Abstract
Background and Aims
Selective feeding by herbivores, especially at the seedling or juvenile phase, has the potential to change plant traits and ultimately the susceptibility of surviving plants to other enemies. Moreover, since hybridization is important to speciation and can lead to introgression of traits between plant species, differential feeding (herbivore-induced mortality) can influence the expression of resistance traits of hybrids and ultimately determine the consequences of hybridization. While it would be expected that herbivore-induced mortality would lead to greater resistance, there may be trade-offs whereby resistance to one herbivore increases susceptibility to others. The hypothesis was tested that the exotic slug, Arion subfuscus, causes non-random survival of hybrid willows and alters plant: (1) susceptibility to slugs; (2) secondary and nutritional chemistry, and growth; and (3) susceptibility to other phytophages.
Methods
Two populations of plants, control and selected, were created by placing trays of juvenile willows in the field and allowing slugs access to only some. When ≤10 individuals/tray remained (approx. 85 % mortality), ‘selected’ and undamaged ‘control’ trays were returned to a common area. Traits of these populations were then examined in year 1 and in subsequent years.
Key Results
The selected population was less palatable to slugs. Surprisingly, foliar concentrations of putative defence traits (phenolic glycosides and tannins) did not differ between treatments, but the selected population had higher foliar nitrogen and protein, lower carbon to nitrogen ratio and greater above-ground biomass, indicating that vigorously growing plants were inherently more resistant to slugs. Interestingly, selected plants were more susceptible to three phytophages: an indigenous pathogen (Melampsora epitea), a native herbivorous beetle (Chrysomela knabi) and an exotic willow leaf beetle (Plagiodera versicolora).
Conclusions
This exotic slug changed the population structure of F2 hybrid willows in unanticipated ways. Defence expression remained unchanged, while nutritional and growth traits changed. These changes caused plants to be more susceptible to other plant enemies. Other exotic herbivore species are anticipated to have similar direct and indirect effects on native plant populations.
Keywords: Exotic species, herbivory, hybridization, slug, Arion subfuscus, trade-offs, willow, Salix
INTRODUCTION
It is well established that herbivores are important agents of mortality, that plant secondary metabolites often determine patterns of susceptibility and that differential survival leads to differential chemical expression in plants (Rosenthal and Janzen, 1979; Orians and Ward, 2010). One important source of chemical variation in plants is generated via hybridization (Orians, 2000). Overall, natural hybridization is increasingly being recognized as a creative process that produces greater genetic variation and potentially novel genotypes and may ultimately lead to the origin of new species, subspecies or local races (Arnold, 1992; Rieseberg, 1995; Rieseberg et al., 1996; Arnold, 1997; Nagy, 1997; Orians, 2000). Whether hybridization provides novel genetic combinations for new adaptations or produces only evolutionary dead-ends depends upon the fitness of hybrids (Emms and Arnold, 1997; Kimball et al., 2008). Exogenous selective agents, such as pathogens and herbivores, are important determinants of fitness and thus have the potential to shape patterns of resistance and subsequent evolutionary dynamics (Anderson and Hubricht, 1938; Anderson, 1949; Heiser, 1973; Rieseberg and Wendel, 1993). Coustau et al. (1991) showed that high parasitism by the trematode, Prosorhynchus squamatus, causes intense selection, via castration, favouring the spread of Mytilus galloproveinialis genes into M. edulis populations. Stutz and Thomas (1964) suggested that selection by grazers favoured introgression of defensive traits from unpalatable Cowania stansburiana into populations of Purshia tridentata (bitterbrush), a heavily grazed plant in the western USA. In addition, introgression of jack pine resistance traits appears to explain the geographical variation in susceptibility of lodgepole pine to several insects and diseases (Wu et al., 1996), and introgression of red spruce into black spruce resulted in reduced susceptibility to spruce budworm (Manley and Fowler, 1969).
While native herbivores can select for changes in resistance traits in hybrids, the ever-increasing abundance of invasive herbivores is expected to exert strong effects on native plant populations. In this study a selection experiment was undertaken to determine if herbivory by an exotic slug, Arion subfuscus (Mollusca: Arionidae), would lead to (1) greater survival of F2 hybrid willows with high concentrations of defensive chemicals; and (2) altered susceptibility to other enemies. Selection experiments are powerful tools to aid in determining the course of selection and its consequences on other traits. One approach to performing selection experiments is to apply artificial selection favouring an increase or a decrease in one or a suite of traits in a population, and then examine the fitness of the resulting population in relation to a non-selected population for the expression of the evolved trait and correlated traits (e.g. Stowe, 1998). The strength of this approach is that selected traits can be specifically identified. An alternative approach is to generate genetically variable progeny, subject these progeny to phenotypic selection for a period of time and then compare selected phenotypes with a non-selected population. For example, using F2 crosses of subspecies of Gilia capitata, Nagy (1997) found that selection favoured multiple traits of the native subspecies in their native habitats. The strength of this approach is that it demonstrates the strength and direction of natural selection. This second approach was used in our study.
Natural selection may be particularly important at the seedling or juvenile stages because they are more susceptible to herbivores than are older plants (Fenner et al., 1999). If natural selection by a herbivore alters genetic variation in a seedling population, then traits expressed in the adult population may also be altered (Stratton, 1992). Terrestrial molluscs are major herbivores in many plant communities, and herbivory by slugs often affects plant productivity, plant distribution and community composition (Hanley et al., 1995a; Rodriguez and Brown, 1998; Bruelheide and Scheidel, 1999; Hanley and Sykes, 2009). Importantly, slugs typically completely consume small plants and thus alter recruitment into plant communities (Hanley et al., 1995b; Rodriguez and Brown, 1998). Slugs often exhibit strong feeding preferences among plant genotypes and among species (Fenner et al., 1999); olfactory cues (Hanley et al., 2011), plant chemistry (e.g. Glen et al., 1990) and growth rate (Albrectsen et al., 2004) play an important role in plant acceptability. Thus slugs and snails are suspected to have influenced the evolution of defences in several plant species (Horrill and Richards, 1986; Westerbergh and Nyberg, 1995). Provided that heritable genetic variation exists, differential survival may cause trait evolution within a single generation (reviewed in Geber and Griffen, 2003).
Arion subfuscus (Mollusca: Arionidae) is an exotic slug species in North America that causes seedling mortality in two native willow species (Salix eriocephala and S. sericea) and their hybrids (Fritz et al., 2001). Arion subfuscus preferentially feeds on young plants of S. eriocephala (a species with high concentrations of condensed tannin in adult plants and older juveniles) over those of S. sericea (a species with high levels of phenolic glycosides in adult plants and older juveniles), and has an intermediate preference for F1 hybrid plants (Fritz et al., 2001). Phenolic glycosides, defensive compounds found in leaves of S. sericea (but not in the leaves of S. eriocephala), appear to be responsible for resistance of young S. sericea seedlings to slugs. Condensed tannins, which occur in high concentrations in the leaves of S. eriocephala, also appear to deter slug damage in older juveniles (Orians and Fritz, 1995; Fritz et al., 2001; Albrectsen et al., 2004). As seedlings grow, their levels of defensive chemicals in leaves increase, and this increase is correlated with a reduction in damage caused by slugs (Fritz et al., 2001; Orians et al., 2010). Based on these findings, herbivory by A. subfuscus was expected to cause a selection differential favouring plants with higher concentrations of defensive chemicals. More specifically, F2 hybrid plants that survived slug herbivory were predicted: (1) to have higher phenolic glycoside and condensed tannin concentrations compared with control plants; (2) to express reduced growth and nutrient chemistry because of expected trade-offs between growth and defensive chemistry; (3) to have decreased susceptibility to A. subfuscus; and (4) to have decreased susceptibility to other phytophages because of increased levels of chemical defences. Hardig et al. (2000) found that plants phenotypically similar to pure S. eriocephala had genetic markers of S. sericea and expressed phenolic glycosides in their leaves, concurring with the prediction that the above pattern of introgression of secondary chemicals was likely.
MATERIALS AND METHODS
Study species
This study was performed at our field site 3 km west of Milford, New York, USA (42°31′N, 75°41′W) where the willow species Salix sericea, S. eriocephala and hybrids (F1, F2 and backcrosses) occur naturally. Salix sericea Marshall is a 0·5–4 m high shrub, while S. eriocephala Michx. reaches 6 m in height. These two willow species are broadly sympatric, co-occurring in swamps and along streams throughout their range. These species commonly hybridize at sites where they co-occur (Argus, 1986) and have a history of hybridization (Hardig et al., 2000).
Arion subfuscus is one of 15 species of slugs that have been introduced to North America from Europe since 1840 (Chichester and Getz, 1969). Arion subfuscus, which is most common in wooded areas, was first reported in the USA in Massachusetts in 1842. Since that initial record, this species has been found in Pensylvania since 1940, in New York since 1941, in New Hampshire since 1962 and in Maine, Vermont and Connecticut since 1969. Now, A. subfuscus has become widespread and very abundant in eastern North America, causing significant damage in native habitats, as well as disturbed areas and agricultural fields (Chichester and Getz, 1969; Robinson, 1999). This species is virtually the only slug present at the field site, with rare occurrences of Deroceras laeve.
Selection experiment
An experimental population of F2 hybrids was used for the phenotypic selection experiment. This hybrid class represents the most common type of adult hybrid willows at our field site (Hardig et al., 2000), and our experimental F2 population has, as expected, increased genetic variation (Hochwender et al., 2000; Lexer et al., 2003; Orians et al., 2010). Fifteen different full-sib F2 families were created in June 2000 using unrelated F1 male and female plants. F1 hybrids had been created by crossing male S. eriocephala with female S. sericea – the reciprocal cross does not produce viable seeds (Mosseler and Papadopal, 1989). F1 hybrid plants had been growing in a breeding garden for at least 2 years before F2 crosses were created. Seeds of all F2 crosses were mixed, germinated in Scott's Metromix 360® potting soil and transplanted to individual cells in 72-cell trays after 10 d.
Seedlings were maintained with water and nutrients (6 g L−1 Peter's Professional NPK 20:20:20) in open-ended greenhouses until they reached the 4–5-leaf stage (i.e. until 5 weeks of age). At this early developmental stage, willow seedlings are highly susceptible to slug herbivory, and variation in secondary chemistry is likely to result in non-random susceptibility to slugs (Fritz et al., 2001). Sixty trays (4320 F2 seedlings in total) were placed in the field among naturally occurring willows in areas where slugs were abundant. Five additional control trays of F2 seedlings were placed in the field and kept free of slugs by placing trays on inverted plastic pots in trays filled with water, thereby creating a moat. Five additional control trays were kept in the greenhouse to ensure that we had control plants available in case the control trays in the field experienced herbivory or unexpected mortality.
Slug herbivory occurs primarily at night when humidity is high (R.S.F. and C.G.H. pers. obs.). Herbivory on seedlings in each tray was monitored daily. Each tray was removed from the field when ≤10 seedlings remained (i.e. when mortality reached approx. 85 %). This level was chosen because it provided a very intensive level of potential phenotypic selection but still left enough surviving seedlings for subsequent studies. All trays were removed from the field within 7 d. Of the 408 seedlings that survived slug herbivory, many lost some leaf tissue, suggesting that slugs sampled and rejected them. Surviving seedlings from slug-selected trays were combined into full trays of 72 seedlings. Both control and slug-selected (those exposed to herbivory by slugs where the surviving plants were avoided) seedlings were allowed to continue growing in the greenhouse for 5 weeks. All seedlings were transplanted to 3·7 L pots in a mixture of topsoil, vermiculite and peat moss (4:1:1) and allowed to overwinter within a fenced-in plot at the field site. In the spring of 2001, overwintered plants were transplanted into 7·8 L pots with the same soil mixture and fertilized with 13 g of Osmocote® N:P:K 10:10:10 per pot. Some plants were used to evaluate susceptibility to differing phytophages (see below), while other plants continued to grow in our fenced area with irrigation and periodic fertilization.
Chemical analyses
Leaf samples for chemical analyses were collected from seedlings before the experiment, 5 weeks following the experiment (August 2000) and in the following year (June 2001). Induction in willows tends to be weak (Fields and Orians, 2006), and thus herbivores cannot cause a plant that produces low phenolic glycoside to produce high concentrations. Moreover, sampling across 2 years allows us to determine if the relative differences between the two populations were able to be evaluated for consistency over time. Samples were returned to the laboratory, vacuum dried, and ground into powder using a Wiley Mill with size 30 mesh. The concentrations of tannins, phenolic glycosides and protein were measured for all samples. Additionally, carbon and nitrogen were measured using samples from June 2001.
Condensed tannins were analysed using standard techniques (Orians, 1995; Hunter and Forkner, 1999; Albrectsen et al., 2004). Briefly, tannins were extracted from ground leaf material (approx. 10 mg), and then analysed using the n-butanol assay for proanthocyanidins (Hagerman and Butler, 1989) using purified tannin standards (0·2–2·0 mg mL−1). Tannin concentration was calculated as mg g−1 dry leaf weight. Phenolic glycosides were analysed using standard methods (Lindroth and Koss, 1996; Albrectsen et al., 2007). Briefly, the phenolic glycosides salicortin and 2'-cinnamoylsalicortin were extracted from ground leaf material (approx. 15 mg) and analyzed by high-performance thin-layer chromatography (HPTLC).
A standard curve for salicortin (0·2–4·0 mg mL−1) and 2′-cinnamoylsalicortin (0·05–1·0 mg mL−1) was also spotted onto each plate. Chromatograms were analysed with Camag TLC software (CATS 3·11). Salicortin and 2′-cinnamoylsalicortin concentrations were calculated as mg g−1 dry leaf weight and the two were summed to give a single value for phenolic glycosides. These compounds are the only phenolic glycosides found in S. sericea or their interspecific hybrids with S. eriocephala (Orians, 2000).
Standard techniques were also used for analysis of protein concentration (Albrectsen et al., 2007). Briefly, ground dry leaf material (0–0·2 mg) was extracted in 1·5 mL of 0·1 m NaOH for 2 h at 100 °C. The protein extracts were combined with the BioRad reagent (Coomassie Brilliant Blue) in 96-well microtitre plates and their absorbances measured at 595 nm. Bovine serum albumin (BSA) was used as a standard to calculate %BSA equivalents per mg dry leaf mass.
Carbon and nitrogen concentrations were determined using a CE Elantech NC 2500 Element Analyzer. For each plant, 12–15 mg of leaf powder was weighed into a 8 × 5 mm tin weighing capsule (EMAL Tech, Inc.). Samples were then combusted at 1000 °C with helium as the carrier gas. Total C and N were measured as released CO2 and N2.
Quantification of biomass
In early March 2002, ramets were created from cuttings of 112 slug-selected plants and 108 control plants. Ramets were planted in Cone-tainers® in the greenhouse (Fritz et al., 2003). Prior to planting, cuttings were weighed to provide a covariate in statistical analyses. After about 6 weeks of growth, plants were transplanted into 7·8 L pots with standard soil mixture. Potted plants were placed randomly in a common garden (1 × 1 m spacing), irrigated, and sprayed with both fungicide (PlantVax PlantVAX75W; Uniroyal Chemical Company, Inc., Middlebury, CT, USA) and carbaryl, a non-systemic insecticide, to prevent rust and insect damage. In August 2002, we harvested and dried (48 h at 60 °C) the above- and below-ground parts of these plants to determine shoot and root biomass. To determine whether selection treatment affected shoot biomass, root biomass or the shoot/root ratio, data were analysed using analysis of covariance (ANCOVA), with initial cutting size included as a covariate. All statistical tests here and below were performed using JMP® 3·2·2 (SAS Institute, 1997). All data were tested for equality of variances and normality of errors, and statistical tests were performed on untransformed data if these assumptions were met. Otherwise, non-parametric statistics were used.
Susceptibility to phytophages
A common garden experiment (2001) and controlled Petri dish assays (2002) were used to compare the susceptibility of selected and control populations to diverse phytophages (slugs, rust fungi and beetles).
Field experiment: rust fungi
The incidence of leaf rust, Melampsora epitea, which forms orange uredinia on the leaves of willows (Roche and Fritz, 1998), was quantified on 60 slug-selected and 60 control plants randomly arranged in a common garden. Five long shoots (>10 cm long) were randomly chosen on each plant and scored for infection level. These shoots were scored repeatedly on five dates (29 June, and 3, 6, 12 and 18 July). For each shoot, each of four leaves was scored for level of infection using a modified Schreiner scoring system (0, 1, 5, 10, >25 uredinea per leaf) (Roche and Fritz, 1998); scores were summed for each shoot and averaged across the five shoots. Mean scores for the five shoots per plant were also used to test for significant differences between slug-selected and control plants using repeated measures ANOVA.
Petri dish assays: slugs and beetles
Slugs (A. subfuscus) (>2·5 cm long) were collected at night when active and kept in groups of ten in 470 mL plastic containers lined with moist paper towels and fed with an artificial diet (Whelan, 1982; Albrectsen et al., 2004). One leaf disc (area = 2·2 cm2) from the first fully expanded leaf was obtained from haphazardly chosen pairs of slug-selected and control plants. Leaf discs from one slug-selected plant and from one control plant were placed in a Petri dish lined with moist filter paper (14·5 cm diameter). A slug was added to the centre of each Petri dish perpendicular to the axis between the leaf discs, with the direction alternated as dishes were prepared. Petri dishes were kept on tables in an open-ended greenhouse overnight. The following morning (about 8 h later), leaf discs were scored for area removed by slugs using a transparent grid cut to the shape of a leaf disc. We predicted that slugs would prefer control plants over slug-selected plants (i.e. plants that had previously undergone phenotypic selection by slugs 2 years prior to this experiment), so a one-tailed paired t-test was used to evaluate whether differences were significantly different from zero.
To test the susceptibility of slug-selected and control plants to beetles, two leaf discs were cut from the first fully expanded leaf from each slug-selected and control plant (area = 1·6 cm2). From these two leaf discs per plant, we made two replicates, such that one Petri dish (10 cm diameter) contained one leaf disc from a slug-selected plant and one leaf disc from a control plant; a second Petri dish contained the remaining leaf disc from each leaf. Adult Plagiodera versicolora beetles, an exotic species (n = 90), were collected from willow plants at the field site. One beetle was added to the centre of each Petri dish. Plates were placed on tables in a shaded, open-ended greenhouse. Beetles were allowed to feed for 24 h, and damage was scored using a transparent grid. To evaluate beetle preference, we analysed the differences in damage between the two discs in each Petri dish for the two replicate sets of leaf discs. Data were non-normally distributed, so a two-tailed, Wilcoxon matched-pairs, signed-rank test was used to analyse the data. Using the same methods described for P. versicolora, we collected 90 adult Chrysomela knabi beetles, a native species, from willow plants at the field site and evaluated their preference for slug-selected vs. control leaves. Data were normally distributed in this second analysis, so data were analysed using a two-tailed, paired t-test to determine whether beetle feeding preference occurred.
RESULTS
Secondary chemistry
Before phenotypic selection occurred, F2 hybrid plants were variable in chemical defences. They displayed a 2- to 3-fold difference in phenolic glycoside and condensed tannin concentrations. In a sample of plants, phenolic glycoside concentration ranged from 40 to 120 mg g−1 and condensed tannin concentration ranged from 58 to 132 mg g−1. The mean concentration of phenolic glycosides was 78·64 ± 6·13 mg g−1 (± s.e.; n = 17), while the mean concentration of condensed tannin was 86·92 ± 4·92 mg g−1 (n = 17).
Slugs did not select for higher concentrations of constitutive chemical defensives. Five weeks after phenotypic selection had taken place, slug-selected and control plants did not differ significantly in phenolic glycoside concentrations. Also, no differences occurred for either salicortin or 2'-cinnamoylsalicortin when tested alone (data not shown). Slug-selected plants had a mean concentration of 79·8 ± 4·7 mg g−1 (n = 75), while control plants had a mean concentration of 81·1 ± 4·8 mg g−1 (n = 75; t = 0·20, P = 0·84). For tannin concentrations, slug-selected plants had a mean concentration of 146·2 ± 12·0 mg g−1 (n = 28), while control plants had a mean concentration of 145·1 ± 11·5 mg g−1 (n = 28; t = 0·07, P = 0·94).
Similarly, 1 year following phenotypic selection by slugs, no significant differences between selected and control plants were observed for either tannins or phenolic glycosides (Table 1). Overall, variation in concentrations of phenolic glycoside and condensed tannin spanned the range between the means of the parental species in the case of both treatments (Fig. 1A, B). Phenolic glycoside concentration in control F2 plants ranged from 0 mg g−1 (as observed in S. eriocephala leaves) to 162·8 mg g−1 (nearly the mean concentration found in mature plants of S. sericea; Fig. 1A). Condensed tannin concentration in control F2 plants ranged from 20·1 mg g−1 (less than the mean concentration found in S. sericea leaves) to 345·1 mg g−1 (greater than the mean concentration observed in S. eriocephala leaves; Fig. 1B).
Table 1.
Comparison of chemical defence, growth and nutrient attributes of selected and control plant populations: mean ± s.e. (n)
| Trait | Control | Selected | Statistic | P-value |
|---|---|---|---|---|
| Phenolic glycosides | 57·2 ± 5·2 (62) | 59·6 ± 5·3 (62) | F = 0·11 | 0·74 |
| Condensed tannin | 181·5 ± 20·0 (52) | 177·4 ± 17·8 (51) | F = 0·03 | 0·87 |
| Protein | 138·4 ± 2·33 (62) | 150·8 ± 2·44 (62) | F = 13·56 | <0·0001 |
| % Nitrogen | 3·24 ± 0·06 (62) | 3·51 ± 0·04 (62) | t = 3·6 | 0·0004 |
| % Carbon | 47·39 ± 0·12 (62) | 48·12 ± 0·11 (62) | t = 4·5 | <0·0001 |
| C/N ratio | 14·94 ± 0·29 (62) | 13·85 ± 0·17 (62) | t = 3·3 | 0·0013 |
| Shoot biomass | 25·16 ± 0·90 (108) | 29·45 ± 0·88 (112) | F = 9·11 | 0·0028 |
| Root biomass | 8·22 ± 0·59 (108) | 7·04 ± 0·44 (112) | F = 2·32 | 0·129 |
| Shoot/root ratio | 5·02 ± 0·43 (108) | 6·40 ± 0·51 (112) | F = 10·70 | 0·0012 |
Comparisons of concentrations (mg g−1) of phenolic glycosides (sum of salicortin and 2'-cinnamoylsalicortin), condensed tannins and protein of selected and control plants 1 year after the two populations were created (June 2001). The percentage of nitrogen (N) and carbon (C) and the C/N ratio of the first fully expanded leaves of selected and control plants sampled in June 2001. Biomass (g) of shoots and roots and the shoot/root ratio of selected and control plants established from cuttings and grown in pots in 2002. Significant differences were tested with ANOVA, with cutting size (g) used as a covariate.
Fig. 1.
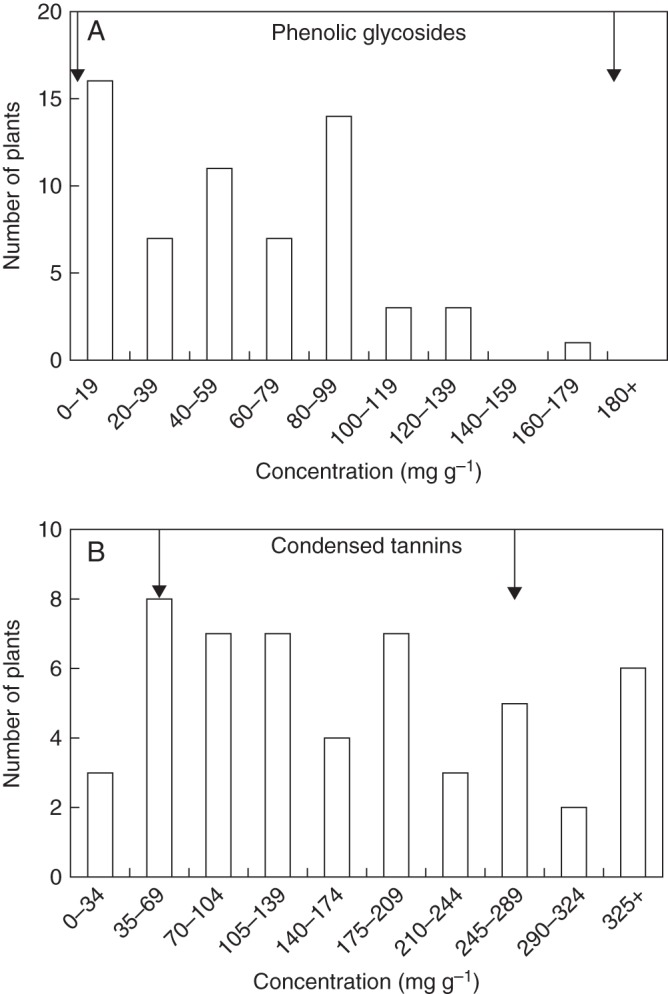
Frequency distributions of (A) phenolic glycosides (salicortin + 2′-cinnamoylsalicortin) and (B) condensed tannins measured on F2 control plants in June 2001 (n = 62). Arrows in (A) indicate mean constitutive levels of phenolic glycosides in S. eriocephala (0·0 mg g−1) and S. sericea (184 ± 7·3 mg g−1; ± s.e.). Arrows in (B) indicate mean constitutive levels of condensed tannins in S. eriocephala (250 ± 11·5 mg g−1) and S. sericea (64·9 ± 8·0 mg g−1).
Nutrient chemistry and plant size
Although slugs did not alter defensive chemistry, nutritive chemistry was affected by slug selection. Even 5 weeks after selection (August 2000), protein levels were significantly higher in slug-selected plants (175·4 ± 2·20) than in control plants (165·4 ± 2·79; F1,72 = 7·90, P = 0·006). In June 2001 (1 year after selection), the concentration of protein was still higher in the selected population (Table 1). Percentage nitrogen and percentage carbon were also higher, while the C/N ratio was lower (Table 1). In addition, for plants measured in 2002, shoot biomass and the shoot/root ratio were significantly greater for slug-selected plants than for control plants (Table 1). However, root biomass did not differ significantly between slug-selected and control plants (Table 1).
Susceptibility to phytophages
Slug-selected plants were more susceptible to naturally dispersed spores of M. epitea; slug-selected plants had significantly higher infection levels compared with control plants at each of the sampling times (Fig. 2). Although infection levels increased significantly over time for both control and selected plants (F4,105 = 80·1, P < 0·001), infection levels were significantly higher on selected plants compared with controls (F1,108 = 4·82, P = 0·03). No significant interaction between treatment and time was detected (F4,105 = 1·689, P = 0·158).
Fig. 2.
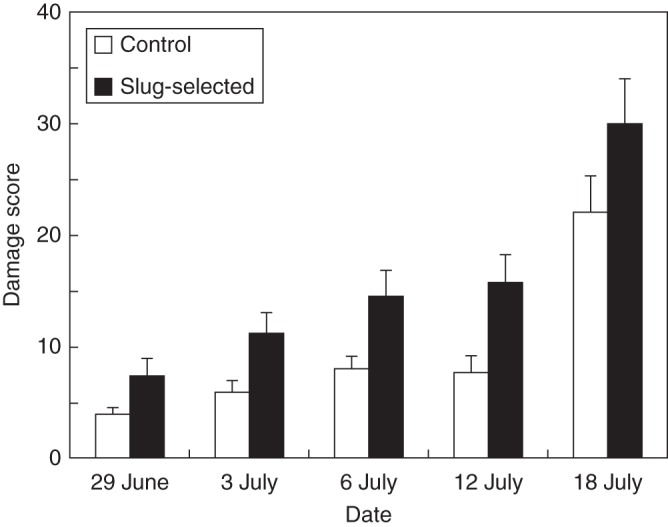
Infection scores of slug-selected and control plants to Melampsora epitea leaf pathogen. Measurements on the first four dates increased significantly over time (F3,118 = 28·06, P < 0·0001) and differed significantly between selected and control plants (F1,120 = 7·73, P = 0·0063). Data on the last date were collected on a different set of leaves and therefore were not included in the repeated analysis. Asterisks indicate significant differences for each date using t-tests (*P < 0·05; **P < 0·01; ***P < 0·001).
Plants that survived the selection experiment in 2000 were still less susceptible to slugs in the 2002 leaf disc choice assay (Fig. 3A). In contrast, susceptibility to the two beetle species was greater for slug-selected plants than for control plants; using leaf discs, both the native C. knabi (Fig. 3B) and the exotic P. versicolora (Fig. 3C) preferred slug-selected plants over control plants. However, for the exotic Japanese beetle. damage scores did not differ significantly between slug-selected plants (0·24 ± 0·03, n = 61) and control plants (0·21 ± 0·03, n = 57) grown in a common garden (t = 0·676, P = 0·25).
Fig. 3.
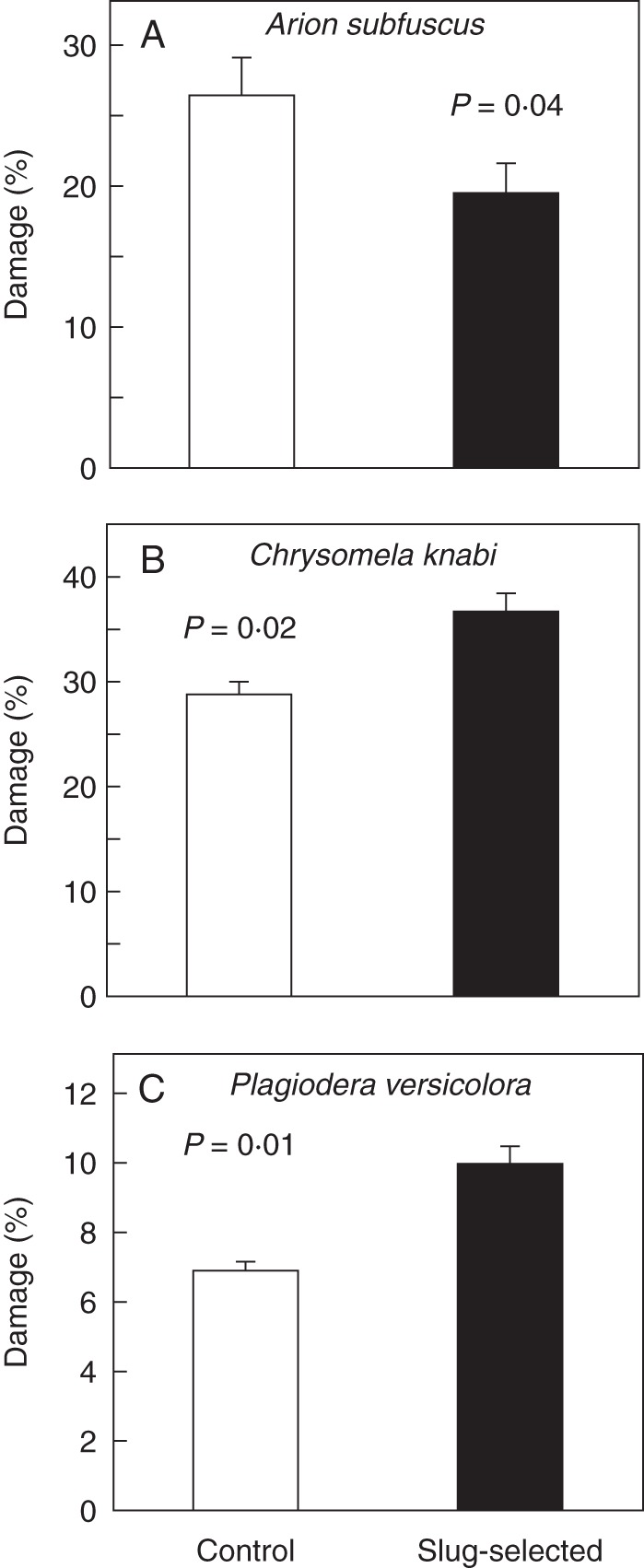
Suitability (mean percentage damage ± s.e.) of leaf discs of control and slug-selected plant to three herbivores. (A) Arion subfuscus slugs. Damage to control discs was significantly greater than to selected discs (one-tailed t-test, t = 1·82; d.f. = 71; P = 0·04). (B) Chrysomela knabi beetles. Damage to control was significantly lower than to selected discs (paired t-test, t = 2·46; d.f. = 44; P = 0·02). (C) Plagiodera versicolora beetles. Damage to control discs was lower than to selected discs (Wilcoxon matched-pairs signed-rank test, S-R value = 225; d.f. = 44; P = 0·01).
DISCUSSION
Contrary to our hypothesis, the concentrations of phenolic glycosides, a known deterrent of slugs and other herbivores (Orians et al., 1997; Fritz et al., 2001), were not higher in the selected population. This is despite that fact that the levels of defences in these plants were such that they could have deterred the slugs (Fritz et al., 2001). Rather, phytochemical responses involved shifts in plant nutritive and growth traits. Slug-selected plants had higher levels of protein, nitrogen and carbon, and a lower C/N ratio than control plants. Slug-selected plants also produced more shoot biomass and had a higher shoot/root ratio than control plants. Interestingly, selection by the exotic slug, A. subfuscus, caused changes in susceptibility in an F2 seedling population; the surviving plant population became less palatable to A. subfuscus. Selection also led to correlated responses; slug-selected plants were more susceptible to three phytophages, i.e. a native pathogen, a native beetle herbivore and an introduced beetle species.
These differences reflect real differences in growth rate; all cuttings were planted synchronously, and shoot and root biomass were corrected for cutting size, so any difference in size was based on differences in the intrinsic growth of control and slug-selected plants. Differences were not due to environmental differences since all plants had been grown from cuttings, had been kept in a common garden and had been sprayed to prevent herbivore damage. Thus, our findings demonstrate that clones of plants that survived phenotypic selection (i.e. plants that survived slug herbivory as seedlings) were physiologically and nutritionally superior compared with control plants.
Overall, we expected plants to be better defended and of lower nutritional value. The fact that surviving plants were nutritionally superior was quite unexpected. Perhaps developmental changes that occur in chemical defence of willow seedlings can help explain why nutritive chemistry changed in response to selection by slugs. Although slugs prefer to feed on willow seedlings with low phenolic glycoside concentrations (S. eriocephala) over those with high concentrations of phenolic glycosides (S. sericea), all seedlings are susceptible to slugs when they are small (Fritz et al., 2001). In this system, as well as for other species, older (and larger) seedlings are less susceptible to herbivores (Fritz et al., 2001; Albrectsen et al., 2004; Goodger et al., 2007; Elger et al., 2009). Moreover, in our system, fast-growing seedlings often produce higher concentrations of secondary chemicals (Albrectsen et al., 2004; Orians et al., 2010). Therefore, selection during this early window of vulnerability could have been on traits associated with plant growth rate and, indirectly, defence. Specifically, we hypothesize that faster growing hybrid willow seedlings reached a size threshold where they could produce higher concentrations of defences (tannins, phenolic glycosides and other unmeasured traits) or greater concentrations of deterrent volatiles [Hanley et al. (2013) report variation in mollusc olfactory selection of plants from different ontogenetic stages]. Importantly, this hypothesis does not require that the final constitutive levels of defence in mature plants differ between the more rapidly maturing seedlings and the more slowly maturing ones. If this hypothesis is correct, the result would be greater consumption of the smaller, slower growing seedlings that had lower levels of defences or less volatile emission. Although still untested, further study in this and other systems to determine whether subtle differences in size can be responsible for the differential seedling survival deserves evaluation.
A consequence of selection favouring higher nutritional quality of surviving plants was that they were more attractive to other herbivores. Although leaves of plants from the selected population were less susceptible to slugs (as might be expected since these were avoided in the selection), they were more susceptible to the rust fungus and the two beetle species. The physiological changes caused by slug selection may explain the increased damage caused by other phytophages. Beetle feeding (Wait et al., 1998; Orians and Fritz, 1996) and Melampsora infection (Desprez-Loustau and Wagner, 1997) are often greater on plants with greater vigour. Because both Melampsora and Chysomela beetles can cause reduced fitness in willows and poplars (Roche and Fritz, 1998, and references therein; Andersen and Nelson, 2002), changes caused by slug herbivory seems to be detrimental to these plants by increasing their susceptibility to other herbivores.
Taken together, these results are consistent with previous work demonstrating that selection on seedlings can dramatically alter the genetic structure of a population (reviewed by Stratton, 1992; Linhart and Grant, 1996). Herbivory has been shown to reduce a seedling's ability to compete, thereby indirectly causing seedling mortality and changing the genetic structure of the population (Prittinen et al., 2003). Although seedlings can experience strong selection (e.g. Stratton, 1992; Hanley et al., 1995a; Fritz et al., 2001), seedlings are generally not considered in estimates of selection in plant populations (Bennington and McGraw, 1995). Provided that physiological changes caused by slug selection are heritable in this willow system, a rapid change in the genetic structure could occur in response to this exotic herbivore species. Selection by this slug at an early stage in plant development may therefore play a significant role in changing the genetic structure of F2 hybrids, ultimately affecting patterns of introgression.
These results also add to the increasing evidence for the importance of exotic herbivores to native plant species. We found that this exotic slug is acting as an important selective force. Moreover, cascading effects are anticipated in response to invasion (Sakai et al., 2001). The extent of the response of these F2 willow seedlings to a single bout of phenotypic selection by an exotic herbivore underscores the potential evolutionary impact exotic species can have on native species. In addition, trade-offs are common in response to specific genetic changes, and can be related to limiting resources, ecological in nature or due to genetic constraints (Strauss et al., 2002; Orians and Ward, 2010). Selection can, as shown here, increase susceptibility to native phytophages, and can result in changes in physiological adaptation to abiotic environmental factors. Each of these could lead to lower plant fitness and reduced competitive ability in natural communities. Finally, selection by exotic herbivores could reduce the genetic diversity of native species.
In conclusion, the present study shows that the exotic slugs clearly changed the population structure of the F2 hybrid willows. However, the findings were quite unexpected in terms of defence expression. We find it striking that this herbivore changed the nutritional and growth traits of the plant population and caused it to become less well adapted to other herbivores. This result is even more interesting in light of the fact that the herbivore is an invasive exotic species. The potentially maladaptive consequences of a response to selection by an exotic species have not previously been observed. Given the recent increase in species invasions in ecosystems around the world, this detrimental outcome may commonly occur in many natural environments, and this deserves more careful analysis.
ACKNOWLEDGEMENTS
This work has been supported by NSF grants BSR 96-15038 and DEB 99-81406 to R.S.F., DEB 99-81568 to C.M.O. and DEB 01-27369 to C.G.H. We thank Len and Ellie Sosnowski and Marcia Membrino, who have permitted us to conduct research on their property. We received logistical support from Rob Hunt and the Biology Department at Hartwick College. B. Crabb, K. Vandenberg, R. D. Fritz, B. Compton, L. Gedmintas, K. Rule, S. Manee, D. Lewkiewicz, D. Willies, C. Lucas, J. Feldman, S. Bothwell, S. Irwin, B. Richmond and B. Krebel assisted us in the field. C.M.O. was assisted in the lab by B. Brannigan, A. Sutton, M. Zaccherio and A. Finzi.
LITERATURE CITED
- Albrectsen BR, Gardfjell H, Orians CM, Murray B, Fritz RS. Slugs, willow seedlings and nutrient fertilization: intrinsic vigor inversely affects palatability. Oikos. 2004;105:268–278. [Google Scholar]
- Albrectsen BR, Gutierrez L, Fritz RS, Fritz RD, Orians CM. Does differential seedling mortality by slugs alter foliar traits and subsequent susceptibility of hybrid willows to a generalist herbivore? Ecological Entomology. 2007;32:211–220. [Google Scholar]
- Andersen DC, Nelson SM. Effects of cottonwood leaf beetle Chrysomela scripta (Coleoptera: Chrysomelidae) on survival and growth of Fremont cottonwood (Populus fremontii) in nortwest Colorado. American Midland Naturalist. 2002;147:189–203. [Google Scholar]
- Anderson E. Introgressive hybridization. New York: John Wiley; 1949. [Google Scholar]
- Anderson E, Hubricht L. Hybridization in Tradescantia. III. The evidence for introgressive hybridization. American Journal of Botany. 1938;25:396–402. [Google Scholar]
- Argus GW. The genus Salix (Salicaceae) in the southeastern United States. Systematic Botany Monographs. 1986;9:1–170. [Google Scholar]
- Arnold ML. Natural hybridization as an evolutionary process. Annual Review of Ecology and Systematics. 1992;23:237–261. [Google Scholar]
- Arnold ML. Natural hybridization and evolution. New York: Oxford University Press; 1997. [Google Scholar]
- Bennington CC, McGraw JB. Phenotypic selection in an artificial population of Impatiens pallida: the importance of the invisible fraction. Evolution. 1995;49:317–324. doi: 10.1111/j.1558-5646.1995.tb02244.x. [DOI] [PubMed] [Google Scholar]
- Bruelheide H, Scheidel U. Slug herbivory as a limiting factor for the geographical range of Arnica montana. Journal of Ecology. 1999;87:839–848. [Google Scholar]
- Chichester LF, Getz LL. The zoogeography and ecology of arionid and limacid slugs introduced into northeastern North America. Malacologia. 1969;7:313–346. [Google Scholar]
- Coustau C, Renaud F, Maillard C, Pasteur N, Delay B. Differential susceptibility to a trematode parasite among genotypes of the Mytilus edulis/galloprovincialis complex. Genetical Research. 1991;57:207–12. doi: 10.1017/s0016672300029359. [DOI] [PubMed] [Google Scholar]
- Desprez-Loustau ML, Wagner K. Influence of silvicultural practices on twisting rust infection and damage in maritime pine, as related to growth. Forest Ecology and Management. 1997;98:135–147. [Google Scholar]
- Elger A, Lemoine DG, Fenner M, Hanley ME. Plant ontogeny and chemical defence: older seedlings are better defended. Oikos. 2009;118:767–773. [Google Scholar]
- Emms SK, Arnold ML. The effect of habitat on parental and hybrid fitness: transplant experiments with Louisiana irises. Evolution. 1997;51:1112–1119. doi: 10.1111/j.1558-5646.1997.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Fenner M, Hanley ME, Lawrence R. Comparison of seedling and adult palatability in annual and perennial plants. Functional Ecology. 1999;13:546–551. [Google Scholar]
- Fields M, Orians CM. Specificity of phenolic glycoside induction in willow seedlings (Salix sericea) in response to herbivory. Journal of Chemical Ecology. 2006;32:2647–2656. doi: 10.1007/s10886-006-9188-7. [DOI] [PubMed] [Google Scholar]
- Fritz RS, Hochwender CG, Lewkiewicz DA, Bothwell S, Orians CM. Seedling herbivory by slugs in a willow hybrid system: developmental changes in damage, chemical defense, and plant performance. Oecologia. 2001;129:87–97. doi: 10.1007/s004420100703. [DOI] [PubMed] [Google Scholar]
- Fritz RS, Hochwender CG, Brunsfeld SJ, Roche BM. Genetic architecture of susceptibility to herbivores in hybrid willows. Journal of Evolutionary Biology. 2003;16:1115–1126. doi: 10.1046/j.1420-9101.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- Geber MA, Griffen LR. Inheritance and natural selection on functional traits. International Journal of Plant Science. 2003;164:S21–S42. [Google Scholar]
- Glen DM, Jones H, Fieldsend JK. Damage to oilseed rape seedlings by the field slug Deroceras reticulatum in relation to glucosinolate concentration. Annals of Applied Biology. 1990;117:197–207. [Google Scholar]
- Goodger JQD, Gleadow RM, Woodrow IE. Growth cost and ontogenetic expression patterns of defence in cyanogenic Eucalyptus spp. Trees. 2006;20:757–765. [Google Scholar]
- Hagerman AE, Butler LG. Choosing appropriate methods and standards for assaying tannin. Journal of Chemical Ecology. 1989;15:1795–1810. doi: 10.1007/BF01012267. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. Impacts of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany. 2009;103:1347–1353. doi: 10.1093/aob/mcp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. An experimental field study of the effects of mollusc grazing on seedling recruitment and survival in grassland. Journal of Ecology. 1995a;83:621–627. [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. The effect of seedling age on the likelihood of herbivory by the slug Deroceras reticulatum. Functional Ecology. 1995b;9:754–759. [Google Scholar]
- Hanley ME, Collins SA, Swann C. Advertising acceptability: is mollusk olfaction important in seedling selection? Plant Ecology. 2011;212:727–731. [Google Scholar]
- Hanley ME, Girling RD, Felix AE, Olliff ED, Newland PL, Poppy GM. Olfactory selection of Plantago lanceolata by snails declines with seedling age. Annals of Botany. 2013;112:671–676. doi: 10.1093/aob/mct003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardig TM, Brunsfeld SJ, Fritz RS, Morgan M, Orians CM. Morphological and molecular evidence for hybridization and introgression in a willow (Salix) hybrid zone. Molecular Ecology. 2000;9:9–24. doi: 10.1046/j.1365-294x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- Heiser CB. Introgression re-examined. Botanical Review. 1973;39:347–366. [Google Scholar]
- Hochwender CG, Fritz RS, Orians CM. Using hybrid systems to explore the evolution of tolerance to damage. Evolutionary Ecology. 2000;14:509–521. [Google Scholar]
- Horrill JC, Richards AJ. Differential grazing by the mollusc Arion hortensis Fér. on cyanogenic and acyanogenic seedlings of the white clover, Trifolium repens L. Heredity. 1986;56:277–281. [Google Scholar]
- Hunter MD, Forkner RE. Hurricane damage influences foliar polyphenolics and subsequent herbivory on surviving trees. Ecology. 1999;80:2676–2682. [Google Scholar]
- Kimball S, Campbell DR, Lessin C. Differential performance of reciprocal hybrids in multiple environments. Journal of Ecology. 2008;96:1306–1318. [Google Scholar]
- Lexer C, Randell RA, Rieseberg LH. Experimental hybridization as a tool for studying selection in the wild. Ecology. 2003;84:1688–1699. [Google Scholar]
- Lindroth RL, Koss PA. Preservation of Salicaceae leaves for phytochemical analyses: further assessment. Journal of Chemical Ecology. 1996;22:765–771. doi: 10.1007/BF02033584. [DOI] [PubMed] [Google Scholar]
- Linhart YB, Grant MC. Evolutionary significance of local genetic differentiation in plants. Annual Review of Ecology and Systematics. 1996;27:237–277. [Google Scholar]
- Manley SAM, Fowler DP. Spruce budworm defoliation in relation to introgression in red and black spruce. Forest Science. 1969;15:365–366. [Google Scholar]
- Mosseler A, Papadopol CS. Seasonal isolation: a reproductive barrier among sympatric Salix species. Canadian Journal of Botany. 1989;67:2563–2570. [Google Scholar]
- Nagy ES. Selection for native characters in hybrids between two locally adapted plant subspecies. Evolution. 1997;51:1469–1480. doi: 10.1111/j.1558-5646.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Orians CM. Preserving leaves for tannin and phenolic glycoside analyses – a comparison of methods using 3 willow taxa. Journal of Chemical Ecology. 1995;21:1235–1243. doi: 10.1007/BF02027558. [DOI] [PubMed] [Google Scholar]
- Orians CM. The effects of hybridization in plants on secondary chemistry: implications for the ecology and evolution of plants and herbivores. American Journal of Botany. 2000;87:1749–1756. [PubMed] [Google Scholar]
- Orians CM, Fritz RS. Secondary chemistry of hybrid and parental willows: phenolic glycosides and condensed tannins in Salix sericea, S. eriocephala, and their hybrids. Journal of Chemical Ecology. 1995;21:1245–1253. doi: 10.1007/BF02027559. [DOI] [PubMed] [Google Scholar]
- Orians CM, Fritz RS. Genetic and soil nutrient effects on the abundance of herbivores on willow. Oecologia. 1996;105:388–396. doi: 10.1007/BF00328742. [DOI] [PubMed] [Google Scholar]
- Orians CM, Ward D. Evolution of plant defenses in non-indigenous environments. Annual Review of Entomology. 2010;55:439–459. doi: 10.1146/annurev-ento-112408-085333. [DOI] [PubMed] [Google Scholar]
- Orians CM, Huang C, Wild A, Dorfman KA, Zee P, Dao MTT, Fritz RS. Willow hybridization differentially affects preference and performance of herbivorous beetles. Entomologia Experimentalis et Applicata. 1997;83:285–294. [Google Scholar]
- Orians CM, Hochwender CG, Fritz RS, Snäll T. Growth and defense in willow seedlings: trade-offs are transient. Oecologia. 2010;163:283–290. doi: 10.1007/s00442-009-1521-8. [DOI] [PubMed] [Google Scholar]
- Prittinen K, Pusenius J, Koivunoro K, Roininen H. Genotypic variation in growth and resistance to insect herbivory in silver birch (Betula pendula) Oecologia. 2003;137:572–577. doi: 10.1007/s00442-003-1384-3. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH. The role of hybridization in evolution: old wine in new skins. American Journal of Botany. 1995;82:944–953. [Google Scholar]
- Rieseberg LH, Wendel JF. Introgression and its consequences in plants. In: Harrison R, editor. Hybrid zones and the evolutionary process. New York: Oxford University Press; 1993. pp. 70–109. [Google Scholar]
- Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM. Role of gene interactions in hybrid speciation: evidence from ancient and experimental hybrids. Science. 1996;272:741–745. doi: 10.1126/science.272.5262.741. [DOI] [PubMed] [Google Scholar]
- Robinson DG. Alien invasions: the effects of the global economy on nonmarine gastropod introductions into the United States. Malacologia. 1999;41:413–438. [Google Scholar]
- Roche BM, Fritz RS. Effects of host plant hybridization on resistance to willow leaf rust caused by Melampsora sp. European Journal of Forest Pathology. 1998;28:259–270. [Google Scholar]
- Rodriguez MA, Brown VK. Plant competition and slug herbivory: effects on the yield and biomass allocation pattern of Poa annua L. Acta Oecologia. 1998;19:37–46. [Google Scholar]
- Rosenthal GA, Janzen DJ, editors. Herbivores: their interaction with secondary plant metabolites. Los Angeles: Academic Press, Inc; 1979. [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, et al. The population biology of invasive species. Annual Review of Ecology and Systematics. 2001;32:305–332. [Google Scholar]
- SAS Institute. JMP® 3·2·2. Cary, NC: 1997. http://www.jmp.com . [Google Scholar]
- Stowe KA. Experimental evolution of resistance in Brassica rapa: correlated response of tolerance in lines selected for glucosinolate content. Evolution. 1998;52:703–712. doi: 10.1111/j.1558-5646.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Stratton DA. Life-cycle components of selection in Erigeron annuus: I. Phenotypic selection. Evolution. 1992;46:92–106. doi: 10.1111/j.1558-5646.1992.tb01987.x. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. Trends in Ecology and Evolution. 2002;17:278–285. [Google Scholar]
- Stutz HC, Thomas LK. Hybridization and introgression in Cowania and Purshia. Evolution. 1964;18:183–195. [Google Scholar]
- Wait DA, Jones CG, Coleman JS. Effects of nitrogen fertilization on leaf chemistry and beetle feeding are mediated by leaf development. Oikos. 1998;82:502–514. [Google Scholar]
- Westerbergh A, Nyberg AB. Selective grazing of hairless Silene dioica plants by land gastropods. Oikos. 1995;73:289–298. [Google Scholar]
- Whelan RJ. An artificial medium for feeding choice experiments with slugs. Journal of Applied Ecology. 1982;19:89–94. [Google Scholar]
- Wu HX, Ying CC, Muir JA. Effect of geographic variation and jack pine introgression on disease and insect resistance in lodgepole pine. Canadian Journal of Forest Research. 1996;26:711–726. [Google Scholar]


