Abstract
Geographic variations in mortality rate in the United States could be due to several hypothesized factors, one of which is exposure to solar ultraviolet radiation (UVR). Limited evidence from previous prospective studies has been inconclusive. The association between ambient residential UVR exposure and total and cause-specific mortality risks in a regionally diverse cohort (346,615 white, non-Hispanic subjects, 50–71 years of age, in the National Institutes of Health (NIH)–AARP Diet and Health Study) was assessed, with accounting for individual-level confounders. UVR exposure (averaged for 1978–1993 and 1996–2005) from NASA's Total Ozone Mapping Spectrometer was linked to the US Census Bureau 2000 census tract of participants' baseline residence. Multivariate-adjusted Cox proportional-hazards models were used to estimate hazard ratios and 95% confidence intervals. Over 12 years, UVR exposure was associated with total deaths (n = 41,425; hazard ratio for highest vs. lowest quartiles (HRQ4 vs. Q1) = 1.06, 95% confidence interval (CI): 1.03, 1.09; Ptrend < 0.001) and with deaths (all Ptrend < 0.05) due to cancer (HRQ4 vs. Q1 = 1.06, 95% CI: 1.02, 1.11), cardiovascular disease (HRQ4 vs. Q1 = 1.06, 95% CI: 1.00, 1.12), respiratory disease (HRQ4 vs. Q1 = 1.37, 95% CI: 1.21, 1.55), and stroke (HRQ4 vs. Q1 = 1.16, 95% CI: 1.01, 1.33) but not with deaths due to injury, diabetes, or infectious disease. These results suggest that UVR exposure might not be beneficial for longevity.
Keywords: epidemiology, mortality, prospective, sunlight, ultraviolet radiation, vitamin D
In the United States, cancer incidence, rates of death due to cancer, and all-cause mortality rates differ by geographic location (1–3). Reasons for such differences remain unclear. Geographic variations in mortality rates could be due to characteristics related to geography and also to demographics. Several determinants of health have been proposed to explain the geographic patterns, including the environment, health care, genetics, socioeconomics, and individual behaviors (4–6). Recently, geospatial analyses have suggested that ultraviolet radiation (UVR) from sunlight exposure could impact health. For example, studies have suggested benefits of UVR exposure for conditions including multiple sclerosis (7, 8), cardiovascular disease (9), diabetes (10), and cancer (11, 12).
The causal relationship between UVR exposure, from sunlight and indoor tanning, and increased skin cancer risk is well known and has stimulated public health campaigns against excessive natural sunlight exposure and nonsolar tanning behaviors (13, 14). The sparse evidence available on the association between sunlight exposure and total and cause-specific mortality risks gives mixed results. Yang et al. (15) found that sunlight exposure might be associated with reduced total and cardiovascular disease–specific mortality risks, whereas artificial UVR exposure might be associated with increased total and cancer-specific mortality risks. Freedman et al. (16) found that, unlike death from nonmelanoma skin cancer, the risk of death from female breast cancer and colon cancer was negatively associated with both residential and occupational sunlight exposure. Berwick et al. (17) suggested that sun exposure is associated with increased risk of survival from melanoma, and Boscoe et al. (11) found that UVR exposure was associated with decreased risk of death from some cancers, including colon, prostate, non-Hodgkin lymphoma, and stomach, but increased risk of death from other cancers, including cervix, oral cavity, and melanoma.
Most of the existing studies investigating the effect of UVR exposure on mortality risk or the health benefits of UVR exposure are ecological studies, in which the unit of analysis is a population rather than a person. These previous studies have relied on crude exposure measurements such as latitude, and they adjusted for aggregated confounding variables rather than individual-level confounders (e.g., Boscoe et al. (11), Garland et al. (18)). A few nonecological studies used questionnaires that collected self-reported sun behavior (e.g., Yang et al. (15)), which might have produced biased estimates. Because of these limitations, uncertainties persist with regard to the relationship between sun exposure and risk of death. Therefore, we aimed to examine the relationship between an objective measure of ambient resident UVR exposure and total mortality risk as well as cause-specific mortality risk in a prospective cohort, the National Institutes of Health (NIH)–AARP Diet and Health Study, and to account for individual-level potential confounders such as diet and lifestyle factors.
MATERIALS AND METHODS
Study population
The details of the NIH-AARP Diet and Health Study have been described (19). Briefly, a self-administered questionnaire was mailed in 1995–1996 to 3.5 million AARP members between 50 and 71 years of age and residing in 6 US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta, Georgia, and Detroit, Michigan). This questionnaire elicited information on demographic characteristics, health-related behaviors, and dietary intake. Of the 617,119 people who returned the questionnaire, 566,398 completed the questionnaire in satisfactory detail and consented to be in the study. We excluded proxy-responders (n = 15,760), subjects with a cancer before baseline (n = 51,234), and those with calorie intake of more than 2 interquartile ranges from the median (n = 4,417). To ensure that individuals in our analysis were healthy at baseline, we excluded those who reported having had heart disease (n = 68,432), stroke (n = 6,363), end-stage renal disease (n = 560), or emphysema (n = 9,356) or who had rated their general health as poor or fair (n = 30,861). We excluded responders who died on the first day of follow-up (n = 10), those who self-identified as any race/ethnicity other than white/non-Hispanic or had missing race/ethnicity information (n = 32,343), and those for whom we had no information on baseline residence (n = 447). The resulting cohort included 346,615 participants: 201,808 men and 144,807 women.
Follow-up and ascertainment of death
The mortality follow-up spanned from the day after study entry until death or current end of mortality follow-up (December 31, 2008). Deaths (n = 41,425) were identified through linkage of the cohort to the Social Security Administration Death Master File and to International Classification of Diseases codes for underlying cause of death from the National Death Index. Cancer mortality (n = 17,611) included all deaths occurring because of invasive, in situ, benign, or unknown-behavior neoplasms; cardiovascular disease mortality (n = 8,854) was classified as deaths due to diseases of the heart, hypertension without heart disease, atherosclerosis, aortic aneurysm and dissection, and other diseases of the arteries, arterioles, and capillaries; respiratory disease mortality (n = 2,077) included deaths from pneumonia, influenza, and chronic obstructive pulmonary disease; stroke mortality (n = 1,765) included death from cerebrovascular diseases; injury mortality (n = 1,359) included deaths from accidents and adverse events, suicide and self-inflicted injury, and homicide and legal intervention; diabetes mortality (n = 787) included deaths due to diabetes mellitus; and infectious disease mortality (n = 689) included deaths from septicemia, tuberculosis, syphilis, human immunodeficiency virus, and other infectious and parasitic diseases. The remaining deaths (n = 8,283) were from other causes. Selected cancer sites were assessed by Surveillance Epidemiology and End Results (SEER) Cause of Death Recode.
UVR exposure assessment
The NASA Total Ozone Mapping Spectrometer (TOMS) database (http://disc.sci.gsfc.nasa.gov/acdisc/toms) provided daily information on a noon-time ground-level erythemal estimate on a 1° latitude–by–1.25° longitude grid in 1978–1993 and 1996–2005. We assigned the ground-level erythemal exposure for each participant by deterministic linkage of the census tract centroid of the residence at baseline to the closest point on the TOMS grid using ArcView version 9.3 (Esri, Redlands, California); the census tract for each subject was assigned spatially on the basis of the longitude and latitude coordinates from geocoding residential addresses. The erythemal exposure was averaged across all available measured days in the month of July because summer is when surface UVR is strongest, when noise factors such as clouds and aerosols are not as influential (20), and when the TOMS UVR data are in better agreement with ground-based data (21). We used both continuous measures and quartiles of the erythemal exposure, defined as biological damage per square meter (BD/m2) (22), with the continuous measure scaled by dividing each averaged dose by half of the interquartile range (33.7 BD/m2). We previously showed that our measure of UVR exposure was associated with self-reported history of nonmelanoma skin cancers (23); thus, we used this measure for other health outcomes.
Covariate assessment
The potential confounders considered in our models were derived from the baseline questionnaire, which inquired about demographics, lifestyle behaviors (including tobacco and alcohol use and physical activity), and diet via a 124-item food frequency questionnaire (19). Age at baseline (continuous variable) and sex were self-reported. Body mass index was calculated from self-reported baseline height and weight and was used as a continuous variable. Intakes of fruits, vegetables, red meat, and white meat were expressed as servings per day, as defined by the US Department of Agriculture's MyPyramid equivalents database. Alcohol consumption was measured as drinks per day. Tobacco smoking was categorized into the following categories: never smokers, former smokers of ≤20 cigarettes/day, former smokers of >20 cigarettes/day, current smokers of ≤20 cigarettes/day, and current smokers of >20 cigarettes/day. Educational level was categorized into 4 ordinal categories: high school graduate or less, technical school or some college, college graduate, and postgraduate education. We included 2 baseline physical activity variables: usual physical routine throughout the day and vigorous physical activity. Usual physical routine throughout the day included the following categories: sit all day, sit much of the day/walk sometimes, stand/walk often/no lifting, lift/carry light loads, and carry heavy loads. Vigorous physical activity included the following categories: never, rarely, 1–3 times/month, 1–2 times/week, 3–4 times/week, and 5 or more times per week. The median household income was estimated for the 2000 US Census tract of each participant. A risk questionnaire was sent to a subset of respondents in 1996–1997, which assessed hypertension with the question, “Have you ever been told by a doctor that you had high blood pressure?” Hypertension was used as reported. Missing indicator variables were used for missing values.
Statistical analysis
We used Cox proportional-hazards models adjusted for age alone and additionally for multiple potential confounders that were considered important a priori (listed in Table 1) to estimate hazard ratios and 95% confidence intervals per half of the interquartile range of UVR (continuous variable) and per quartile of exposure. The timeline for the Cox models was based on person-years. Trends were measured on the basis of ordinal quartiles. To examine the proportionality assumption, we used models that allowed time-dependent relative risks and found no significant violations. To examine residual confounding due to smoking, we further adjusted for smoking by including time since quitting and number of cigarettes per day, and we also stratified by smoking. We examined potential residual confounding by socioeconomic status by conducting stratified analyses on income and educational level. To examine the potential for reverse causation, we performed a lag analysis by excluding the first 2 years of follow-up. We also accounted for potential residual correlations due to geography by including a random effect for census tract in our Cox models. All tests of statistical significance were 2 sided. All analyses were conducted in R (http://www.r-project.org/) or SAS (SAS Institute, Cary, North Carolina). Figures were made in GraphPad Prism 5.00 (GraphPad Software, San Diego, California). We interpreted P < 0.05 and 95% confidence intervals excluding 1.00 as statistically significant.
Table 1.
Characteristics of Subjects in the National Institutes of Health-AARP Study of Diet and Health Cohort, 1995–2008
| Quartiles of July UVR exposure,a BD/m2 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort (n = 346,615) |
176.1–186.2 (n = 89,876) |
186.3–236.7 (n = 81,172) |
236.8–253.6 (n = 82,233) |
253.7–289.5 (n = 93,334) |
|||||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Sex, male | 201,808 | 58 | 54,239 | 60 | 47,628 | 59 | 47,200 | 57 | 52,741 | 57 | |||||
| Age at entry | 61.69 (5.40) | 61.45 (5.46) | 61.48 (5.42) | 61.70 (5.36) | 62.11 (5.34) | ||||||||||
| Body mass indexb | 26.74 (4.52) | 27.11 (4.57) | 26.68 (4.46) | 26.68 (4.55) | 26.49 (4.46) | ||||||||||
| Calories intake, kcal/day | 1,826.45 (784.82) | 1,874.67 (792.80) | 1,848.28 (793.89) | 1,790.65 (769.26) | 1,792.56 (779.60) | ||||||||||
| Alcohol, drinks/day | 0.95 (2.35) | 0.86 (2.22) | 0.86 (2.25) | 1.00 (2.38) | 1.06 (2.54) | ||||||||||
| Fruit, servings/day | 1.16 (0.80) | 1.18 (0.80) | 1.10 (0.77) | 1.15 (0.80) | 1.19 (0.83) | ||||||||||
| Vegetables, servings/day | 1.13 (0.59) | 1.12 (0.57) | 1.09 (0.56) | 1.14 (0.60) | 1.16 (0.63) | ||||||||||
| Red meat, servings/day | 1.08 (0.66) | 1.11 (0.66) | 1.10 (0.64) | 1.06 (0.65) | 1.05 (0.67) | ||||||||||
| White meat, servings/day | 0.63 (0.54) | 0.64 (0.54) | 0.59 (0.50) | 0.64 (0.53) | 0.67 (0.58) | ||||||||||
| Smokingc | |||||||||||||||
| Never | 129,420 | 37 | 34,727 | 39 | 30,801 | 38 | 30,840 | 38 | 33,052 | 35 | |||||
| Former | |||||||||||||||
| ≤20 cigarettes/day | 92,923 | 26 | 23,884 | 27 | 21,572 | 27 | 22,194 | 27 | 25,273 | 27 | |||||
| >20 cigarettes/day | 67,073 | 19 | 16,479 | 18 | 15,105 | 19 | 16,272 | 20 | 19,217 | 21 | |||||
| Current | |||||||||||||||
| ≤20 cigarettes/day | 29,345 | 8 | 7,827 | 9 | 6,940 | 9 | 6,576 | 8 | 8,002 | 9 | |||||
| >20 cigarettes/day | 15,589 | 5 | 3,623 | 4 | 3,918 | 5 | 3,525 | 4 | 4,523 | 5 | |||||
| Educational levelb | |||||||||||||||
| High school or less | 82,435 | 24 | 27,581 | 31 | 20,627 | 25 | 15,588 | 19 | 18,639 | 20 | |||||
| Technical/some college | 113,802 | 33 | 25,959 | 29 | 25,767 | 32 | 28,387 | 35 | 33,689 | 36 | |||||
| College | 68,379 | 20 | 16,089 | 18 | 16,498 | 20 | 17,151 | 21 | 18,641 | 20 | |||||
| Postgraduate | 74,146 | 21 | 18,366 | 20 | 16,428 | 20 | 19,264 | 23 | 20,088 | 22 | |||||
| Physical activity throughout the dayc | |||||||||||||||
| Sit during the day, not much walking | 23,502 | 7 | 6,341 | 7 | 5,053 | 6 | 5,835 | 7 | 6,273 | 7 | |||||
| Sit much of the day, walk a fair amount | 110,403 | 32 | 29,117 | 32 | 24,414 | 30 | 27,150 | 33 | 29,722 | 32 | |||||
| Stand/walk a lot, no lifting | 132,141 | 38 | 31,624 | 35 | 30,600 | 38 | 32,188 | 39 | 37,729 | 40 | |||||
| Lift/carry light loads | 63,967 | 18 | 18,329 | 20 | 16,834 | 21 | 13,439 | 16 | 15,365 | 16 | |||||
| Heavy work | 10,766 | 3 | 2,785 | 3 | 2,906 | 4 | 2,387 | 3 | 2,688 | 3 | |||||
| Vigorous physical activityc | |||||||||||||||
| Never | 10,391 | 3 | 3,131 | 3 | 2,385 | 3 | 2,230 | 3 | 2,645 | 3 | |||||
| Rarely | 41,578 | 12 | 12,199 | 14 | 9,507 | 12 | 9,213 | 11 | 10,659 | 11 | |||||
| 1–3 times/month | 48,045 | 14 | 13,635 | 15 | 11,456 | 14 | 11,025 | 13 | 11,929 | 13 | |||||
| 1–2 times/week | 78,481 | 23 | 21,303 | 24 | 19,040 | 23 | 18,176 | 22 | 19,962 | 21 | |||||
| 3–4 times/week | 96,375 | 28 | 23,465 | 26 | 22,824 | 28 | 23,662 | 29 | 26,424 | 28 | |||||
| ≥5 times/week | 69,551 | 20 | 15,522 | 17 | 15,430 | 19 | 17,435 | 21 | 21,164 | 23 | |||||
| Hypertension | 73,491 | 37 | 19,966 | 38 | 17,073 | 36 | 16,999 | 36 | 19,453 | 36 | |||||
| Census tract median household income, USD | 55,444 (23,988) | 61,522 (25,190) | 50,429 (19,883) | 58,275 (26,859) | 51,458 (21,591) | ||||||||||
Abbreviations: BD/m2, biological damage per square meter; SD, standard deviation; USD, US dollars; UVR, ultraviolet radiation.
a The July UVR exposure was calculated as the averaged dose across all available measured days in the month of July in 1978–1993 and 1996–2005.
b Weight (kg)/height (m)2.
c Because of missing data, percentages do not total 100%.
RESULTS
Table 1 presents the characteristics of the 346,615 participants by UVR exposure quartiles (176.1–186.2, 186.3–236.7, 236.8–253.6, and 253.7–289.5 BD/m2). There were more men than women (58% vs. 42%, respectively) in the cohort.
During a mean follow-up period of 12.06 (standard deviation, 3.55) years, 41,425 deaths (11.95%) occurred. In age-adjusted models (Table 2), UVR exposure (continuous) was positively associated with the total risk of death as well as with risk of death due to cancer and respiratory disease; UVR exposure was inversely associated with risk of death due to infectious disease. In quartile analyses, UVR exposure was positively associated with the total risk of death and the risk of death of death due to cancer and respiratory disease.
Table 2.
Age-adjusteda Association Between Ambient Residential Ultraviolet Radiation Exposure and Total Mortality and Cause-Specific Mortality in the National Institutes of Health-AARP Study of Diet and Health Cohort, 1995–2008
| Cause of Death | July UVR Exposure,b BD/m2 |
||||
|---|---|---|---|---|---|
| No. | % | HR | 95% CI | P Value | |
| Total | |||||
| Continuousc | 41,425 | 100 | 1.02 | 1.01, 1.03 | <0.001 |
| Quartile 1 | 10,246 | 100 | 1.00 | Referent | |
| Quartile 2 | 9,394 | 100 | 1.02 | 0.99, 1.04 | |
| Quartile 3 | 9,928 | 100 | 1.05 | 1.02, 1.08 | |
| Quartile 4 | 11,857 | 100 | 1.06 | 1.03, 1.09 | |
| P for trend | <0.001 | ||||
| Cancer | |||||
| Continuousc | 17,611 | 43 | 1.02 | 1.01, 1.04 | 0.003 |
| Quartile 1 | 4,351 | 42 | 1.00 | Referent | |
| Quartile 2 | 3,962 | 42 | 1.01 | 0.97, 1.05 | |
| Quartile 3 | 4,244 | 43 | 1.06 | 1.01, 1.10 | |
| Quartile 4 | 5,054 | 43 | 1.07 | 1.03, 1.12 | |
| P for trend | <0.001 | ||||
| Cardiovascular disease | |||||
| Continuousc | 8,854 | 21 | 1.02 | 1.00, 1.04 | 0.089 |
| Quartile 1 | 2,254 | 22 | 1.00 | Referent | |
| Quartile 2 | 1,967 | 21 | 0.97 | 0.91, 1.03 | |
| Quartile 3 | 2,061 | 21 | 0.99 | 0.93, 1.05 | |
| Quartile 4 | 2,572 | 22 | 1.04 | 0.98, 1.10 | |
| P for trend | 0.139 | ||||
| Respiratory disease | |||||
| Continuousc | 2,077 | 5 | 1.18 | 1.13, 1.23 | <0.001 |
| Quartile 1 | 418 | 4 | 1.00 | Referent | |
| Quartile 2 | 431 | 5 | 1.14 | 1.00, 1.31 | |
| Quartile 3 | 563 | 6 | 1.45 | 1.28, 1.65 | |
| Quartile 4 | 665 | 6 | 1.43 | 1.27, 1.62 | |
| P for trend | <0.001 | ||||
| Stroke | |||||
| Continuousc | 1,765 | 4 | 1.05 | 1.00, 1.10 | 0.051 |
| Quartile 1 | 402 | 4 | 1.00 | Referent | |
| Quartile 2 | 421 | 4 | 1.16 | 1.01, 1.33 | |
| Quartile 3 | 432 | 4 | 1.15 | 1.01, 1.32 | |
| Quartile 4 | 510 | 4 | 1.14 | 1.00, 1.30 | |
| P for trend | 0.081 | ||||
| Injury | |||||
| Continuousc | 1,359 | 3 | 1.06 | 1.00, 1.12 | 0.046 |
| Quartile 1 | 314 | 3 | 1.00 | Referent | |
| Quartile 2 | 308 | 3 | 1.09 | 0.93, 1.27 | |
| Quartile 3 | 341 | 3 | 1.18 | 1.01, 1.37 | |
| Quartile 4 | 396 | 3 | 1.17 | 1.01, 1.36 | |
| P for trend | 0.024 | ||||
| Diabetes | |||||
| Continuousc | 787 | 2 | 0.97 | 0.90, 1.04 | 0.366 |
| Quartile 1 | 212 | 2 | 1.00 | Referent | |
| Quartile 2 | 179 | 2 | 0.94 | 0.77, 1.14 | |
| Quartile 3 | 186 | 2 | 0.95 | 0.78, 1.16 | |
| Quartile 4 | 210 | 2 | 0.91 | 0.75, 1.10 | |
| P for trend | 0.363 | ||||
| Infectious disease | |||||
| Continuousc | 689 | 2 | 0.91 | 0.84, 0.98 | 0.012 |
| Quartile 1 | 185 | 2 | 1.00 | Referent | |
| Quartile 2 | 175 | 2 | 1.05 | 0.85, 1.29 | |
| Quartile 3 | 160 | 2 | 0.93 | 0.76, 1.15 | |
| Quartile 4 | 169 | 1 | 0.83 | 0.67, 1.02 | |
| P for trend | 0.047 | ||||
Abbreviations: BD/m2, biological damage per square meter; CI, confidence interval; HR, hazard ratio.
a Hazard ratios and 95% confidence intervals were calculated with Cox regression models adjusted for age at study entry.
b The July UVR exposure was calculated as the averaged dose across all available measured days in the month of July in 1978–1993 and 1996–2005.
c The continuous July UVR exposure was scaled by 33.7 BD/m2 (half of the interquartile range for the cohort).
With the addition of multiple potential individual-level confounders in the multivariate-adjusted models (Table 3), UVR exposure was positively associated with the total risk of death and the risk of death due to cancer, cardiovascular disease, respiratory disease, and stroke. UVR exposure was inversely associated with death due to infectious diseases, although the association was borderline statistically significant in the multivariate model. In quartile analyses, UVR exposure was positively associated with the total risk of death and the risk of death due to cancer, cardiovascular disease, respiratory disease, and stroke. We further explored the association with selected cancer site–specific mortality risk in our cohort (Appendix Table 1) and found that deaths from lung, liver, and prostate cancer and from melanoma were associated with UVR exposure.
Table 3.
Multivariate-adjusteda Association Between Ambient Residential Ultraviolet Radiation Exposure and Total Mortality and Cause-Specific Mortality in the National Institutes of Health-AARP Study of Diet and Health Cohort, 1995–2008
| Cause of Death | July UVR Exposure,b BD/m2 |
||||
|---|---|---|---|---|---|
| No. | % | HR | 95% CI | P Value | |
| Total | |||||
| Continuousc | 41,425 | 100 | 1.03 | 1.02, 1.04 | <0.001 |
| Quartile 1 | 10,246 | 100 | 1.00 | Referent | |
| Quartile 2 | 9,394 | 100 | 1.00 | 0.98, 1.03 | |
| Quartile 3 | 9,928 | 100 | 1.08 | 1.05, 1.11 | |
| Quartile 4 | 11,857 | 100 | 1.06 | 1.03, 1.09 | |
| P for trend | <0.001 | ||||
| Cancer | |||||
| Continuousc | 17,611 | 43 | 1.03 | 1.01, 1.04 | 0.001 |
| Quartile 1 | 4,351 | 42 | 1.00 | Referent | |
| Quartile 2 | 3,962 | 42 | 0.99 | 0.95, 1.04 | |
| Quartile 3 | 4,244 | 43 | 1.07 | 1.03, 1.12 | |
| Quartile 4 | 5,054 | 43 | 1.06 | 1.02, 1.11 | |
| P for trend | <0.001 | ||||
| Cardiovascular disease | |||||
| Continuousc | 8,854 | 21 | 1.04 | 1.02, 1.07 | <0.001 |
| Quartile 1 | 2,254 | 22 | 1.00 | Referent | |
| Quartile 2 | 1,967 | 21 | 0.97 | 0.91, 1.03 | |
| Quartile 3 | 2,061 | 21 | 1.04 | 0.98, 1.11 | |
| Quartile 4 | 2,572 | 22 | 1.06 | 1.00, 1.12 | |
| P for trend | 0.010 | ||||
| Respiratory disease | |||||
| Continuousc | 2,077 | 5 | 1.18 | 1.13, 1.24 | <0.001 |
| Quartile 1 | 418 | 4 | 1.00 | Referent | |
| Quartile 2 | 431 | 5 | 1.09 | 0.96, 1.26 | |
| Quartile 3 | 563 | 6 | 1.47 | 1.30, 1.67 | |
| Quartile 4 | 665 | 6 | 1.37 | 1.21, 1.55 | |
| P for trend | <0.001 | ||||
| Stroke | |||||
| Continuousc | 1,765 | 4 | 1.07 | 1.01, 1.12 | 0.012 |
| Quartile 1 | 402 | 4 | 1.00 | Referent | |
| Quartile 2 | 421 | 4 | 1.16 | 1.01, 1.34 | |
| Quartile 3 | 432 | 4 | 1.21 | 1.05, 1.38 | |
| Quartile 4 | 510 | 4 | 1.16 | 1.01, 1.33 | |
| P for trend | 0.035 | ||||
| Injury | |||||
| Continuousc | 1,359 | 3 | 1.06 | 1.00, 1.12 | 0.065 |
| Quartile 1 | 314 | 3 | 1.00 | Referent | |
| Quartile 2 | 308 | 3 | 1.03 | 0.88, 1.21 | |
| Quartile 3 | 341 | 3 | 1.18 | 1.01, 1.37 | |
| Quartile 4 | 396 | 3 | 1.12 | 0.97, 1.31 | |
| P for trend | 0.057 | ||||
| Diabetes | |||||
| Continuousc | 787 | 2 | 1.00 | 0.93, 1.08 | 0.984 |
| Quartile 1 | 212 | 2 | 1.00 | Referent | |
| Quartile 2 | 179 | 2 | 0.96 | 0.78, 1.18 | |
| Quartile 3 | 186 | 2 | 1.03 | 0.85, 1.26 | |
| Quartile 4 | 210 | 2 | 0.94 | 0.77, 1.15 | 0.702 |
| P for trend | |||||
| Infectious disease | |||||
| Continuousc | 689 | 2 | 0.92 | 0.85, 1.00 | 0.046 |
| Quartile 1 | 185 | 2 | 1.00 | Referent | |
| Quartile 2 | 175 | 2 | 1.04 | 0.84, 1.28 | |
| Quartile 3 | 160 | 2 | 0.98 | 0.79, 1.22 | |
| Quartile 4 | 169 | 1 | 0.84 | 0.68, 1.04 | |
| P for trend | 0.089 | ||||
Abbreviations: BD/m2, biological damage per square meter; CI, confidence interval; HR, hazard ratio; UVR, ultraviolet radiation.
a Hazard ratios and 95% confidence intervals were calculated with Cox regression models adjusted for the following covariates: age at study entry; sex; body mass index; caloric intake; intake of fruit, vegetables, and red and white meat; alcohol consumption; tobacco smoking; educational level; physical activity; median household income; and hypertension.
b The July UVR exposure was calculated as the averaged dose across all available measured days in the month of July in 1978–1993 and 1996–2005.
c The continuous July UVR exposure was scaled by 33.7 BD/m2 (half of the interquartile range for the cohort).
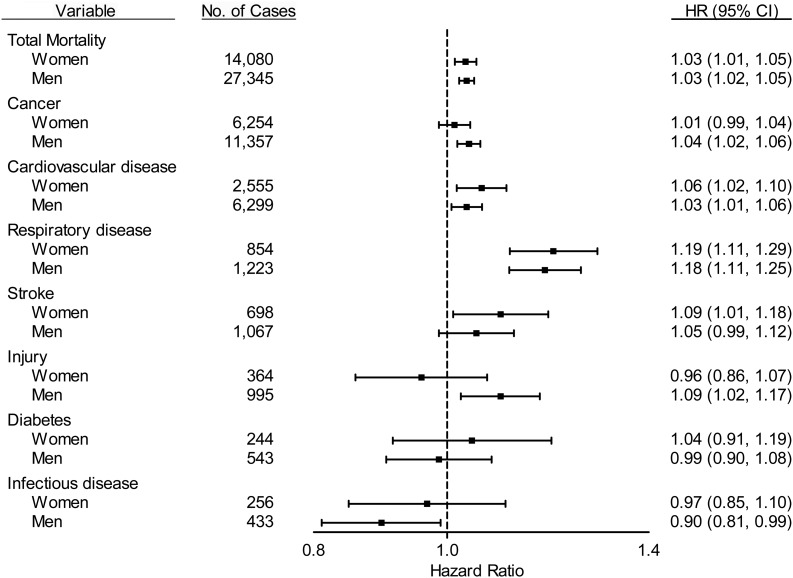
We examined the sex-stratified multivariable-adjusted associations between continuous UVR exposure and total mortality risk and cause-specific mortality risk (Figure 1). UVR exposure was positively associated with total mortality risk in both sexes (Pinteraction = 0.722). Similar positive associations were observed for death due to cardiovascular disease and respiratory disease. UVR exposure was significantly associated with increased risk of death due to stroke in women but not in men. Conversely, the positive associations with death due to cancer and injury were significant in men but not women. The associations with diabetes were similarly null in men and women. For all mortality outcomes, we found no significant interactions between UVR exposure and sex (Pinteraction > 0.05).
Figure 1.
Stratified analysis examining the association between ambient residential ultraviolet radiation (UVR) exposure (continuous, scaled by 33.7 biological damage per square meter [BD/m2]) and mortality outcomes by sex. Cox regression models were adjusted for the following covariates: age at study entry; body mass index; caloric intake; intake of fruit, vegetables, and red and white meat; alcohol consumption; tobacco smoking; educational level; physical activity; median household income; and hypertension. All Pinteraction > 0.05. HR, hazard ratio; CI, confidence interval.
To examine the possibility of residual confounding due to smoking, we further adjusted for time since quitting and number of cigarettes per day but observed no change in the risk estimates; in addition, we stratified by smoking (never vs. ever smoking) and saw no evidence for residual confounding. To examine the potential for residual confounding by socioeconomic status, we conducted stratified analyses on income and educational level and saw no evidence for residual confounding. Upon excluding the first 2 years of follow-up in a lag analysis to exclude the mortality outcomes of persons who might have been unhealthy or those who had undiagnosed or unreported health conditions at the start of the study, we did not observe evidence of reverse causation. Moreover, the addition of a random effect for census tract to address residual confounding at the geographic-area level did not change our main results (data not shown).
DISCUSSION
We examined total and cause-specific mortality risks in relation to ambient current residential UVR exposure in a large prospective study in the United States. After adjusting for multiple individual-level potential confounders, including educational level, smoking, diet, alcohol intake, and physical activity, we found that UVR exposure in the range of 176.1–289.5 BD/m2 was positively but weakly associated with total deaths as well as with several specific causes of death, including cancer, cardiovascular disease, respiratory disease, and stroke.
Little evidence is available from epidemiologic studies on UVR exposure and its association with all-cause death after accounting for individual-level potential confounders. Most studies of UVR exposure and mortality risk have focused on total cancer-specific deaths or cancer site–specific deaths. In contrast to results from previous ecological studies that found a protective effect of sunlight or UVR exposure against cancer-specific death (11, 24–28), the results from our prospective study suggested a weak positive association with cancer-specific mortality risk, which could be driven partly by positive associations with deaths from lung, prostate, and liver cancer and from melanoma (upon excluding deaths from these 4 cancers, the association becomes nonsignificant). A previous prospective study of UVR exposure and mortality risk among Swedish women also found an increased risk of cancer-specific death with UVR exposure assessed as self-reported indoor tanning, whereas natural sun exposure, assessed as the number of sunburns and time spent on sunbathing vacations, was associated with decreased overall mortality risk as well as cardiovascular disease–specific mortality risk (15).
We observed that deaths from lung cancer, which accounted for approximately 30% of the cancer deaths in this cohort, were positively associated with current UVR exposure. This result is in contrast to previous ecological work showing that regional ambient UVR estimates were inversely correlated with deaths due to several malignancies, including the lung (26). To account for the known influential role of tobacco smoking in lung cancer, we extensively examined smoking as a potential confounder but did not observe evidence for residual confounding. However, we did not have information on environmental or secondhand tobacco smoke and could not account for such exposure. We also found that prostate and liver cancers were positively associated with current UVR exposure; these results are in contrast to those of previous studies that suggested an inverse relationship (11, 29, 30). Results for death from melanoma have been mixed (11, 17, 31), and in our study, we observed a positive association. Additional studies suggested that exposure to sunlight might be correlated with decreased rates of death from breast and colon cancer (11, 16, 32), but in our cohort, we see a borderline increased risk of death from breast cancer and no association with death from colon cancer. Many studies suggest that the molecular actions of vitamin D contribute to cancer prevention (33), and therefore, exposure to solar UVR, which results in the cutaneous synthesis of most of the vitamin D requirements in the human body, is important. Interestingly, in our previous study of cancer risk in this same cohort, UVR exposure was positively associated with incident melanoma, not associated with risk of incident lung or liver cancers, and inversely associated with risk of incident colon and prostate cancers (23). The associations we observe with ambient UVR exposure could be due to several mechanisms, including vitamin D synthesis, although these mechanisms might be different between the initiation and progression of cancer. The analyses performed in this cohort were not designed to determine the definitive role of vitamin D in cancer prevention or mortality risk. Evidence of a protective effect of UVR against some site-specific incident cancers but not against death from cancer could imply geographic differences in diagnostic practices or healthcare utilization.
In our study, we found a positive but weak association between UVR exposure and death due to cardiovascular disease, respiratory disease, and stroke. These results differ from those of previous studies, which have focused mostly on the importance of sun exposure as the major source of vitamin D, and these studies found that serum vitamin D levels were inversely associated with cardiovascular disease and stroke incidence and mortality risk (reviewed in Zittermann and Gummert (9)). Interestingly, seasonal fluctuations are observed for cardiovascular disease, respiratory disease, and stroke, with higher rates during winter months (34–36). Many factors (such as temperature) could contribute to the disease rate patterns, and the synthesis of vitamin D through sun exposure is hypothesized to be one of the factors contributing to the seasonality (9, 35). Some studies suggest that respiratory infections such as influenza and pneumonia are seasonal because of the annual cycle of UVR (37, 38). Contrary to these hypotheses, our results suggest that higher UVR exposure is significantly associated with increased risks of death from cardiovascular disease, respiratory disease, and stroke. The mechanism, which might or might not involve the synthesis of vitamin D, remains unknown. In fact, a recent Mendelian randomization study found no causal relationship between similar outcomes (myocardial infarction, diabetes, cancer, and total mortality) and the genetic variants that confer higher vitamin D status (39). Thus, the relationship between sun exposure and these mortality outcomes could be independent of vitamin D. A possible alternative mechanism might be UVR-induced immune modulation, including enhanced T-helper 2–mediated immune activity (40–42) and immunosuppression (43–45).
We did not find any association between ambient UVR exposure and death due to injury, diabetes, and infectious disease. Previous studies have suggested that UVR exposure and vitamin D might be inversely associated with diabetes risk (10, 46–48), but none have examined the risk of diabetes-specific death. Neither animal nor human studies have definitively determined whether UVR exposure is beneficial for infectious diseases (8), although many have suggested UVR exposure to be associated with less effective control of some infectious diseases (43, 45).
Our study has several strengths. We used a very large prospective study with a geographically diverse population and an objective measure of UVR exposure to avoid potential biases such as recall bias or self-report of sun behaviors. We used the TOMS dataset for assigning UVR exposure, which is an improvement over other studies that used latitude as the surrogate for ground-level UVR exposure. The TOMS dataset takes into account a variety of environmental factors that affect UVR dose, including cloud cover and automobile exhaust. We used the July estimate because summer is when surface UVR is strongest and noise factors such as clouds and aerosols are not as influential (20). Averaging the TOMS-estimated doses over longer periods of time improves the agreement with measured ground-level UVR (21), so we used the TOMS-estimated doses over several years. In addition to the valid exposure assessment, we were able to adjust for a large number of potential confounders, including tobacco use and physical activity. Moreover, dietary habits throughout the United States can vary, so we adjusted for food intake in broad dietary categories.
This study also has several limitations. We had only the residence at enrollment and therefore had insufficient information to consider population mobility or early life exposures. We could not take into account seasonal migrations (i.e., time spent in winters or summers in locations other than the baseline residence); migrating persons might adopt different behaviors while at their alternative residence(s). Moreover, the spatial resolution of the UVR exposure was limited in the TOMS dataset to grid cells of 1° latitude by 1.25° longitude, which represents about 111 km north to south and 75–101 km east to west, depending on latitude. Participants were assigned the same UVR exposure dose if they lived within the same grid cell; therefore, our UVR exposure is a residential exposure rather than an individual-level exposure. Residual confounding by neighborhood or geographic factors that are related to residence, such as socioeconomic status, healthcare access, healthcare practices, other health behaviors, culture, and climate, could be possible; however, we stratified by income and educational level and also included a random effect for census tract in our models to account for regional differences and did not find evidence for such residual confounding. Generalizability to other populations is limited because this analysis was conducted in white non-Hispanic persons.
As in other epidemiologic studies, incomplete control for confounders might have influenced our results. We had no information on the range of skin pigmentation or skin types among our participants or on lifetime sun-related behaviors, such as time spent outdoors, sunscreen use, and other sun-seeking or sun-protective activities. We could not adjust for vitamin D supplementation or estimate dietary vitamin D intake, although dietary intake is not as well correlated with serum vitamin D (49). Moreover, we had no information on occupation, which could be an important determinant of sun behavior.
Beyond the known role of UVR exposure in skin cancer, the impact of UVR exposure on other health outcomes could be limited. If UVR exposure does indeed play a role in mortality risk, the effect is quite small. Unlike many previous studies, our study suggests that UVR exposure might not be beneficial for longevity. We found that ambient residential UVR exposure was positively associated with total mortality risk and some cause-specific mortality outcomes in a prospective cohort in the United States. These results add to the conflicting evidence for the role of UVR exposure in risk of death. Future longitudinal studies that reliably assess lifetime UVR exposure or measure personal UVR exposure could help to better assess the association with death and other health outcomes.
ACKNOWLEDGMENTS
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Shih-Wen Lin, Yikyung Park, D. Michal Freedman, and Christian C. Abnet); Department of Biostatistics, School of Medicine, Virginia Commonwealth University, Richmond, Virginia (David C. Wheeler); Information Management Services, Inc., Silver Spring, Maryland (Michael Spriggs); and AARP, Washington, District of Columbia (Albert R. Hollenbeck).
This research was supported (in part) by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the Cancer Surveillance Section, California Department of Health Services, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Michigan Department of Community Health, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System, Miami, Florida, under contract with the Florida Department of Health. The views expressed herein are solely those of the authors and do not necessarily reflect those of the Florida Cancer Data System or Florida Department of Health. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, School of Public Health, Louisiana State University, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services, Trenton, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas.
We thank Sigurd Hermansen and Kerry Grace Morrissey from Westat, Rockville, Maryland, for study outcomes ascertainment and management and Leslie Carroll at Information Management Services, Silver Spring, Maryland, for data support.
Conflict of interest: none declared.
Appendix Table 1.
Multivariate-adjusteda Association Between Ambient Residential UVR Exposure and Cancer Site–specific Mortality Risk in the National Institutes of Health-AARP Study of Diet and Health Cohort, 1995–2008
| Cancer Siteb | July UVR Exposure,c BD/m2 |
|||
|---|---|---|---|---|
| No. | % | HR | 95% CI | |
| Lung | ||||
| Continuousd | 5,327 | 1.05 | 1.02, 1.08 | 0.002 |
| Quartile 1 | 1,224 | 1.00 | Referent | |
| Quartile 2 | 1,214 | 1.03 | 0.95, 1.12 | |
| Quartile 3 | 1,273 | 1.11 | 1.03, 1.21 | |
| Quartile 4 | 1,616 | 1.12 | 1.04, 1.21 | |
| P for trend | <0.001 | |||
| Pancreas | ||||
| Continuousd | 1,416 | 0.97 | 0.92, 1.03 | 0.305 |
| Quartile 1 | 399 | 1.00 | Referent | |
| Quartile 2 | 295 | 0.80 | 0.69, 0.94 | |
| Quartile 3 | 298 | 0.80 | 0.69, 0.93 | |
| Quartile 4 | 424 | 0.95 | 0.82, 1.09 | |
| P for trend | 0.526 | |||
| Colon | ||||
| Continuousd | 1,235 | 1.02 | 0.96, 1.08 | 0.629 |
| Quartile 1 | 317 | 1.00 | Referent | |
| Quartile 2 | 271 | 0.97 | 0.82, 1.14 | |
| Quartile 3 | 316 | 1.12 | 0.96, 1.31 | |
| Quartile 4 | 331 | 1.00 | 0.85, 1.17 | |
| P for trend | 0.590 | |||
| Non-Hodgkin Lymphoma | ||||
| Continuousd | 771 | 0.99 | 0.92, 1.07 | 0.825 |
| Quartile 1 | 195 | 1.00 | Referent | |
| Quartile 2 | 192 | 1.09 | 0.89, 1.34 | |
| Quartile 3 | 198 | 1.13 | 0.93, 1.38 | |
| Quartile 4 | 186 | 0.91 | 0.74, 1.12 | |
| P for trend | 0.454 | |||
| Leukemia | ||||
| Continuousd | 755 | 1.02 | 0.95, 1.10 | 0.566 |
| Quartile 1 | 205 | 1.00 | Referent | |
| Quartile 2 | 168 | 0.94 | 0.76, 1.15 | |
| Quartile 3 | 157 | 0.90 | 0.73, 1.11 | |
| Quartile 4 | 225 | 1.08 | 0.89, 1.31 | |
| P for trend | 0.518 | |||
| Prostatee | ||||
| Continuousd | 621 | 1.14 | 1.05, 1.24 | 0.002 |
| Quartile 1 | 141 | 1.00 | Referent | |
| Quartile 2 | 131 | 1.02 | 0.80, 1.30 | |
| Quartile 3 | 175 | 1.41 | 1.13, 1.77 | |
| Quartile 4 | 179 | 1.19 | 0.95, 1.49 | |
| P for trend | 0.026 | |||
| Brain | ||||
| Continuousd | 590 | 1.06 | 0.97, 1.15 | 0.208 |
| Quartile 1 | 155 | 1.00 | Referent | |
| Quartile 2 | 117 | 0.88 | 0.69, 1.13 | |
| Quartile 3 | 146 | 1.05 | 0.83, 1.31 | |
| Quartile 4 | 172 | 1.11 | 0.89, 1.39 | |
| P for trend | 0.196 | |||
| Breaste | ||||
| Continuousd | 550 | 1.09 | 0.99, 1.19 | 0.070 |
| Quartile 1 | 126 | 1.00 | Referent | |
| Quartile 2 | 117 | 1.01 | 0.78, 1.31 | |
| Quartile 3 | 139 | 1.17 | 0.91, 1.49 | |
| Quartile 4 | 178 | 1.26 | 0.99, 1.60 | |
| P for trend | 0.028 | |||
| Kidney | ||||
| Continuousd | 420 | 0.97 | 0.88, 1.08 | 0.577 |
| Quartile 1 | 108 | 1.00 | Referent | |
| Quartile 2 | 100 | 0.99 | 0.75, 1.30 | |
| Quartile 3 | 119 | 1.19 | 0.92, 1.55 | |
| Quartile 4 | 93 | 0.78 | 0.59, 1.04 | |
| P for trend | 0.241 | |||
| Melanoma | ||||
| Continuousd | 417 | 1.13 | 1.02, 1.25 | 0.016 |
| Quartile 1 | 95 | 1.00 | Referent | |
| Quartile 2 | 92 | 1.18 | 0.88, 1.58 | |
| Quartile 3 | 94 | 1.14 | 0.85, 1.52 | |
| Quartile 4 | 136 | 1.48 | 1.13, 1.94 | |
| P for trend | 0.007 | |||
| Bladder | ||||
| Continuousd | 382 | 1.05 | 0.95, 1.17 | 0.362 |
| Quartile 1 | 83 | 1.00 | Referent | |
| Quartile 2 | 100 | 1.31 | 0.97, 1.76 | |
| Quartile 3 | 95 | 1.27 | 0.94, 1.70 | |
| Quartile 4 | 104 | 1.14 | 0.85, 1.53 | |
| P for trend | 0.543 | |||
| Liver | ||||
| Continuousd | 292 | 1.18 | 1.05, 1.34 | 0.007 |
| Quartile 1 | 58 | 1.00 | Referent | |
| Quartile 2 | 70 | 1.40 | 0.98, 2.00 | |
| Quartile 3 | 68 | 1.42 | 1.00, 2.03 | |
| Quartile 4 | 96 | 1.73 | 1.23, 2.41 | |
| P for trend | 0.002 | |||
Abbreviations: BD/m2, biological damage per square meter; CI, confidence interval; HR, hazard ratio; UVR, ultraviolet radiation.
a Hazard ratios and 95% confidence intervals were calculated with Cox regression models adjusted for the following covariates: age at study entry; sex; body mass index; caloric intake; intake of fruit, vegetables, and red and white meat; alcohol consumption; tobacco smoking; educational level; physical activity; median household income; and hypertension.
b Surveillance Epidemiology and End Results (SEER) Cause of Death Recode for these cancer sites: lung 22030; pancreas 21100; colon 21040; NHL 33040; leukemia 35011-13, 35021, 35031, 35022-23, 35041, 35043; prostate 28010; kidney 29020; melanoma 25010; brain 31010; breast 26000; bladder 29010; and liver 21071.
c The July UVR exposure was calculated as the averaged dose across all available measured days in the month of July in 1978–1993 and 1996–2005.
d The continuous July UVR exposure was scaled by 33.7 BD/m2 (half of the interquartile range for the cohort).
e The association for prostate cancer was estimated for men only (201,808 men); the association for breast cancer was estimated for women only (144,807).
REFERENCES
- 1.Murray CJL, Michaud CM, McKenna M, et al. US Patterns of Mortality by County and Race: 1965–1994. Cambridge, MA: Harvard Center for Population and Development Studies; 1998. [Google Scholar]
- 2.Kindig DA, Seplaki CL, Libby DL. Death rate variation in US subpopulations. Bull World Health Organ. 2002;80(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2008 Incidence and Mortality Web-based Report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2012. www.cdc.gov/uscs. ). (Accessed October 15, 2012) [Google Scholar]
- 4.Kitagawa EM, Hauser PM. Differential Mortality in the United States: A Study in Socioeconomic Epidemiology. Vital and Health Statistics Monographs. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- 5.Morrill R. Development, diversity, and regional demographic variability in the United States. Ann Assoc Am Geogr. 1993;83(3):406–433. [Google Scholar]
- 6.Evans RG, Barer ML, Marmor TR,, editors. Why Are Some People Healthy and Others Not? The Determinants of Health of Populations. New York, NY: Aldine de Gruyter; 1994. [Google Scholar]
- 7.Beretich BD, Beretich TM. Explaining multiple sclerosis prevalence by ultraviolet exposure: a geospatial analysis. Mult Scler. 2009;15(8):891–898. doi: 10.1177/1352458509105579. [DOI] [PubMed] [Google Scholar]
- 8.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011;11(9):584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 9.Zittermann A, Gummert JF. Sun, vitamin D, and cardiovascular disease. J Photochem Photobiol B. 2010;101(2):124–129. doi: 10.1016/j.jphotobiol.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Elliott JC, Lucas RM, Clements MS, et al. Population density determines the direction of the association between ambient ultraviolet radiation and type 1 diabetes incidence. Pediatr Diabetes. 2010;11(6):394–402. doi: 10.1111/j.1399-5448.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 11.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant WB. Ecological studies of the UVB–vitamin D–cancer hypothesis. Anticancer Res. 2012;32(1):223–236. [PubMed] [Google Scholar]
- 13.Greinert R, Boniol M. Skin cancer—primary and secondary prevention (information campaigns and screening)—with a focus on children & sunbeds. Prog Biophys Mol Biol. 2011;107(3):473–476. doi: 10.1016/j.pbiomolbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Wehner MR, Shive ML, Chren M-M, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909. doi: 10.1136/bmj.e5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Lof M, Veierod MB, et al. Ultraviolet exposure and mortality among women in Sweden. Cancer Epidemiol Biomarkers Prev. 2011;20(4):683–690. doi: 10.1158/1055-9965.EPI-10-0982. [DOI] [PubMed] [Google Scholar]
- 16.Freedman DM, Dosemeci M, McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med. 2002;59(4):257–262. doi: 10.1136/oem.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berwick M, Armstrong BK, Ben-Porat L, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97(3):195–199. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- 18.Garland FC, Garland CF, Gorham ED, et al. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19(6):614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 19.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 20.Ziemke JR, Chandra S, Herman J, et al. Erythemally weighted UV trends over northern latitudes derived from Nimbus 7 TOMS measurements. J Geophys Res: Atmospheres. 2000;105(D6):7373–7382. [Google Scholar]
- 21.Kalliskota S, Kaurola J, Taalas P, et al. Comparison of daily UV doses estimated from Nimbus 7/TOMS measurements and ground-based spectroradiometric data. J Geophys Res: Atmospheres. 2000;105(D4):5059–5067. [Google Scholar]
- 22.Herman JR, Krotkov N, Celarier E, et al. Distribution of UV radiation at the Earth's surface from TOMS-measured UV-backscattered radiances. J Geophys Res: Atmospheres. 1999;104(D10):12059–12076. [Google Scholar]
- 23.Lin SW, Wheeler DC, Park Y, et al. Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. Int J Cancer. 2012;131(6):E1015–E1023. doi: 10.1002/ijc.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim H-S, Roychoudhuri R, Peto J, et al. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int J Cancer. 2006;119(7):1530–1536. doi: 10.1002/ijc.22052. [DOI] [PubMed] [Google Scholar]
- 25.Apperly FL. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941;1(3):191–195. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 26.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94(6):1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 27.Porojnicu AC, Dahlback A, Moan J. Sun exposure and cancer survival in Norway: changes in the risk of death with season of diagnosis and latitude. In: Reichrath J, editor. Sunlight, Vitamin D and Skin Cancer. New York, NY: Springer; 2008. pp. 43–54. [DOI] [PubMed] [Google Scholar]
- 28.Ainsleigh HG. Beneficial effects of sun exposure on cancer mortality. Prev Med. 1993;22(1):132–140. doi: 10.1006/pmed.1993.1010. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Clements M, Rahman B, et al. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control. 2010;21(10):1701–1709. doi: 10.1007/s10552-010-9599-1. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz G, Hanchette C. UV, latitude, and spatial trends in prostate cancer mortality: all sunlight is not the same (United States) Cancer Causes Control. 2006;17(8):1091–1101. doi: 10.1007/s10552-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 31.Fears TR, Tucker MA. Re: sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97(23):1789–1790. doi: 10.1093/jnci/dji410. [DOI] [PubMed] [Google Scholar]
- 32.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9(3):227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 33.Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29(6):388–396. doi: 10.1016/j.mam.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douglas AS, Allan TM, Rawles JM. Composition of seasonality of disease. Scott Med J. 1991;36(3):76–82. doi: 10.1177/003693309103600304. [DOI] [PubMed] [Google Scholar]
- 35.Pell JP, Cobbe SM. Seasonal variations in coronary heart disease. QJM. 1999;92(12):689–696. doi: 10.1093/qjmed/92.12.689. [DOI] [PubMed] [Google Scholar]
- 36.Tsementzis SA, Kennet RP, Hitchcock ER, et al. Seasonal variation of cerebrovascular diseases. Acta Neurochirurgica. 1991;111(3):80–83. doi: 10.1007/BF01400492. [DOI] [PubMed] [Google Scholar]
- 37.Juzeniene A, Ma L-W, Kwitniewski M, et al. The seasonality of pandemic and non-pandemic influenzas: the roles of solar radiation and vitamin D. Int J Infect Dis. 2010;14(12):e1099–e1105. doi: 10.1016/j.ijid.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 38.White AN, Ng V, Spain CV, et al. Let the sun shine in: effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect Dis. 2009;9:196. doi: 10.1186/1471-2334-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorde R, Schirmer H, Wilsgaard T, et al. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromsø Study. PLoS ONE. 2012;7(5):e37295. doi: 10.1371/journal.pone.0037295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullrich SE. Does exposure to UV radiation induce a shift to a Th-2–like immune reaction? Photochem Photobiol. 1996;64(2):254–258. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 41.Duthie MS, Kimber I, Norval M. The effects of ultraviolet radiation on the human immune system. Br J Dermatol. 1999;140(6):995–1009. doi: 10.1046/j.1365-2133.1999.02898.x. [DOI] [PubMed] [Google Scholar]
- 42.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 43.Norval M. The challenges of UV-induced immunomodulation for children's health. Prog Biophys Mol Biol. 2011;107(3):323–332. doi: 10.1016/j.pbiomolbio.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz T, Schwarz A. Molecular mechanisms of ultraviolet radiation–induced immunosuppression. Eur J Cell Biol. 2011;90(6-7):560–564. doi: 10.1016/j.ejcb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Termorshuizen F, Garssen J, Norval M, et al. A review of studies on the effects of ultraviolet irradiation on the resistance to infections: evidence from rodent infection models and verification by experimental and observational human studies. Int Immunopharmacol. 2002;2(2-3):263–275. doi: 10.1016/s1567-5769(01)00178-3. [DOI] [PubMed] [Google Scholar]
- 46.Mohr S, Garland C, Gorham E, et al. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51(8):1391–1398. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 47.Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24(8):1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 48.Ishida H, Seino Y, Matsukura S, et al. Diabetic osteopenia and circulating levels of vitamin D metabolites in type 2 (noninsulin-dependent) diabetes. Metabolism. 1985;34(9):797–801. doi: 10.1016/0026-0495(85)90101-5. [DOI] [PubMed] [Google Scholar]
- 49.McCullough ML, Weinstein SJ, Freedman DM, et al. Correlates of circulating 25-hydroxyvitamin D. Am J Epidemiol. 2010;172(1):21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]