Abstract
OBJECTIVES
Although evidence suggests that aspirin and celecoxib may reduce the risk of esophageal adenocarcinoma (EAC) in patients with Barrett’s esophagus (BE), these drugs can also cause harmful side effects. Our aim was to determine and characterize preferences for these two drugs in patients with BE.
METHODS
Preferences data were collected from recruited BE patients using a customized questionnaire, which incorporated standard risk communication techniques. Summary profiles outlined the benefits and harms of celecoxib and aspirin presented anonymously. Both drugs were portrayed as reducing the risk of EAC and increasing the risk of GI events. However, celecoxib increased the risk of myocardial infarction (MI) while aspirin reduced the risk. Factors influencing patient acceptance of each drug were analyzed.
RESULTS
One hundred of 109 (92%) subjects completed the study. Under base case conditions, 15% stated that they would take celecoxib and 76% aspirin (P < 0.0001). Patients identified the greater risk of MI as the primary reason for their unwillingness to take celecoxib and the lower risk of EAC for aspirin. Even in scenarios in which the benefits of celecoxib were improved and the harms reduced, a majority continued to find it unacceptable.
CONCLUSIONS
A majority of those surveyed stated that they would take aspirin but would not take celecoxib. Most patients are interested in EAC chemoprevention, but the amount of protection and the side effect profile of a drug determine its acceptability. These data can inform physicians regarding the tradeoffs patients are willing to consider for chemoprevention.
INTRODUCTION
There is evidence suggesting that both aspirin (1) and selective cyclooxygenase-2 (COX-2) inhibitors (coxibs) (2, 3) can prevent esophageal adenocarcinoma (EAC) in patients with Barrett’s esophagus (BE). However, this benefit may come with a greater risk of specific diseases or adverse events. Although initial reports of a greater risk of cardiovascular events were for rofecoxib, this risk has also been documented in celecoxib (4, 5), albeit at a potentially lower risk ratio (6). In comparison, aspirin may not only reduce future EAC risk, but also prevent primary cardiovascular events (7, 8). The principal risk associated with aspirin use is gastrointestinal bleeding (9).
The decision to take either coxibs or aspirin to prevent esophageal adenocarcinoma by patients with BE is a preference-sensitive one. In these decisions, the benefits of the treatments do not clearly outweigh the risks involved for all patients. Therefore, each decision must be based on individual patient preferences regarding the benefits and risks faced both with treatment and without. Information regarding patient attitudes toward the benefits and harms of these drugs could provide useful information to inform both health-care providers and patients as they consider these specific drugs and other interventions.
The purpose of this study was to determine and explore patient preferences for celecoxib and aspirin for the chemoprevention of EAC in BE patients. This included an evaluation of the tradeoffs patients were willing to consider to reduce their future cancer risk.
METHODS
Overview of Study Design
The study was approved by our institutional review board. Subject recruitment was done in person by a member of the study staff. The questionnaire was administered over the telephone, but the subject had a paper copy of the questionnaire and accompanying visual aids with him/her during the interview. All interviews were conducted by the same investigator (D.E.B.).
Recruitment
Recruitment took place in the Massachusetts General Hospital’s endoscopy suite (Boston, MA). Target subjects were patients with a diagnosis of Barrett’s esophagus confirmed by histology. Eligible participants were at least 18 yr of age and able to read and understand information presented in English. Patients with a diagnosis of esophageal adenocarcinoma or carcinoma in situ on pathology were excluded. Potential subjects who were scheduled for an endoscopy or clinic visit were identified using the electronic scheduling system and approached by a study investigator in the waiting room. Those who agreed to participate were given the paper questionnaire and visual aids to take home and a follow-up telephone appointment with the investigator was arranged.
Survey Description
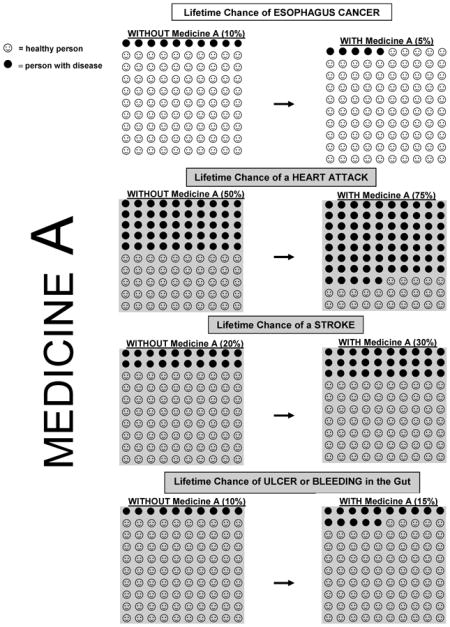
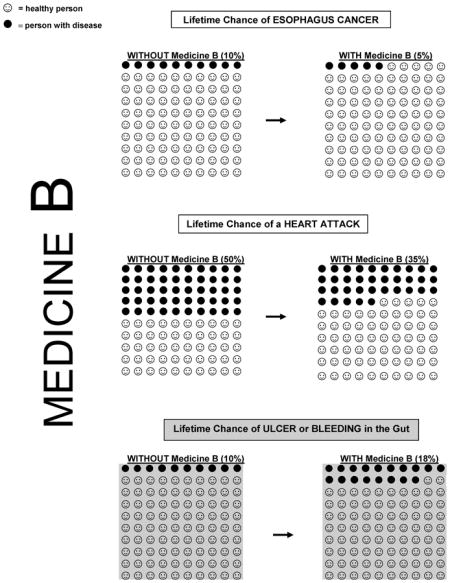
Representative portions of the questionnaire and visual aids are available in the Appendix. Summary benefits and harms profiles of celecoxib and aspirin (described as ‘Medicine A’ and ‘Medicine B’) were based on the current published data (see Table 1). Studies have suggested that aspirin use is associated with an EAC risk reduction of approximately 50%, which we extrapolated to a change in lifetime risk from 10% to 5%. Although the data for celecoxib are less robust (see Discussion), we assumed a similar reduction in lifetime risk with celecoxib use.
Table 1.
Descriptions of Disease Risks and Effect of Chemoprevention
| Esophageal Adenocarcinoma | Men | Women | Reference(s) |
|---|---|---|---|
| Lifetime risk* | 10% | 10% | Shaheen (25, 26) |
| With celecoxib | 5% | 5% | Jacobson (2), Buttar (3, 27), |
| With aspirin | 5% | 5% | Corley (1), Farrow (28) |
| Heart attack | |||
| Lifetime risk | 50% | 30% | Lloyd-Jones (10) |
| With celecoxib | 75% | 45% | Kearney (11), Arber (4), Bertagnolli (5) |
| With aspirin | 35% | 20% | Hayden (9), Sanmuganathan (8) |
| Stroke | |||
| Lifetime risk | 20% | 20% | Seshadri (29) |
| With celecoxib | 30% | 30% | Kearney (11), Arber (4), Bertagnolli (5) |
| With aspirin | 20% | 20% | Hayden (9), Sanmuganathan (8) |
| Ulcer/GI bleeding | |||
| Lifetime risk | 10% | 10% | Graham (30) |
| With celecoxib | 15% | 15% | Simon (12), Deeks (13) |
| With aspirin | 18% | 18% | Sanmuganathan (8), Hayden (9) |
Average lifetime risk in patients with Barrett’s esophagus.
All percentages are lifetime risks.
The summary data presented to patients were drawn from the current literature. Because the lifetime risk of myocardial infarction (MI) is different for men and women (10), male and female versions of the questionnaire were created. We portrayed celecoxib as increasing the lifetime risk of cardiovascular events (MI and stroke) by 50%. This value is consistent with a recent meta-analysis that reported a 40% increase in the risk of serious vascular events (11) and within the range of values reported in other studies ranging from 30% to 160% for a dose of 400 mg per day (4, 5).
Aspirin was not portrayed as affecting the overall risk of stroke, as a potential increase in risk of hemorrhagic strokes could be offset by a lower risk of ischemic strokes (8, 9). Both celecoxib (12, 13) and aspirin (8, 9) were presented as increasing the risk of gastrointestinal events (GIE; ulcer or gastrointestinal bleeding) by the same amount.
Descriptions of celecoxib and aspirin were presented sequentially, in random order, as anonymous “Medicine A” and “Medicine B.” After a profile of a drug was presented, preferences were elicited. Benefits and harms were presented as absolute lifetime chances which is normative in risk communication methodology (14).
Concise textual descriptions of EAC and one of the two medicines were followed by a depiction of the potential harms associated with the drug. The risk of developing esophageal adenocarcinoma (“esophagus cancer” in the survey), MI (“heart attack” in the survey), stroke, and gastrointestinal events (GIE; ulcer or gastrointestinal bleeding) were presented as absolute lifetime risks along with an explanation of how these values would change if one of the chemopreventive medicines was taken (e.g., esophagus cancer risk would be reduced from 10% to 5%). The participant was instructed by the interviewer to view a visual representation of the percentages (15) provided on a separate sheet of colored paper (see Fig. 1 for example). The “Benefits” and “Harms” of each drug were summarized in a table (see Appendix).
Figure 1.
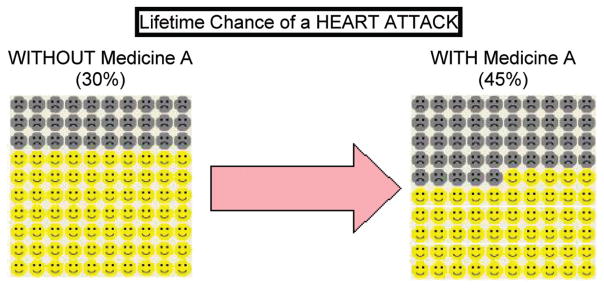
Visual aid, representation of percentages. Patients were given visual representations of the risk and benefit percentages using a 10 by 10 icon grid format, with the “unhappy faces” representing heart attacks and the “smiley faces” representing no heart attack. The 30% absolute lifetime risk is Without Medicine A (average population risk for a women) while the 45% risk is the presumed risk if one were to take celecoxib (increase risk by 50%).
After each drug profile was presented, participants were asked if they would be willing to take the drug. After making their decision, the interviewer asked the subjects which characteristic of the drug was most influential in their decision making process and why.
To further explore patient preferences regarding EAC chemoprevention, subjects were asked to “re-consider” celecoxib or aspirin if a particular drug benefit was maximized or if a particular harm was minimized, while all of the other benefits and harms were held constant (“Improved Drug Profile Analysis”).
Risk perception questions asked subjects about their fear and perceived risk of heart attack and esophageal cancer. Demographic and medical history data were also collected. Finally, subjects were asked to rate their understanding of the questionnaire on a 5-point scale. The interviewer also rated each subject’s comprehension upon completion of the survey.
Statistics
TEST OF SIGNIFICANCE OF PRIMARY END POINT
A comparison of the proportion of patients who would be willing to take celecoxib versus aspirin was made using the McNemar’s test.
POWER
Power calculations were based on an anticipated difference in the proportion of subjects’ willingness to take celecoxib versus aspirin. Assuming independence of responses for the two drugs and using the McNemar’s test, at an alpha error rate of 5%, 100 participants had 80% power to detect a 2-sided difference in responses (willingness to take the drug) of 60% versus 40%.
REGRESSION MODEL
Univariate analysis was performed with test of significance using a Student’s t-test for continuous predictor (age), and either χ2 test or Fisher’s exact test for all binary or categorical predictors. All reported P values are 2-sided. Multivariate logistic regression analysis was performed to identify independent predictors of willingness to take aspirin or celecoxib, respectively. For each of the two models, seven covariates were included as potential predictors. Age was treated as a continuous variable, and highest education level completed was treated as a class variable with four strata. The remainder of the covariates (gender, history of cancer, history of heart disease, presence of Barrett’s with dysplasia, and history of current or prior use of either aspirin or COX-2 inhibitor) were treated as dichotomous variables. The model was constructed without use of a variable selection algorithm. Statistical analysis was performed using SAS v 9.1.3 (SAS Carey Institute, Inc., Cary, NC).
RESULTS
Participant Characteristics
See Table 2 for detailed patient characteristics. Of the 109 patients asked to participate, 100 (92%) completed the survey and were included in the analysis. The mean age was 64.5 with 72% of the participants male and 28% female. The group was predominantly white (97%) and highly educated, with 70% reporting at least some college education. Of those surveyed, 37% were taking aspirin regularly while only 2% stated that they were currently taking celecoxib (Celebrex) and 27% reported ever having taken celecoxib or rofecoxib (Vioxx).
Table 2.
Patient Characteristics
| % or # | (SD) | |
|---|---|---|
| Number of subjects (N) | 100 | |
| Gender, % male | 72.0% | |
| Mean age, yr | 64.5 | (11.3) |
| Ethnicity | ||
| White | 97% | |
| Asian or Pacific Islander | 3% | |
| Education level | ||
| Some high school or less | 4% | |
| High school/GED | 26% | |
| College or some college | 44% | |
| Postgraduate | 26% | |
| NSAID history | ||
| Current aspirin usage | ||
| Dose/week | ||
| None | 61% | |
| 1–5 | 2% | |
| 6–14 | 37% | |
| Currently taking Celebrex | 2% | |
| Ever taken Celebrex/Vioxx | 27% | |
| Barrett’s esophagus history | ||
| History of dysplasia | ||
| None | 50% | |
| Low-grade | 5% | |
| High-grade | 45% | |
| Current PPI Use | 93% | |
| Average time since BE Dx | 6.1 yr | (4.4) |
| Unknown (referral) | 44% | |
| Average number of EGDs | 7.6 | (6.2) |
| Other medical history | ||
| History of cancer | 16% | |
| History of heart condition | 28% | |
| Risk perceptions (1- strongly agree, 5- strongly disagree) | ||
| Esophageal cancer | ||
| Afraid of getting EAC | 2.2 | (1.4) |
| At risk of getting EAC | 1.9 | (1.1) |
| Lifestyle choices affect risk | 1.9 | (1.1) |
| Heart attack | ||
| Afraid of getting HA | 2.7 | (1.3) |
| At risk of getting HA | 2.8 | (1.3) |
| Lifestyle choices affect risk | 1.4 | (0.8) |
SD = standard deviation; PPI = proton pump inhibitor; BE Dx = diagnosis of Barrett’s esophagus; EAC = esophageal adenocarcinoma; EGD = upper endoscopy; GED = graduate equivalency diploma
Regarding BE history, 50% of subjects reported never having had any dysplasia, while 45% reported a history of high-grade dysplasia (HGD) and 5% reported low-grade dysplasia (LGD). The majority (93%) were currently on proton pump inhibitor therapy.
Base Case Results
Results of the base-case portion of the study are presented in Table 3. After participants were provided a base-case description of celecoxib’s potential benefits and harms, 15% stated that they would be willing to take the drug (14% of women and 15% of men). Seventy-six percent of those surveyed were willing to take aspirin (82% of women and 74% of men). Alternatively stated, participants were 5 times more likely to agree to take aspirin than celecoxib. The difference in the proportion of participants willing to take the two drugs was highly statistically significant (P < 0.0001).
Table 3.
Results of Base Analysis
| Percentage Willing to Take the Drug Under Base Conditions | |||
|---|---|---|---|
| All | F | M | |
| Results stratified by gender | |||
| Number of subjects (N) | 100 | 28 | 72 |
| Celecoxib | 15.0% | 14.3% | 15.3% |
| Aspirin | 76.0% | 82.1% | 73.6% |
|
| |||
| All | HGD | No HGD | |
|
| |||
| Results stratified by history of HGD | |||
| Number of subjects (N) | 100 | 45 | 55 |
| Celecoxib | 15.0% | 20.0% | 10.9% |
| Aspirin | 76.0% | 68.9% | 81.8% |
HGD = history of high-grade dysplasia.
P < 0.0001; difference in percentage willing to take the two drugs.
The average time to complete the survey was 20.7 min (SD = 5.3). The average self-reported survey comprehension rating was 4.6 (5-point scale, 5 = best; SD = 1.0) and average rating of the investigator’s perception of subject comprehension was 4.4 (SD = 0.7).
Univariate and Multivariate Regression Analysis
None of the demographic and medical history variables predicted a patient’s willingness to take celecoxib in univariate analysis (see Table 4). However, younger age (P = 0.009) and more education (P = 0.01) were positively associated with a willingness to take aspirin. When these seven variables were incorporated into a multivariate logistic regression model, none of the variables independently predicted a willingness to take either celecoxib or aspirin.
Table 4.
Univariate Predictors
| Willing | Not Willing | P Value | |
|---|---|---|---|
| Univariate analysis, factors associated with willingness to take celecoxib | |||
| Baseline | 15% | 85% | |
| Age (mean) | 65.2 | 64.4 | 0.81 |
| Gender | |||
| Male | 15% (11/72) | 85% (61/72) | 1.0 |
| Female | 14% (4/28) | 86% (24/28) | |
| Current/prior COX-2 | |||
| Yes | 11% (3/27) | 89% (24/27) | 0.75 |
| No | 16% (12/73) | 84% (61/73) | |
| Hx cancer | |||
| Yes | 6% (1/16) | 94% (15/16) | 0.45 |
| No | 17% (14/84) | 83% (70/84) | |
| Hx heart disease | |||
| Yes | 14% (4/28) | 86% (24/28) | 1.0 |
| No | 15% (11/72) | 85% (61/72) | |
| Barrett’s dysplasia | |||
| Yes | 18% (9/50) | 82% (41/50) | 0.40 |
| No | 12% (6/50) | 88% (44/50) | |
| Education level | |||
| Less than HS | 20% (1/5) | 80% (4/5) | 0.45 |
| HS or GED | 19% (5/26) | 81% (21/26) | |
| College | 9% (4/43) | 91% (39/43) | |
| Postgraduate | 19% (5/26) | 81% (21/26) | |
| Univariate analysis, factors associated with willingness to take ASA | |||
| Baseline | 76% | 24% | |
| Age (mean) | 62.9 | 69.8 | 0.009 |
| Gender | |||
| Male | 74% (53/72) | 27% (19/72) | 0.37 |
| Female | 82% (23/28) | 18% (5/28) | |
| Current ASA use | |||
| Yes | 69% (27/39) | 31% (12/39) | 0.21 |
| No | 80% (49/61) | 20% (12/61) | |
| Hx cancer | |||
| Yes | 63% (10/16) | 38% (6/16) | 0.20 |
| No | 79% (66/84) | 21% (18/84) | |
| Hx heart disease | |||
| Yes | 64% (18/28) | 36% (10/28) | 0.09 |
| No | 81% (58/72) | 19% (14/72) | |
| Barrett’s dysplasia | |||
| Yes | 70% (35/50) | 30% (15/50) | 0.16 |
| No | 82% (41/50) | 18% (9/50) | |
| Education level | |||
| Less than HS | 20% (1/5) | 80% (4/5) | 0.01 |
| HS or GED | 69% (18/26) | 31% (8/26) | |
| College | 86% (37/43) | 14% (6/43) | |
| Postgraduate | 77% (20/26) | 23% (6/26) | |
ASA = aspirin; Hx = history; HS = high school; GED = graduate equivalency diploma.
Percentages do not always add to 100% because of rounding.
Most Important Factor Analysis
When participants who were not initially willing to take celecoxib were asked what characteristic of the drug profile was most important to their decision, a majority identified the greater risk of MI (59%; see Table 5). Participants who were willing to take celecoxib (15 subjects) cited the reduced risk of esophageal cancer as the most important factor in their decision.
Table 5.
Most Important Factor (Benefit or Harm) in Decision Making: Celecoxib
| All | F | M | HGD | No HGD | |
|---|---|---|---|---|---|
| All participants | |||||
| Number of subjects (N) | 100 | 28 | 72 | 45 | 55 |
| EAC | 18% | 14% | 19% | 27% | 11% |
| MI | 59% | 50% | 63% | 40% | 75% |
| Stroke | 19% | 32% | 14% | 27% | 13% |
| GI event | 3% | 4% | 3% | 7% | 2% |
| Among those willing to take celecoxib | |||||
| Number of subjects (N) | 15 | 4 | 11 | 9 | 6 |
| EAC | 100% | 100% | 100% | 100% | 100% |
| MI | 0 | 0 | 0 | 0 | 0 |
| Stroke | 0 | 0 | 0 | 0 | 0 |
| GI event | 0 | 0 | 0 | 0 | 0 |
| Among those unwilling to take celecoxib: | |||||
| Number of subjects (N) | 85 | 24 | 61 | 36 | 49 |
| EAC | 4% | 0 | 5% | 8% | 0 |
| MI | 69% | 58% | 74% | 50% | 90% |
| Stroke | 22% | 38% | 16% | 33% | 8% |
| GI event | 4% | 4% | 3% | 8% | 2% |
All = total group surveyed; HGD = history of high-grade dysplasia.
Percentages do not always add to 100% because of rounding.
EAC = esophageal adenocarcinoma; MI = myocardial infarction; GI event = ulcer or gastrointestinal bleeding.
Patients who were willing to take aspirin identified the lower risk of esophageal cancer as the most important factor affecting their decision (86%; see Table 6). Among patients who were initially unwilling to take aspirin, the greater risk of GIE was the most cited reason (96%).
Table 6.
Most Important Factor (Benefit or Harm) in Decision Making: Aspirin
| All | F | M | HGD | No HGD | |
|---|---|---|---|---|---|
| All participants | |||||
| Number of subjects | 100 | 28 | 72 | 45 | 55 |
| EAC | 66% | 68% | 65% | 64% | 67% |
| MI | 11% | 18% | 8% | 7% | 15% |
| GI event | 23% | 14% | 26% | 29% | 18% |
| Among those willing to take aspirin | |||||
| Number of subjects | 76 | 23 | 53 | 31 | 45 |
| EAC | 86% | 78% | 89% | 90% | 94% |
| MI | 14% | 22% | 11% | 10% | 16% |
| GI event | 0 | 0 | 0 | 0 | 0 |
| Among those unwilling take aspirin | |||||
| Number of subjects | 24 | 5 | 19 | 14 | 10 |
| EAC | 4% | 20% | 0 | 7% | 0 |
| MI | 0 | 0 | 0 | 0 | 0 |
| GI event | 96% | 80% | 100% | 93% | 100% |
All = total group surveyed; HGD = history of high-grade dysplasia.
Percentages do not always add to 100% because of rounding.
EAC = esophageal adenocarcinoma; MI = myocardial infarction; GI event = ulcer or gastrointestinal bleeding.
Improved Drug Profile Scenarios
To further evaluate the factors affecting patient decision making, we presented participants with hypothetical scenarios in which each of the drugs became perfect EAC prevention agents (eliminated all future risk of EAC). When the revised profile of celecoxib had the maximum potential benefit but the baseline profile for harms, the percentage of participants who were willing to take the improved celecoxib was 43%, compared to the initial 15% response (see Table 7). Willingness to take aspirin also increased when aspirin was presented as able to eliminate the risk of EAC (from 76% to 87%).
Table 7.
Improved Drug Profile Scenarios “Would you be willing to take this drug if …”
| Celecoxib | Aspirin | |
|---|---|---|
| % Willing to take drug, base case | 15.0% | 76.0% |
| Scenario 1: Improved EAC risk (EAC risk eliminated, ▼100%; MI, stroke, GIE risks unchanged) | 43.0% | 87.0% |
| Scenario 2: Improved MI risk (MI risk, no increase, ▲0%; EAC; stroke; GIE unchanged) | 42.0% | – |
| Scenario 3: Improved MI risk (MI risk eliminated, ▼100%; EAC, GIE risks unchanged) | – | 86.0% |
| Scenario 4: Improved stroke risk (Stroke risk, no increase, ▲0%; EAC; MI; GIE unchanged) | 29.4% | – |
| Scenario 5: Improved GIE risk (GIE risk, no increase, ▲0%; EAC; MI; stroke unchanged | 25.0% | 97.0% |
EAC = esophageal adenocarcinoma; MI = myocardial infarction; GIE = gastrointestinal event, which includes ulcer or gastrointestinal bleeding; ▼ = decrease; ▲ = increase.
We also presented participants with scenarios in which the future lifetime risk of EAC was returned to the initial baseline benefit value and the magnitude of risk for each harm was reduced in turn. In a scenario in which celecoxib did not increase the future risk of heart attack at all, only 42% were willing to take the drug. When it had no effect on stroke and GIE risk, 29.4% and 25.0% of patients were willing to take the improved celecoxib, respectively. For aspirin, the elimination of MI risk and no increase in GIE risk would result in 86% and 97% acceptance of the drug.
DISCUSSION
Patients with Barrett’s esophagus face a steady increase in both the number and complexity of available medical management options. The decision to take either aspirin or coxibs to prevent esophageal adenocarcinoma by patients with Barrett’s esophagus is a preference-sensitive decision. In these decisions, the benefits of the treatments do not clearly outweigh the risks involved for all patients. Therefore, each decision must be based on individual patient preferences regarding the benefits and risks faced both with treatment and without. Many prevention decisions are preference sensitive. For example, chemoprevention with tamoxifen decreases the short-term risk of developing breast cancer by approximately 50%, yet the potential side effects include endometrial cancer, stroke, and pulmonary embolism (16). Similar tradeoffs are present in the decision to take hormone replacement therapy. Because patients’ preferences regarding the outcomes associated with such decisions may vary widely, it is important to understand patient preferences regarding prevention interventions.
Our study analyzed patient preferences for two esophageal adenocarcinoma risk-reducing medications. When patients were informed about the benefits and harms of celecoxib and aspirin for the chemoprevention of esophageal adenocarcinoma, the majority stated that they would not take celecoxib, but would take aspirin. Participants were five times more willing to take aspirin than celecoxib, with similar preferences in women and men. These findings are consistent with what we intuitively hypothesized, as the salient difference between the two drugs’ effect on future risk of MI makes aspirin a more attractive chemopreventive choice to prevent EAC.
Our analysis found that the pivotal issue affecting a subject’s willingness to take celecoxib was the greater risk of MI, while the lower risk of EAC was most cited for aspirin. Improved drug profile scenarios for celecoxib found that even if specific benefits and harms were improved, the majority (57–75%) stated that they still would not be willing to take the drug. With numerous reports documenting the greater risk of MI with celecoxib, its use for EAC chemoprevention would appear highly unlikely and perhaps ill-advised. Although we studied patient preferences for two specific drugs, our analysis of important factors and improved drug profile scenarios are applicable to other drugs not expressly studied. For example, our findings regarding patient preferences for celecoxib may be relevant as newer coxibs in development are likely to have an overall similar profile to celecoxib but with improvements in a particular characteristic (e.g., improved cardiac safety). For aspirin, if the greater risk of GIE were eliminated in an improved drug, a majority (97%) stated that they would be willing to take it. Although we are not endorsing such a practice, combination therapy with prophylactic proton pump inhibitor therapy could significantly diminish GIEs (17).
Our results are not intended to be used as clinical guidelines. Furthermore, clinical guidelines for these types of prevention interventions typically recommend that patients make decisions based on individual preferences rather than on population-based standards, placing a significant responsibility on physicians to effectively counsel patients and on patients to identify their preferences. We present data to aid in this process of shared decision making. A natural corollary to our study would be the construction of a decision aid to guide patients with BE considering chemo-prevention against esophageal adenocarcinoma. Such an instrument could be valuable in a real-world clinical setting where patients and physicians are making decision regarding chemoprevention.
Cost-effectiveness analyses (CEA) that have studied aspirin for EAC chemoprevention in BE have found that it can be a cost-effective therapy (18, 19). A similar cost-effectiveness analysis for celecoxib in BE patients has not been published. A colorectal cancer chemoprevention CEA comparing celecoxib and aspirin incorporated the cardiovascular benefits of aspirin, but not the harms of celecoxib, and found that aspirin was the preferred choice (20). Another CEA studied the use of aspirin for primary cardiovascular prevention and found that it could be cost-saving (more effective and less costly) compared to no aspirin in a high-risk group of middle-aged men (21). None of the aforementioned studies incorporated patient adherence to the chemoprevention drug into their models, which could have significantly affected the results of their analyses.
The Chemoprevention for Barrett’s Esophagus Trials Research Group (CBET) recently published the results of a randomized trial of celecoxib or placebo in patients with BE and low- or high-grade dysplasia. After 48 wk, no statistically significant difference in the rate of dysplasia was observed (22). This negative study’s suggestion that celecoxib does not prevent progression from dysplasia to cancer must be viewed with some caution as the study had an inadequate follow-up period, sample size, and the use of a surrogate end point in a specific subpopulation (those with dysplasia) of Barrett’s patients.
Our research had several limitations. The questionnaire was not a validated instrument. However, we incorporated established concepts and techniques from the disciplines of risk communication and survey science. Furthermore, the effort and cost required to formally validate a survey instrument is prohibitive for highly specific clinical issues such as chemoprevention in Barrett’s esophagus.
Our questionnaire design attempted to achieve the difficult balance between medical accuracy while avoiding cognitive burden (23). The descriptions, particularly the quantitative values, were based on the published literature but were still simplifications of medical knowledge. The process of distilling large amounts of complex medical data into information that is comprehensible and does not overwhelm an individual without medical training could lead to biases, or “framing effects.” For example, the order of the questions asked could have influenced the subjects’ perception of risks, with earlier questions and data affecting subsequent responses. We followed published recommendations on methods to minimize “framing manipulation” (24) such as presenting benefits and harms in the absolute risk format as well as the use of adjunctive visual aids.
Our study population, although demographically diverse, may not be representative of the general public. In particular, the level of education was much higher than the population average. Univariate analysis found that more educated patients were more likely to take aspirin, although this effect did not persist in the multivariate model. Because the study was performed at a tertiary referral center, 45% of the study group had a history of high-grade dysplasia, which may have led to some patient biases (possibly overestimates) regarding future EAC risk in particular. All subjects were patients who were recruited prior to an endoscopy or clinic visit, selecting out a more compliant and potentially sicker group of individuals.
In summary, a majority of those surveyed stated that they would take aspirin but not celecoxib. The greater risk of MI and the lower risk of EAC were the most important factors determining celecoxib and aspirin use, respectively. Our study finds that most patients are interested in EAC chemoprevention, but that the amount of protection offered and the side-effect profile of a drug determine whether or not it is acceptable. These data provide insights into the tradeoffs patients are willing to consider and can inform physicians regarding the variability and factors that affect patient preferences for chemoprevention.
STUDY HIGHLIGHTS.
What Is Current Knowledge
Data suggest that aspirin and nonsteroidal anti-inflammatory drug (NSAID) use in Barrett’s esophagus (BE) may prevent progression to esophageal adenocarcinoma.
Aspirin chemoprevention in BE could be cost-effective.
What Is New Here
Patients are more willing to take aspirin than celecoxib for chemoprevention of esophageal adenocarcinoma.
The greater risk of myocardial infarction and the lower risk of esophageal adenocarcinoma are the most important factors determining a drug’s acceptability.
Most BE patients are interested in esophageal adenocarcinoma (EAC) chemoprevention, depending on the amount of benefit provided and side-effect profile.
Acknowledgments
The authors would like to thank Ms. Lauren E. Cipriano for her numerous suggestions and thoughtful editing of our manuscript.
Financial support: Grant support: NIH K07CA107060 (C.H.) and American Gastroenterological Association Research Scholar Award (C.H.)
APPENDIX. Barrett’s Esophagus and Esophagus Cancer Survey (Male Version)
Scenario
We will ask you to think about your health and esophagus cancer prevention and then ask you to make some choices.
Barrett’s Esophagus and Esophagus Cancer
As a patient with Barrett’s esophagus, you are at higher risk for getting cancer of your esophagus. There are medicines available that may help prevent Barrett’s from progressing to cancer. Each medicine has its own risks and side effects. Not everything is known about these medicines. Please read some of the best information we have available about one of these medicines, Medicine A, and answer the imaginary choices in the questions.
Medicine A
Medicine A is a pill that you take once a day.
Your doctor would give you a prescription for this medicine.
Medicine A reduces your risk of getting esophagus cancer by 50%. As a person with Barrett’s, your lifetime chance of getting esophagus cancer is about 10%, so this medicine reduces your lifetime risk to 5%. (See pictures on green sheet.)
Harms and Side Effects
Medicine A increases your chance of having a heart attack by 50%. Over a lifetime, 50% of men will have a heart attack, so Medicine A increases your chance of having a heart attack to 75%.
Medicine A increases your chance of having a stroke by 50%. Since 20% of men will have a stroke in their lifetime, Medicine A increases your lifetime chance of having a stroke to 30%.
Medicine A increases the chance of you having problems in your gut (stomach or intestines) by 50%. This may include getting an open sore in the gut, called an ulcer, or having bleeding in your stomach or intestines. The lifetime risk of having an ulcer or bleeding in your gut is 10%, so Medicine A increases your chance of having these problems to 15%.
Medicine A increases your chance of having stomach upset or indigestion. About 10% of people taking Medicine A will stop taking it because of stomach upset.
Medicine A Summary
| Benefits | Harms |
|---|---|
|
|
Scenario
Now please think about a DIFFERENT medicine that helps prevent esophagus cancer, Medicine B.
Medicine B
Medicine B is a pill that you take once a day.
You can buy Medicine B over the counter (you don’t need a prescription).
Medicine B reduces your risk of getting esophagus cancer by 50%. Your lifetime chance of getting esophagus cancer is 10%, so this medicine reduces your lifetime risk to 5%.
Medicine B reduces your chance of heart attack by 30%. Since 50% of men will have a heart attack in their lifetime, Medicine B lowers your chance of having a heart attack to 35%.
Harms and Side Effects
Medicine B increases the chance of you having problems in your gut (stomach or intestines) by 75%. This may include getting an ulcer or having bleeding in your stomach or intestines. Over a lifetime, 10% of people will have an ulcer or bleeding in their gut. Medicine B increases your chance of having these problems to 18%.
Medicine B Summary
| Benefits | Harms |
|---|---|
|
|
Footnotes
Guarantor of the article: Chin Hur, M.D., M.P.H.
Specific author contributions: Chin Hur: Study conception, design, funding, IRB, patient recruitment, data collection, results interpretation, statistical analysis, manuscript preparation; Darcy E. Broughton: Study conception, design, IRB, patient recruitment, data collection, results interpretation, manuscript preparation; Elissa Ozanne: Study design, results interpretation, statistical analysis, manuscript preparation; Patrick Yachimski: Statistical analysis, manuscript preparation; Norman S. Nishioka: Study design, patient recruitment, manuscript preparation; G. Scott Gazelle: Study funding, manuscript preparation.
Potential competing interests: No financial support for the research. Dr. Norman Nishioka reports having received speaking honoraria from Axcan Pharma. None of the other authors have any potential competing interests to disclose.
References
- 1.Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson GA, Narkowicz C, Lord R, et al. Effect of celecoxib on cyclooxygenase-2 expression and possible variants in a patient with Barrett’s esophagus. Dis Esophagus. 2007;20:265–8. doi: 10.1111/j.1442-2050.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Buttar NS, Wang KK, Anderson MA, et al. The effect of selective cyclooxygenase-2 inhibition in Barrett’s esophagus epithelium: An in vitro study. J Natl Cancer Inst. 2002;94:422–9. doi: 10.1093/jnci/94.6.422. [DOI] [PubMed] [Google Scholar]
- 4.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 5.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 6.Kimmel SE, Berlin JA, Reilly M, et al. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med. 2005;142:157–64. doi: 10.7326/0003-4819-142-3-200502010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 8.Sanmuganathan PS, Ghahramani P, Jackson PR, et al. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart. 2001;85:265–71. doi: 10.1136/heart.85.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden M, Pignone M, Phillips C, et al. Aspirin for the primary prevention of cardiovascular events: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:161–72. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Larson MG, Beiser A, et al. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 11.Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: A randomized controlled trial. JAMA. 1999;282:1921–8. doi: 10.1001/jama.282.20.1921. [DOI] [PubMed] [Google Scholar]
- 13.Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: Systematic review of randomised controlled trials. BMJ. 2002;325:619. doi: 10.1136/bmj.325.7365.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malenka DJ, Baron JA, Johansen S, et al. The framing effect of relative and absolute risk. J Gen Intern Med. 1993;8:543–8. doi: 10.1007/BF02599636. [DOI] [PubMed] [Google Scholar]
- 15.Lenert LA, Sturley A, Watson ME. iMPACT3: Internet-based development and administration of utility elicitation protocols. Med Decis Making. 2002;22:464–74. doi: 10.1177/0272989X02238296. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study [see comments] J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 17.Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033–8. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg A, Fennerty MB. Medical decision analysis of chemoprevention against esophageal adenocarcinoma. Gastroenterology. 2003;124:1758–66. doi: 10.1016/s0016-5085(03)00393-7. [DOI] [PubMed] [Google Scholar]
- 19.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of aspirin chemoprevention for Barrett’s esophagus. J Natl Cancer Inst. 2004;96:316–25. doi: 10.1093/jnci/djh039. [DOI] [PubMed] [Google Scholar]
- 20.Hur C, Simon LS, Gazelle GS. The cost-effectiveness of aspirin versus cyclooxygenase-2-selective inhibitors for colorectal carcinoma chemoprevention in healthy individuals. Cancer. 2004;101:189–97. doi: 10.1002/cncr.20329. [DOI] [PubMed] [Google Scholar]
- 21.Pignone M, Earnshaw S, Tice JA, et al. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: A cost-utility analysis. Ann Intern Med. 2006;144:326–36. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 22.Heath EI, Canto MI, Piantadosi S, et al. Secondary chemo-prevention of Barrett’s esophagus with celecoxib: Results of a randomized trial. J Natl Cancer Inst. 2007;99:545–57. doi: 10.1093/jnci/djk112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlong WFD, Torrance GW, Barr R, et al. Centre for Health Economics and Policy Analysis Working Paper Series #90–9. Hamilton, Ontario, Canada: McMaster University; 1990. Guide to design and development of health-state unitility instrumentation. [Google Scholar]
- 24.Edwards A, Elwyn G, Mulley A. Explaining risks: Turning numerical data into meaningful pictures. BMJ. 2002;324:827–30. doi: 10.1136/bmj.324.7341.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology. 2000;119:333–8. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 26.Shaheen NJ, Green B, Medapalli RK, et al. The perception of cancer risk in patients with prevalent Barrett’s esophagus enrolled in an endoscopic surveillance program. Gastroenterology. 2005;129:429–36. doi: 10.1016/j.gastro.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 27.Buttar NS, Wang KK, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–12. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 28.Farrow DC, Vaughan TL, Hansten PD, et al. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97–102. [PubMed] [Google Scholar]
- 29.Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: Estimates from the Framingham Study. Stroke. 2006;37:345–50. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 30.Graham DY, Rakel RE, Fendrick AM, et al. Scope and consequences of peptic ulcer disease. How important is asymptomatic Helicobacter pylori infection? Postgrad Med. 1999;105:100–2. 105–8, 110. doi: 10.3810/pgm.1999.03.593. [DOI] [PubMed] [Google Scholar]


