Abstract
Background and Aims
The lack of studies assessing the simultaneous expression of tolerance and resistance traits during seedling development and overall seedling defences as compared with adult plants, in general, constitutes a significant research need that can greatly improve our understanding of overall investment in defences during plant ontogeny.
Methods
Using two seedling and two juvenile stages of the perennial herb Penstemon virgatus (Plantaginaceae) evaluations were made of (a) patterns of investment in constitutive chemical defences [i.e. iridoid glycosides (IGs)], and (b) simultaneous variation in the short-term ability of seedling and juvenile stages to induce resistance traits, measured as induced chemical defences, or tolerance traits, measured as compensatory re-growth following moderate levels of damage by a specialist insect herbivore.
Key Results
Plants were highly defended during most of their transition from seedling to early juvenile stages, reaching a constant approx. 20 % dry weight total IGs. Furthermore, following 30 % above-ground tissue damage, seedlings and juvenile stages were equally able to induce resistance, by raising their IG concentration by approx. 8 %, whereas compensatory re-growth was only achieved at young juvenile but not seedling stages.
Conclusions
Two major trends emerged from this study: (1) in contrast to expected and previously observed trends, in this perennial plant species, seedlings seem to be one of the most well-defended stages as compared with adult ones; (2) high levels of constitutive defences did not limit the ability of young developmental stages to induce resistance following damage, although this response may come with a cost (i.e. decreased compensation) in young seedling stages. Hence, as has been previously demonstrated in few other systems, these results points towards an indirect evidence for a trade-off between tolerance and resistance traits at some, but not all, developmental stages; making them often difficult to detect.
Keywords: Constitutive defences, compensation, development, herbivory, induced resistance, iridoid glycosides, Penstemon virgatus, plant ontogeny, seedling, tolerance, trade-offs
INTRODUCTION
As plants undergo their transition from seeds to mature stages, their resistance traits (Barton and Koricheva, 2010) and the identity of their herbivores and mutualists (Fenner et al., 1999; Boege and Marquis, 2005; C. Quintero, K. E. Barton and K. Boege, unpubl. res.) often change in a rather predictable way. Such stage-specific interactions can alter the transition rate among stages as well as demographic processes such as survival, growth and reproduction (Miller and Rudolf, 2011). A particularly critical period during a plant's life cycle is the seedling stage, given that seedlings are typically the stage most vulnerable to inter- and intra-specific competition (Fayolle et al., 2009), nutrient limitation (Santiago et al., 2012), and top-down controls (Terborgh, 2012), driven by pathogens (Mangan et al., 2010) and, foremost, herbivory (Moles and Westoby, 2004; Alvarez-Clare and Kitajima, 2009). Beyond the obvious effects that herbivores have on seedling survival and demography (Buschmann et al., 2005), the selective loss of plant species and genotypes at the seedling stage can lead to long-term effects on plant community composition and species interactions (Hanley and Sykes, 2009; Terborgh, 2012), highlighting the central role of seedling defensive traits in structuring complex communities.
Plant physical and chemical defences, expressed constitutively or induced by damage, can effectively protect seedlings against herbivores (e.g. Fenner et al., 1999; Hanley and Lamont, 2001). Seedlings have been shown to actively synthesize chemical defences soon after germination and/or following the depletion of defences present in seeds, which often coincides with herbivore preference and damage (Barton and Koricheva, 2010). Yet, seedling investment in chemical defences varies among species and, more critically, during the course of seedling development. In most cases, constitutive chemical defences tend to increase during seedling development (Barton and Koricheva, 2010), as has been described in a diversity of plant species and groups of secondary compounds such as alkaloids (Schaffner et al., 2003; Gregianini et al., 2004; Elger et al., 2009), phenolics (Fritz et al., 2001; Albrectsen et al., 2004; Elger et al., 2009), defensive proteins and protease inhibitors (Doan et al., 2004), cyanogenic glycosides (Goodger et al., 2007; Elger et al., 2009) and iridoid glycosides (Barton, 2007). Nonetheless, there are examples where seedling defences decreased or did not change over time (Liu et al., 1998; Wallace and Eigenbrode, 2002; Elger et al., 2009), showed a non-linear pattern (Cipollini and Bergelson, 2000; Gianoli, 2002), or cases where trajectories vary among individual secondary compounds as seedlings age (Cipollini and Redman, 1999; Elger et al., 2009). This variation in resource allocation to constitutive chemical defences during seedling development may be due to disparity among studies in what is considered a seedling stage (see Hanley et al., 2004), or it may reflect diverse strategies in resource investment between constitutive and induced defences.
In addition to variation in constitutive chemical defences, conferring baseline resistance to antagonists, plants deploy two basic types of induced defences: (1) resistance traits that aim to reduce subsequent herbivore damage, by generally increasing baseline levels of physical and chemical defences (i.e. induced defences); and (2) tolerance traits that minimize the negative effects of damage on plant fitness by, for example, replacing the tissues lost to herbivores (i.e. compensatory re-growth) (Karban and Baldwin, 1997; Stowe et al., 2000). In particular, during seedling development, pronounced changes in induced defences should be expected given that seedling resource acquisition and storage might be particularly limited after damage, constraining the ability to simultaneously induce chemical defences (leading to induced resistance) and to grow (leading to compensation). In general, it has been predicted that younger stages should be better at inducing chemical defences than at compensating for lost tissue, while older stages are more likely to tolerate damage but show decreased induced resistance capabilities (Karban and Baldwin, 1997; Strauss and Agrawal, 1999; Haukioja and Koricheva, 2000; Cipollini et al., 2003). However, research testing these predictions is inconclusive (Barton and Koricheva, 2010) and trade-offs between resistance and tolerance traits have not been detected in the only study assessing both lines of defence during seedling development (Barton, 2008). Instead, studies examining induced resistance during seedling development have shown all possible responses with some plant species showing induced defences to decrease (Cipollini and Bergelson, 2000; Barton, 2008), increase (Gianoli, 2002) or show compound-specific trends (Cipollini and Redman, 1999) as seedlings age. In contrast, damage early during seedling development seems to have a more consistent long-term impact on plant growth and seed production as compared with damage occurring later during seedling development (Wallace and Eigenbrode, 2002; Hanley and Fegan, 2007) or at later developmental stages (Warner and Cushman, 2002; Del-Val and Crawley, 2005; Hodar et al., 2008). Yet, few studies have simultaneously tested induced resistance and tolerance traits in response to herbivore damage during this vulnerable stage of a plant life cycle.
To investigate the role of seedling developmental stage in constitutive chemical defence, seedling ability to induce chemical defences (induced resistance trait) in response to herbivory, and seedling ability to compensate for leaf tissue lost to herbivory (tolerance trait), we used seedlings to young juvenile stages of the perennial herb, Penstemon virgatus (Plantaginaceae). With >270 described species, the genus Penstemon is one of the largest genera endemic to North America (Wolfe et al., 2006). Widely distributed across most of North America and encompassing a group of morphologically diverse species, adapted to a wide range of environments (Wolfe et al., 2006), Penstemon species have been recognized as a valuable source of novel and common iridoid glycosides (IGs) (Foderaro and Stermitz, 1992; Abdelkader and Stermitz, 1993; Stermitz et al., 1994a, b) among other chemical defences (e.g. Lindroth et al., 1986). Yet, beyond phytochemical description, few studies have focused on the chemical ecology of Penstemon species (but see Lindroth et al., 1986; Stermitz et al., 1988; L'empereur and Stermitz, 1990). In this study, we specifically ask the following two questions: (1) How do constitutive chemical defences (i.e. IGs) vary across seedling development and early juvenile stages of P. virgatus? (2) How does plant age influence the short-term ability of P. virgatus seedlings to induce chemical defences or compensate for moderate levels of damage by a IG-specialist insect herbivore? Finally, we explored whether there was any indirect evidence for a potential trade-off between investment in constitutive and induced defences or between induced defences and compensation in this herbaceous, perennial plant species.
MATERIALS AND METHODS
Study system
Penstemon virgatus A. Gray (Plantaginaceae), upright blue beardtongue, is a long-lived, herbaceous perennial plant native to the south-western United States (i.e. Colorado, Arizona and New Mexico; http://plants.usda.gov/java/profile?symbol=pevi4). This species, mostly found in mountain meadows, pine woods and road cuts, often grows in dry sandy soils. The tall, smooth stems can reach 25–80 cm, with several linear and opposite stem leaves, and its long inflorescences bear several small to medium purple flowers, blooming from June to late August or September. Like other Penstemon species (Foderaro and Stermitz, 1992; Abdelkader and Stermitz, 1993; Stermitz et al., 1994a, b), P. virgatus produces IGs as their primary chemical defences (L'empereur and Stermitz, 1990). Presence of these compounds in plant tissues of related plant species has been shown to influence feeding preferences of a wide range of generalist and specialist insect herbivores (reviewed in Bowers, 1991; Dobler et al., 2011), and several herbivores have been observed to be associated with Penstemon species in natural populations (Stermitz et al., 1988; L'empereur and Stermitz, 1990; Stermitz et al., 1994a; C. Quintero and M. D. Bowers, unpubl. res.). In P. virgatus, two major IGs have been found, catalpol and scutellarioside, with much smaller amounts of globularin and globularicisin (<10 % of total) (L'empereur and Stermitz, 1990). Variation in production of IGs (with an average of 10 % dry weight) has been documented within and among mature individuals of P. virgatus in natural populations (L'empereur and Stermitz, 1990), but IG content has not been documented in seedlings of this long-lived species and the relationship between IGs and plant ontogeny has never been examined.
The buckeye butterfly, Junonia coenia (Nymphalidae), is an oligophagous New World butterfly that can have one to three broods per year under temperate conditions. It has been described as a specialist herbivore of plants containing IGs, commonly feeding on members of six plant families: Cornaceae, Acanthaceae, Plantaginaceae, Scrophulariaceae, Phrymaceae and Verbenaceae (Bowers, 1984). Adult female butterflies use IGs as oviposition stimulants (Pereyra and Bowers, 1988; Klockars et al., 1993; Prudic et al., 2005), and caterpillars not only use IGs as feeding stimulants (Bowers, 1984), but they are also able to sequester these compounds in their hemolymph, rendering them unpalatable to potential predators (Dyer and Bowers, 1996; Theodoratus and Bowers, 1999). Although buckeyes have not been recorded feeding on P. virgatus in nature, they do feed on other Penstemon species (Robinson et al., 2002) and will readily eat P. virgatus in the laboratory. We used this species for the experiment due to its wide availability and ease of rearing under laboratory conditions.
Plant and caterpillar colony maintenance
The study was performed at the University of Colorado during spring–summer 2007. Seeds, obtained from Western Native Seeds (http://www.westernnativeseed.com/seedlist.html), were stratified for 45 d at 5 °C and dark conditions before germination. Four sets of seeds, spaced 1 month apart, from May to August, were used to obtain approx. 50 individuals per age class. The seeds germinated and the plants were grown in a greenhouse, using a growth medium of equal parts Metro Mix 350, sterilized sand and turface. Plants were grown under natural light (i.e. no supplemental lighting was used), and the temperature fluctuated between 18 and 28 °C, following daily and seasonal cycles. After transplanting, plants were grown in 1-L pots to minimize root-binding effects. Thus, ontogenetic changes in constitutive and induced defences were analysed simultaneously across stages, in order to control for potential environmental factors that could influence plant performance such as photoperiod and temperature. This experimental design may have some disadvantages such as dissimilar average environmental conditions from sowing to harvest. Yet, similar to previous studies, this design ensured that all individuals were exposed to the same conditions at the time of harvest (see Quintero and Bowers, 2012). Given that IG concentrations can vary substantially over time (Hogedal and Molgaard, 2000; Fuchs and Bowers, 2004; Quintero and Bowers, 2012) or with environmental factors such as temperature (Tamura, 2001), simultaneous harvests should minimize variables other than plant age that could influence IG content.
Hence, by 28 September, there were four stages: 1-month-old plants with an average of six leaves and with cotyledons still attached; 2-month-old plants with an average of eight leaves and mostly with cotyledons still attached; and 3- and 4-month-old plants with an average of 14 and 20 leaves, respectively, without attached cotyledons. The decision to designate the first two stages as seedlings (1- and 2-month-old plants) and the last two stages as young juvenile stages (3- and 4-month-old plants) is partially based on the work of Hanley et al. (2004), where they proposed the point of attainment, maximum relative growth rate (RGRmax), as the most reliable marker for defining the end of the seedling stage, which may coincide with the exhaustion of cotyledon reserves and the attainment of independence from cotyledons. In our system, as is the case of other epigeal species, the cotyledons act both as a nutrient store and as a photosynthesizing leaf; thus, complete independence may be achieved even after plants have surpassed the seedling stage. Yet, given that we did not measure RGRmax, we decided to use the natural time of cotyledon excision as a defining developmental transition. By taking this approach, we might be overestimating the seedling stage but we are very conservative in defining the young juvenile stages, which certainly are independent from cotyledon reserves and/or function.
Buckeye larvae used in this study were from a laboratory colony reared at the University of Colorado at Boulder. Prior to their use in the experiment, larvae were fed on a mix of wild Plantago lanceolata (Plantaginaceae) leaves harvested from local populations around the University of Colorado and kept in growth chambers with a photoperiod of 14-h day/10-h night, and day/night temperatures of 27/22 °C.
Biomass and constitutive defences across seedling to juvenile development
Above-ground biomass and constitutive defences during seedling to juvenile development were assessed at four age classes: 1-, 2-, 3- and 4-month-old stages. Ten plants per ontogenetic stage were randomly selected and harvested before the induction experiment started (see below). All above-ground tissues were harvested, weighed fresh immediately after being cut, oven-dried at 50 °C for 48 h to a constant mass, and weighed again to the nearest hundredth of a gram. Changes in total dry weight biomass and percentage water content were analysed by a one-way ANOVA, followed by Bonferroni post hoc tests to assess significant differences among the four age classes. Biomass was transformed using the natural logarithm and water content was arcsine square-root transformed to meet assumptions of normality.
To assess variation in concentrations of IGs, dried leaves were ground into a fine powder, and subsamples of 5–30 mg were processed for IG extraction, the entire plant in the case of some seedlings, and analysed by gas chromatography following previously described methods (Bowers and Stamp, 1993; Jarzomski et al., 2000). Briefly, samples were extracted overnight in 95 % methanol, and then partitioned between water and ether to remove hydrophobic compounds, using phenyl-β-d-glucose as an internal standard. An aliquot of the solution was derivatized with Tri-Sil-Z™ (Pierce Chemical Company) and injected into an HP 7890A gas chromatograph (Agilent Technology) using an Agilent DB-1 column (30 m, 0·320 mm, 0·25-μm particle size). We only had standards of catalpol and scutellarioside-II (hereafter scutellarioside), which compose 90 % or more of the total IG content of P. virgatus, thus we were only able to quantify these two compounds. Amounts of catalpol and scutellarioside were quantified using ChemStation B-03-01 software and data were analysed as proportions of dry mass (concentration) and were arcsine transformed to meet assumptions of normality. Given that amounts of catalpol and scutellarioside were not significantly correlated (r = –0·21, n = 40, P = 0·19), changes in constitutive defences across seedling stages, measured as total IGs (= catalpol + scutellarioside) as well as separately for catalpol and scutellarioside, were analysed by a one-way analysis of variance (ANOVA), followed by Bonferroni post hoc tests to assess significant differences among the four age classes.
In addition, we also assessed IG defences in seeds. To have samples large enough to detect trace amounts of IGs in seeds, two sets of seeds from multiple maternal plants were ground together and IG extraction and quantification were performed as described above. Given the small sample size, these data were not statistically analysed and are only presented for qualitative comparative purposes.
Response to herbivory: compensation and induced defences across seedling to juvenile development
To test for compensation and the induction of IGs in P. virgatus during early plant development, 50 individuals per age class (1-, 2- and 3-month-old plants) were randomly assigned to one of two possible treatments: no herbivory (control) or herbivory by J. coenia caterpillars. To account for potential differences among treatments before the experiment started, we measured initial plant size as total number of leaves. Germination rate of the oldest plants (4 months old) was very low as compared with the following groups (3, 2 and 1 month old; data not shown). Therefore, as the sample size of 4-month-old plants was too small, we excluded this age class from this second experiment. All plants, across ages and treatments, were randomly distributed in three 1·2 × 4 m greenhouse benches, with plants being at least 20 cm apart from each other.
Field herbivory rates in adult P. virgatus plants are highly variable depending on the herbivore community present, with field estimates ranging from 5 % to 100 %, if the herbivore community is mostly dominated by generalists or specialist herbivores such as Euphydryas anicia (Nymphalidae), respectively (C. Quintero, unpubl. res.). Therefore, we chose to impose an intermediate level of damage (i.e. 30 %) as a meaningful level of damage probably experienced in natural populations. The herbivory treatment consisted of one to three newly moulted 3rd or 4th instar larvae added to the plants and kept there until they had consumed approx. 30 % of the plant tissues. Larvae were placed on a single plant from 1000 to 1800 h and were visually monitored by six observers who prevented caterpillars from leaving their assigned individual plant until they achieved the desired level of damage, at which time the larvae were removed. If 30 % damage was not achieved during the first day, larvae were removed during the night, and placed back again on the same treatment plant as the previous day until 30 % damage was achieved. The longest time caterpillars were on the plant was 3 d. Damage was estimated to the nearest 10 %, and any plants receiving <20 or >40 % damage were excluded from the analysis.
Because induction of IGs in another IG-containing plant species, Plantago lanceolata, had been shown to reach its highest point 6–7 d after damage (Fuchs and Bowers, 2004), to test for induction, all plants per ontogenetic stage and treatment were harvested 1 week after herbivore removal. Harvested tissues, which comprised all above-ground tissues including damaged leaves and new leaves emerging after the damage treatment, were weighed fresh immediately after being cut, oven-dried at 50 °C for 48 h to a constant mass, and weighed again to the nearest hundredth of a gram. Tissues for IG extraction and quantification were processed as described above.
Independent sample t-tests were used to compare initial plant size (measured as number of leaves) between control (C) and herbivory (H) treatments in each age class, before the herbivory treatments were applied. Following damage, plant responses to herbivory (C versus H) at the three developmental stages were assessed as changes in above-ground biomass (dry mass), percentage water content, total concentration of IGs (% dry weight), and proportion of scutellarioside/total IGs, using a series of two-way analyses of variance (ANOVA) with age and treatment as the main effects and an interaction term included. Given that amounts of catalpol and scutellarioside were not significantly correlated with each other (r = 0·07, n = 134, P = 0·44), we performed separate analyses for each of these dependent variables. In the case of a significant interaction effect, mean group differences among treatments were tested by a priori single degree of freedom contrasts. Biomass was transformed to its natural logarithm, whereas water and IG concentration data were arcsine-square root transformed to meet assumptions of normality.
RESULTS
Biomass and constitutive defences across seedling to juvenile development
Dry weight above-ground biomass (F3,36 = 94·21, P < 0·0001) and water content (F 3,36 = 8·78, P < 0·0001) significantly varied among developmental stages. In particular, biomass increased exponentially as seedlings developed, while water content showed an increasing trend from 1- to 3-month-old stages but decreased back again at 4-month-old juvenile stages (Fig. 1A, B). For IGs, there was no effect of plant age on percentage dry weight scutellarioside (F3,36 = 1·05, P = 0·38) or total IGs (F3,36 = 0·21, P = 0·88), with all stages showing approx. 19 % dry weight total IGs. However, catalpol varied significantly among stages (F3,36 = 2·95, P =0·046) and the proportion of total IGs that was scutellarioside also significantly changed among stages (F3,36 = 3·54, P =0·024), with seedlings investing slightly more in scutellarioside than catalpol at younger than older stages (Fig. 1C). Interestingly, seeds completely lacked scutellarioside, and catalpol levels were on average 4·5 times less in seeds than in young seedling stages (Fig. 1C).
Fig. 1.
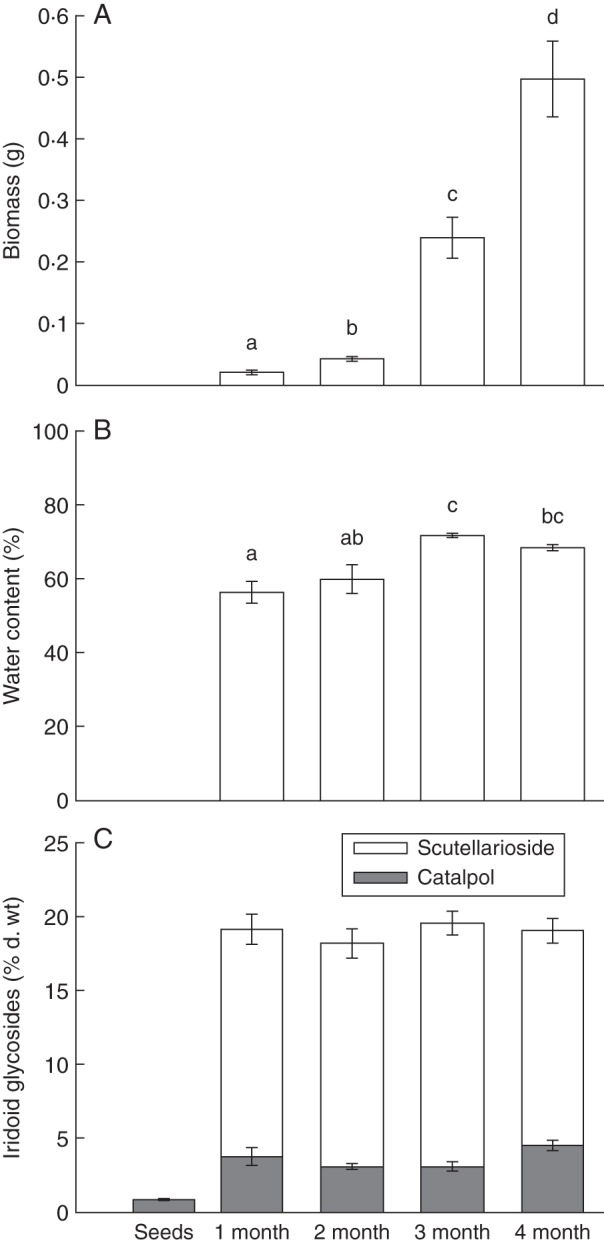
Penstemon virgatus ontogenetic variation (mean ± s.e.) in above-ground biomass (A) and water content (B) across four seedling developmental stages. (C) Percentage dry weight of iridoid glycosides, divided into the two major individual compounds, catalpol and scutellarioside, across four early plant developmental stages and seeds (i.e. 1- and 2-month-old plants are considered seedlings and 3- and 4-month-old plants are considered early juvenile stages). Given the small sample size, seeds (n = 2) were not included in the analyses – the data are shown for illustrative purposes. Letters indicate mean group differences as tested by a Bonferroni post hoc test (P < 0·05).
Response to herbivory: compensation and induced defences across seedling to juvenile development
As expected, at the start of the experiment, size of seedlings assigned to the two herbivory treatments was not significantly different for any of the three developmental stages (P > 0·05 in each case). One week after herbivores were removed, there was a significant effect of both age and herbivory on seedling above-ground biomass, as well as a significant interaction (Table 1). The significant interaction resulted from the fact that, while damaged 1- and 2-month-old seedlings showed no compensatory re-growth, the biomass of damaged 3-month-old juvenile stages did not differ from the biomass achieved by control plants, indicating that young juvenile stages were able to compensate for the 30 % lost tissue in the week following damage while seedlings could not (Fig. 2A). Water content was also significantly affected by plant age and decreased in plants exposed to herbivores (Fig. 2B); however, there was no interaction (Table 1).
Table 1.
Summary of statistical results (ANOVA) comparing Penstemon virgatus responses in above-ground biomass, water content and iridoid glycosides, across three plant developmental stages, 1 week after the herbivores were removed from treatment plants. Significant results (P < 0·05) are highlighted in bold
| Source of variation | d.f. | Biomass (g) |
Water content (%) |
Catalpol (% d. wt) |
Scutellarioside (% d. wt) |
Total IGs (% d. wt) |
Scutellarioside/
total IGs |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | ||
| Herbivory | 1 | 24·1 | 0·0001 | 21·9 | 0·0001 | 0·11 | 0·74 | 8·54 | 0·004 | 6·14 | 0·015 | 1·72 | 0·19 |
| Age | 2 | 550·9 | 0·0001 | 10·7 | 0·0001 | 11·59 | 0·0001 | 10·24 | 0·0001 | 17·83 | 0·0001 | 5·32 | 0·006 |
| Age × herbivory | 2 | 3·33 | 0·04 | 0·32 | 0·73 | 1·37 | 0·26 | 0·44 | 0·64 | 0·07 | 0·93 | 1·77 | 0·17 |
| Error | 128 | ||||||||||||
Fig. 2.
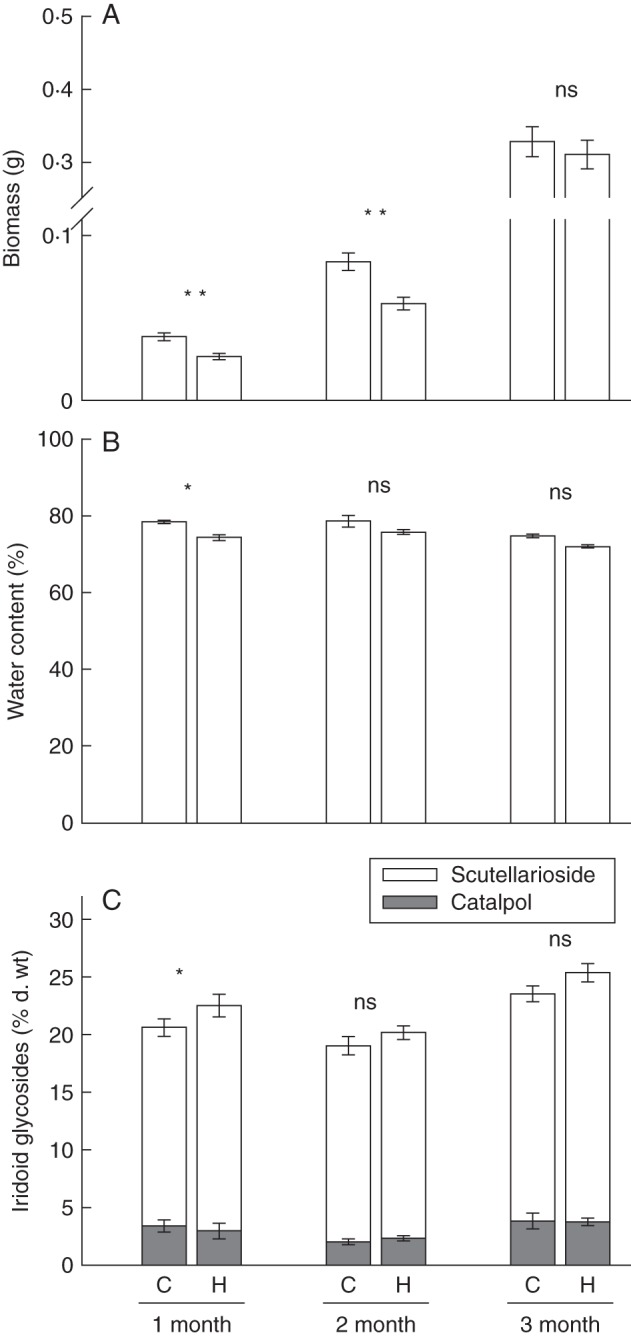
Plant responses to herbivory in Penstemon virgatus above-ground biomass (A) and water content (B). (C) Percentage dry weight of iridoid glycosides, divided into the two major individual compounds, catalpol and scutellarioside, for control (C) and herbivore-damaged plants (H) across three early plant developmental stages (i.e. 1- and 2-month-old plants are considered seedlings and 3-month-old plants are considered early juvenile stages). Asterisks represent mean group differences among treatments as tested by single degree of freedom contrasts. Significance is displayed as: ***, P < 0·001; **, P < 0·01; *, P < 0·05. In panel (C) significance is displayed only for scutellarioside, as the percentage of catalpol did not vary among treatments (see Table 1).
In terms of chemical defences, there was a significant effect of plant age on percentage dry weight of catalpol, scutellarioside, total IGs and proportion of total IGs that was scutellarioside (Fig. 2C and Table 1). This is particularly interesting, because it differs from the results of the first experiment, which showed no effect of plant age on IGs. This implies that, as plants grow, the plant age effect may become more or less detectable as a source of variation driving plant resource allocation to chemical defences. In this case, 1 week after we assessed constitutive defences, we saw that some stages invested more in IG production (e.g. at 3 months old) than others (e.g. at 2 months old). Yet, given the relatively small difference in IG production among the three developmental stages (i.e. average of 3 % d. wt difference), we believe the statistical significance in this second experiment might be due to a larger sample size (n = 50 individuals/stage) than in the previous experiment (n = 10 individuals/stage). In turn, herbivory resulted in an increase in scutellarioside and total IGs, but not catalpol or the proportion of total IGs that was scutellarioside (Table 1). These increases were seen across all age classes (Fig. 2C). Hence, there were no significant interactions between herbivory and plant age, indicating that all age classes showed a similar response in terms of induced chemical defences after herbivory.
DISCUSSION
This study uncovered interesting patterns in plant investment in constitutive and induced defensive traits during one of the most vulnerable stages of a plants' life cycle. Importantly, our results demonstrated that Penstemon virgatus seedlings are highly defended (approx. 20 % dry weight total IGs) and that, in general, investment in constitutive defences did not vary throughout the observed period of seedling to juvenile development, while responses to herbivore damage did vary slightly during the same period. Particularly, in this perennial plant species, high levels of constitutive defences did not limit the ability of young developmental stages to induce chemical defences following damage, although this response may come with a cost (i.e. decreased compensation) in younger seedling stages.
Constitutive defences during seedling to juvenile development
A recent review paper assessing ontogenetic patterns in plant defence by means of meta-analysis concluded that one of the clearest trends across ontogeny, for both woody plants and herbs, was the dramatic increase in constitutive levels of all classes of chemical defences through the seedling stage (Barton and Koricheva, 2010). In contrast, here we showed that, while P. virgatus seedlings increased their above-ground biomass 50 times from 1- to 4-month-old stages, investment in constitutive defences did not vary, showing an average of total IGs slightly below 20 % dry weight across stages. A few other perennial herbs have also shown no change in concentrations of constitutive chemical defences over seedling development such as pyrrolizidine alkaloids in Senecio jacobaea (Schaffner et al., 2003), alkaloids in Lotus corniculatus and Trifolium repens (Elger et al., 2009) and phenolics in Leucanthemum vulgare and Taraxacum officinale (Elger et al., 2009). Nonetheless, what is more surprising is the exceptionally high levels of defences during this early developmental phase (20 % d. wt), which is only comparable with a few other woody boreal species that experience high rates of winter mammalian herbivory (Swihart and Bryant, 2001). This surprisingly high investment in early constitutive resistance may have been favoured under scenarios with significant rates of herbivore damage, especially during early spring, and/or with high inter-/intra-specific competition (see, for example, Boege, 2010).
Synthesizing and maintaining a high concentration of chemical defences throughout most of this vulnerable stage might be highly adaptive in the case of long-lived perennial plant species, such as P. virgatus. Besides the damage caused by generalist herbivores (mainly deer and generalist insect herbivores such as grasshoppers and aphids; C. Quintero, pers. obs.), Penstemon species are often damaged by IG-specialist herbivores such as the checkerspot butterflies [Poladryas spp. and Euphydryas spp. (Nymphalidae); L'empereur and Stermitz, 1990; Stermitz et al., 1994a, and references therein]. These species, which are univoltine or bivoltine, often overwinter as immature larvae (typically 4th instar) and so, when they emerge from diapause in the spring, these larvae consume young Penstemon plants (L'empereur and Stermitz, 1990). Hence, achieving higher levels of constitutive defences early in development might be critical for this plant species exposed to highly mobile specialist caterpillars searching for hosts. In addition, seedlings consistently invested more in scutellarioside than catalpol, showing a 3 : 1 ratio (Fig. 1C), which might also be adaptive. For both generalist and specialist herbivores, qualitative changes in plant defence compounds can be as important as quantitative changes (Dobler et al., 2011). Thus, lower levels of catalpol may alter the attractiveness of these seedlings for specialist caterpillars.
Although this trend needs further field and laboratory support, preliminary data suggest that key changes in constitutive ontogenetic trajectories in plant defences may occur before and after the developmental window observed here. First, a small sample size looking at seed defences showed that P. virgatus seeds are defended by low levels of catalpol, and completely lacked the catalpol ester, scutellarioside (Fig. 1C). Hence, it would be particularly interesting to study the period between seed germination and 1-month-old seedlings to assess the point at which defences in seeds are depleted and seedlings become biosynthetically active, as well as when seedlings start to invest significantly more in scutellarioside as compared with catalpol. In addition, the pattern shown for the early development of P. virgatus does not agree with the non-linear model proposed by Boege and Marquis (2005), nor does it agree with the overall trends reported by Barton and Koricheva (2010). This may be due to the fact that we were only looking at a small portion of the life cycle of this long-lived perennial, or it may reflect the importance of a high investment in defence of the early developmental stages of such long-lived herbaceous plants. Also, another change in ontogenetic trajectories is possible between seedling and mature stages, since leaves of P. virgatus reproductive plants in the field have shown to contain approx. 10 % total IGs (L'Empereur and Stermitz, 1990). Further research is needed to confirm this potential decrease in IG content as P. virgatus age, since current knowledge about adult defences is based on just three field individuals (L'empereur and Stermitz, 1990). Nonetheless, potential non-linear changes in IGs in P. virgatus over its entire life-cycle is highly likely given that several IG-containing plant species have shown such trends (Hogedal and Molgaard, 2000; Beninger et al., 2009; Quintero and Bowers, 2012).
Induced defences during seedling to juvenile development
In contrast to previous studies that have focused on either induced resistance (Cipollini and Redman, 1999; Cipollini and Bergelson, 2000; Gianoli, 2002) or tolerance traits (Weltzin et al., 1998; Wallace and Eigenbrode, 2002; Del-Val and Crawley, 2005; Hanley and Fegan, 2007; Hodar et al., 2008) during the critical phase of seedling development, our study is one of the first ones to report the simultaneous expression of resistance (i.e. induced chemical defences) and tolerance (i.e. compensatory re-growth) traits across seedling to young juvenile stages (see also Barton, 2008; Muola et al., 2010). Moreover, our results provide mixed support for the overall trends described by Barton and Koricheva (2010), which concluded that, while the ability to induce chemical defences decreases during seedling development in herbs, tolerance capabilities did not vary for either herbs or woody species.
In accordance with these overall trends, our study showed that the compensatory ability of P. virgatus increased only after the transition from seedling to juvenile stages. In particular, 1 week following 30 % above-ground tissue loss, 1- and 2-month-old seedlings exposed to herbivores were still approx. 30 % smaller than control plants whereas herbivore-damaged 3-month-old juvenile stages did not significantly vary in total above-ground biomass from controls (Fig. 2A). This higher tolerance capability in older stages agrees with previous expectations (Strauss and Agrawal, 1999; Haukioja and Koricheva, 2000), and is interpreted as being a consequence of a greater number of available meristems, smaller root to shoot ratios, greater capacity to acquire resources, and higher probability of mobilization of stored reserves at older developmental stages. Yet, because we only evaluated above-ground biomass, we cannot neglect the possibility that (a) in seedlings, resources were allocated to root biomass following damage or that (b) in young juvenile stages, compensation was achieved at the expense of root biomass. Allocation of resources to root tissues to enhance nutrient uptake needed for the production of new photosynthetic tissue has been reported previously (Orians et al., 2011), as well as above-ground compensation at the expense of belowground biomass (e.g. Barton, 2008); hence, future studies should confirm these possibilities.
In terms of induced chemical defences, we did not observe the predicted decrease in induced defences with plant age. Instead, we observed that damaged plants in all three stages increased their IG concentration by approx. 8 % compared with control plants, and that this induced response was driven by an increase in concentrations of scutellarioside but not catalpol across stages. Compound-specific trends following damage as seedlings age was previously reported in tomato plants (Cipollini and Redman, 1999). Yet, a similar capability to induce defences from seedling to juvenile stages has not been previously reported. In general, earlier studies showed that seedlings tended to increase (Gianoli, 2002; Doan et al., 2004) or decrease (Cipollini and Bergelson, 2000; Barton, 2008) their induced response as they age. Nonetheless, it is important to note that all the above studies differ in the developmental window assessed compared with the present study, making comparisons among them hard to interpret. Hence, given the reduced number of studies looking at herbivore-induced defences during early plant development as compared with adult stages, the uniqueness of this observed trend or its adaptive significance warrants further testing.
It has been long proposed that plants often incur trade-offs between investment in constitutive and induced defences or between resistance and tolerance traits. While the former had received some support (reviewed in Koricheva et al., 2004; Kempel et al., 2011), it is becoming clear that defensive strategies following damage might not be mutually exclusive (reviewed in Leimu and Koricheva, 2006; Nunez-Farfan et al., 2007). In seedlings, there is some evidence that such trade-offs do occur (Gianoli, 2002; Kuhlmann and Muller, 2009; Orians et al., 2010; Zust et al., 2011), but assessment of trade-offs following damage, between chemical defences (leading to induced resistance) and growth (leading to compensation) are scarce. To date, only one study has tested both induction and compensation across seedling stages, and that did not find a trade-off between these two lines of defence (Barton, 2008). Our experimental design, which lacks within genetic-family variation (e.g. Boege et al., 2007; Barton, 2008) and uses destructive sampling, precluded us from properly evaluating the association between different lines of defences (Mole, 1994). Nonetheless, our results provide indirect evidence suggesting that, while no trade-off between constitutive and induced IG defences during the developmental window assessed here is likely (given no statistical difference in either constitutive or induced IGs across stages), trade-offs between resistance and tolerance traits may arise at some but not all developmental stages. In our study, P. virgatus seedling to juvenile stages showed a similar level of constitutive defences and a comparable induced resistance response across stages, but the ability to compensate for the lost tissue increased as plants aged. In particular, we showed that while young juvenile stages were able to increase chemical defences and compensate for the 30 % lost tissue 1 week following damage, seedlings were incapable of investing in both defensive strategies, prioritizing chemical defences over compensatory re-growth. Hence, this comparable ability to induce defences across stages may limit resource allocation to growth and, thus, compensation to damage early but not late during development, indicating that often trade-offs may be transient (see, for example, Orians et al., 2010) and, thus, difficult to detect.
In conclusion, the present work illustrated that P. virgatus plants were well defended during most of the transition from seedling to early juvenile stages, reaching a constant approx. 20 % dry weight total IGs. These surprisingly high levels of defence in young stages, expected to be allocating most of their resources towards growth and stored reserves for overwintering and future reproduction, highlights the potential role of herbivores in shaping ontogenetic trajectories in plant defences, as seen in other woody species exposed to high levels of damage early on in their life cycles (Swihart and Bryant, 2001). Furthermore, following moderate damage by specialist herbivores, all seeding and juvenile stages were able to increase their IG concentration by approx. 8 %, while compensatory re-growth was only achieved at later stages but not in seedlings, suggesting that induction during early plant development may come with a cost, and trade-offs between these two strategies may arise at some but not all developmental stages. More empirical studies looking at several constitutive and induced defensive traits over this critical phase of plant development would help us to understand the selective pressures shaping early ontogenetic trajectories. Specifically, studies addressing how natural selection acts on particular plastic responses and on their combinations, depending on the ecological context in which plants grow (e.g. competition and herbivory; Boege, 2010), will shed light on what might be the combination of plastic responses most favoured by selection during plant establishment across plant species.
ACKNOWLEDGEMENTS
We thank K. E. Barton, M. E. Hanley and two anonymous reviewers for valuable comments and suggestions on this manuscript. In addition, we gratefully acknowledge L. Mulder, A. Hill, M. Tapy, E. Lynch and T. J. Lemieux for greenhouse and laboratory assistance. This work was supported by the Undergraduate Research Opportunity Program, the Department of Ecology and Evolutionary Biology at the University of Colorado, and a National Science Foundation grant (DEB 0909717).
LITERATURE CITED
- Abdelkader MS, Stermitz FR. Iridoid and other glycosides from Penstemon species. Phytochemistry. 1993;34:1367–1371. [Google Scholar]
- Albrectsen BR, Gardfjell H, Orians CM, Murray B, Fritz RS. Slugs, willow seedlings and nutrient fertilization: intrinsic vigor inversely affects palatability. Oikos. 2004;105:268–278. [Google Scholar]
- Alvarez-Clare S, Kitajima K. Susceptibility of tree seedlings to biotic and abiotic hazards in the understory of a moist tropical forest in Panama. Biotropica. 2009;41:47–56. [Google Scholar]
- Barton KE. Early ontogenetic patterns in chemical defense in Plantago (Plantaginaceae): genetic variation and trade-offs. American Journal of Botany. 2007;94:56–66. doi: 10.3732/ajb.94.1.56. [DOI] [PubMed] [Google Scholar]
- Barton KE. Phenotypic plasticity in seedling defense strategies: compensatory growth and chemical induction. Oikos. 2008;117:917–925. [Google Scholar]
- Barton KE, Koricheva J. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. American Naturalist. 2010;175:481–493. doi: 10.1086/650722. [DOI] [PubMed] [Google Scholar]
- Beninger CW, Cloutier RR, Grodzinski B. A comparison of antirrhinoside distribution in the organs of two related Plantaginaceae species with different reproductive strategies. Journal of Chemical Ecology. 2009;35:1363–1372. doi: 10.1007/s10886-009-9715-4. [DOI] [PubMed] [Google Scholar]
- Boege K. Induced responses to competition and herbivory: natural selection on multi-trait phenotypic plasticity. Ecology. 2010;91:2628–2637. doi: 10.1890/09-0543.1. [DOI] [PubMed] [Google Scholar]
- Boege K, Marquis RJ. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Boege K, Dirzo R, Siemens D, Brown P. Ontogenetic switches from plant resistance to tolerance: minimizing costs with age? Ecology Letters. 2007;10:177–187. doi: 10.1111/j.1461-0248.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- Bowers MD. Iridoid glycosides and host-plant specificity in larvae of the Buckeye butterfly, Junonia coenia (Nymphalidae) Journal of Chemical Ecology. 1984;10:1567–1577. doi: 10.1007/BF00988425. [DOI] [PubMed] [Google Scholar]
- Bowers MD. Iridoid glycosides. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: their interactions with secondary plant metabolites. San Diego, CA: Academic Press; 1991. pp. 297–326. [Google Scholar]
- Bowers MD, Stamp NE. Effects of plant-age, genotype, and herbivory on Plantago performance and chemistry. Ecology. 1993;74:1778–1791. [Google Scholar]
- Buschmann H, Keller M, Porret N, Dietz H, Edwards PJ. The effect of slug grazing on vegetation development and plant species diversity in an experimental grassland. Functional Ecology. 2005;19:291–298. [Google Scholar]
- Cipollini DF, Bergelson J. Environmental and developmental regulation of trypsin inhibitor activity in Brassica napus. Journal of Chemical Ecology. 2000;26:1411–1422. [Google Scholar]
- Cipollini DF, Redman AM. Age-dependent effects of jasmonic acid treatment and wind exposure on foliar oxidase activity and insect resistance in tomato. Journal of Chemical Ecology. 1999;25:271–281. [Google Scholar]
- Cipollini D, Purrington CB, Bergelson J. Costs of induced responses in plants. Basic and Applied Ecology. 2003;4:79–89. [Google Scholar]
- Del-Val EK, Crawley MJ. Are grazing increaser species better tolerators than decreasers? An experimental assessment of defoliation tolerance in eight British grassland species. Journal of Ecology. 2005;93:1005–1016. [Google Scholar]
- Doan AT, Ervin G, Felton G. Temporal effects on jasmonate induction of anti-herbivore defense in Physalis angulata: seasonal and ontogenetic gradients. Biochemical Systematics and Ecology. 2004;32:117–126. [Google Scholar]
- Dobler S, Petschenka G, Pankoke H. Coping with toxic plant compounds: the insect's perspective on iridoid glycosides and cardenolides. Phytochemistry. 2011;72:1593–1604. doi: 10.1016/j.phytochem.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Dyer LA, Bowers MD. The importance of sequestered iridoid glycosides as a defense against an ant predator. Journal of Chemical Ecology. 1996;22:1527–1539. doi: 10.1007/BF02027729. [DOI] [PubMed] [Google Scholar]
- Elger A, Lemoine DG, Fenner M, Hanley ME. Plant ontogeny and chemical defence: older seedlings are better defended. Oikos. 2009;118:767–773. [Google Scholar]
- Fayolle A, Violle C, Navas ML. Differential impacts of plant interactions on herbaceous species recruitment: disentangling factors controlling emergence, survival and growth of seedlings. Oecologia. 2009;159:817–825. doi: 10.1007/s00442-008-1254-0. [DOI] [PubMed] [Google Scholar]
- Fenner M, Hanley ME, Lawrence R. Comparison of seedling and adult palatability in annual and perennial plants. Functional Ecology. 1999;13:546–551. [Google Scholar]
- Foderaro TA, Stermitz FR. Iridoid glycosides from Penstemon species: a C-5, C-9 trans iridoid and C-8 epimeric pairs. Phytochemistry. 1992;31:4191–4195. [Google Scholar]
- Fritz RS, Hochwender CG, Lewkiewicz DA, Bothwell S, Orians CM. Seedling herbivory by slugs in a willow hybrid system: developmental changes in damage, chemical defense, and plant performance. Oecologia. 2001;129:87–97. doi: 10.1007/s004420100703. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Bowers MD. Patterns of iridoid glycoside production and induction in Plantago lanceolata and the importance of plant age. Journal of Chemical Ecology. 2004;30:1723–1741. doi: 10.1023/b:joec.0000042398.13765.83. [DOI] [PubMed] [Google Scholar]
- Gianoli E. A phenotypic trade-off between constitutive defenses and induced responses in wheat seedlings. Ecoscience. 2002;9:482–488. [Google Scholar]
- Goodger JQD, Choo TYS, Woodrow IE. Ontogenetic and temporal trajectories of chemical defence in a cyanogenic eucalypt. Oecologia. 2007;153:799–808. doi: 10.1007/s00442-007-0787-y. [DOI] [PubMed] [Google Scholar]
- Gregianini TSA, Porto DD, Do Nascimento NC, Fett JP, Henriques AT, Fett-Neto AG. Environmental and ontogenetic control of accumulation of brachycerine, a bioactive indole alkaloid from Psychotria brachyceras. Journal of Chemical Ecology. 2004;30:2023–2036. doi: 10.1023/b:joec.0000045592.24785.33. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Fegan EL. Timing of cotyledon damage affects growth and flowering in mature plants. Plant, Cell and Environment. 2007;30:812–819. doi: 10.1111/j.1365-3040.2007.01671.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB. Herbivory, serotiny and seedling defence in Western Australian Proteaceae. Oecologia. 2001;126:409–417. doi: 10.1007/s004420000538. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. Impacts of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany. 2009;103:1347–1353. doi: 10.1093/aob/mcp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Whibley H, Darvill B. Early plant growth: identifying the end point of the seedling phase. New Phytologist. 2004;163:61–66. doi: 10.1111/j.1469-8137.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- Haukioja E, Koricheva J. Tolerance to herbivory in woody vs. herbaceous plants. Evolutionary Ecology. 2000;14:551–562. [Google Scholar]
- Hodar JA, Zamora R, Castro J, Gomez JM, Garcia D. Biomass allocation and growth responses of Scots pine saplings to simulated herbivory depend on plant age and light availability. Plant Ecology. 2008;197:229–238. [Google Scholar]
- Hogedal BD, Molgaard P. HPLC analysis of the seasonal and diurnal variation of iridoids in cultivars of Antirrhinum majus. Biochemical Systematics and Ecology. 2000;28:949–962. doi: 10.1016/s0305-1978(00)00045-4. [DOI] [PubMed] [Google Scholar]
- Jarzomski CM, Stamp NE, Bowers MD. Effects of plant phenology, nutrients and herbivory on growth and defensive chemistry of plantain. Plantago lanceolata. Oikos. 2000;88:371–379. [Google Scholar]
- Karban R, Baldwin IT. Induced responses to herbivory. Chicago, IL: University of Chicago Press; 1997. [Google Scholar]
- Kempel A, Schadler M, Chrobock T, Fischer M, van Kleunen M. Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proceedings of the National Academy of Sciences of the USA. 2011;108:5685–5689. doi: 10.1073/pnas.1016508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockars GK, Bowers MD, Cooney B. Leaf variation in iridoid glycoside content of Plantago lanceolata (Plantaginaceae) and oviposition of the buckeye, Junonia coenia (Nymphalidae) Chemoecology. 1993;4:72–78. [Google Scholar]
- Koricheva J, Nykanen H, Gianoli E. Meta-analysis of trade-offs among plant antiherbivore defenses: are plants jacks-of-all-trades, masters of all? American Naturalist. 2004;163:E64–E75. doi: 10.1086/382601. [DOI] [PubMed] [Google Scholar]
- Kuhlmann F, Muller C. Development-dependent effects of UV radiation exposure on broccoli plants and interactions with herbivorous insects. Environmental and Experimental Botany. 2009;66:61–68. [Google Scholar]
- Leimu R, Koricheva J. A meta-analysis of tradeoffs between plant tolerance and resistance to herbivores: combining the evidence from ecological and agricultural studies. Oikos. 2006;112:1–9. [Google Scholar]
- L'empereur KM, Stermitz FR. Chemistry of the Scrophulariaceae. 14. Iridoid glycoside metabolism and sequestration by Poladryas minuta (Lepidoptera, Nymphalidae) feeding on Penstemon virgatus (Scriphulariaceae) Journal of Chemical Ecology. 1990;16:1495–1506. doi: 10.1007/BF01014084. [DOI] [PubMed] [Google Scholar]
- Lindroth RL, Batzli GO, Seigler DS. Patterns in the phytochemistry of three prairie plants. Biochemical Systematics and Ecology. 1986;14:597–602. [Google Scholar]
- Liu ZJ, Carpenter SB, Bourgeois WJ, et al. Variations in the secondary metabolite camptothecin in relation to tissue age and season in Camptotheca acuminata. Tree Physiology. 1998;18:265–270. doi: 10.1093/treephys/18.4.265. [DOI] [PubMed] [Google Scholar]
- Mangan SA, Schnitzer SA, Herre EA, et al. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature. 2010;466:752–756. doi: 10.1038/nature09273. [DOI] [PubMed] [Google Scholar]
- Miller TEX, Rudolf VHW. Thinking inside the box: community-level consequences of stage-structured populations. Trends in Ecology and Evolution. 2011;26:457–466. doi: 10.1016/j.tree.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Mole S. Trade-offs and constraints in plant-herbivore defense theory: a life-history perspective. Oikos. 1994;71:3–12. [Google Scholar]
- Moles AT, Westoby M. What do seedlings die from and what are the implications for evolution of seed size? Oikos. 2004;106:193–199. [Google Scholar]
- Muola A, Mutikainen P, Laukkanen L, Lilley M, Leimu R. Genetic variation in herbivore resistance and tolerance: the role of plant life-history stage and type of damage. Journal of Evolutionary Biology. 2010;23:2185–2196. doi: 10.1111/j.1420-9101.2010.02077.x. [DOI] [PubMed] [Google Scholar]
- Nunez-Farfan J, Fornoni J, Valverde PL. The evolution of resistance and tolerance to herbivores. Annual Reviews of Ecology, Evolution, and Systematics. 2007;38:541–566. [Google Scholar]
- Orians CM, Hochwender CG, Fritz RS, Snall T. Growth and chemical defense in willow seedlings: trade-offs are transient. Oecologia. 2010;163:283–290. doi: 10.1007/s00442-009-1521-8. [DOI] [PubMed] [Google Scholar]
- Orians CM, Thorn A, Gomez S. Herbivore-induced resource sequestration in plants: why bother? Oecologia. 2011;167:1–9. doi: 10.1007/s00442-011-1968-2. [DOI] [PubMed] [Google Scholar]
- Pereyra PC, Bowers MD. Iridoid glycosides as oviposition stimulants for the Buckeye butterfly, Junonia coenia (Nymphalidae) Journal of Chemical Ecology. 1988;14:917–928. doi: 10.1007/BF01018783. [DOI] [PubMed] [Google Scholar]
- Prudic KL, Oliver JC, Bowers MD. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia. 2005;143:578–587. doi: 10.1007/s00442-005-0008-5. [DOI] [PubMed] [Google Scholar]
- Quintero C, Bowers MD. Changes in plant chemical defenses and nutritional quality as a function of ontogeny in Plantago lanceolata (Plantaginaceae) Oecologia. 2012;168:471–481. doi: 10.1007/s00442-011-2114-x. [DOI] [PubMed] [Google Scholar]
- Robinson GS, Ackery PR, Kitching IJ, Beccaloni GW, Hernandez LM, editors. Hostplants of the moth and butterfly caterpillars of America North of Mexico. Gainesville, FL: The American Entomological Institute; 2002. [Google Scholar]
- Santiago LS, Wright SJ, Harms KE, et al. Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. Journal of Ecology. 2012;100:309–316. [Google Scholar]
- Schaffner U, Vrieling K, van der Meijden E. Pyrrolizidine alkaloid content in Senecio: ontogeny and developmental constraints. Chemoecology. 2003;13:39–46. [Google Scholar]
- Stermitz FR, Gardner DR, McFarland N. Iridoid glycoside sequestration by two aposematic Penstemon-feeding goemetrid larvae. Journal of Chemical Ecology. 1988;14:435–441. doi: 10.1007/BF01013895. [DOI] [PubMed] [Google Scholar]
- Stermitz FR, Abdelkader MS, Foderaro TA, Pomeroy M. Chemistry of the Scrophulariaceae. 29. Iridoid glycosides from some butterflies and their larval food plants. Phytochemistry. 1994a;37:997–999. [Google Scholar]
- Stermitz FR, Blokhin A, Poley CS, Krull RE. Iridoid glycosides of additional Penstemon species. Phytochemistry. 1994b;37:1283–1286. [Google Scholar]
- Stowe KA, Marquis RJ, Hochwender CG, Simms EL. The evolutionary ecology of tolerance to consumer damage. Annual Review of Ecology and Systematics. 2000;31:565–595. [Google Scholar]
- Strauss SY, Agrawal AA. The ecology and evolution of plant tolerance to herbivory. Trends in Ecology & Evolution. 1999;14:179–185. doi: 10.1016/s0169-5347(98)01576-6. [DOI] [PubMed] [Google Scholar]
- Swihart RK, Bryant JP. Importance of biogeography and ontogeny of woody plants in winter herbivory by mammals. Journal of Mammalogy. 2001;82:1–21. [Google Scholar]
- Tamura Y. Effects of temperature, shade, and nitrogen application on the growth and accumulation of bioactive compounds in cultivars of Plantago lanceolata L. Japanese Journal of Crop Science. 2001;70:548–553. [Google Scholar]
- Terborgh J. Enemies maintain hyperdiverse tropical forests. American Naturalist. 2012;179:303–314. doi: 10.1086/664183. [DOI] [PubMed] [Google Scholar]
- Theodoratus DH, Bowers MD. Effects of sequestered iridoid glycosides on prey choice of the prairie wolf spider, Lycosa carolinensis. Journal of Chemical Ecology. 1999;25:283–295. [Google Scholar]
- Wallace SK, Eigenbrode SD. Changes in the glucosinolate-myrosinase defense system in Brassica juncea cotyledons during seedling development. Journal of Chemical Ecology. 2002;28:243–256. doi: 10.1023/a:1017973005994. [DOI] [PubMed] [Google Scholar]
- Warner PJ, Cushman JH. Influence of herbivores on a perennial plant: variation with life history stage and herbivore species. Oecologia. 2002;132:77–85. doi: 10.1007/s00442-002-0955-z. [DOI] [PubMed] [Google Scholar]
- Weltzin JE, Archer SR, Heitschmidt RK. Defoliation and woody plant (Prosopis glandulosa) seedling regeneration: potential vs realized herbivory tolerance. Plant Ecology. 1998;138:127–135. [Google Scholar]
- Wolfe AD, Randle CP, Datwyler SL, Morawetz JJ, Arguedas N, Diaz J. Phylogeny, taxonomic affinities, and biogeography of Penstemon (Plantaginaceae) based on ITS and cpDNA sequence data. American Journal of Botany. 2006;93:1699–1713. doi: 10.3732/ajb.93.11.1699. [DOI] [PubMed] [Google Scholar]
- Zust T, Joseph B, Shimizu KK, Kliebenstein DJ, Turnbull LA. Using knockout mutants to reveal the growth costs of defensive traits. Proceedings of The Royal Society B – Biological Sciences. 2011;278:2598–2603. doi: 10.1098/rspb.2010.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]


