Abstract
Background and Aims
Plant defence metabolites are considered costly due to diversion of energy and nutrients away from growth. These costs combined with changes in resource availability and herbivory throughout plant ontogeny are likely to promote changes in defence metabolites. A comprehensive understanding of plant defence strategy requires measurement of lifetime ontogenetic trajectories – a dynamic component largely overlooked in plant defence theories. This study aimed to compare ontogenetic trajectories of foliar phenolics and terpenoids. Phenolics are predicted to be inexpensive to biosynthesize, whereas expensive terpenoids also require specialized, non-photosynthetic secretory structures to avoid autotoxicity. Based on these predicted costs, it is hypothesized that phenolics would be maximally deployed early in ontogeny, whereas terpenoids would be maximally deployed later, once the costs of biosynthesis and foregone photosynthesis could be overcome by enhanced resource acquisition.
Methods
Leaves were harvested from a family of glasshouse-grown Eucalyptus froggattii seedlings, field-grown saplings and the maternal parent tree, and analysed for total terpenoids and phenolics.
Key Results
Foliar phenolics were highest in young seedlings and lowest in the adult tree. Indeed the ratio of total phenolics to total terpenoids decreased in a significantly exponential manner with plant ontogeny. Most individual terpene constituents increased with plant ontogeny, but some mono- and sesquiterpenes remained relatively constant or even decreased in concentration as plants aged.
Conclusions
Plant ontogeny can influence different foliar defence metabolites in directionally opposite ways, and the contrasting trajectories support our hypothesis that phenolics would be maximally deployed earlier than terpenoids. The results highlight the importance of examining ontogenetic trajectories of defence traits when developing and testing theories of plant defence, and illustrate an advantage of concurrently studying multiple defences.
Keywords: Chemical defence cost, Eucalyptus froggattii, herbivory, ontogeny, ontogenetic trajectory, monoterpene, monoterpene acid glucose ester, phenolic, secondary metabolite, sesquiterpene, terpenoid
INTRODUCTION
Plants deploy a diverse array of toxic and anti-nutritional defence metabolites in order to reduce tissue loss to herbivory. This deployment comes at a cost – the allocation cost – because metabolite synthesis, storage and maintenance require, both directly and indirectly, energy and nutrients that could otherwise be used to sustain growth and other fitness-enhancing processes (McKey, 1974; Mooney and Gulmon, 1982). In addition, allocation costs incurred early in plant ontogeny may result in opportunity costs, which involve loss of growth and competitive status with effects that are evident later in life (Coley et al., 1985; Sagers and Coley, 1995). Indeed, the assumption that plant defence metabolites are costly forms the foundation upon which many theories on the evolution and distribution of defence metabolites are based (Feeny, 1976; Rhoades and Cates, 1976; Coley et al., 1985; Herms and Mattson, 1992; Strauss et al., 2002).
As plants develop from seed through to reproductively mature adults, their defence strategy will be shaped not only by allocation costs and consequent opportunity costs, but also by environmental variables. For example, plants are typically exposed to different levels and types of herbivory, whilst encountering markedly different levels of resource availability throughout their lives (Farnsworth, 2004; Weiner, 2004). Acting concomitantly or independently, these selective pressures may also promote changes in the amount and type of defence metabolites deployed during a plant's lifetime. Given the likelihood that deployment of plant defence metabolites will be dynamic in nature, a comprehensive understanding of the cost of their production requires measurement not only of their effect on growth and fitness, but also of their lifetime ontogenetic trajectories (i.e. how they change through time as plants develop; Boege and Marquis, 2005).
Many defence theories inadequately consider the likelihood of changes to allocation and opportunity costs that result from ontogenetic changes in defence metabolite concentrations or types. This is probably because the vast majority of research used as the basis for such theories has not dealt with the possibility that plant defence metabolites can change with ontogeny. Indeed, in their seminal review, Boege and Marquis (2005) noted that with few exceptions, measurements of a chosen defence metabolite have been made at a single point in the life cycle of plants, often with the implicit assumption that it does not change. Furthermore, in those studies that have examined two or more distinct ontogenetic stages (e.g. seedlings, saplings and adults; Bryant and Julkunen-Tiitto, 1995; Busk and Møller, 2002; Del-Val and Dirzo, 2003; Yan et al., 2003; Boege, 2005; Goodger et al., 2006), the results have been variable, with some species displaying higher amounts of a given defence metabolite when plants are young, and others displaying more when plants are mature. In order to make appropriate extensions to defence theories, there is a need for more detailed studies of how defence metabolites change throughout plant ontogeny.
It is also noteworthy that few studies have examined the deployment of multiple classes of plant defence metabolites within a species to determine if their ontogenetic trajectories differ (Boege et al., 2011). Within a given plant it is possible that different classes of defence metabolite are maximally deployed at different ontogenetic stages. Such contrasting trajectories may reflect the relative effectiveness of each class against changes in herbivores that occur with ontogeny (e.g. terrestrial herbivory of seedlings vs. arboreal folivory of trees). They may also reflect the relative cost of each class in relation to the resources available at each ontogenetic stage. The relative costs of the carbon-based defences of phenolics and terpenoids make an excellent case in point.
Phenolic defence metabolites are ubiquitous in plants (Boudet, 2007) and relatively inexpensive to biosynthesize (average cost = 2·11 g glucose g−1 metabolite; Gershenzon, 1994b). Furthermore, phenolics do not require specialized storage structures as they are broadly distributed within a variety of plant tissues and cells, often accumulating in cells on or near external surfaces of plant organs, in keeping with their defensive role (Hutzler et al., 1998). In contrast, terpenoids are among the most expensive defence metabolites for plants to synthesize (average cost = 3·11 g glucose g−1 metabolite) due to their high level of chemical reduction (Gershenzon, 1994b). In addition, the storage costs of lipophilic terpenoids can be especially high because they are generally sequestered in complex secretory structures to avoid autotoxicity (Gershenzon, 1994a). Not only are such structures expensive to build in terms of energy and resources, they are also generally composed of non-photosynthetic cells, therefore reducing the amount of photosynthetically productive tissue. If the relative costs of phenolics and terpenes can constrain their production at ontogenetic stages where resources are limited (e.g. seedlings) then they will show different ontogenetic trajectories. It should be noted, however, that general ontogenetic patterns for phenolics and terpenoids were not detected in a recent meta analysis (Barton and Koricheva, 2010). Nonetheless, this was probably due to the vast diversity of compounds within these broad classes and the dearth of studies simultaneously comparing both classes.
An excellent system for comparing ontogenetic changes in terpenoids and phenolics are plants from Eucalyptus, which grow from remarkably small seeds and seedlings to very large and long-lived trees whilst deploying a complex array of foliar defence metabolites throughout their life span. In particular, Eucalyptus trees are characteristically high in terpenoids due to the presence of abundant foliar secretory cavities that house a complex mixture of mono- and sesquiterpenes (Brophy et al., 1991), and a recently identified suite of monoterpene acid glucose esters (MAGEs; Heskes et al., 2012). Terpenes are well characterized as defence metabolites (Harborne, 2001) and, in particular, Eucalyptus terpenes are known for their potent defensive activities (Batish et al., 2008). Although the function of MAGEs is largely unknown, a number exhibit biological activities that are also suggestive of a role in plant defence (Goodger and Woodrow, 2011).
Previous research within Eucalyptus suggests that plant ontogeny can influence foliar monoterpene accumulation, resulting in a delay to the maximum total concentration until saplings are ≥2 years old (Barton et al., 1991; Eastham et al., 1993; King et al., 2006; Goodger and Woodrow, 2009), but the influence of plant ontogeny on the concentration of sesquiterpenes and MAGEs is unknown. The influence of ontogeny on total phenolics in Eucalyptus has not been studied in detail, although higher foliar concentrations were reported in adult trees from natural populations compared with glasshouse-grown seedlings of three species at 4 months of age (Goodger et al., 2006).
The aim of this study was to make detailed measurements of the ontogenetic trajectories of total phenolics and total secretory cavity constituents (terpenoids) in Eucalyptus froggattii, a species particularly high in defensive sesquiterpenes as an adult, and whose MAGEs can comprise >50 % of cavity lumen volume in adult leaves (Goodger et al., 2010). We hypothesized that the low biosynthesis and storage costs of total phenolics would enable their ontogenetic trajectory to reach its maximum in early ontogeny. Then as older seedlings become established and continue to develop their photosynthetic capacity and nutrient acquisition capabilities, the relative cost of foregone photosynthesis due to the presence of secretory cavities would diminish and the available resources would increase sufficiently to enable secretory cavities and their terpenoid constituents to accumulate in ever increasing amounts with tontogeny, reaching maximum levels in adult trees.
MATERIALS AND METHODS
Plant material and growth conditions
A single half-sibling family of Eucalyptus froggattii Blakely was used in an attempt to limit potential genetic variation and thereby maximize the ability to detect changes across ontogenetic stages. Genetic variation is known to influence both terpene composition (Barton et al., 1991; Doran and Matheson, 1994) and foliar secretory cavity size (Goodger and Woodrow, 2012) in Eucalyptus, and the use of half-sibling families of the related mallee species E. polybractea has been shown to reduce such variation (see King et al., 2004; Goodger and Woodrow, 2008). Mature seed capsules and fully expanded leaves were harvested from an adult tree (estimated to be at least 10 years old) growing in a natural population (Greater Bendigo National Park, Victoria, Australia 36°30·04′S, 144°22·24′E). Leaves were stored at –70 °C prior to chemical analyses. Seed capsules were dried at 50 °C to facilitate dehiscence and seeds were sown in bulk in June 2009. Individual half-sibling seedlings were transferred to 1 L pots containing top soil:sand (2:1, v/v) 60 d after sowing (DAS), placed in a glasshouse and supplied with 5 g of slow-release fertilizer (Osmocote PLUS, The Scotts Company, Marysville, OH, USA). Pots were watered daily to field capacity with nutrient solution as previously described (Goodger et al., 2007). At 365 DAS, seedlings were transferred to 9 L pots and an additional 5 g of slow-release fertilizer applied. The air temperature and relative humidity of the glasshouse were measured every 60 min with a Spectrum temperature/humidity sensor (Veriteq Instruments, Richmond, Canada). The day/night mean (± s.e.) air temperature and relative humidity over the course of the growth period were 23·2 ± 0·1 °C/19·8 ± 0·1 °C and 55·5 ± 0·2 %/63·4 ± 0·1 %, respectively. At 500 DAS, four randomly selected seedlings were transferred to the System Garden at the University of Melbourne (Parkville, Australia 37°47·81′S, 144°57·53′E) and given supplemental watering for 4 weeks, and relied on rainfall thereafter.
Leaf sampling
In total, 40 individuals from a single cohort of half-sibling seedlings were harvested over the course of the experiment. Four different glasshouse-grown plants were randomly chosen and sampled from the cohort each month from 222 to 475 DAS (nine harvests, 36 plants in total). Sampling commenced when plants produced large enough leaves to provide sufficient material for all chemical analyses to be performed on a single leaf. At each harvest date, the most recently fully expanded leaf was removed from each of four randomly selected plants, none of which had been sampled previously to avoid potentially inducing metabolites. In addition, a single harvest was made in the same manner at 1090 DAS from four saplings transferred to the System Garden. Each harvested leaf was weighed, its area determined using a flat bed scanner, and thickness measured using a micrometer, before being ground to a fine powder in a mortar under liquid N2. The ground tissue was then immediately divided into pre-weighed vials and weighed prior to use in three different chemical metabolite analyses, after which vials and extracted tissue were dried at 50 °C and weighed for dry weight calculations.
Chemical analyses
All chemical analyses were carried out on each individual leaf. Total sesquiterpenes (C15) and monoterpenes (C10) were extracted from fresh ground leaf tissue (350 mg) in 3 mL of hexane containing 100 µg mL−1 tri-decane as an internal standard and incubated at 50 °C for 4 d. Constituents were identified using gas chromatography–mass spectrometry (GC-MS) comparison with the Wiley Registry 8th edn./NIST 2005 Mass Spectral Library (John Wiley & Sons, Hoboken, NJ, USA) and quantified by GC-flame ionization detection (FID) using standards as described in Goodger et al. (2010), with the additional use of standard series of sabinene, verbenone, cis-carveol, trans-caryophyllene, caryophyllene oxide and α-humulene (Sigma-Aldrich, St. Louis, MO, USA).
Monoterpene acid glucose esters were extracted from fresh ground leaf tissue (350 mg) in 30 % acetonitrile for 24 h at 25 °C. Extracts were filtered and fractionated by reverse phase high-perfomance liquid chromatography (RP-HPLC) as described in Heskes et al. (2012) using a gradient of 30–50 % acetonitrile over 7 min. The two most abundant MAGEs in E. froggattii, cuniloside B and froggattiside A, were identified and quantified as described in Hakki et al. (2010).
Total phenolics were extracted from fresh ground leaf tissue (60 mg) with 1 mL of 50 % acetone three times, with the extracts pooled and analysed for total phenolics using the Folin–Ciocalteu Assay according to the method of Cork and Krockenburger (1991) with modifications described in Burns et al. (2002).
Microscopy
Leaf strips (2 mm wide) were dehydrated in ethanol, infiltrated with LR White resin (Sigma-Aldrich) in ethanol and embedded in gelatine capsules prior to polymerization (60 °C for 24 h). Embedded leaf strips were sectioned (500 µm) using a microtome, stained with 0·5 % toluidine blue (in 0·1 % sodium carbonate) and imaged at ×100 magnification with a compound microscope. The leaf sections were used for illustrative purposes only, and not secretory cavity size or density determinations.
Statistical analyses
Non-linear regressions (three-parameter exponential rise to maximum and three-parameter exponential decay) were performed using Sigmaplot Version 12·3 (Systat Software, Chicago, IL, USA).
RESULTS
Eucalyptus froggattii is a ‘mallee’ eucalypt – an informal grouping within the genus, with members having a multistemmed habit from ground level and relatively slow growth (Walker et al., 1989; Brooker and Kleinig, 2006). The half-sibling family grew very slowly and, by 222 DAS, the mean (± 1 s.e.) height of seedlings was only 8·4 ± 0·5 cm (Fig. 1A). Plant height, leaf mass per unit area (LMA) and leaf thickness increased at a relatively constant rate with plant ontogeny (Fig. 1). The apparent slowing of height growth in the final three glasshouse harvests (DAS 420–475) probably reflects the decreased daylength of winter over most of that period. Ontogenetic increases in leaf thickness appeared to be associated with changes in mesophyll cells, cuticle thickness and also secretory cavity size and number, as illustrated in Fig. 2, although these anatomical parameters were not quantified. The average size of leaves also increased with plant ontogeny in the seedlings and saplings, but then showed a marked decrease in leaves from the maternal adult (Fig. 1B).
Fig. 1.
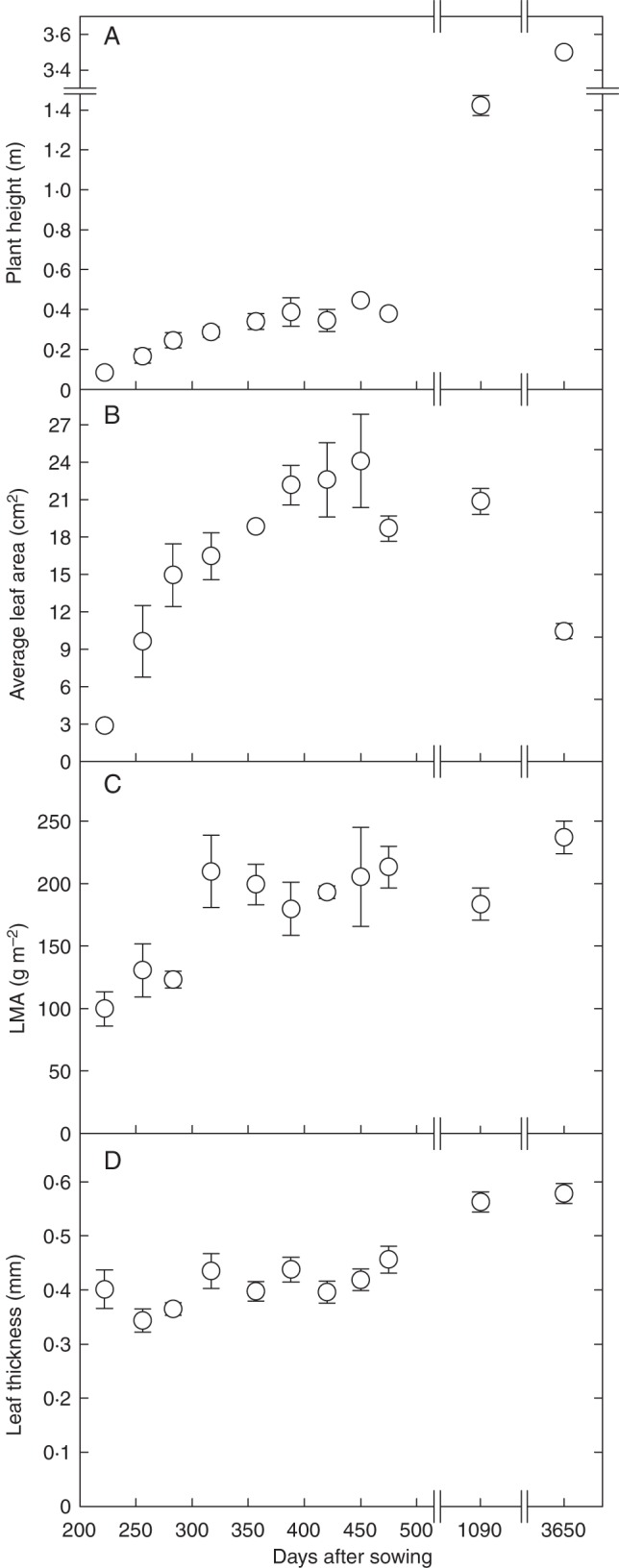
Growth parameters for Eucalyptus froggattii throughout plant ontogeny. Plant height (A), leaf area per unit leaf mass (LMA; C) and leaf thickness (D) increase in a relatively linear fashion with plant ontogeny, whereas average leaf area (B) increases to an apparent maximum in saplings before decreasing in adult tree leaves. Values are the mean (± s.e.) of four different half-siblings destructively harvested each day after sowing, except for harvest day approx. 3650 which represents the mean of five leaves sampled from the maternal tree.
Fig. 2.
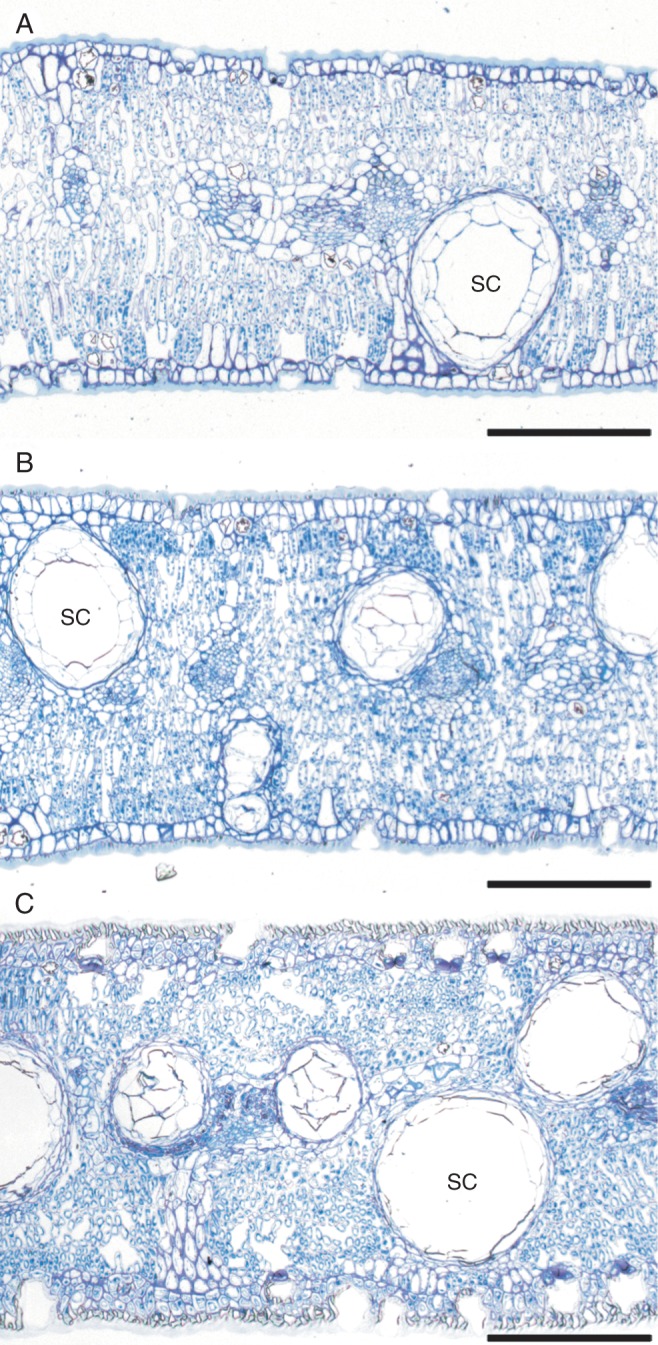
Sections through resin-embedded leaves of Eucalyptus froggattii harvested at different stages of plant ontogeny. (A) Glasshouse-grown seedling leaf at 317 DAS; (B) field-grown sapling leaf 1090 DAS; (C) maternal tree leaf. SC denotes secretory cavities. Sections were stained with toluidine blue, and scale bars = 200 µm.
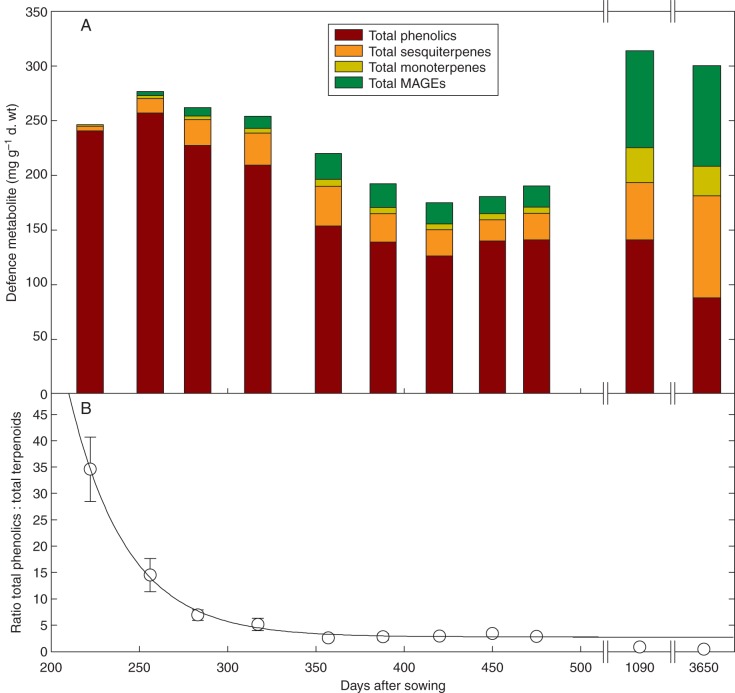
The influence of plant ontogeny on the concentration of foliar defence metabolites was substantial in terms of both abundance and direction of change. The maximum concentration of total phenolics was 257 mg g−1 d. wt in the second youngest cohort of plants (DAS 256) whereas the minimum of 88 mg g−1 d. wt was observed in leaves from the adult tree (Fig. 3A). An exponential decay regression applied to the phenolic concentration data with increasing plant ontogeny was significant (F = 23, P = 0·0005, r2 = 0·85). In contrast, the minimum concentrations of 6 mg g−1 d. wt total sesquiterpenes, 1 mg g−1 d. wt total monoterpenes and zero MAGEs were each observed in the youngest plants (DAS 222), whereas the respective highest concentrations of 93, 27 and 92 mg g−1 d. wt for each of these secretory cavity constituents were found in foliage of the adult tree (Fig. 3A). It should be noted that the level of HPLC detection of MAGEs is not as low as GC-FID detection of terpenes, thus the youngest plants (DAS 222) may have contained some MAGEs albeit at very low levels. Exponential rises to maximum regressions applied to the secretory cavity constituent data through time were significant for sesquiterpenes (F = 40, P < 0·0001, r2 = 0·91), monoterpenes (F = 27, P = 0·0003, r2 = 0·87) and MAGEs (F = 60, P < 0·0001, r2 = 0·94). Interestingly, the relaxation time (the inverse of the rate constant which equates to the days when some two-thirds of the maximum metabolite concentration is reached) was 1563 ± 794 d (P = 0·09) for sesquiterpenes, but was identical for both monoterpenoid classes: 500 ± 165 d (t = 2·7, P = 0·03) for monoterpenes and 500 ± 104 d (t = 3·9, P = 0·004) for MAGEs. This remarkable similarity may reflect the rate of biosynthesis of the common monoterpene constituents. The ratio of total phenolics to total secretory cavity terpenoids (total sesquiterpenes + total monoterpenes + total MAGEs) decreased in a significantly exponential manner with plant ontogeny from an average maximum of 34·6:1 in the youngest plants to a minimum of 0·4:1 in the leaves of the adult tree (F = 149, P < 0·0001, r2 = 0·88; Fig. 3B).
Fig. 3.
Differential production of foliar defence metabolites with Eucalyptus froggattii plant ontogeny. (A) The concentration of secretory cavity-housed sesquiterpenes, monoterpenes and monoterpene acid glucose esters (MAGEs) increases with plant ontogeny, whereas total phenolic concentration decreases. This differential pattern is exemplified in (B) where the mean (± 1 s.e.) proportion of total phenolics to total secretory cavity-housed defence metabolites (terpenoids) decreased in a significantly exponential manner with plant ontogeny (F = 149, P < 0·0001, r2 = 0·88).
Terpene accumulation with plant ontogeny
Up to 66 different sesquiterpenes and 44 different monoterpenes were detected in the E. froggattii leaves harvested for this study. An examination of how individual terpenes varied with plant ontogeny showed some interesting patterns. For example, the sesquiterpene β-eudesmol (the most abundant terpene in adult foliage) increased from a mean of 2 mg g−1 d. wt in the leaves of the youngest plants to 24 mg g−1 d. wt in leaves of the adult, whereas the sesquiterpene spathulenol also had a mean concentration of 2 mg g−1 d. wt in the youngest plants, but only 1 mg g−1 d. wt was detected in the leaves of the adult tree (Fig. 4A). The sesquiterpenes α-elemol, γ-eudesmol and rosifoliol showed similar increases with ontogeny to β-eudesmol, whereas α-caryophyllene, ledene and globulol showed similar patterns to spathulenol. Directionally different patterns of accumulation with ontogeny were also observed for some monterpene constituents. For example, limonene (the most abundant monoterpene in foliage from the adult) increased from a mean of 0·03 mg g−1 d. wt in the leaves of the youngest plants to 7 mg g−1 d. wt in the adult, whereas cryptone decreased from a mean of 0·2 mg g−1 d. wt in the youngest plants to 0·04 mg g−1 d. wt in the adult (Fig. 4B). The monoterpenes sabinene, myrcene, 1,8-cineole, α-pinene and β-pinene showed similar increases with ontogeny to limonene, whereas p-cymene and iso-pinocarveol showed similar patterns to cryptone.
Fig. 4.
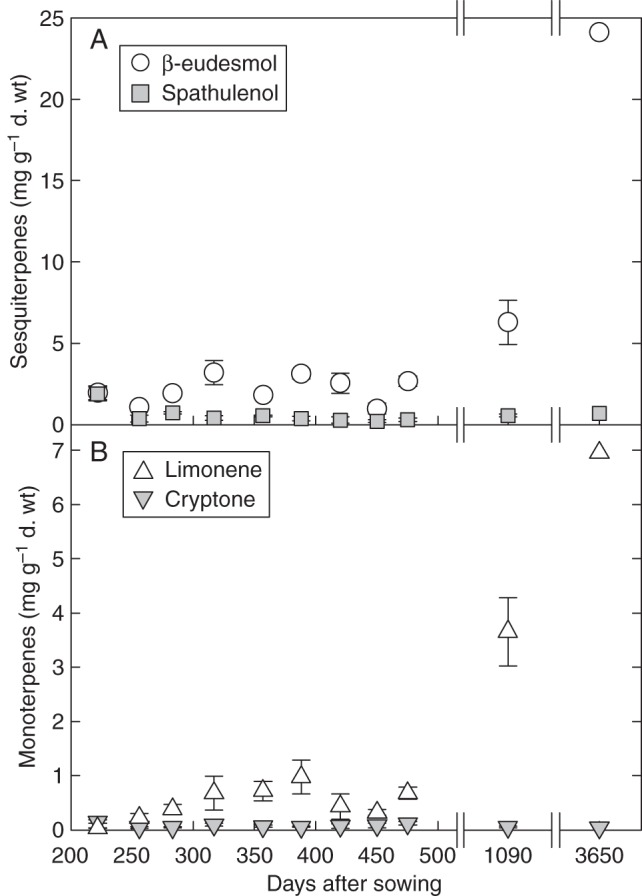
Terpene constituents showing different concentration patterns with plant ontogeny in Eucalyptus froggattii. Sesquiterpenes β-eudesmol and spathulenol (A) show different patterns with plant ontogeny. Similarly, monoterpenes limonene and cryptone also show different ontogenetic patterns (B).
DISCUSSION
Ontogenetic trajectories of defence metabolites
The results of this study show that directionally opposite ontogenetic trajectories exist for total foliar phenolics and terpenoid defence metabolites in E. froggattii. In particular, the total phenolic concentration significantly decreased, whereas total terpenoids (including MAGEs) significantly increased with plant ontogeny. These contrasting trajectories are in support of our hypothesis that phenolics – most probably the cheapest defence metabolite in terms of biosynthesis and storage – would predominate in resource-limited seedlings, and that the expensively housed and biosynthesized terpenoids would show an ontogenetic delay to maximum accumulation in parallel with increasing resource acquisition capabilities as seedlings develop into mature trees. The negative relationship observed between phenolics and terpenoids with plant ontogeny suggests a possible resource trade-off or spatial constraint to the total defence metabolite complement in E. froggattii leaves, i.e. at no ontogenetic stage did any trees deploy both high phenolics and high terpenoids.
The increase in terpenoids with plant ontogeny is consistent with the model proposed by Boege and Marquis (2005) in which the resources required for defence metabolites become more readily available as plants grow. In this case, it may be photosynthetic resources, or more specifically the potential cost of foregoing photosynthesis due to the presence of large and abundant secretory cavities (see Fig. 2), that is constraining the ontogenetic deployment of terpenoids. A recent study in E. polybractea found that the amount of foregone photosynthesis is likely to be greater than the photosynthetic mesophyll space lost to cavities alone as there is also a minimum volume of photosynthetic tissue that is apparently dedicated to the maintenance of secretory cavities (Goodger and Woodrow, 2012). Hence in the small, slow growing E. froggattii seedlings, there may be insufficient photosynthetic productivity to produce costly terpenoids, and available carbon resources appear to be used for production of high phenolic defence and limited above-ground growth. High phenolic defence has been predicted to be an optimal strategy in slow-growing species as the potential impact of replacing leaves lost to herbivory is high, even though the cost of phenolic production further reduces the realized growth rate (Coley et al., 1985; Endara and Coley, 2011). As seedlings develop, leaf photosynthetic resources (i.e. leaf area ratio) may increase until they are sufficient to offset the costs not only of producing secretory cavities, but also foregoing photosynthesis due to their presence, and biosynthesizing the terpenoids they house.
Similar to the delayed accumulation of terpenoids in the E. froggattii half-siblings studied here, ontogenetic delays to the accumulation of purportedly costly foliar defence metabolites have recently been observed in other species of Eucalyptus. For example, total monoterpenes in E. polybractea (Goodger and Woodrow, 2009) and the cyanogenic glycoside prunasin in E. yarraensis (Goodger et al., 2007) and E. camphora (Neilson et al., 2011) showed ontogenetic delays to maximal accumulation. Interestingly, ontogenetic trajectories of monoterpenes in E. polybractea (Goodger and Woodrow, 2009) and prunasin in E. yarraensis (Goodger et al., 2007) showed peak concentrations within the first year or two of growth, and declined thereafter. In contrast, prunasin in E. camphora showed maximum concentrations in adult foliage (Neilson et al., 2011) and, in a similar manner, abundant terpenes such as β-eudesmol and limonene, and the MAGE cuniloside B showed maximum concentrations in adult E. froggattii foliage. The different ontogenetic strategies for defence metabolites in Eucalyptus may reflect a balance between growth in environments with differing nutrient availabilities and the production of costly defence metabolites under changing herbivore pressures as each species develops from seedling to adult.
The optimal defence hypothesis (McKey, 1974; Rhoades, 1979) predicts concentrations of plant defence metabolites to reflect a balance between two principal selective forces – the effectiveness of a metabolite in reducing herbivory and the allocation cost of its deployment. The results of this study have largely been presented in the context of the latter, but the importance of the former in shaping the contrasting ontogenetic trajectories observed in E. froggattii must also be considered. Indeed herbivory has been argued to be the main selective force affecting defence metabolite production in plants (McKey, 1974), and therefore the effectiveness of each metabolite class against the prevailing herbivores at a given ontogenetic stage is likely to promote ontogenetic variation in the defences. For example, during the seedling stage, high foliar phenolics may be more effective against terrestrial grazing marsupials such as kangaroos and wallabies, but, once saplings grow beyond the reach of these herbivores, high terpenoid levels (and lower phenolics) may be more effective against folivorous arboreal marsupials such as possums and koalas. In support of this notion, total phenolics have been shown to be the major factor determining feeding preference by western grey kangaroos on a range of Hakea species differing in phenolic content (Rafferty et al., 2005). In addition, selection of Eucalyptus leaves by arboreal marsupials has been shown to be influenced by both the amount of terpenes and the proportions of terpene constituents in foliage (Hume and Esson, 1993; McLean and Foley, 1997). It is noteworthy that the lower total phenolics of older E. froggattii trees observed here may continue to exhibit defensive properties, depending on the specific phenolic constituents that are present. For example, the purification of the phenolic macrocarpal G from E. ovata foliage and its addition to an artificial diet at 2 % of dry weight resulted in a 90 % reduction in voluntary food intake by the common ringtail possum (Pass et al., 1998). Future work profiling the ontogenetic trajectories of specific metabolites within the total phenolic pool would provide a greater understanding of phenolic defences in E. froggattii.
Phytophagous insects are also major pests of Eucalyptus, and their behavioural responses to terpenes are well characterized (Edwards et al., 1993; Östrand et al., 2008; Matsuki et al., 2011). Small seedlings may be less likely targets for flying insects to land on and also provide inadequate targets for oviposition and larval feeding, perhaps supporting the delayed accumulation of terpenes until a stage when insect herbivory is likely to be more prevalent. Similarly, LMA and leaf thickness were also observed to increase with plant ontogeny in this study, to reach maxima in older plants. LMA is the product of leaf thickness and leaf density, and increases in either of these parameters can lead to an increase in physical resistance to herbivory (Coley, 1983), especially insect herbivory in Eucalyptus (Landsberg and Cork, 1997). This observation supports the delayed deployment of insect defences until a time when they may be more likely to be of use.
Optimal defence theory also predicts natural selection to favour young plants being more highly defended than older plants. This is based on the assumption that the impact of herbivory on plant fitness decreases as plants age (Herms and Mattson, 1992; Haukioga and Koricheva, 2000; Stowe et al., 2000; Warner and Cushman, 2002; Boege et al., 2011). The observed maximum concentration of total phenolics in the youngest E. froggattii plants supports such predictions. Studies on birch (Reichardt et al., 1984) and aspen (Basey et al., 1988) have also found negative relationships for foliar phenolics between juvenile and mature trees. Nonetheless, the deployment of high levels of foliar phenolics is apparently not a consistent feature of early tree ontogeny as even within the genus Eucalyptus there are examples of species with greater phenolics in adult populations compared with half-sibling families of young saplings derived from those populations (Goodger et al., 2006). The factors that determine a plant's defence strategy are undoubtedly complex and will relate to both the cost of producing each class of metabolite in a given environment and the effectiveness of each defence against the prevailing herbivores in that environment and at each ontogenetic stage.
Mono- and sesquiterpene synthesis
Not only were differing ontogenetic trajectories observed between total phenolics and total terpenoids, but even within the mono- and sesquiterpene classes differing trajectories were observed. In particular, there were two apparent groupings within each terpene class – one that increased with ontogeny and one that remained relatively constant. Terpenes showing similar ontogenetic patterns may each be produced by a single, specific enzyme, and well-correlated terpenes may group due to genetic linkage (Wilderman et al., 2004). Alternatively, and arguably more probably, there may be at least two different sesquiterpene synthases and two different monoterpene synthases in E. froggattii, each capable of catalysing the formation of multiple products. Multiproduct enzymes are well known from plant terpenoid research. For example, recombinant sesquiterpene synthases from Zingibar zerumbet (Yu et al., 2008), Oryza sativa (Cheng et al., 2007) and Medicago truncatula (Garms et al., 2010) have been shown to catalyse the formation of six, 14 and 27 sesquiterpenes, respectively from the single substrate farnesyl diphosphate. In particular, the Z. zerumbet synthase produced β-, γ- and α-eudesmol (Yu et al., 2008), a finding consistent with the eudesmane-type compounds showing similar ontogenetic increases in E. froggattii. A single multiproduct synthase for the other group of sesquiterpenes that did not increase with ontogeny in E. froggattii is also possible given the structural similarities of the aromadendrane- and caryophyllane-type compounds in that group. Recent studies of recombinant 1,8-cineole (monoterpene) synthase from species including Arabidopsis thaliana (Chen et al., 2004), Salvia pomifera (Kampranis et al., 2007) and Nicotiana suaveolens (Roeder et al., 2007) have also shown it to be capable of producing up to ten monoterpene products from geranyl pyrophosphate. Importantly, these products include limonene, sabinene, myrcene, 1,8-cineole, α-terpineol, α-pinene and β-pinene, all of which showed similar ontogenetic increases in E. froggattii. Differential ontogenetic trajectories of terpene synthases will ensure young plants have a different overall terpene profile from that of older plants, which may make them less palatable to herbivores adapted to the terpene profiles of adult trees.
The similar ontogenetic increases in the eudesmane sesquiterpenes and many of the monoterpene constituents (limonene, α-pinene, etc.) suggest that the two groups may be co-regulated ontogenetically. Evidence for such co-regulation of mono- and sesquiterpenes comes from work on Picea abies where application of methyl jasmonate induced mono- and sesquiterpene synthesis simultaneously (Martin et al., 2003). A correlative analysis of terpene constituents in M. alternifolia chemotypes also found a positive correlation between a monoterpene and sesquiterpene group, but this was only detected in one of three chemotypes (Keszei et al., 2010). From an ecological stand point, co-regulating multiproduct sesquiterpene and monoterpene synthases ontogenetically will ensure the production of terpene mixtures. Such terpene mixtures may aid the defence of E. froggattii foliage in a number of ways. As reviewed by Gershenzon and Dudareva (2007), complex mixtures of terpenes can confound the capacity for herbivores to evolve resistance to all constituents, may help achieve simultaneous protection against numerous herbivores and can increase the probability that individuals in a population will have a unique terpene profile – an advantage when herbivores are already adapted to overcome the more common profile found in a given population. In addition, individual terpenes can act synergistically to provide greater defence than the equivalent amount of a single constituent, and mixtures of terpenes may also be a deterrent to herbivores for longer periods than single constituents (Gershenzon and Dudareva, 2007). With respect to allocation costs, the use of multiproduct enzymes to produce such complex mixtures of mono- or sesquiterpenes has been posited as a cost amelioration strategy because only a limited number of enzymes are required (Gershenzon, 1994b).
Conclusions
The results of this study show that plant ontogeny can influence the accumulation of foliar defence metabolites in directionally opposite ways. Such variable ontogenetic trajectories may result from plants maintaining the optimal balance between three factors: the resources required (and foregone) to produce and store a given defence metabolite; the resources available for production at each life stage; and potential changes to the effectiveness of defences as herbivore types and amounts change throughout plant ontogeny. The dynamic nature of this balance means that defence metabolite concentrations, and indeed types, are likely to change during ontogeny. Our results highlight the importance of examining ontogenetic trajectories of defence traits when developing and testing theories of plant defence. Moreover, the contrasting trajectories of phenolics and terpenoids illustrate a potential shortcoming of studying a single defence trait in isolation, and an advantage of concurrently studying multiple traits.
ACKNOWLEDGEMENTS
J.Q.D.G. and I.E.W. were supported by a grant from the Australian Research Council (Project DP1094530). A.M.H. was supported by the Holsworth Wildlife Research Endowment (managed by ANZ Trustees). We thank Metabolomics Australia for GC-MS assistance, and Dr Simon Crawford, School of Botany for microscopy preparation and sectioning.
LITERATURE CITED
- Barton AFM, Cotterill PP, Brooker MIH. Heritability of cineole yield in Eucalyptus kochii. Silvae Genetica. 1991;40:37–38. [Google Scholar]
- Barton KE, Koricheva J. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. American Naturalist. 2010;175:481–493. doi: 10.1086/650722. [DOI] [PubMed] [Google Scholar]
- Basey MB, Jenkins SH, Busher PE. Optimal central-place foraging by beavers: tree-size selection in relation to defensive chemicals of quaking aspen. Oecologia. 1988;76:278–282. doi: 10.1007/BF00379963. [DOI] [PubMed] [Google Scholar]
- Batish DR, Singh HP, Kohli RK, Kaur S. Eucalyptus essential oil as a natural pesticide. Forest Ecology and Management. 2008;256:2166–2174. [Google Scholar]
- Boege K. Herbivore attack in Casearia nitida influenced by plant otogenetic variation in foliage quality and plant architecture. Oecologia. 2005;143:117–125. doi: 10.1007/s00442-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Boege K, Marquis RJ. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Boege K, Barton KE, Dirzo R. Influence of tree ontogeny on plant–herbivore interactions. In: Meinzer FC, Lachenbruch B, Dawson TE, editors. Size- and age-related changes in tree structure and function. Dordrecht: Springer; 2011. pp. 193–214. [Google Scholar]
- Boudet AM. Evolution and current status of research in phenolic compounds. Phytochemistry. 2007;68:2722–2735. doi: 10.1016/j.phytochem.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Brooker MIH, Kleinig DA. Field guide to eucalypts: South-eastern Australia. Melbourne: Bloomings Books Pty Ltd; 2006. [Google Scholar]
- Brophy JJ, House APN, Boland DJ, Lassak EV. Digests of the essential oils of 111 species from northern and eastern Australia. In: Boland DJ, Brophy JJ, House APN, editors. Eucalyptus leaf oils: use, chemistry, distillation and marketing. Melbourne: Inkata Press; 1991. pp. 29–155. [Google Scholar]
- Bryant JP, Julkunen-Tiitto R. Ontogenic development of chemical defense by seedling resin birch: energy cost of defense production. Journal of Chemical Ecology. 1995;21:883–896. doi: 10.1007/BF02033796. [DOI] [PubMed] [Google Scholar]
- Burns A, Gleadow RM, Woodrow IE. Light alters the allocation of nitrogen to cyanogenic glycosides in Eucalyptus cladocalyx. Oecologia. 2002;133:288–294. doi: 10.1007/s00442-002-1055-9. [DOI] [PubMed] [Google Scholar]
- Busk PK, Møller BL. Dhurrin synthesis in Sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiology. 2002;129:1222–1231. doi: 10.1104/pp.000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Ro D-K, Petri J, et al. Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiology. 2004;135:1956–1966. doi: 10.1104/pp.104.044388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A-X, Xiang C-Y, Li J-X, et al. The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry. 2007;68:1632–1641. doi: 10.1016/j.phytochem.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Coley PD. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecological Monographs. 1983;53:209–233. [Google Scholar]
- Coley PD, Bryant JP, Chapin FSI. Resource availability and plant antiherbivore defence. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- Cork SJ, Krockenburger AK. Methods and pitfalls of extracting condensed tannins and other phenolics from plants: insights from investigations on Eucalyptus leaves. Journal of Chemical Ecology. 1991;17:123–134. doi: 10.1007/BF00994426. [DOI] [PubMed] [Google Scholar]
- Del-Val E, Dirzo R. Does ontogeny cause changes in the defensive strategies of the myrmecophyte Cecropia peltata? Plant Ecology. 2003;169:35–41. [Google Scholar]
- Doran JC, Matheson AC. Genetic parameters and expected gains from selection for monoterpene yields in Petford Eucalyptus camaldulensis. New Forests. 1994;8:155–167. [Google Scholar]
- Eastham J, Scott PR, Steckis RA, Barton AFM, Hunter LJ, Sudmeyer RJ. Survival, growth and productivity of tree species under evaluation for agroforestry to control salinity in the Western Australian wheatbelt. Agroforestry Systems. 1993;21:223–237. [Google Scholar]
- Edwards PB, Wanjura WJ, Brown WV. Selective herbivory by Christmas beetles in response to intraspecific variation in Eucalyptus terpenoids. Oecologia. 1993;95:551–557. doi: 10.1007/BF00317440. [DOI] [PubMed] [Google Scholar]
- Endara M-J, Coley PD. The resource availability hypothesis revisited: a meta-analysis. Functional Ecology. 2011;25:389–398. [Google Scholar]
- Farnsworth E. Hormones and shifting ecology throughout plant development. Ecology. 2004;85:5–15. [Google Scholar]
- Feeny PP. Plant apparency and chemical defense. In: Wallace J, Mansell RL, editors. Biochemical interactions between plants and insects. Recent advances in phytochemistry. Vol. 10. New York: Plenum Press; 1976. pp. 1–40. [Google Scholar]
- Garms S, Köllner TG, Boland W. A multiproduct terpene synthase from Medicago truncatula generates cadalene sesquiterpenes via two different mechanisms. Journal of Organic Chemistry. 2010;75:5590–5600. doi: 10.1021/jo100917c. [DOI] [PubMed] [Google Scholar]
- Gershenzon J. The cost of plant chemical defense against herbivory: a biochemical perspective. In: Bernays EA, editor. Insect–plant interactions. Boca Raton: CRC Press; 1994a. pp. 105–173. [Google Scholar]
- Gershenzon J. Metabolic costs of terpenoid accumulation in higher plants. Journal of Chemical Ecology. 1994b;20:1281–1328. doi: 10.1007/BF02059810. [DOI] [PubMed] [Google Scholar]
- Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nature Chemical Biology. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Woodrow IE. Selection gains for essential oil traits using micropropagation of Eucalyptus polybractea. Forest Ecology and Management. 2008;255:3652–3658. [Google Scholar]
- Goodger JQD, Woodrow IE. The influence of ontogeny on essential oil traits when micropropagating Eucalyptus polybractea. Forest Ecology and Management. 2009;258:650–656. [Google Scholar]
- Goodger JQD, Woodrow IE. α, β-unsaturated monoterpene acid glucose esters: structural diversity, bioactivities and functional roles. Phytochemistry. 2011;72:2257–2264. doi: 10.1016/j.phytochem.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Woodrow IE. Genetic determinants of oil yield in Eucalyptus polybractea R.T. Baker. Trees. 2012;26:1951–1956. [Google Scholar]
- Goodger JQD, Gleadow RM, Woodrow IE. Growth cost and ontogenetic expression patterns of defence in cyanogenic Eucalyptus spp. Trees. 2006;20:757–765. [Google Scholar]
- Goodger JQD, Heskes AM, Mitchell MC, King DJ, Neilson EH, Woodrow IE. Isolation of intact sub-dermal secretory cavities from Eucalyptus. Plant Methods. 2010;6:20. doi: 10.1186/1746-4811-6-20. http://dx.doi.org/10.1186/1746-4811-6-20 http://dx.doi.org/10.1186/1746-4811-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodger JQD, Choo TYS, Woodrow IE. Ontogenetic and temporal trajectories of chemical defence in a cyanogenic eucalypt. Oecologia. 2007;153:799–808. doi: 10.1007/s00442-007-0787-y. [DOI] [PubMed] [Google Scholar]
- Hakki Z, Cao B, Heskes AM, Goodger JQD, Woodrow IE, Williams SJ. Synthesis of the monoterpenoid esters cypellocarpin C and cuniloside B and evidence for their widespread occurrence in Eucalyptus. Carbohydrate Research. 2010;345:2079–2084. doi: 10.1016/j.carres.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Twenty-five years of chemical ecology. Natural Product Reports. 2001;18:361–379. doi: 10.1039/b005311m. [DOI] [PubMed] [Google Scholar]
- Haukioga E, Koricheva J. Tolerance to herbivory in woody vs. herbaceous plants. Evolutionary Ecology. 2000;14:551–562. [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Quarterly Review of Biology. 1992;67:283–335. [Google Scholar]
- Heskes AM, Goodger JQD, Tsegay S, Quach T, Williams SJ, Woodrow IE. Localization of oleuropeyl glucose esters and a flavanone to secretory cavities of Myrtaceae. PLoS One. 2012;7:e40856. doi: 10.1371/journal.pone.0040856. http://dx.doi.org/10.1371/journal.pone.0040856 http://dx.doi.org/10.1371/journal.pone.0040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume ID, Esson C. Nutrients, antinutrients and leaf selection by captive koalas (Phascolarctos cinereus) Australian Journal of Zoology. 1993;41:379–392. [Google Scholar]
- Hutzler P, Fischbach R, Heller W, et al. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. Journal of Experimental Botany. 1998;49:953–965. [Google Scholar]
- Kampranis SC, Ioannidis D, Purvis A, et al. Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: structural insights into the evolution of terpene synthase function. The Plant Cell. 2007;19:1994–2005. doi: 10.1105/tpc.106.047779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszei A, Brubaker CL, Carter R, Köllner T, Degenhardt J, Foley WJ. Functional and evolutionary relationships between terpene synthases from Australian Myrtaceae. Phytochemistry. 2010;71:844–852. doi: 10.1016/j.phytochem.2010.03.013. [DOI] [PubMed] [Google Scholar]
- King DJ, Gleadow RM, Woodrow IE. Terpene deployment in Eucalyptus polybractea; relationships with leaf structure, environmental stresses, and growth. Functional Plant Biology. 2004;31:451–460. doi: 10.1071/FP03217. [DOI] [PubMed] [Google Scholar]
- King DJ, Gleadow RM, Woodrow IE. The accumulation of terpenoid oils does not incur a growth cost in Eucalyptus polybractea seedlings. Functional Plant Biology. 2006;33:497–505. doi: 10.1071/FP05304. [DOI] [PubMed] [Google Scholar]
- Landsberg JJ, Cork SJ. Herbivory: interactions between eucalypts and the vertebrates and invertebrates that feed on them. In: Williams JE, Woinarski JCZ, editors. Eucalypt ecology. Cambridge: Cambridge University Press; 1997. pp. 342–372. [Google Scholar]
- Martin DM, Gershenzon J, Bohlmann J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiology. 2003;132:1586–1599. doi: 10.1104/pp.103.021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki M, Foley WJ, Floyd RB. Role of volatile and non-volatile plant secondary metabolites in host tree selection by Christmas beetles. Journal of Chemical Ecology. 2011;37:286–300. doi: 10.1007/s10886-011-9916-5. [DOI] [PubMed] [Google Scholar]
- McKey D. Adaptive patterns in alkaloid physiology. American Naturalist. 1974;108:305–320. [Google Scholar]
- McLean S, Foley WJ. Metabolism of Eucalyptus terpenes by herbivorous marsupials. Drug Metabolism Reviews. 1997;29:213–218. doi: 10.3109/03602539709037582. [DOI] [PubMed] [Google Scholar]
- Mooney HA, Gulmon SL. Constraints on leaf structure and function in reference to herbivory. Bioscience. 1982;32:198–206. [Google Scholar]
- Neilson EH, Goodger JQD, Motawia MS, et al. Phenylalanine derived cyanogenic diglucosides from Eucalyptus camphora and their abundances in relation to ontogeny and tissue type. Phytochemistry. 2011;72:2323–2332. doi: 10.1016/j.phytochem.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Östrand F, Wallis IR, Davies NW, Matsuki M, Steinbauer MJ. Causes and consequences of host expansion by Mnesampela privata. Journal of Chemical Ecology. 2008;34:153–167. doi: 10.1007/s10886-007-9422-y. [DOI] [PubMed] [Google Scholar]
- Pass DM, Foley WJ, Bowden B. Vertebrate herbivory on Eucalyptus – identification of specific feeding deterrents for common ringrail possums (Psudocheirus peregrinus) by bioassay-guided fractionation of Eucalyptus ovata foliage. Journal of Chemical Ecology. 1998;24:1513–1527. [Google Scholar]
- Rafferty C, Lamont BB, Hanley ME. Selective feeding by kangaroos (Macropus fuliginosus) on seedlings of Hakea species: effects of chemical and physical defences. Plant Ecology. 2005;177:201–208. [Google Scholar]
- Reichardt PB, Bryant JP, Clausen TP, Wieland GD. Defense of winter-dormant Alaska paper birch against snowshoe hares. Oecologia. 1984;65:58–69. doi: 10.1007/BF00384463. [DOI] [PubMed] [Google Scholar]
- Rhoades DF. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH, editors. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press; 1979. pp. 3–54. [Google Scholar]
- Rhoades DF, Cates RG. Toward a general theory of plant antiherbivore chemistry. Recent Advances in Phytochemistry. 1976;10:168–213. [Google Scholar]
- Roeder S, Hartmann AM, Effmert U, Piechulla B. Regulation of simultaneous synthesis of floral scent terpenoids by the 1,8-cineole synthase of Nicotiana suaveolens. Plant Molecular Biology. 2007;65:107–124. doi: 10.1007/s11103-007-9202-7. [DOI] [PubMed] [Google Scholar]
- Sagers C, Coley PD. Benefits and costs of defense in a neotropical shrub. Ecology. 1995;76:1835–1843. [Google Scholar]
- Stowe KA, Marquis RJ, Hochwender CG, Simms EL. The evolutionary ecology of tolerance to consumer damage. Annual Review of Ecology and Systematics. 2000;31:565–595. [Google Scholar]
- Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. Trends in Ecology and Evolution. 2002;17:278–284. [Google Scholar]
- Walker CD, Brunel JP, Dunin FX, et al. A joint project on the water use of a mallee community in late summer. In: Noble JC, Joss PJ, Jones GK, editors. The mallee lands – a conservation perspective. Melbourne: CSIRO; 1989. pp. 114–119. [Google Scholar]
- Warner PJ, Cushman JH. Influence of herbivores on a perennial plant: variation with life history stage and herbivore species. Oecologia. 2002;132:77–85. doi: 10.1007/s00442-002-0955-z. [DOI] [PubMed] [Google Scholar]
- Weiner J. Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology, Evolution and Systematics. 2004;6:207–215. [Google Scholar]
- Wilderman PR, Xu M, Jin Y, Coates RM, Peters RJ. Identification of syn-pimara-7,15-diene synthase reveals functional clustering of terpene synthases involved in rice phytoalexin/allelochemical biosynthesis. Plant Physiology. 2004;135:2098–2105. doi: 10.1104/pp.104.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XF, Wang Y, Yu T, Zhang YH, Dai SJ. Variation in camptothecin content in Camptotheca acuminata leaves. Botanical Bulletin of Academia Sinica. 2003;44:99–105. [Google Scholar]
- Yu F, Harada H, Yamasaki K, et al. Isolation and functional characterization of a β-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet Smith. FEBS Letters. 2008;585:565–572. doi: 10.1016/j.febslet.2008.01.020. [DOI] [PubMed] [Google Scholar]