disease is highly age related. For example, the Japanese have a life expectancy of 82 years, while Russians have a life expectancy of 65 years. Differences related to distances from the Equator could be due to differences in cancer verification between countries. However, reports from Japan, a country with a large range in latitude, showed that the incidence of pancreatic cancer varies by region, and the variation is inversely related to the amount of global solar radiation or daily maximum temperature for both males and females.3 These findings are supported by worldwide studies4 showing a negative correlation between amounts of solar radiation and serum 25-(OH) vitamin D levels and pancreatic cancer incidence. Findings showing relationships between solar radiation and cancer incidence, as well as vitamin D status, have been reported for other malignancies, including colorectal, prostate, breast, and renal cancers.3
Risk factors
Environmental factors are likely responsible for the majority of cases. Age is a key risk factor for pancreatic cancer, with the median age at diagnosis of pancreatic cancer at 72 years. Less than 10% of patients develop pancreatic cancer before the age of 50, and this younger group is likely to include a higher proportion of patients with underlying predisposing genetic disorders.5 There is a male predominance of the disease that is likely explained by higher smoking rates in men than women.2,5
Chronic pancreatitis represents the greatest risk for the development of pancreatic cancer, as indicated by several studies.6 This disorder commonly results from alcohol abuse and smoking, but also from rare hereditary forms (5–10% of the total group of patients with chronic pancreatitis). There is a 14-fold risk of developing pancreatic cancer in patients with chronic pancreatitis of at least 5 years’ duration.6 The risk for those with hereditary forms of pancreatitis is approximately 70 times greater than the normal population, giving this group a lifetime risk of 40–55% for the development of cancer.
Cigarette smoking represents an independent risk for pancreatic cancer, resulting in an approximate doubling of the risk.7 The increased risk of cancer abates following the cessation of smoking, diminishing over a 10-year period. Because of the high prevalence of smoking in the world, it is estimated that approximately 25% of the burden of pancreatic cancer is due to smoking.2,8
A recent meta-analysis convincingly showed that moderate alcohol use is not associated with increased risk of pancreatic cancer, but that there is a 22% increased risk in those with heavy alcohol consumption (i.e. 3 or more drinks per day).9 The specific role of alcohol drinking in pancreatic carcinogenesis is complicated by the high percentage of smokers between drinkers and recent findings showing that smoking accelerates alcoholic chronic pancreatitis.10 These results suggest the possibility of a complex interrelationship between alcohol abuse and smoking in the initiation and promotion of pancreatic cancer.
Case-control and prospective studies demonstrate an increased risk of pancreatic cancer in patients with long-standing type-2 diabetes,11,12 type-1 diabetes,13 or abnormal glucose tolerance.14 There is a high prevalence of diabetes in patients with pancreatic cancer (up to 40%), and is often new in onset.15 Finally, there is increasing recognition that abnormal glucose metabolism, insulin resistance, and higher insulin levels are positively associated with pancreatic cancer risk.16
There are several meta-analyses indicating a positive association between body mass index (BMI) and pancreatic cancer.17 These studies indicate the development of pancreatic cancer at a younger age in patients who are overweight or obese compared to those with BMI values in the normal range.17 Of note, obesity and diabetes are linked by way of the metabolic syndrome, a cluster of medical disorders, including obesity, insulin resistance and diabetes mellitus, raised blood pressure, dyslipidemia, and fatty liver disease.18 The incidence of metabolic syndrome is increasing worldwide due to the proliferation of high-fat, high-calorie diets. Although the percentage increased risk with diabetes, overweight, and obesity is relatively small, the high prevalence of the metabolic syndrome risk factors suggests that they are major contributors to overall risk for pancreatic cancer.
Ethnicity
There is a 50–90% increase in the incidence of pancreatic cancer in African Americans compared to the general population.19 The reasons for the higher incidence can be largely explained by the increased prevalence of known risk factors in this population, including cigarette smoking, BMI, diabetes, and moderate to heavy alcohol consumption.19 In addition, considering the epidemiology related to sunlight and the vitamin D system described previously in this article, low levels of serum 25-(OH) vitamin D in African Americans compared to white Americans might explain the increased incidence of pancreatic cancer in the African American population.20
Summary of epidemiology and risk factors
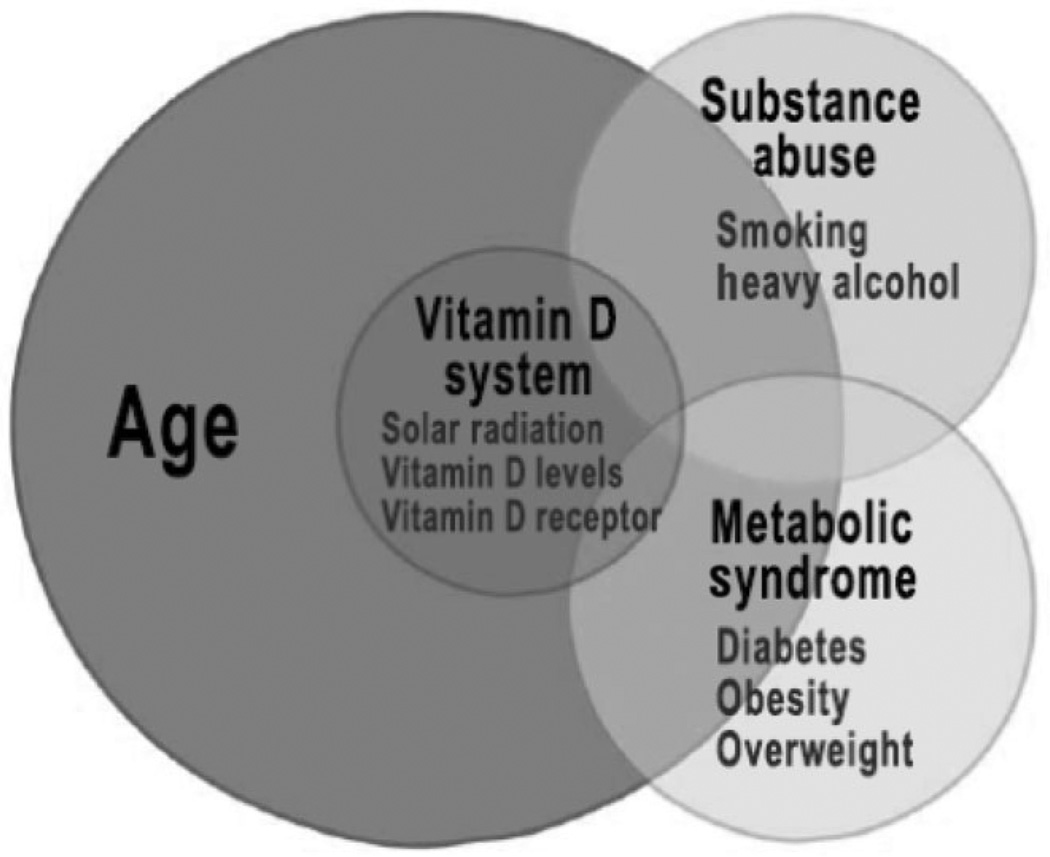
A conceptual framework and summary of the information provided in the literature related to epidemiology and environmental risk factors for pancreatic cancer is provided in Figure 1. The Figure emphasizes that the greatest risk for this cancer is advancing age. Other risk factors include substance abuse, the metabolic syndrome, and the vitamin D system. We have condensed the risk factors into these larger categories because of the likelihood that there is a cooperative interaction between smoking and alcohol abuse, as has been demonstrated for other gastrointestinal cancers,21 as well as pancreatitis,10 which is a risk factor of cancer, because diabetes, overweight, obesity, and insulin resistance are all part of the metabolic syndrome, and because solar radiation, vitamin D levels, and vitamin D receptor variants likely work through the same or similar pathways to promote pancreatic cancer. The likelihood of interactions between the categories of risk factors is shown by overlaps in the Figure.
Figure 1.
Age and environmental risk factors for pancreatic cancer. Greatest risk factor for pancreatic cancer is age. Risk further increases with smoking and alcohol abuse, the metabolic syndrome, and solar radiation/vitamin D system.
Desmoplasia and the pancreatic stellate cell in the promotion of pancreatic cancer
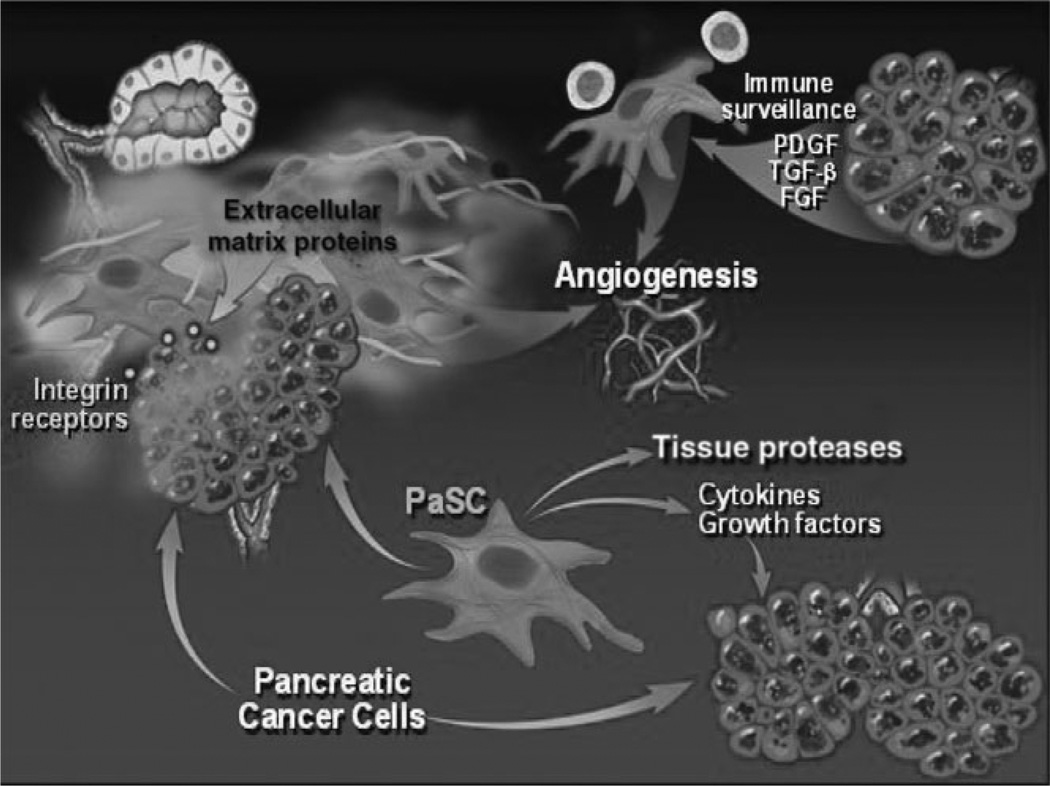
Pancreatic cancer is unique among solid tumors because of the extremely dense desmoplastic reaction that surrounds the cancer cell glands of this tumor. The desmoplasia, containing myofibroblastic pancreatic stellate cells (PaSC), extracellular matrix (ECM) proteins, and immune cells, modulates the growth of the cancer by providing a scaffold for the cancer cells to grow, as well as growth factors, angiogenesis factors, and immune modulators (Fig. 2).
Figure 2.
Components of desmoplasia in pancreatic cancer. Pancreatic cancer cells and pancreatic stellate cells (PaSC) promote each others’ growth and proliferation, and together regulate processes of extracellular matrix proteins deposition, angiogenesis, and disordered immune surveillance. FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β.
Composition of pancreatic cancer desmoplasia
In pancreatic cancer desmoplasia, the major ECM proteins are type I, III, and V collagens, and fibronectin.22 The cell responsible for in the desmoplasia is the PaSC. Although the cancer cells are able to produce ECM proteins, recent evidence demonstrates that the PaSC is the main producer of ECM proteins, as well as cytokines, chemokines, and growth factors during the development and progression of pancreatic cancer.
PaSC and desmoplasia
The potential role of the PaSC in pancreatic cancer development is receiving increased attention.23–25 PaSC are normally located in the peri-acinar space in a “quiescent” state, having long cytoplasmic projections that encircle the base of the pancreatic acinus. Quiescent PaSC have a low rate of proliferation and production of ECM proteins, growth factors, and cytokines; they contain lipid droplets rich in vitamin A, and can be identified by their expression of selective markers, such as desmin and glial fibrillary acid protein. PaSC participate in disease pathogenesis after transforming from their normal “quiescent” state into an “activated” state (also known as a “myofibroblastic” state). Activated PaSC proliferate, migrate, and produce large amounts of ECM proteins, cytokines, chemokines, and growth factors. During disease pathogenesis, the PaSC maintain the “activated” state through exposure to cytokines, growth factors, and other ECM factors likely produced by acinar cells, cancer cells, inflammatory cells, platelets, endothelial cells, and PaSC themselves. Evidence that the stromal cells in cancer are “activated” PaSC comes from findings indicating that cells expressing α-smooth muscle actin (α-SMA), a specific marker of the “activated” state of the PaSC, colocalize with mRNA, encoding procollagen α1 type-I in the stromal component of the cancer.26
The complete networks of mechanisms by which PaSC and other components of desmoplasia enhance the growth of tumor cells in pancreatic cancer are complex and barely understood. A potential mechanism by which desmoplasia promotes cancer is through the direct action of ECM proteins and growth factors coming from PaSC on the cancer cells. For example, we have found that both ECM proteins (such as laminin and fibronectin) and growth factors (such as insulin-like growth factor-1 [IGF-1]) promote the survival of pancreatic cancer cells through their interactions with integrins in the cancer cells.27,28 When bound to ECM proteins, the integrin receptors transactivate the IGF-1 receptor, which in turn mediates the intracellular events that promote cancer cell survival and growth. Furthermore, our studies revealed that both ECM proteins and growth factors promote the survival of cancer cells through the activation of intracellular reactive oxygen species (ROS)-generating systems. The ROS mediate prosurvival and proproliferative effects in cancer cells through their regulation of phosphatases and kinases. Key generators of ROS in pancreatic cancer cells are the NADPH oxidase enzymes.
Evidence is emerging that there is a symbiotic relationship between pancreatic cancer cells and PaSC of the tumor that results in an overall increase in the rate of growth of the tumor and possibly metastasis. For example, culture supernatants from human pancreatic tumor cell lines stimulate PaSC proliferation and the production of ECM proteins via the ability of the cancer cells to produce and secrete PDGF, transforming growth factor-β1, and fibroblast growth factor-2.26,29 The growth rate of tumor cells injected s.c. into nude mice (mice that lack T cells and are severely immune compromised) is markedly increased when PaSC are included in the inoculum.29 In addition, the tumors that form when both cancer cells and PaSC are used have a desmoplasia similar to that observed in human pancreatic cancer.29 In a “converse” approach, conditioned media taken from PaSC cultures promoted pancreatic cancer cell proliferation, migration, invasion, and anchorage-independent growth.30,31
In sum, PaSC are the major contributor to the tumor microenvironment, and as such, are likely key participants in carcinogenesis for pancreatic cancer. Thus, it is likely that information about epidemiology and risk factors and their potential impact on PaSC will lead to a better understanding of pancreatic carcinogenesis.
Risk factors and PaSC
The fibrosis of chronic pancreatitis from alcohol abuse smoking has the same characteristics as the desmoplasia of pancreatic cancer.24,25 Of particular importance for the present discussion is the fact that alcohol and its metabolites, mainly acetaldehyde, promote the activation of pancreatic stellate cells,32,33 and the finding that lipopolysaccharide (LPS), a gut microflora metabolite that increases in the blood of alcoholics, causes activation of pancreatic stellate cells both in vitro and in vivo.34 Alcohol abuse is also associated with increased circulating levels of oxidized lowdensity lipoprotein (oxLDL)35 and tumor necrosis factor-α (TNF-α),36 both having potential effects on stellate cell activation, as described later. Of note, there have been no studies reported on the effect of smoking compounds alone or in combination with alcohol metabolites on PaSC activation. However, there is information from pulmonary fibroblasts that nicotine causes transdifferentiation of pulmonary fibroblasts to myofibroblasts involved in pulmonary fibrosis.37
The metabolic syndrome is associated with increased circulating levels of leptin, IGF-1, and oxLDL.38 A high-fat, high-calorie diet39,40 and type II obesity-induced diabetes mellitus41 are associated with increased circulating levels of LPS. Patients with type II obesity-induced diabetes mellitus have increased secretion of TNF-α from their adipose tissue.42
How might these circulating factors that increase in alcoholism and disorders of the metabolic syndrome lead to the activation of PaSC to promote pancreatic carcinogenesis? Although there is some information on the effects of LPS and TNF-α on PaSC responses, most of the information on receptors and responses for the agents that are increased in alcohol abuse and metabolic syndrome come from hepatic stellate cells (HSC; likely identical to PaSC).24 Both HSC and PaSC contain Toll-like receptors (TLR) (e.g. TLR-4) that interact with LPS and receptors responding to TNF-α, stimulation of which might result in activation responses.43 Leptin has profibrogenic effects in the liver.38 IGF-1 has been demonstrated to have a proliferative effect in HSC.44 There are different receptors identified that interact with oxLDL, and the specificity of interaction might depend upon the extent of oxidation of the LDL.45 HSC express oxLDL receptors, and respond to oxLDL with increased production of ECM proteins.46,47
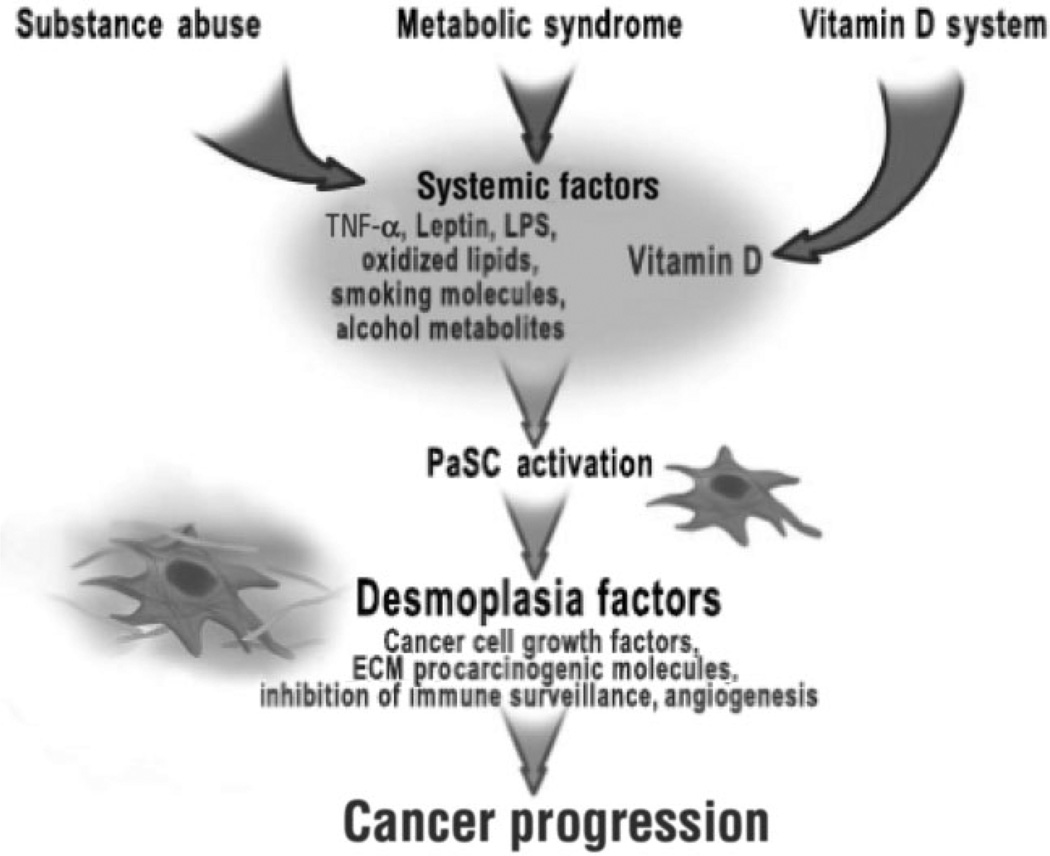
Finally, a recent report indicated that vitamin D inhibits HSC activation and the production of the ECM in an animal model of liver fibrosis.48 In combination, the experimental results described in this section suggest the possibility that the mechanism by which environmental factors affect pancreatic carcinogenesis involves PaSC. Our hypothesis of the effects of environmental factors on PaSC and pancreatic carcinogenesis is represented in Figure 3.
Figure 3.
Mechanisms of regulation of pancreatic stellate cells (PaSC) and pancreatic carcinogenesis by environmental risk factors. Potential pathways emanating from the environmental risk factors that regulate the activation state of PaSC and emphasizes the emerging role of PaSC in pancreatic carcinogenesis are shown. LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
PaSC in early lesions of pancreatic cancer
The earliest lesions in the progression of pancreatic neoplasia are called pancreatic intra-epithelial neoplasias (PanIN). We posited that if PaSC play an important role in the development and progression of pancreatic cancer, they should be present in PanIN.
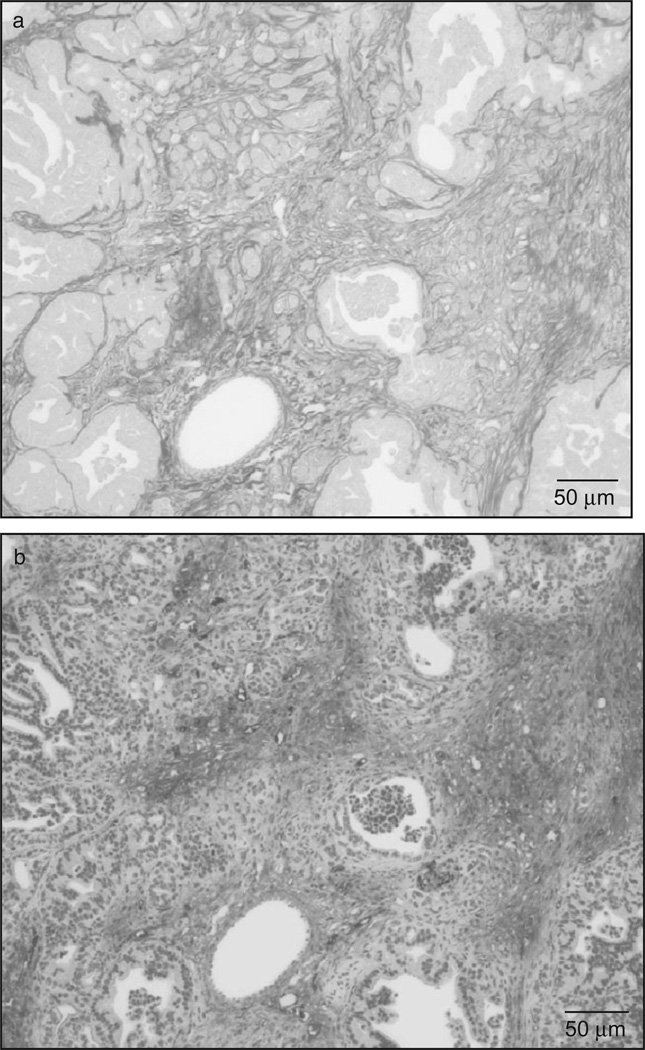
We found that PanIN lesions in mice with pancreas-specific and conditional overexpression of KrasG120 that leads to pancreatic carcinogenesis49 were accompanied by a marked desmoplastic reaction characterized by extensive deposition of collagens, as visualized by Sirius red staining and the high proliferation of active PaSC (α-SMA staining) (Fig. 4). These data suggest a close relationship between the progression of PanIN lesions and PaSC activation.
Figure 4.
Mice with pancreas-specific overexpression of Kras (KrasG120) display abundant desmoplasia surrounding pancreatic intra-epithelial neoplasias (PanIN). Representative images show Sirius red (a) and a-smooth muscle actin (α-SMA) staining (b) in serial sections of pancreas from 8-month-old, conditional KrasG120 mice. As illustrated in (a), tumor cells are surrounded by abundant collagen fibers (red staining). Moreover, α-SMA staining (brown staining), representing active pancreatic stellate cells, is abundant in areas with marked accumulation of collagen fibers.
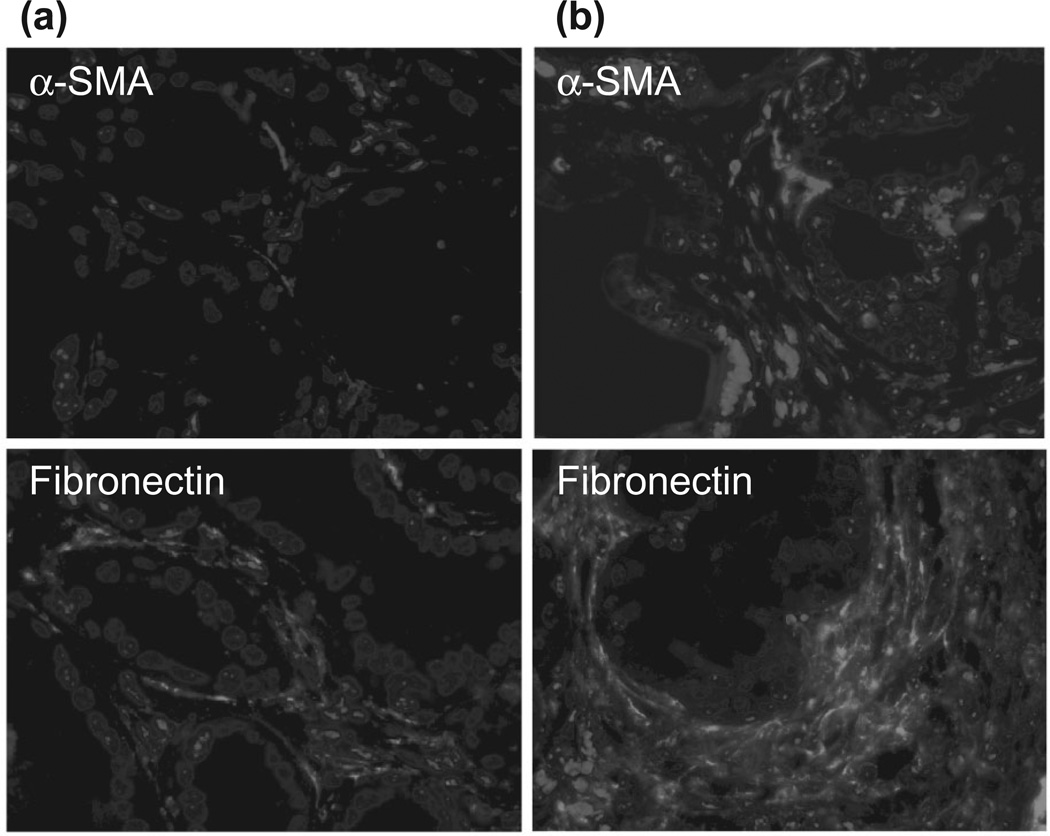
In order to determine the effects of diet on both PaSC and pancreatic carcinogenesis, we treated mice with the pancreas-specific and conditional overexpression of KrasG120 for 3 months with either a standard diet or a high-fat, high-calorie diet for 6–9 months. We found that the high-fat, high-calorie diet increased the number and advanced state of PanIN lesions (data not shown). Further, we observed a greater number of active PaSC and extensive deposition of fibronectin in pancreata of KrasG120 mice fed the high-fat, high-calorie diet compared to the standard diet (Fig. 5).
Figure 5.
High-fat diets promote desmopla-sia in mice with pancreas-specific overexpression of Kras (KrasG120). Conditional KrasG120 mice were fed either a standard diet (a) or a high-fat, high-calorie (HFCD) diet (b) for 3 months. Mice also received a daily injection of cerulein to promote pancreatic inflammation. Five-month-old mice fed the HFCD diet displayed a greater number of active pancreatic stellate cells (α-smooth muscle actin marker, red staining), and deposition of extracellular matrix proteins (fibronectin, green staining) than mice fed the standard diet. Blue staining represents DAPI-positive nuclei.
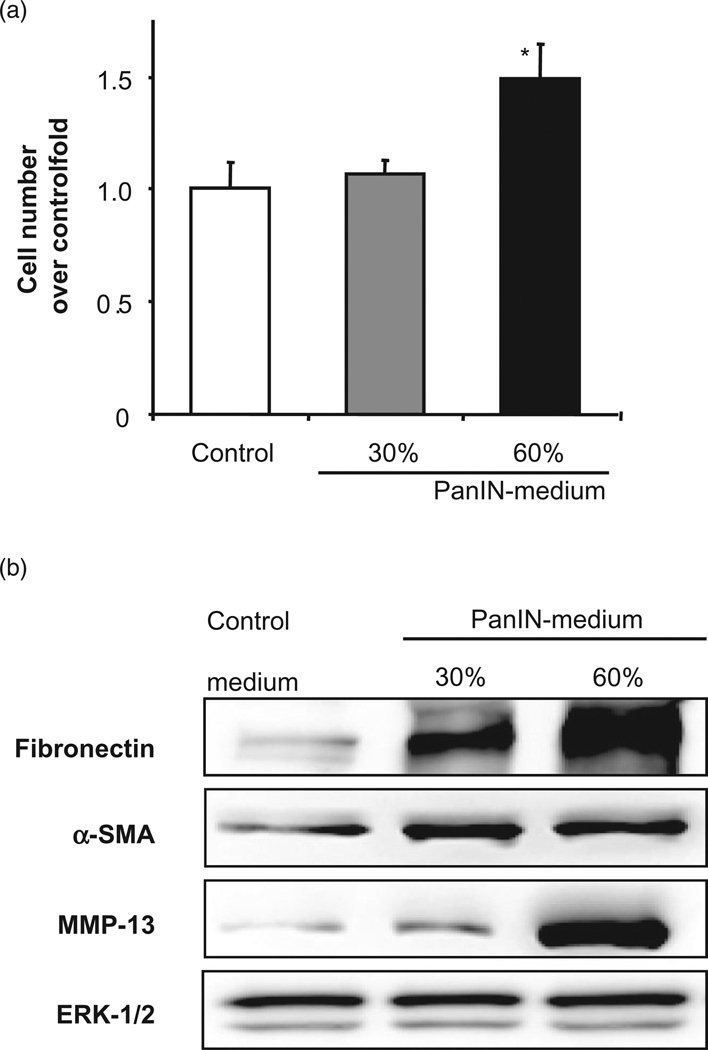
Further, we found that conditioned medium from cultured pancreatic PanIN KrasG120 cells, isolated from our conditional Kras mice, promoted the activation of quiescent mouse PaSC. As shown in Figure 6, PanIN-conditioned medium stimulated PaSC proliferation, and markedly increased the protein levels of α-SMA, fibronectin, and the key metalloproteinase (MMP), MMP-13. In addition, freshly-isolated PaSC cultured in the presence of PanIN-conditioned medium acquired early in culture a myofibroblast-like phenotype characterized by elongated cytoplasm with stress fibers and focal adhesions (data not shown). It is worth noting that previous studies demonstrated that conditioned media taken from PaSC cultures promoted pancreatic cancer cell proliferation and migration,30 indicating that a symbiotic relationship exits between the PaSC and the cancer cell.
Figure 6.
Conditioned medium from pancreatic intra-epithelial neopla-sias (PanIN) cells stimulates pancreatic stellate cell (PaSC) activation. Mouse PanIN cells were cultured for 2 days in 10% fetal bovine serum medium, and subsequently, aliquots of conditioned medium were transferred onto freshly isolated mouse PaSC. PaSC were cultured for 3 days in 1% serum medium in the presence of PanIN-conditioned media (up to 60% of the total culture medium) or equal volume of cell-free 10% media as the control. PaSC activation was evaluated by measuring (a) cell proliferation (total cell number) and (b) protein expression levels of fibronectin, α-smooth muscle actin (α-SMA), and metalloproteinase-13 (MMP-13) (proform) in cell lysates (Western blot analysis). ERK, extracellular signal-regulated kinase.
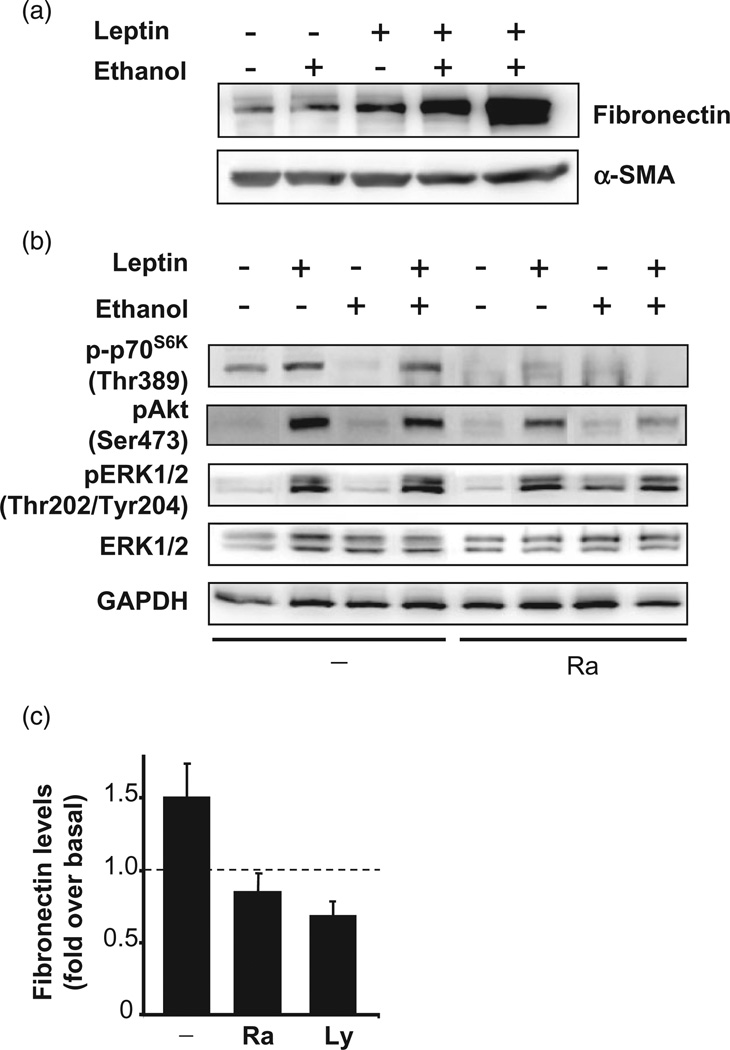
Studies using human and rat PaSC have examined a variety of factors known to be systemically elevated during alcohol abuse as potential regulators of PaSC activation and fibrogenesis. Circulating levels of the adipocyte-derived hormone leptin are elevated in individuals who are obese and those with high-fat diets. We tested whether ethanol and leptin potentiate PaSC responses. Leptin and ethanol in combination markedly induced PaSC activation, as indicated by a marked increase in fibronectin production (Fig. 7). This fibrogenic effect of leptin and alcohol was mediated by the PI3K/ AKT/mTOR signaling pathway, a key signaling system that regulates metabolism and cellular survival in many cells, including cancer cells. As illustrated in Figure 7, we found that the fibrotic response induced by the combination of leptin and ethanol was significantly reduced in the presence of the mTOR inhibitor rapamycin, and the PI3K inhibitor LY294002. We also found that other PaSC activators, such as IGF-1, TNF-α, and LPS, all elevated systemically in mice fed ethanol and high-fat diets, and activated the PI3K/AKT/mTOR pathway (data not shown). These studies demonstrate that PaSC certainly respond by activation to circulating factors occurring in patients with risk factors for pancreatic cancer. Considering the symbiotic relationship between PaSC and the pancreatic cancer cell, these results indicate that PaSC participate in the promotion of pancreatic cancer.
Figure 7.
Combination of ethanol and leptin treatment increases extracellular matrix deposition via the PI3K/AKT/mTOR signaling in cultured activated mouse pancreatic stellate cells (PaSC). (a) PaSC cultured in 1% fetal bovine serum medium were either untreated or treated with leptin (0.5 mg/mL) and/or ethanol (50 mmol/mL) for 24 h. Immunoblots show cellular levels of fibronectin and α-smooth muscle actin (α-SMA). (b) Immunoblots show levels of phosphorylated p70S6K, an mTOR substrate, p-Akt, and extracellular signal-regulated kinase-1/2 in activated PaSC untreated or treated for 30 min with leptin (0.5 mg/mL) alone or in combination with ethanol (50 mmol/mL). GAPDH was used as loading control. Pretreatment with rapamycin (Ra) or LY294002 (Ly) (data not shown) inhibited p70S6K phosphorylation. (c) Graphs show fibronectin levels in PaSC treated for 24 h with 0.5 mg/mL leptin and 50 mmol/mL ethanol in the presence or absence of Ra or Ly.
Conclusion
The epidemiology and environmental risk factors for pancreatic cancer provide important clues for mechanisms underlying pancreatic carcinogenesis. In the present study, we show data providing support for a role for the PaSC in mediating carcino-genesis resulting from environmental risk factors. Because this cancer is resistant to current therapies, and because part of this resistance is a result of the PaSC, an enhanced research effort on PaSC functions in carcinogenesis is a necessary step to improve outcomes.
Acknowledgments
The authors wish to thank the Department of Veterans Affairs and Southern California Research Center for Alcoholic Liver and Pancreatic Diseases (grant no. P50 AA011999).
Biography

Stephen Pandol
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig. Dis. 2010;28:645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita S, Wagatsuma Y, Okada M. Geographical distribution for malignant neoplasm of the pancreas in relation to selected climatic factors in Japan. Int. J. Health Geogr. 2007;6:34. doi: 10.1186/1476-072X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Ultraviolet B irradiance and vitamin D status are inversely associated with incidence rates of pancreatic cancer worldwide. Pancreas. 2010;39:669–674. doi: 10.1097/MPA.0b013e3181ce654d. [DOI] [PubMed] [Google Scholar]
- 5.Raimondi S, Maisonneuve P, Lohr JM, Lowenfels AB. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol. Biomarkers Prev. 2007;16:1894–1897. doi: 10.1158/1055-9965.EPI-07-0341. [DOI] [PubMed] [Google Scholar]
- 6.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis, the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 7.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int. J. Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 8.Vrieling A, Bueno-de-Mesquita HB, Boshuizen HC, et al. Cigarette smoking, environmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer. 2010;126:2394–2403. doi: 10.1002/ijc.24907. [DOI] [PubMed] [Google Scholar]
- 9.Tramacere I, Scotti L, Jenab M, et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int. J. Cancer. 2010;126:1474–1486. doi: 10.1002/ijc.24936. [DOI] [PubMed] [Google Scholar]
- 10.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Iwasaki M, Inoue M, et al. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large-scale population-based cohort study in Japan—the JPHC study. Cancer Causes Control. 2007;18:603–612. doi: 10.1007/s10552-007-9002-z. [DOI] [PubMed] [Google Scholar]
- 12.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens RJ, Roddam AW, Beral V. Pancreatic cancer in type 1 and young-onset diabetes: systematic review and meta-analysis. Br. J. Cancer. 2007;96:507–509. doi: 10.1038/sj.bjc.6603571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 15.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stolzenberg-Solomon RZ, Graubard BI, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day C. Metabolic syndrome, or what you will: definitions and epidemiology. Diab. Vasc. Dis. Res. 2007;4:32–38. doi: 10.3132/dvdr.2007.003. [DOI] [PubMed] [Google Scholar]
- 19.Silverman DT, Hoover RN, Brown LM, et al. Why do black Americans have a higher risk of pancreatic cancer than white Americans? Epidemiology. 2003;14:45–54. doi: 10.1097/00001648-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J. Am. Med. Dir. Assoc. 2010;11:617–628. doi: 10.1016/j.jamda.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Seitz HK, Cho CH. Contribution of alcohol and tobacco use in gastrointestinal cancer development. Methods Mol. Biol. 2009;472:217–241. doi: 10.1007/978-1-60327-492-0_9. [DOI] [PubMed] [Google Scholar]
- 22.Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Vonlaufen A, Phillips PA, Xu Z, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 24.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J. Clin. Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandol S, Edderkaoui M, Gukovsky I, Lugea A, Gukovskaya A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2009;7(Suppl.):S44–S47. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188–1202. doi: 10.1016/s0016-5085(03)01203-4. [DOI] [PubMed] [Google Scholar]
- 28.Edderkaoui M, Nitsche C, Zheng L, Pandol SJ, Gukovsky I, Gukovskaya AS. NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox. J. Biol. Chem. 2011;286:7779–7787. doi: 10.1074/jbc.M110.200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu R, Wang YL, Edderkaoui M, Lugea A, Apte MV, Pandol SJ. Ethanol augments PDGF-induced NADPH oxidase activity and proliferation in rat pancreatic stellate cells. Pancreatology. 2007;7:332–340. doi: 10.1159/000105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apte MV, Phillips PA, Fahmy RG, et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780–794. doi: 10.1016/s0016-5085(00)70148-x. [DOI] [PubMed] [Google Scholar]
- 34.Vonlaufen A, Xu Z, Daniel B, et al. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology. 2007;133:1293–1303. doi: 10.1053/j.gastro.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 35.Alho H, Sillanaukee P, Kalela A, Jaakkola O, Laine S, Nikkari ST. Alcohol misuse increases serum antibodies to oxidized LDL and C-reactive protein. Alcohol Alcohol. 2004;39:312–315. doi: 10.1093/alcalc/agh059. [DOI] [PubMed] [Google Scholar]
- 36.Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav. Immun. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Krebs M, Sakurai R, Torday JS, Rehan VK. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp. Lung Res. 2010;36:390–398. doi: 10.3109/01902141003714023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanthier N, Horsmans Y, Leclercq IA. The metabolic syndrome: how it may influence hepatic stellate cell activation and hepatic fibrosis. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:404–411. doi: 10.1097/MCO.0b013e32832c7819. [DOI] [PubMed] [Google Scholar]
- 39.Erridge C. The roles of pathogen-associated molecular patterns in atherosclerosis. Trends Cardiovasc. Med. 2008;18:52–56. doi: 10.1016/j.tcm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 42.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 43.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J. Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 44.Gentilini A, Lottini B, Brogi M, et al. Evaluation of intracellular signalling pathways in response to insulin-like growth factor I in apoptotic-resistant activated human hepatic stellate cells. Fibrogenesis Tissue Repair. 2009;2:1–11. doi: 10.1186/1755-1536-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiesner P, Choi SH, Almazan F, et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ. Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang Q, Chen A. Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1. Lab. Invest. 2009;89:1275. doi: 10.1038/labinvest.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneiderhan W, Schmid-Kotsas A, Zhao J, et al. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology. 2001;34(Pt 1):729–737. doi: 10.1053/jhep.2001.27828. [DOI] [PubMed] [Google Scholar]
- 48.Abramovitch S, Dahan-Bachar L, Sharvit E, et al. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–1737. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 49.Bai H, Li H, Zhang W, et al. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis. 2011;32:1689–1696. doi: 10.1093/carcin/bgr191. [DOI] [PMC free article] [PubMed] [Google Scholar]