Abstract
Background
Many tropical forest tree species delay greening their leaves until full expansion. This strategy is thought to provide newly flushing leaves with protection against damage by herbivores by keeping young leaves devoid of nutritive value. Because young leaves suffer the greatest predation from invertebrate herbivores, delayed greening could prevent costly tissue loss. Many species that delay greening also produce anthocyanin pigments in their new leaves, giving them a reddish tint. These anthocyanins may be fungicidal, protect leaves against UV damage or make leaves cryptic to herbivores blind to the red part of the spectrum.
Methods
A comprehensive survey was undertaken of seedlings, saplings and mature trees in two diverse tropical forests: a rain forest in western Amazonia (Yasuní National Park, Ecuador) and a deciduous forest in Central America (Barro Colorado Island, Panamá). A test was made of whether individuals and species with delayed greening or red-coloured young leaves showed lower mortality or higher relative growth rates than species that did not.
Key results
At both Yasuní and Barro Colorado Island, species with delayed greening or red young leaves comprised significant proportions of the seedling and tree communities. At both sites, significantly lower mortality was found in seedlings and trees with delayed greening and red-coloured young leaves. While there was little effect of leaf colour on the production of new leaves of seedlings, diameter relative growth rates of small trees were lower in species with delayed greening and red-coloured young leaves than in species with regular green leaves, and this effect remained when the trade-off between mortality and growth was accounted for.
Conclusions
Herbivores exert strong selection pressure on seedlings for the expression of defence traits. A delayed greening or red-coloured young leaf strategy in seedlings appears to be associated with higher survival for a given growth rate, and may thus influence the species composition of later life stages.
Keywords: Delayed greening, seedling herbivory, young leaf colour, seedlings, tropical forest
INTRODUCTION
Viewed at most periods of the growing season, many of the world's terrestrial ecosystems appear green and verdant. However, this lush veneer belies the intense pressure that many biomes face from herbivores and other primary consumers that consume vast amounts of plant material – upwards of 20 % of net primary productivity every year (Agrawal, 2011). Seedlings are particularly vulnerable to attack because they are often less defended and lignified than mature plants, and the loss of even one leaf has a greater impact than in larger plants (Eichhorn et al., 2010), especially in low-light environments such as a forest understorey. Understanding how life history strategies and seedling traits drive the transition from seedling to adult remains a key question in evolution and ecology (Harms et al., 2000; Wright et al., 2010).
Although all plant parts are potential meals, the leaves, often the softest parts of a seedling, are attacked by a myriad of organisms, from sap-sucking, chewing and mining insects (Novotny et al., 2010), to terrestrial mammals (Bodmer, 1989; Leigh, 1997) and pathogens (Barone, 1994). This ‘predation’ is a strong selective pressure that has led to the evolution of a wide array of mechanisms in seedlings (and adult plants) aimed at preventing or inhibiting tissue loss (Agrawal et al., 2012). These mechanisms include physical defences such as hairs and spines to discourage herbivores from accessing leaves (Gowda, 1996; Hanley et al., 2007), mechanical defences such as toughened leaf tissues to slow chewing and digestion (Coley, 1983; Wright and Vincent, 1996; Lucas et al., 2000), chemical defences to slow growth or even directly poison herbivores (Kursar and Coley, 2003; Kursar et al., 2009), as well as mutualisms with animals that protect their host plant from herbivores (Janzen, 1966; Brenes-Arguedas et al., 2008). Furthermore, leaves vary in their defences during ontogeny (Lee and Collins, 2001), but it is when they are newly flushed and expanding that leaves are at their most vulnerable because few of the defences listed above are available and the young leaves are relatively nutrient rich (Coley, 1983; Coley and Kursar, 1996). As such, the young expanding leaves of seedlings often endure the highest levels of herbivory of any plant part, suffering up to 100 times greater levels of damage than mature leaves (Coley and Aide, 1996).
A different suite of defences have evolved to decrease leaf loss at this critical period of leaf expansion. Many species show wide variation in phenology, abundance, and speed of new leaf development and expansion. Some quickly expand and toughen up their new leaves (Coley, 1983; Kursar and Coley, 2003). Other species withhold greening their leaves until full expansion, either by delayed chlorophyll synthesis or by delayed development of the chloroplasts themselves (Whatley, 1992; Coley and Kursar, 1996; Hughes et al., 2007). This strategy is thought to minimize the impact of herbivore damage since leaves with delayed greening have less energy and nitrogen resources compared with a normally greening leaf (Kursar and Coley, 2003). Furthermore, many woody tropical species (that delay greening or not) also produce anthocyanin pigments in their new leaves, giving them a red or occasionally blue colour (Dominy et al., 2002; Kursar and Coley, 1992a). Various hypotheses have been put forward to explain this phenomenon, including rendering leaves cryptic to herbivores blind to red wavelengths of the spectrum, a fungicidal effect or even protection against UV damage (Dominy et al., 2002). While a number of studies have examined the physiological and leaf-level differences between species that flush green, pale or red leaves (Coley and Aide, 1989; Kursar and Coley, 1992a, b, c; Coley and Kursar, 1996), whether leaf colour is correlated with plant performance is unknown.
Although leaf colour is unlikely actually to protect the plant from UV damage (Dominy et al., 2002), incident light has other profound effects on plant life history. In light-limited environments such as tropical forests, a strong axis of niche differentiation with respect to growth in high light vs. survival in deep shade has been demonstrated for a large number of tree species (Swaine and Whitmore, 1988; Welden et al., 1991; Brokaw and Busing, 2000). Relatively few species are true pioneers, only germinating and growing in high light (e.g. Cecropia), but many species persist at some point along the light-demanding–shade-tolerant axis (Wright et al., 2003). A number of traits are predicted to be correlated with this light availability axis. Among these traits, seed size, leaf mass per area, wood density and maximum height are all predicted to be lower for fast-growing light-demanding species compared with slow-growing shade-tolerant species (Wright et al., 2010). It is likely that defence traits are no exception. Leaves should be well defended if the cost of loss is greater than the cost of replacement or the cost of decreased photosynthetic capacity (Coley et al., 1985). Supporting this prediction, forest understorey species, as well as those of nutrient-poor soils, generally have long-lived and tough leaves (Coley, 1983; Turner et al., 1993; Fine et al., 2006; Kitajima and Poorter, 2010; Kitajima et al., 2012). However, few authors have considered how other defence traits such as leaf colour vary between species along this spectrum of growth and mortality, apart from the original study of Kursar and Coley (1992a). Specifically, there has been no exploration of how the dramatic difference in coloration of expanding leaves is associated with the overall performance of plants – in the low-light environment of a forest understorey is flushing red leaves correlated with high survival and lower growth, similar to seedling physical defences (Kitajima and Poorter, 2010; Kitajima et al., 2012)? In this study we examined this question for communities of woody species in two different tropical forests, an aseasonal rain forest in western Amazonia (Yasuní National Park, Ecuador) and a seasonal moist forest in Central America [Barro Colorado Island (BCI), Panamá], and posed the following questions. (1) Is a delayed greening or red-coloured leaf flushing strategy correlated with higher survival and lower growth rates in seedlings, saplings and trees? (2) Does the proportion of species and/or individuals with delayed greening or red-coloured young leaves increase in the community from seedlings to large trees?
MATERIALS AND METHODS
Study site
Our work was carried out in and around two large permanent Forest Dynamic Plots (FDPs), one in Yasuní National Park, Ecuador and the other on BCI, Panamá.
The Yasuní 25 ha FDP (0 °41'S, 76 °24'W; Valencia et al., 2004) is located in largely pristine tropical lowland aseasonal rain forest in eastern Ecuador (Finer et al., 2008; Bass et al., 2010). The National Park and adjacent Huaorani territory comprise 1 600 000 ha. The field station is located in the north-western corner of the park, in terra-firme, mature forest bordering the Tiputini River. The 25 ha plot lies along two smaller ridges dominated by red clays and separated by a valley characterized by brown or grey alluvium. The climate is aseasonal, the mean annual rainfall is 2830 mm and no month has <100 mm of mean precipitation. Mean daytime temperature in the shade is 25·2 °C (Valencia et al., 2004). The species count in 25 mapped hectares places Yasuní as one of the highest alpha-diversity forests in the world (Valencia et al., 2004). The field station and the plot are managed by the Pontificia Universidad Católica del Ecuador.
The BCI 50 ha FDP (9 °09'N, 79 °51'W) is situated within the Barro Colorado National Monument, a 6500 ha reserve comprised of BCI and its surrounding mainland peninsulas: Bohío, Buena Vista, Peña Blanca and Gigante. Barro Colorado Island is a 1592-ha island in the Gatún Lake, which was created when the Panamá Canal was flooded in 1914. The vegetation is typical of a lowland semi-deciduous moist tropical forest. While part of BCI contains secondary forest that has been recovering since the 1880s, all our measurements were carried out in the 50 ha plot, where the absence of phytoliths of agricultural species and the continuous presence of phytoliths of forest species indicate that agriculture was absent for the past 1500 years (Piperno, 1990). The climate of the area is seasonal. The mean total yearly rainfall is 2600 mm, 90 % of which falls during the rainy season from May to December, and the mean daily temperature is 27 °C (Leigh et al., 1996). The FDP was established by the Center for Tropical Forest Science of the Smithsonian Tropical Research Institute.
Within both FDPs, all stems ≥1 cm diameter at breast height (DBH; measured at 1·3 m) of all woody tree species are tagged, mapped, and measured every 5–6 years (Condit, 1998; Valencia et al., 2004).
Demographic data
Demographic data for seedlings <1 cm DBH have been collected in long-term projects at both sites (Wright et al., 2005; Metz et al., 2010). A total of 600 (Yasuní) or 800 (BCI) 1 m2 seedling plots were laid out in sets of three (or four, BCI) at 200 census stations along trails within the central area of the FDP (excluding a 50 m buffer zone within which no seedling plot is located). Growth, survival and recruitment of all seedlings in the plots were recorded annually since 2002 (Yasuní) and 1994 (BCI).
Demographic data for individual free-standing woody stems (hereafter, trees) >1 cm DBH were also available for Yasuní from 1997 and from BCI from 1980. In addition, mean mortality and diameter relative growth rates for almost all species have been estimated using Bayesian hierarchical models (allowing estimation of demographic rates for even the rare species) from both FDPs (Condit et al., 2006; data available from www.ctfs.si.edu).
Leaf colour surveys
During 2008–2012, young leaves of seedlings, saplings and trees growing in and around the Yasuní FDP were visually scored for colour. In 2010–2012, during the annual seedling census, every seedling present in six hunderd 1 m2 seedling plots was censused for the presence of new leaves and visually scored for their colour.
During 1991–1992, the young leaves of seedlings, saplings and trees of species growing throughout BCI were scored for colour by Kursar and Coley (1992a).
In all cases, young leaves were placed in one of the following colour categories: green, pale (i.e. delayed greening), red, or (rarely) white or blue.
Data analyses
We tested for a correlation of young leaf colour with two demographic parameters (predicting lower mortality rate and lower growth rate in species with delayed greening and red-coloured young leaves) in the seedling and tree life history stages separately for Yasuní and BCI.
Seedlings
We examined mortality (0/1) and growth rate (net change in the number of leaves) of individual seedlings as a function of young leaf colour for two age cohorts: (1) from recruitment to their first year and; (2) from 1 to 4 years. In all cases we used a generalized linear mixed effects model. To account for spatial autocorrelation in mortality and growth and spatiotemporal variation in the environment, we included 1 m2 seedling plot, census station and recruitment year as random effects. To account for differences among species in functional traits and defence strategies, we also included species as a random effect. Specifically for mortality, we included young leaf colour as the only fixed effect, and used a binomial error distribution. For growth, we included leaf colour and the ln(number of leaves +1 at the first census) as fixed effects and used a Poisson error distribution. Growth rates were taken solely from seedlings that survived the entire duration of the 4 year census interval.
Trees
Abundance and demographic data were available for two size classes of tree: 1–10 cm DBH and >10 cm DBH. We used data from the first censuses in Yasuní and BCI to test for differences in ln-transformed tree abundance, using analysis of variance (ANOVA). Then we tested for differences in ln-transformed mortality rate and ln-transformed diameter relative growth rate as a function of young leaf colour, using ANOVA. Finally, to examine how young leaf colour related to the growth–mortality trade-off in tropical trees, we included diameter relative growth rate as a covariate in a set of three models of mortality rate as a function of leaf colour for trees 1–10 cm DBH for each site, and used AIC (Akaike information criterion) values to determine whether including an interaction or additive term for relative growth rate improved the fit of the model.
RESULTS
Incidence of delayed greening in the woody plant communities of Yasuní and BCI
In the seedling community at Yasuní we documented the young leaf colour of 588 species. Delayed greening was found in 136 species (23 %) and young red leaves in 128 species (22 %). Within the new recruit community (seedlings <1 year old between 2003 and 2009), species with delayed greening comprised 22 % of individuals, and species with red leaves 26 % of individuals. For established seedlings 1–4 years old, species with delayed greening comprised 24 % of individuals, and species with red leaves 39 % of individuals.
In the tree community at Yasuní, we documented young leaf colour for 342 species. Normal green leaves were found in 161 species. Delayed greening was found in 65 species (19 %) and young red leaves in 112 species (32 %). We also found two species [Moutabea aculeata (Polygonaceae) and Ampelocera edentula (Ulmaceae)] with blue young leaves and two species [Gustavia longifolia (Lecythidaceae) and Quiina florida (Quiinaceae)], with virtually white young leaves; Fig. 1. Within the FDP, species with green leaves comprised 19 % of individuals >1 cm DBH, species with delayed greening 12 % of individuals, species with red leaves 21 % of individuals and species with blue or white leaves <0·1 % of individuals. The young leaf colour of the remaining 54 % of individuals and 762 species is unknown.
Fig. 1.
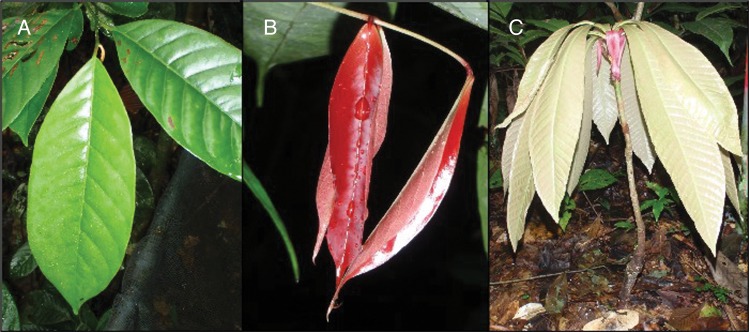
Examples of species from Yasuní with different young leaf colours. (A) Virola elongata Warb. (Myristicaceae). (B) Rourea camptoneura Radlk. (Connaraceae). (C) Gustavia hexapetala Aubl. J.E. Smith (Lecythidaceae). Images: Simon A. Queenborough.
At BCI, Kursar and Coley (1992a) documented 53 species with delayed greening and 48 species with red leaves out of 175 tree species surveyed (their data set also included an extra 35 liana species). Species with white young leaves at BCI included Gustavia superba (Lecythidaceae) and Ouratea lucens (Ochnaceae). Within the seedling new recruit community, species with delayed greening comprised 41 % of individuals and species with red leaves 39 % of individuals. For established seedlings 1–4 years old, species with delayed greening comprised 38 % of individuals and species with red leaves 43 % of individuals.
Within the tree community of the BCI FDP, species with normally greening leaves comprised 19 % of individuals >1 cm DBH, species with delayed greening 38 % of individuals and species with red leaves 29 % of individuals. The young leaf colour of the remaining 11 % of individuals and 186 species is unknown (most of these unknowns are rare: 60 % had <50 individuals).
The proportions of individuals with different coloured juvenile leaves varied significantly between seedlings and trees for both Yasuní (χ2 = 1224, d.f. = 2, P < 0·001) and BCI (χ2 = 1282, d.f. = 2, P < 0·001), but in opposite directions. At Yasuní, individuals with normal coloured leaves were more abundant than expected in the seedling community, but less abundant than expected in the tree community, indicating that individuals with delayed greening or red leaves formed a greater proportion of the trees; for BCI this situation was exactly reversed. However, species with delayed greening or red leaves were common at both sites in both life history stages and formed a large component of both communities, suggesting that the strategy is successful.
Association of delayed greening with seedling performance
Mortality
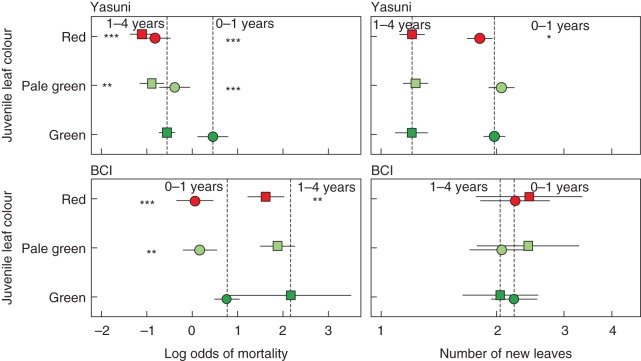
Seedlings of species with delayed greening and red young leaves had a significantly lower probability of mortality within 1 year of recruitment at both Yasuní and BCI (Fig. 2). At Yasuní, individuals with pale green leaves had a 30 % lower probability of mortality within 1 year of recruitment than individuals with regular green leaves, and individuals with red leaves had a 22 % lower probability of mortality within 1 year of recruitment. At BCI, individuals with delayed greening leaves had a 35 % lower probability of mortality within 1 year of recruitment than individuals with regular green leaves, and individuals with red leaves had a 33 % lower probability of mortality within 1 year.
Fig. 2.
The association of young leaf colour with seedling demography in two Neotropical forest plots (Yasuni, top, and BCI, bottom). Each panel describes a different measure of performance: mortality (log odds, left) and growth rate (number of new leaves, right). In each panel, we show seedling recruits to 1 year of age (circles) and established seedlings from 1 to 4 years (squares), according to young leaf colour. Points are group means ±95 % confidence intervals; significant differences from the baseline (green young leaves, vertical dashed line) are indicated by asterisks on the appropriate side of the figure (**P < 0·01; ***P < 0·001). Panels represent the 25 ha Yasuní and 50 ha BCI Forest Dynamics Plots.
Seedlings of species with delayed greening leaves had a significantly lower probability of mortality at between 1 and 4 years of age at Yasuní, while individuals with red-coloured young leaves had a significantly lower probability of mortality at both sites (Fig. 2). At Yasuní, individuals with delayed greening leaves had a 42 % lower probability of mortality at between 1 and 4 years than individuals with regular green leaves, and individuals with red leaves had a 37 % lower probability of mortality. At BCI, the probability of mortality for individuals with delayed green leaves was not significantly different from that of individuals with regular green leaves, but individuals with red leaves also had a 37 % lower probability of mortality at between 1 and 4 years.
Growth
The number of new leaves produced by seedling recruits that survived 1 year showed a significant association with leaf colour in only one case (Fig. 2), but negatively so. Seedling recruits with red-coloured leaves at Yasuní had just under two new leaves after 1 year, compared with two new leaves for seedlings with green leaves. Seedling recruits at BCI showed no significant differences in numbers of leaves among young leaf colours.
Established seedlings that survived 4 years showed no significant difference in the number of leaves among young leaf colours at either Yasuní or BCI (Fig. 2).
Association of delayed greening with sapling and tree performance
Abundance
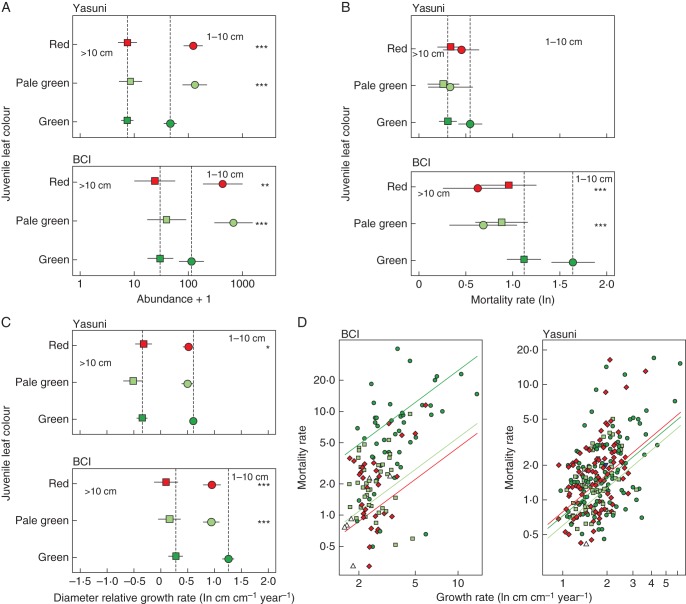
In the 1–10 cm DBH size class, species with delayed greening or red leaves had significantly higher abundances than species with regular green leaves [mean ± s.e. abundance for Yasuní (25 ha), green leaves 171 ± 35 individuals, pale 312 ± 63, red 278 ± 44; and BCI (50 ha), green leaves 693 ± 247, pale 2175 ± 1011, red 1914 ± 741; Fig. 3A]. There were no significant differences in abundance among trees in the >10 cm DBH size class (mean abundance for Yasuní, green leaves 20 ± 3 individuals, pale 25 ± 9, red 26 ± 44; and BCI, green leaves 103 ± 24, pale 140 ± 31, red 173 ± 72).
Fig. 3.
The association of young leaf colour with tree abundance and demography in two Neotropical forest plots. Each panel details a different measure of plant performance: (A) abundance, (B) mortality rate, (C) diameter growth rate, and (D) the trade-off between mortality and growth rate. For (A–C) we show individuals 1–10 cm DBH (circles) and individuals >10 cm DBH (squares). Symbols are mean abundance or demographic rate ±95 % confidence intervals; significant differences from the baseline (green leaves, vertical dashed line) are indicated by asterisks on the appropriate side of the figure (**P < 0·01; ***P < 0·001). Panels represent the 25-ha Yasuní and 50-ha BCI Forest Dynamics Plots. For (D) we show species-level demographic rates, with symbols coloured according to young leaf colour (green, circles; red, diamonds; pale green, squares; white, triangles; each symbol represents one species). Coloured lines are fitted regression lines from an ANCOVA with different intercepts but identical slopes for each leaf colour (see Table 1).
Mortality
At Yasuní, differences between mortality rates of tree species with regular green, delayed greening or red leaves were not significant. At BCI, trees in the 1–10 cm DBH size class with delayed greening or red-coloured young leaves had significantly lower mortality rates than species with green leaves, but there were no significant differences among the larger size class (Fig. 3B).
Growth
Tree species in the 1–10 cm DBH size class with red leaves showed significantly lower diameter relative growth rates than species with green leaves at both sites (Fig. 3C). At BCI, small trees with delayed greening also showed significantly lower growth rates than species with green leaves. However, trees >10 cm DBH showed no significant differences at either site.
The mortality–growth trade-off
Including diameter relative growth rate as a covariate in the mortality model to account for a growth–survival trade-off further highlighted the importance of leaf colour (Table 1). The best model with the lowest AIC value for both sites was the additive model of leaf colour + growth rate. Thus, leaf colour adds further explanatory power to the relationship, over and above the trade-off between low mortality and low growth (Fig. 3D). We found a similar pattern at both sites. Trees with pale green young leaves had significantly lower intercepts than trees with green leaves, and, at BCI, we also found a significantly lower intercept for species with red leaves. Thus, for a given growth rate, mortality was lower in these species with chromatic leaf defences. At Yasuní, the intercept for species with red leaves was not significantly different from that of those species with green leaves.
Table 1.
AIC values for three logistic models of mortality as a function of relative growth rate and young leaf colour for species in the Yasuní and BCI forest plots
| Model | Yasuní | BCI |
|---|---|---|
| Growth × leaf colour | 1176·00 | 765·60 |
| Growth + leaf colour | 1173·20 | 760·50 |
| Growth only | 3381·20 | 877·90 |
DISCUSSION
The performance of seedlings and small trees varied dramatically with young leaf colour. Seedling recruits with delayed greening had a lower probability of mortality than seedlings with regular greening or red-coloured leaves, although these effects decreased in older established seedlings. Trees with delayed greening and red-coloured leaves had significantly lower mortality but also lower relative growth rates in the 1–10 cm DBH size class at BCI (and for species with red leaves at Yasuní), although there no significant associations with leaf colour in trees >10 cm DBH. These results suggest that herbivory of young expanding leaves exerts strong selective pressure on seedlings and trees, and a delayed greening or red-coloration strategy appears to be associated with higher seedling survival and influences the species composition of later life stages. However, the selection for delayed greening, which appears to improve mortality, may come at an allocation cost, evidenced by the reduction in growth for red-leaved plants.
Defence mechanisms vary during ontogeny
What might explain the difference in the association of leaf colour and performance between seedlings and juveniles on one hand and trees on the other? Allocation to defence varies during not only the life of the individual leaf (herbivory rates drop an order of magnitude once leaves are fully expanded), but also the life of the whole plant (Coley, 1983; Lee and Collins, 2001; Boege and Marquis, 2005; Barton and Koricheva, 2010). For example, Ceiba pentandra (Malvaceae sensu lato) has spines on the trunk and branches as a juvenile tree but loses them as an adult. In part, this type of variation must reflect the change in herbivore pressure as the plant matures. The kinds of herbivores that can attack seedlings on the forest floor are probably very different from those that can potentially attack a sub-canopy, canopy or emergent tree. Deer are notorious ‘terminal shoot removers’ of seedlings, but cannot have the same effect on large trees (Zamora et al., 2001). Further, several authors have found evidence for stratum specialization among insect herbivores and leaf fungi in central Panamá. The species that attack leaves in the canopy are absent from the understorey, and vice versa (Basset, 2001; Basset et al., 2003; Gilbert et al., 2007).
Moreover, different herbivores will exert different selection pressures. In terms of leaf colour, most folivorous insects and mammals lack long-wave (i.e. red) receptors in their eyes (Dominy et al., 2002); in mammals hese are found only in howler monkeys [Alouatta spp. Dominy and Lucas (2001)]. As such, red-coloured leaves will be seen as darker or even dead by the majority of potential terrestrial and arboreal herbivores (Stone, 1979; Lucas et al., 1998).
Finally, our results (especially those concerning the abundances of seedlings) are probably driven in part by fluctuations in seedling recruitment and/or the timing of the annual seedling censuses (which may occur directly following germination of particular species). This is much more likely in Yasuní, where germination occurs throughout the year, than in BCI, where germination for many species occurs early in the wet season (Garwood, 1983).
Trade-offs between defence and growth and survival
Understanding life history trade-offs is a fundamental task of evolutionary ecology (Obeso, 2002). In a world of limited resources, plants face a dilemma (Bell, 1980; Reznick, 1985). Resources allocated to one function cannot usually be assigned to another, and so trade-offs, or costs, among the primary components of plant fitness imply that increased allocation to one leads to decreased allocation to the others (Levins, 1968; Bell, 1980). This situation is not always obvious though, as structures may have dual roles. For example, in terms of defence, many spines and thorns contain photosynthetic tissue. For species with young leaves that delay greening, plants appear to trade-off present photosynthesis with defence and future potential growth, although given our result of fewer leaves per seedling in red-leaved species after 1 year, this trade-off may be very long term. Further, investing in coloured pigments may be the only possible way to defend young leaves cheaply before they expand and toughen up.
This question leads us to consider how leaf colour and delayed greening interact with growth and survival as well as other defences. Are the differences in mortality that we have documented driven by leaf colour, or is leaf colour correlated with another trait? If we assume that a trade-off between allocation to defence and resource acquisition underlies the growth–mortality trade-off, then solutions to this underlying trade-off may vary with light environment. The costs of delayed greening increase rapidly with light availability; however, the availability of carbon for carbon-based defences should also increase rapidly with light availability. Furthermore, the delayed greening strategy comes with a cost in high-light environments because developing leaves cannot pay for their own development. In pioneer species, photosynthetic rates of still-expanding leaves pay for a large proportion of the carbon cost of leaf development (Terwilliger et al., 2001). In contrast, leaves with delayed greening must rely on stored carbohydrates. Carbohydrate storage is part of the shade-tolerant syndrome, and it incurs a growth cost (Myers and Kitajima, 2007). As a consequence, carbon-based defences are probably more important among light-demanding species whereas delayed greening is more important among shade-tolerant species. None of the light-demanding pioneer species has been documented to delay greening. Thus, delayed greening or red leaf colour may not control the species differences that we have documented. Rather, these strategies may be a cost-effective defence among species that happen to have high survival and slow growth because they are shade tolerant.
Conclusions
Using comprehensive surveys of young leaf colour in two Neotropical forests, we examined differences in growth and mortality at the seedling, sapling and tree life stages for hundreds of woody plant species. We found lower mortality in species with delayed greening and red-coloured leaves at all life stages, and lower growth rates in species with delayed greening and red-coloured leaves for seedlings and small trees. Thus, assuming that herbivory in expanding leaves exerts a strong selective pressure on young seedlings and saplings, the delayed greening or chromatic defence strategy appears to correlate with lower seedling mortality and lower growth rates, and to influence the species composition of later life stages.
ACKNOWLEDGEMENTS
We are extremely grateful to Lissy Coley and Tom Kursar for allowing us to use data they collected in Panamá, as well as providing many insightful comments on the manuscript. We further thank Kasey Barton and two anonymous reviewers for many helpful comments on the manuscript, and Kasey Barton and Mick Hanley for inviting M.R.M. and S.A.Q. to the seedling herbivory special symposium at ESA 2012. We thank Laura Mason for help with database management. R.V. thanks PUCE for granting him a sabbatical year and the University of Aarhus for offering research facilities during this year. We appreciate the hard work of the many field assistants who have diligently surveyed the seedling and tree communities in Yasuní and BCI for many years, especially Anelio Loor, Everaldo Zambrano and Milton Zambrano at Yasuní, and A. Hernandez and R. Gonzalez at BCI. We thank the Ministerio del Medioambiente of Ecuador for permission to carry out fieldwork in Yasuní National Park and within the Yasuní Forest Dynamics Plot. Both the Yasuní and BCI Forest Dynamics Plots are associated to the Center for Tropical Forest Science, a global network of large-scale demographic tree plots. The Yasuní seedling census is supported via the National Science Foundation's LTREB programme under grants DEB-1122634 and DEB-0614525, as well as grants from the Center for Tropical Forest Science at the Smithsonian Tropical Research Institute and from the University of California, Berkeley. The Yasuní FDP was supported by SENESCYT (project 300) in 2011. The Forest Dynamics Plot of Yasuní National Park has been made possible through the generous support of the Pontifical Catholic University of Ecuador (PUCE) funds of donaciones del impuesto a la renta, the government of Ecuador, the US National Science Foundation, the Andrew W. Mellon Foundation, the Smithsonian Tropical Research Institute and the University of Aarhus of Denmark. The Environmental Sciences Program of the Smithsonian Institution supported the seedling studies at BCI.
LITERATURE CITED
- Agrawal AA. Current trends in the evolutionary ecology of plant defence. Functional Ecology. 2011;25:420–432. [Google Scholar]
- Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen J-P. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science. 2012;338:113–116. doi: 10.1126/science.1225977. [DOI] [PubMed] [Google Scholar]
- Barone JA. 1st International Canopy Conference. Sarasota, FL, USA: Selby Botanical Gardens; 1994. Herbivores and herbivory in the canopy and understory on Barro Colorado Island, Panama. [Google Scholar]
- Barton KE, Koricheva J. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. American Naturalist. 2010;175:481–493. doi: 10.1086/650722. [DOI] [PubMed] [Google Scholar]
- Bass MS, Finer M, Jenkins CN, et al. Global conservation significance of Ecuador's Yasuní National Park. PLoS One. 2010;5:22. doi: 10.1371/journal.pone.0008767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset Y. Communities of insect herbivores foraging on saplings versus mature trees of Pourouma bicolor (Cecropiaceae) in Panama. Oecologia. 2001;129:253–260. doi: 10.1007/s004420100724. [DOI] [PubMed] [Google Scholar]
- Basset Y, Hammond PM, Barrios H, Holloway JD, Miller SE. Vertical stratification of arthropod assemblages. In: Basset Y, Novotny V, Miller SE, Kitching RL, editors. Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge: Cambridge University Press; 2003. pp. 17–27. [Google Scholar]
- Bell G. The costs of reproduction and their consequences. American Naturalist. 1980;116:45–76. [Google Scholar]
- Bodmer RE. Ungulate biomass in relation to feeding strategy within Amazonian forests. Oecologia. 1989;81:547–450. doi: 10.1007/BF00378967. [DOI] [PubMed] [Google Scholar]
- Boege K, Marquis RJ. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brenes-Arguedas T, Coley PD, Kursar TA. Divergence and diversity in the defensive ecology of Inga at two Neotropical sites. Journal of Ecology. 2008;96:127–135. [Google Scholar]
- Brokaw N, Busing RT. Niche versus chance and tree diversity in forest gaps. Trends in Ecology and Evolution. 2000;15:183–188. doi: 10.1016/s0169-5347(00)01822-x. [DOI] [PubMed] [Google Scholar]
- Coley PD. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecological Monographs. 1983;53:209–233. [Google Scholar]
- Coley PD, Aide TM. Red coloration of tropical young leaves: a possible antifungal defence? Journal of Tropical Ecology. 1989;5:293–300. [Google Scholar]
- Coley PD, Aide TM. Comparison of herbivory and plant defenses in temperate and tropical broad-leaved forests. In: Price PW, Lewinsohn TM, Fernandes WW, Benson WW, editors. Plant–animal interactions: evolutionary ecology in tropical and temperate regions. New York: John Wiley & Sons; 1996. pp. 25–49. [Google Scholar]
- Coley PD, Kursar TA. Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological tradeoffs. In: Mulkey SS, Chazdon R, Smith AP, editors. Tropical forest plant ecophysiology. New York: Chapman and Hall; 1996. pp. 305–366. [Google Scholar]
- Coley PD, Bryant JP, Chapin FS. Resource availability and plant anti-herbivore defense. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- Condit R. Tropical forest census plots. Georgetown, TX and Berlin: R.G. Landes Company and Springer-Verlag; 1998. [Google Scholar]
- Condit R, Ashton PMS, Bunyavejchewin S, et al. The importance of demographic niches to tree diversity. Science. 2006;313:98–101. doi: 10.1126/science.1124712. [DOI] [PubMed] [Google Scholar]
- Dominy NJ, Lucas PW, Ramsden LW, Riba-Hernandez P, Stoner KE, Turner IM. Why are young leaves red? Oikos. 2002;98:163–176. [Google Scholar]
- Dominy NJ, Lucas PW. Ecological importance of trichromatic vision to primates. Nature. 2001;410:363–366. doi: 10.1038/35066567. [DOI] [PubMed] [Google Scholar]
- Eichhorn MP, Nilus R, Compton SG, Hartley SE, Burslem DFRP. Herbivory of tropical rain forest tree seedlings correlates with future mortality. Ecology. 2010;91:1092–1101. doi: 10.1890/09-0300.1. [DOI] [PubMed] [Google Scholar]
- Fine PVA, Miller ZJ, Mesones I, et al. The growth–defense trade-off and habitat specialization by plants in Amazonian forests. Ecology. 2006;87:S150–S162. doi: 10.1890/0012-9658(2006)87[150:tgtahs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Finer M, Jenkins CN, Pimm SL, Keane B, Ross C. Oil and gas projects in the western Amazon: threats to wilderness, biodiversity, and indigenous peoples. PLoS One. 2008;3:e2932. doi: 10.1371/journal.pone.0002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood NC. Seed germination in a seasonal tropical forest in Panama: a community study. Ecological Monographs. 1983;53:159–181. [Google Scholar]
- Gilbert GS, Reynolds DR, Bethancourt A. The patchiness of epifoliar fungi in tropical forests: host range, host abundance, and environment. Ecology. 2007;88:575–581. doi: 10.1890/05-1170. [DOI] [PubMed] [Google Scholar]
- Gowda JH. Spines of Acacia tortilis: what do they defend and how? Oikos. 1996;77:279–284. [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics. 2007;8:157–178. [Google Scholar]
- Harms KE, Wright SJ, Calderón O, Hernández A, Herre EA. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature. 2000;404:493–495. doi: 10.1038/35006630. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Morley CB, Smith WK. The coordination of anthocyanin decline and photosynthetic maturation in developing leaves of three deciduous tree species. New Phytologist. 2007;175:675–685. doi: 10.1111/j.1469-8137.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Coevolution of mutualism between ants and acacias in Central America. Evolution. 1966;20:249–275. doi: 10.1111/j.1558-5646.1966.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Llorens AM, Stefanescu C, Timchenko MV, Lucas PW, Wright SJ. How cellulose-based leaf toughness and lamina density contribute to long leaf lifespans of shade-tolerant species. New Phytologist. 2012;195:640–652. doi: 10.1111/j.1469-8137.2012.04203.x. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Poorter L. Tissue-level leaf toughness, but not lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. New Phytologist. 2010;186:708–721. doi: 10.1111/j.1469-8137.2010.03212.x. [DOI] [PubMed] [Google Scholar]
- Kursar TA, Coley PD. Delayed greening in tropical leaves: an anti-herbivore defense? Biotropica. 1992a;24:256–262. [Google Scholar]
- Kursar TA, Coley PD. The consequences of delayed greening during leaf development for light absorption and light use efficiency. Plant, Cell, and Environment. 1992b;15:901–909. [Google Scholar]
- Kursar TA, Coley PD. Delayed development of the photosynthetic apparatus in tropical rainforest species. Functional Ecology. 1992c;6:411–422. [Google Scholar]
- Kursar TA, Coley PD. Convergence in defense syndromes of young leaves in tropical rainforests. Biochemical Systematics and Ecology. 2003;21:929–949. [Google Scholar]
- Kursar TA, Dexter KG, Lokvam J, et al. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proceedings of the National Academy of Sciences, USA. 2009;106:18073–18078. doi: 10.1073/pnas.0904786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Collins TM. Phylogenetic and ontogenetic influences on the distribution of anthocyanins and betacyanins in leaves of tropical plants. International Journal of Plant Science. 2001;162:1141–1153. [Google Scholar]
- Leigh EG. Ecology of tropical forests: the view from Barro Colorado. New York: Oxford University Press; 1997. [Google Scholar]
- Leigh EG, Rand AS, Windsor DM. The ecology of a tropical forest: seasonal rhythms and long-term changes. Washington, DC: Smithsonian Institution Press; 1996. [Google Scholar]
- Levins R. Evolution in changing environments. Princeton, NJ: Princeton University Press; 1968. [Google Scholar]
- Lucas PW, Darvell BW, Lee PK, Yuen TD, Choong MF. Colour cues for leaf food selection by long-tailed macaques (Macaca fascicularis) with a new suggestion for the evolution of trichromatic colour vision. Folia Primatologica. 1998;69:139–152. doi: 10.1159/000021576. [DOI] [PubMed] [Google Scholar]
- Lucas PW, Turner IM, Dominy NJ, Yamshita N. Mechanical defences to herbivory. Annals of Botany. 2000;86:913–920. [Google Scholar]
- Metz MR, Sousa WP, Valencia LR. Community-wide, density-dependent seedling mortality promotes species coexistence in a highly diverse Amazonian rainforest. Ecology. 2010;91:3675–3685. doi: 10.1890/08-2323.1. [DOI] [PubMed] [Google Scholar]
- Myers JA, Kitajima K. Carbohydrate storage enhances seedling shade and stress tolerance in a Neotropical forest. Journal of Ecology. 2007;95:383–395. [Google Scholar]
- Novotny V, Miller SE, Baje L, et al. Guild-specific patterns of species richness and host specialization in plant–herbivore food webs from a tropical forest. Journal of Animal Ecology. 2010;79:1193–1203. doi: 10.1111/j.1365-2656.2010.01728.x. [DOI] [PubMed] [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Piperno D. Fitolitos, arquelogía y cambios prehistoricos de la vegetacion en un lote de cincuenta hectáreas de la isla de Barro Colorado. In: Leigh EG, Rand AS, Windsor DM, editors. Ecología de un Bosque Tropical. Washington, DC: Smithsonian Institution Press; 1990. pp. 153–156. [Google Scholar]
- Reznick D. Cost of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Stone BC. Protective coloration of young leaves in certain Malaysian palms. Biotropica. 1979;11:126. [Google Scholar]
- Swaine MD, Whitmore TC. On the definition of ecological species groups in tropical rain forests. Vegatatio. 1988;75:81–86. [Google Scholar]
- Terwilliger VJ, Kitajima K, Le Roux-Swarthout DJ, Mulkey S, Wright SJ. Intrinsic water-use efficiency and heterotrophic investment in tropical leaf growth of two Neotropical pioneer tree species as estimated from δ13C values. New Phytologist. 2001;152:267–281. [Google Scholar]
- Turner IM, Choong MF, Tan HTW, Lucas PW. How tough are sclerophylls. Annals of Botany. 1993;71:343–345. [Google Scholar]
- Valencia R, Foster RB, Villa G, et al. Tree species distributions and local habitat variation in the Amazon: large forest plot in eastern Ecuador. Journal of Ecology. 2004;92:214–229. [Google Scholar]
- Welden CW, Hewett SW, Hubbell SP, Foster RB. Sapling survival, growth, and recruitment: relationship to canopy height in a Neotropical forest. Ecology. 1991;72:35–50. [Google Scholar]
- Whatley JM. Plastid development in distinctively coloured juvenile leaves. New Phytologist. 1992;120:417–426. [Google Scholar]
- Wright SJ, Muller-Landau HC, Condit R, Hubbell SP. Gap-dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology. 2003;84:3174–3185. [Google Scholar]
- Wright SJ, Muller-Landau HC, Calderón O, Hernandéz A. Annual and spatial variation in seedfall and seedling recruitment in a Neotropical forest. Ecology. 2005;86:848–860. [Google Scholar]
- Wright SJ, Kitajima K, Kraft NJB, et al. Functional traits and the growth–mortality trade-off in tropical trees. Ecology. 2010;91:3664–3674. doi: 10.1890/09-2335.1. [DOI] [PubMed] [Google Scholar]
- Wright W, Vincent JFV. Herbivory and the mechanics of fracture in plants. Biological Reviews. 1996;71:401–413. [Google Scholar]
- Zamora R, Gómez JM, Hódar JA, Castro J, García D. Effect of browsing by ungulates on sapling growth of Scots pine in a Mediterranean environment: consequences for forest regeneration. Forest Ecology and Management. 2001;144:33–42. [Google Scholar]