Abstract
Rationale
Increased neutrophil and monocyte counts are often associated with an increased risk of atherosclerosis, but their relationship to insulin sensitivity is unknown.
Objective
To investigate the contribution of forkhead transcription factors (FoxO) in myeloid cells to neutrophil and monocyte counts, atherosclerosis, and systemic insulin sensitivity.
Methods and Results
Genetic ablation of the three genes encoding FoxO isoforms 1, 3a, and 4, in myeloid cells resulted in an expansion of the granulocyte/monocyte progenitor compartment, and was associated with increased atherosclerotic lesion formation in Ldl receptor knockout mice. In vivo and ex vivo studies indicate that FoxO ablation in myeloid cells increased generation of reactive oxygen species. Accordingly, treatment with the antioxidant N-acetyl-L-cysteine reversed the phenotype, normalizing atherosclerosis.
Conclusions
Our data indicate that myeloid cell proliferation and oxidative stress can be modulated via the FoxO branch of insulin receptor signaling, highlighting a heretofore-unknown link between insulin sensitivity and leukocytosis that can affect the predisposition to atherosclerosis.
Keywords: Atherosclerosis, neutrophils, macrophages, insulin resistance, oxidative stress, antioxidant enzymes, stem cell
INTRODUCTION
Atherosclerotic cardiovascular disease is the leading cause of death of type 2 diabetic patients 1, possibly owing to its refractoriness to glucose control 2–4. Insulin resistance can also account for the increased vulnerability of diabetic patients to atherosclerosis, but its pathogenetic mechanism is not completely understood, and is likely to involve multiple target organs of insulin action.5
In the liver, for example, alterations of insulin receptor (InsR) signaling result in changes of hepatocellular triglyceride content and assembly into or export as apolipoprotein B (ApoB)-containing, very low-density lipoproteins 6, 7 that are typically elevated in the plasma of type 2 diabetic patients 8. In addition, hepatic InsR signaling also regulates LDL receptor turnover9, possibly contributing to the lower than expected LDL-cholesterol levels in these patients.
In the arterial wall, the role of insulin resistance in different cell types and at different stages of disease progression is controversial. In endothelial cells, a burgeoning consensus supports the conclusion that augmenting insulin signaling through Irs2/Akt/FoxO prevents atherosclerosis by pleiotropic mechanisms10–12. In macrophages, another insulin-sensitive cell type with critical functions in disease progression13, the data are mixed. At the cellular level, InsR signaling in macrophages modulates inflammation in a context-specific fashion, as well as apoptosis and ER stress14. These signals appear to be largely mediated through FoxO15. It’s unclear how anti-atherogenic InsR signals are mediated14, 16.
To study the role of the FoxO branch of InsR signaling in macrophages on atherosclerosis, we generated mice lacking the three FoxO isoforms (1, 3a, and 4) in this cell type. Our data provide evidence for a dual role of FoxO-dependent signaling in monocyte/macrophages and their progenitors in the pathogenesis of atherosclerosis. First, we show that that FoxO ablation increases proliferation of granulocyte-monocyte progenitors, resulting in neutrophilia with monocytosis, a predisposing factor in both human 17 and murine atherosclerosis 18. Second, myeloid FoxO ablation also increases iNOS expression and oxidative stress in macrophages, possibly contributing to endothelial dysfunction. As a result, mice lacking the three FoxO proteins in myeloid cells develop larger atherosclerotic lesions than WT controls, with an increased number of intra-lesional macrophages, but a decreased percentage of apoptotic macrophages.
METHODS
We generated myeloid-specific Foxo knockout mice (MYFKO) by mating Foxo1lox/lox3alox/lox4lox/lox mice 19 with Lysozyme M (LysM)-Cre mice (Jackson Laboratories). We then crossed MYFKO mice and Ldlr−/− mice to generate Ldlr−/−: MYFKO mice. We fed animals Western diet (WTD, 0.2% cholesterol, 42% from fat adjusted calorie diet, TD 88137, Harlan Tekland) for the indicated times. We conducted experiments in male Cre(+) and littermate Cre(−) (control) mice. Macrophage isolation and manipulations have been described 20. Blood and bone marrow analysis using flow cytometry were performed as previously described 18, 21,22 (Online Figure IA and IB). The Columbia University Animal Care and Utilization Committee approved all procedures. An expanded Methods section is available as Online Data Supplement.
RESULTS
MYFKO mice display neutrophilia and monocytosis
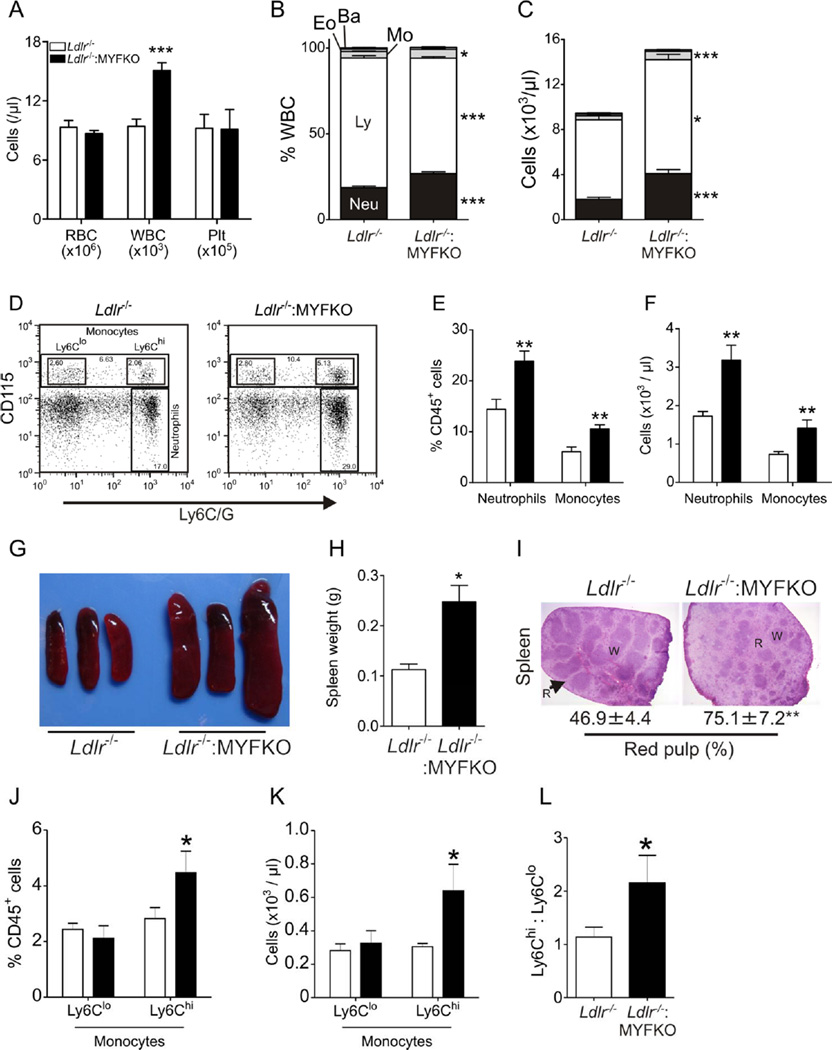
Thioglycollate-elicited peritoneal or bone marrow-derived macrophages from myeloid-specific triple Foxo-knockout mice (MYFKO) showed successful ablation of FoxO1, 3a, and 4 mRNA and/or protein (Online Figure IIA–IID). We determined the cellular composition of peripheral blood and bone marrow in MYFKO mice. Peripheral blood counts showed increased number of white cells in Ldlr−/−: MYFKO mice, with normal numbers of erythrocytes and platelets (Figure 1A). Differential white cell counts showed an increased percentage of neutrophils and monocytes, and decreased lymphocytes (Figure 1B), accompanied by increased total numbers of neutrophils, monocytes, and lymphocytes (Figure 1C). These findings were corroborated by flow cytometry analysis of peripheral blood that showed increased relative and absolute numbers of neutrophils (defined as CD45+ CD115– Ly6C/Ghi cells) and monocytes (CD45+ CD115+) (Figure 1D–1F). Ldlr−/−: MYFKO mice showed splenomegaly, secondary to red pulp hypertrophy (Figure 1G–1I), as well as increased Ly6Chi monocytes, a key contributing population to murine atherosclerosis 23 (Figure 1J–1L). Neutrophilia and monocytosis are associated with increased cardiovascular disease risk in humans 17 and in animal models of atherosclerosis 23.
Figure 1. Peripheral blood cell and monocyte count.
(A) Complete blood counts (RBC, WBC, and Plt) in 6-week-old Ldlr−/− and Ldlr−/−: MYFKO mice (n=9–10). (B) Percentage and (C) absolute number of neutrophils (Neu), lymphocytes (Ly), monocytes (Mo), eosinophils (Eo), and basophils (Ba) (n=9–10). (D) Representative scatter plots, (E) percentage and (F) absolute number of CD45+ CD115– Ly6Chi (neutrophils) and CD45+ CD115+ cells (monocytes) (n=5–6). (G) Representative picture and (H) weight of spleens, and (I) percentage of red pulp in H&E section of spleens (n=5). (J) Percentage and (K) absolute number of CD45+ CD115+ Ly6Clo and CD45+ CD115+ Ly6Chi monocytes (n=5–6). (L) Ly6Clo/Ly6Chi monocyte ratio (n=5–6). R: red pulp, W: white pulp. * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. Ldlr−/−.
These abnormalities of blood cell composition are likely determined by the combination of triple Foxo knockout and Ldlr nullizygosity, as they were not observed in single LysM-Cre-Foxo1−/− mice (Online Figure IIIA–IIIC), and were considerably less marked in Ldlr-competent MYFKO mice (Online Figure IIID–IIIF).
Altered turnover of Foxo-deficient granulocyte-macrophage progenitors and macrophages
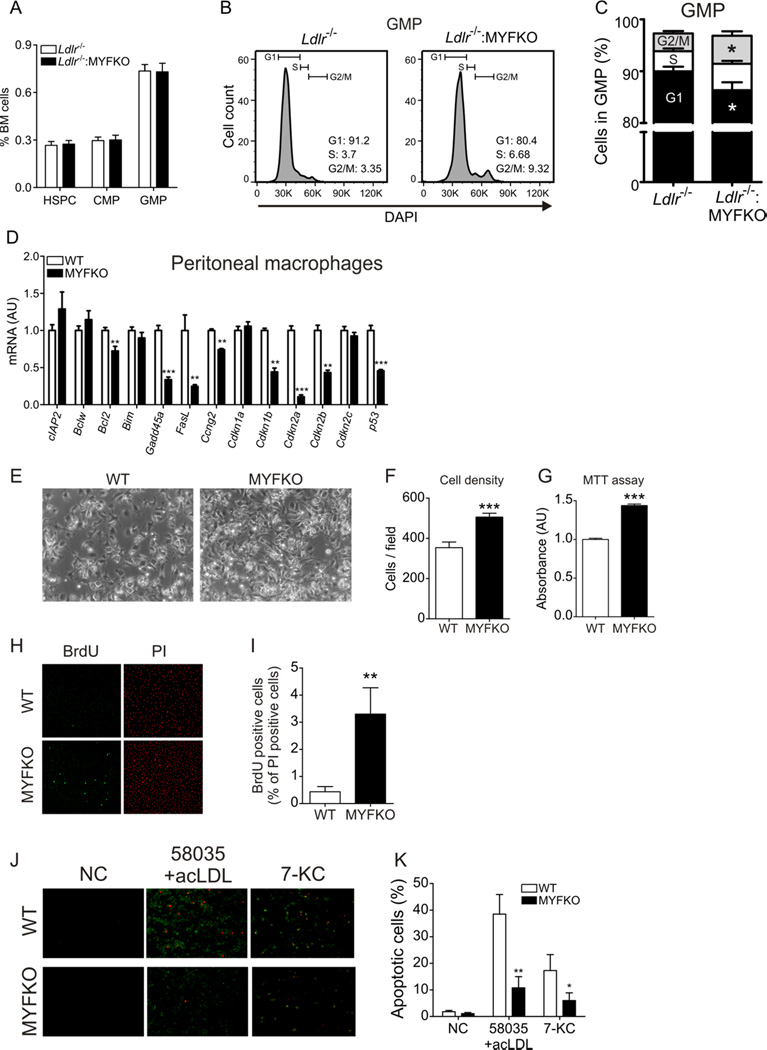
Granulocytes and monocytes develop from a common myeloid granulocyte-macrophage progenitor cell (GMP). Bone marrow (BM) analysis revealed that the abundance of hematopoietic stem and progenitor cells (HSPC), common myeloid progenitor (CMP), and GMP cells were unchanged in Ldlr−/−: MYFKO mice (Figure 2A). However, cell cycle analysis of BM-derived cells showed an increased proportion of GMP cells from Ldlr−/−: MYFKO mice in G2/M phase, and a decreased proportion in G1 phase (Figure 2B and 2C). In contrast, proportion of cells in G1, S, and G2/M were comparable in HSPC and CMP (Online Figure IVA and IVB). Gene expression analysis revealed the expected reduction of FoxO3a in CMP, and modest reduction of FoxO1, FoxO3a, FoxO4, Cdkn1b and 2a in GMP of Ldlr−/−: MYFKO mice (Online Figure IVC–IVF). Peritoneal macrophages from MYFKO mice showed a marked reduction of cell cycle inhibitor genes Ccng2, Cdkn1b, 2a 2b, and p53, as reported in another triple Foxo knockout 24 (Figure 2D). Consistently, cultured peritoneal macrophages from MYFKO mice showed increased proliferation (Figure 2E–2G) and BrdU staining (Figure 2H and 2I). These data indicate that FoxO ablation impairs cell cycle arrest, resulting in increased proliferation.
Figure 2. Proliferation and apoptosis of BM and macrophages.
(A) Percentage of hematopoietic stem and progenitor cells (HSPC), common myeloid progenitors (CMP), and granulocyte-myeloid progenitors (GMP) in BM (n=5). (B) Representative scatter plots and (C) quantification of cell cycle progression in HSPC, CMP, and GMP using DAPI staining (n=5). (D) Expression of apoptosis- and cell cycle-related genes in cultured peritoneal macrophages (n=5–6). (E) Representative pictures and quantification of cell densities assessed by (F) counting and (G) MTT assay of peritoneal macrophages after 32 h of culture (n=4). (H) Representative pictures of BrdU and propidium iodide (PI) staining and (I) percentage of BrdU-positive cells of peritoneal macrophages after 32 h of culture (n=4). (J) Representative pictures and (K) quantification of Annexin V (green) and PI staining (red) in macrophages loaded with free-cholesterol (58035 10µg/ml + acLDL 100µg/ml) or 7-ketocholesterol (10µM) for 20 h. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. WT or Ldlr−/−.
We have shown that FoxO ablation protects macrophages from free-cholesterol-induced apoptosis15. Consistently, peritoneal macrophages from MYFKO mice were refractory to free-cholesterol-and 7-ketocholesterol-induced apoptosis compared to WT (Figure 2J and 2K). These data suggest that neutrophilia and monocytosis in Ldlr−/−: MYFKO mice result from increased proliferation and decreased apoptosis in FoxO-deficient GMP cells.
Increased atherosclerosis and macrophage accumulation in Ldlr−/−: MYFKO mice
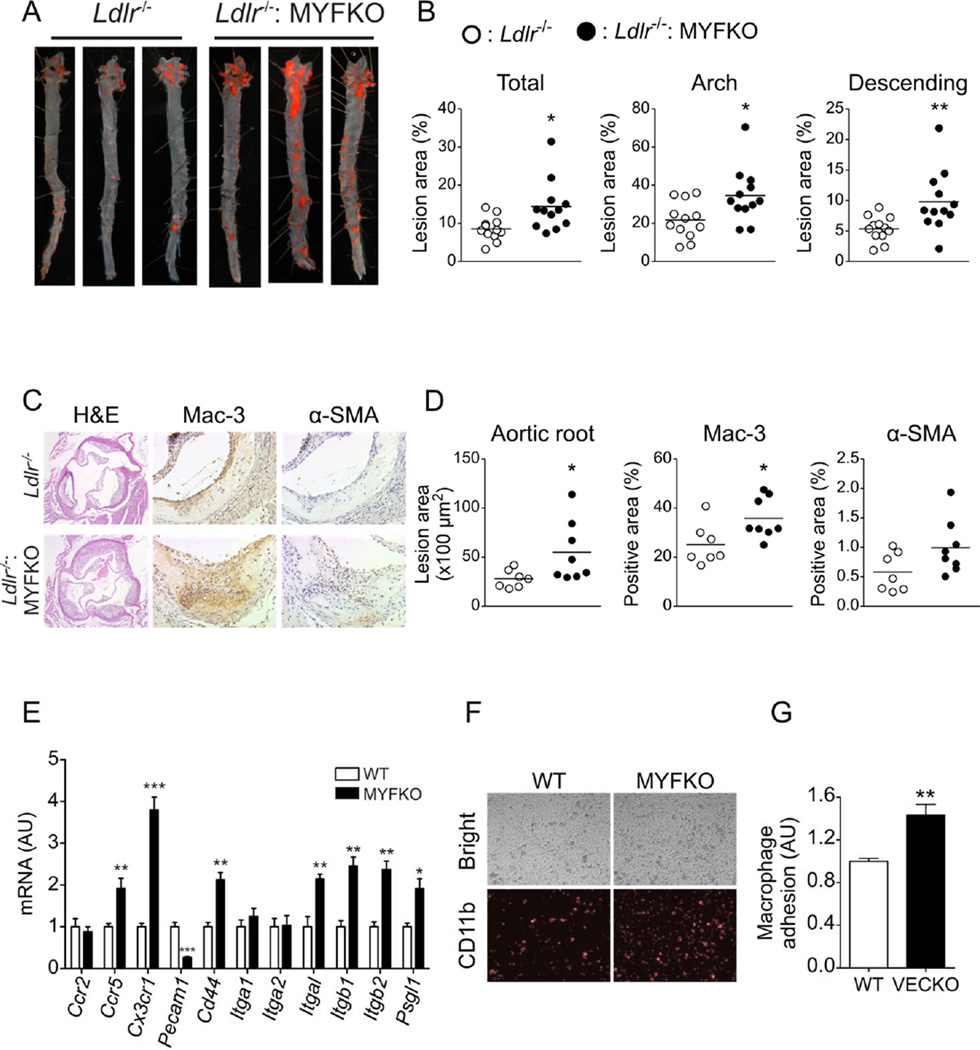
We analyzed atherosclerotic lesions in Ldlr−/−: MYFKO mice after 14 weeks of WTD. Analyses of en face aorta preparations revealed a 68% increase of lesion area in Ldlr−/−: MYFKO compared to Ldlr−/− mice (Figure 3A and 3B). RNA extracted from whole aortae showed increased expression of macrophage marker Emr1, Il1b, and NADPH oxidase components p47phox and p60phox in Ldlr−/−: MYFKO mice (Online Figure VA). Histological analysis of aortic roots also showed increased lesion area, as well as Mac-3 and α-SMA immunoreactivities in Ldlr−/−: MYFKO mice (Figure 3C and 3D). Collagen content and necrotic lesion area ware similar (data not shown). In contrast, we observed decreased active caspase-3 immunoreactivity in aortic root plaques of Ldlr−/−: MYFKO mice (Online Figure VB and VC). Peritoneal macrophages from MYFKO mice showed increased expression of chemokine receptors Ccr5 and Cx3cr1, and adhesion molecule Cd44, as reported in regulatory T-cells lacking FoxO1 and 3a 25 (Figure 3E), as well as integrins Itgal, Itgb1 and 2, and Psgl1. In contrast, Pecam1, which suppresses atherosclerosis in BM 26, was decreased in macrophages from MYFKO mice. Consistently, adhesion assays using peritoneal macrophages and LPS-stimulated MS-1 endothelial cells showed increased adhesion of MYFKO macrophages to endothelial cells (Figure 3F and 3G). Expression of markers of classic (M1) and alternative (M2) peritoneal macrophages was unaltered (Online Figure VD). LPS-induced p105 degradation and p65 phosphorylation were comparable between WT and MYFKO macrophages (Online Figure VE).
Figure 3. Atherosclerotic lesion analysis in WTD-fed mice.
(A) Representative en face Oil Red-O staining and (B) quantification of total, aortic arch, and descending thoracic aorta lesion area in 20-week-old Ldlr−/− and Ldlr−/−: MYFKO mice after 14 weeks on WTD (n=12). (C) Representative pictures and (D) quantification of aortic root with hematoxylin and eosin (H&E), Mac-3, and α-smooth muscle cell actin (α-SMA) (n=7–8). (E) Gene expression of peritoneal macrophages (n=4–5). (F) Representative pictures and (G) quantification of adherent CD11b-labelled peritoneal macrophages to MS1 cells (n=4).* p < 0.05, ** p < 0.01, *** p < 0.001 vs. WT or Ldlr−/−.
These data indicate that ablation of the three FoxO in myeloid cells exacerbates WTD-induced atherosclerosis in Ldlr−/− mice. This appears to be due to macrophage accumulation owing to decreased macrophage apoptosis and proliferation 21, 27.
FoxO inactivation in macrophages reduces Akt signaling
Next, we examined the consequence of FoxO ablation on macrophage insulin signaling. We and others have described an auto-regulatory FoxO/Akt loop, whereby increased FoxO activity begets a compensatory increase in Akt phosphorylation 28, and vice versa 12. Similar to prior observations in other conditional knockouts 12, 29, we detected decreased InsR and Irs1 levels, as well as decreased phospho-Akt (S473 and T308) generation in response to insulin in FoxO-deficient macrophages (Online Figure VIA). These data phenocopy the decrease in insulin signaling observed following chronic exposure of primary peritoneal macrophages to pharmacological concentrations of insulin, with impaired insulin-stimulated Akt phosphorylation (S473 and T308) associated with marked reduction in InsR and Irs1 levels (Online Figure VIB). It should be pointed out that, despite impaired Akt activation, basal phosphorylation of FoxO1 and 3a was increased in this model, resulting in decreased FoxO activity. These data are consistent with the observation that FoxO phosphorylation is extremely sensitive to basal levels of Akt activity 30, and allow us to infer that the triple FoxO knockout mimics the biochemical effects of in vivo insulin resistance, thus validating the genetic model as a surrogate of the effects of hyperinsulinemia in vivo.
Increased oxidative stress and NO production in MYFKO macrophages
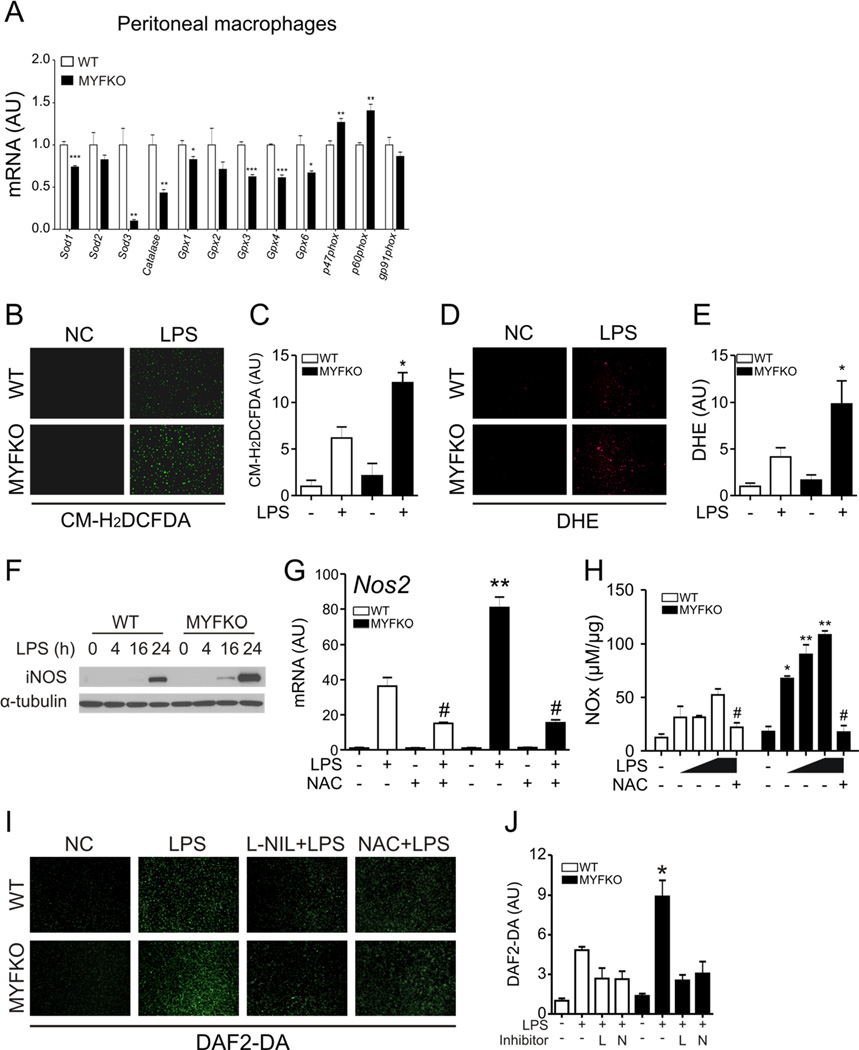
Next, we analyzed the consequences of the triple FoxO knockout on macrophage function. Expression of antioxidant enzymes superoxide dismutase (Sod), catalase (Cat), and Glutathione peroxidase (Gpx) was lower in peritoneal macrophages from MYFKO mice compared to WT (Figure 4A). Genes encoding catalytic subunits of NADPH oxidase (p22phox and p47phox) were expressed at higher levels in MYFKO macrophages. Consistently, LPS-induced reactive oxygen species and superoxide production were enhanced in macrophages from MYFKO mice, as assessed by CM-H2DCFDA (Figure 4B and 4C) and DHE (Figure 4D and 4E), respectively. Elevated iNOS expression is a marker of Ly6Chi monocytes 31, and is induced by oxidative stress in macrophages 32. Accordingly, LPS-dependent iNOS protein and mRNA induction were enhanced in MYFKO macrophages (Figure 4F and 4G), as were palmitate- or H2O2-dependent Nos2 inductions (data not shown). Pretreatment with N-Acetyl-L-Cysteine (NAC), an antioxidant that promotes GSH synthesis, partly inhibited LPS-induced Nos2 expression, reversing the difference between WT and MYFKO mice (Figure 4G), and normalized NO production, measured by NOx (nitrate and nitrite) concentration in conditioned media (Figure 4H). Pretreatment with the iNOS inhibitor, L-NIL, also blunted LPS-induced NO production (Figure 4I and 4J). The combined reductions of Sod and Gpx, along with the increase of NADPH oxidase subunits, can increase oxidative stress in MYFKO mice. These data suggest that increased oxidative stress mediates iNOS-derived NO overproduction in macrophages from MYFKO mice.
Figure 4. Oxidative stress or iNOS induction in peritoneal macrophages.
(A) Expression of oxidative stress-related genes in peritoneal macrophages (n=4). Representative images and quantification of (B and C) CM-H2DCFDA and (D and E) DHE fluorescence in peritoneal macrophages with or without LPS stimulation (10ng/ml) for 30 min (n=4). (F) Representative iNOS immunoblot in peritoneal macrophages after LPS stimulation. (G) Nos2 levels after 6-hr incubation with LPS (10ng/ml) with or without NAC (10mM) pretreatment (n=4). (H) NOx (nitrate and nitrite) concentration in media from peritoneal macrophages after 16-hr LPS (0, 1, 5, or 10ng/ml) incubation with or without NAC (10mM) pretreatment (n=3–4). (I) Representative pictures and (J) quantification of LPS (10ng/ml, 30min)-stimulated NO production visualized by DAF2-DA fluorescence in cultured peritoneal macrophages from WT and MYFKO mice pretreated with or without L-NIL (250µM) or NAC (10mM) (n=4). * p < 0.05, ** p < 0.01 vs. WT. # p < 0.05 vs. LPS.
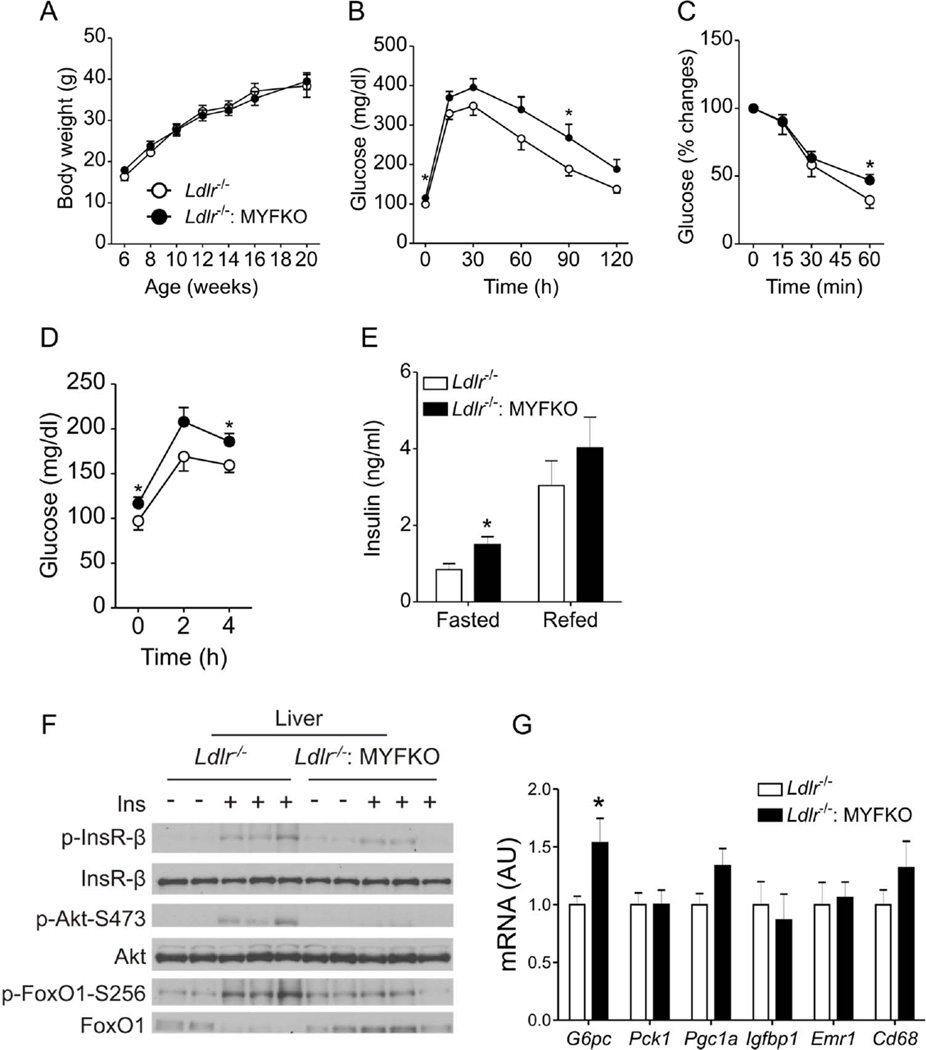
Reduced hepatic insulin signaling in WTD-fed Ldlr−/−: MYFKO mice
Altered insulin signaling in macrophages can affect systemic insulin sensitivity 33. This, in turn, could affect atherosclerosis development in MYFKO mice. Therefore, we investigated the effect of the triple myeloid FoxO knockout on insulin action. MYFKO mice fed standard (SD) or high-fat diet (HFD) showed no differences in body weight, fasted or re-fed glucose and insulin levels, or glucose tolerance (Online Figure VIIA–VIIH). In contrast, WTD-fed Ldlr−/−: MYFKO displayed normal body weight (Figure 5A) and composition (not shown), but borderline glucose tolerance without significant alterations of insulin tolerance (Figure 5B and 5C), and modest but significant elevations of fasting and fed glucose, as well as fasting insulin levels (Figure 5D and 5E). Total serum cholesterol (TC), triglyceride (TG), and non-esterified fatty-acid (NEFA) levels were comparable to Ldlr−/− mice (Online Figure VIIIA–VIIIC).
Figure 5. Metabolic characterization of WTD-fed mice.
(A) Body weights of Ldlr−/− and Ldlr−/−: MYFKO mice fed WTD starting at 6 weeks of age. Intraperitoneal (B) glucose and (C) insulin tolerance tests in 14- or 15-week-old WTD-fed mice after 18-hr or 4-hr fast, respectively (n=8–10). Serum (D) glucose and (E) insulin levels in WTD-fed, 19-week-old mice fasted for 16 hr (0h, Fasted), or fasted for 16 hr and refed for 4 hr (Refed) (n=6–8). (F) Immunoblots of liver insulin signaling of 14-week-old mice fed WTD for 8 weeks. After a 16-hr fast, mice were injected with insulin or PBS and livers were collected 3 min later (n=3). (G) Liver gene expression in 14-week-old Ldlr−/− and Ldlr−/−: MYFKO mice fed WTD for 8 weeks and fasted for 16 hr (n=6–8). * p < 0.05, ** p < 0.01 vs. Ldlr−/−.
When we analyzed hepatic insulin signaling after administration of insulin in the portal vein, we detected an attenuation of insulin-induced InsR, Akt (S473), and FoxO1 (S256) phosphorylation (Figure 5F, Online Figure VIIID–VIIIF). Changes in hepatic gene expression were limited to a modest increase of G6pc (Figure 5G). Liver TG and cholesterol contents were comparable between Ldlr−/− and Ldlr−/−: MYFKO mice (data not shown). WTD-fed Ldlr−/−: MYFKO mice did not show liver, pancreas, and kidney dysfunction by serum chemistry (Online Table I). These data are consistent with a mild impairment of hepatic insulin signaling in WTD-fed Ldlr−/−: MYFKO mice.
Analysis of gene expression in epidydimal adipose tissue showed no differences in Nos2, macrophage markers Emr1 and Cd68, and other inflammatory genes (data not shown). Immunostaining with Mac-3 in epididymal adipose tissue did not show any difference either (data not shown). Thus, it appears unlikely that activated tissue macrophages contribute to the mild metabolic defect of these mice.
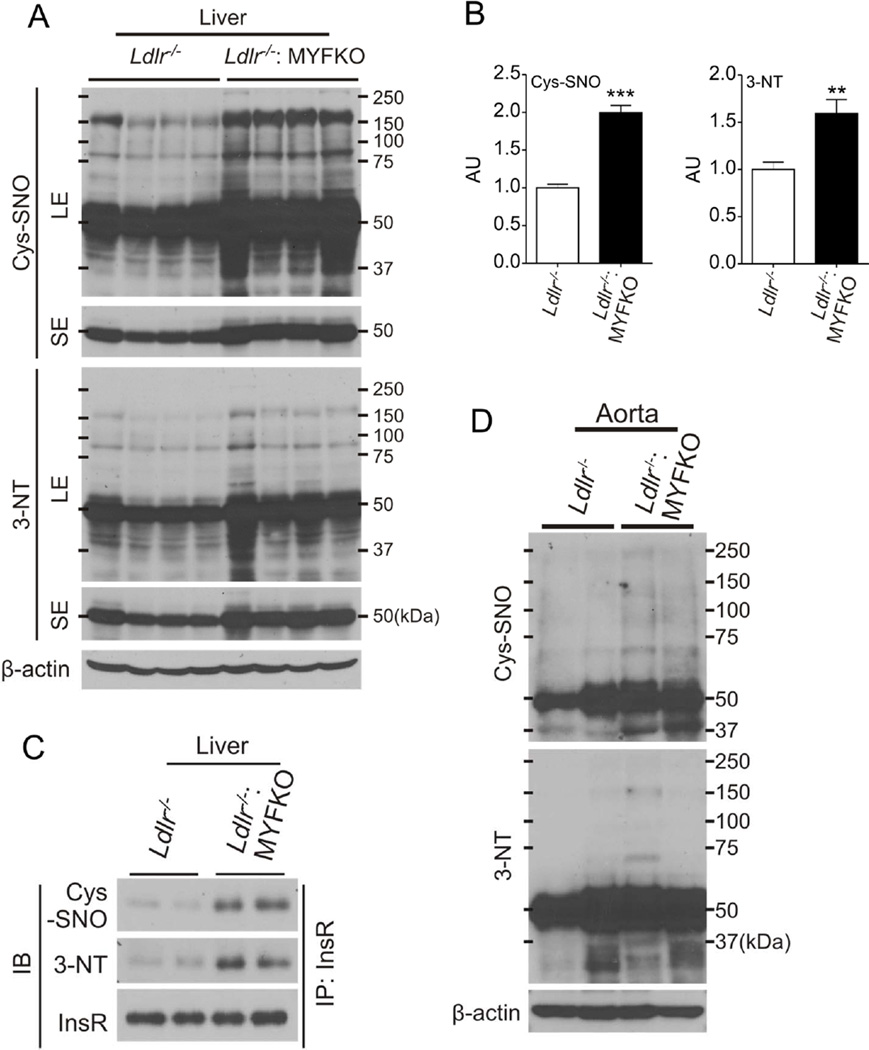
Increased cysteine nitrosylation and tyrosine nitration of InsR in WTD-fed Ldlr−/−: MYFKO mice
To understand the causes of the mild impairment of hepatic insulin signaling, we explored the hypothesis that increased iNOS-dependent NO production in liver caused protein nitrosylation as well as cysteine and tyrosine nitration 34. In fact, nitrosylation and/or nitration of InsR, Irs1, and Akt have been proposed to contribute to insulin resistance 35, 36. Indeed, immunoblotting analysis demonstrated a generalized increase of protein cysteine nitrosylation (Cys-SNO) and tyrosine nitration (3-NT) in livers of WTD-fed Ldlr−/−: MYFKO mice (Figure 6A and 6B). Immunoblotting of InsR immunoprecipitates showed increased Cys-SNO and 3-NT content of InsR from WTD-fed Ldlr−/−: MYFKO liver (Figure 6C). Cys-SNO and 3-NT in aortae of WTD-fed Ldlr−/−: MYFKO mice were also increased (Figure 6D). In contrast, MYFKO mice fed SD or HFD showed normal levels of hepatic Cys-SNO and 3-NT (data not shown). These data suggest that increased Cys-SNO and 3-NT of InsR, possibly caused by iNOS-derived NO from macrophages, contribute to the impairment of hepatic insulin signaling in WTD-fed Ldlr−/−: MYFKO mice.
Figure 6. Protein nitrosylation/nitration and insulin signaling in WTD-fed mice.
(A) Representative liver immunoblots and (B) quantification of S-Nitroso-Cysteine (Cys-SNO) and 3-Nitro-Tyrosine (3-NT) in 20-week-old mice after 14 weeks on WTD (n=4). (C) Immunoblots (IB) of Cys-SNO, 3-NT and InsR after immunoprecipitation (IP) of liver extracts with InsR. (D) Representative immunoblots with Cys-SNO and 3-NT in aortic extracts from 20-week-old mice after 14 weeks on WTD (n=4). SE: short exposure, LE: long exposure. ** p < 0.01, *** p < 0.001 vs. Ldlr−/−.
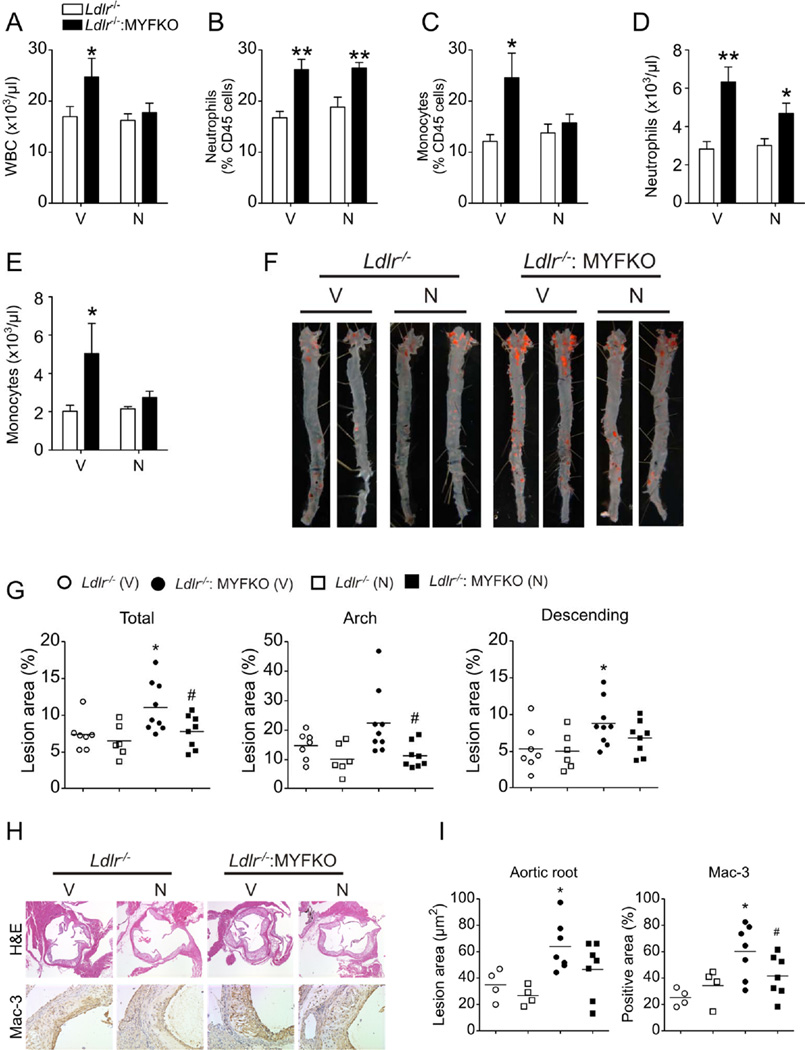
Antioxidant treatment reduces atherosclerosis in Ldlr−/−: MYFKO mice
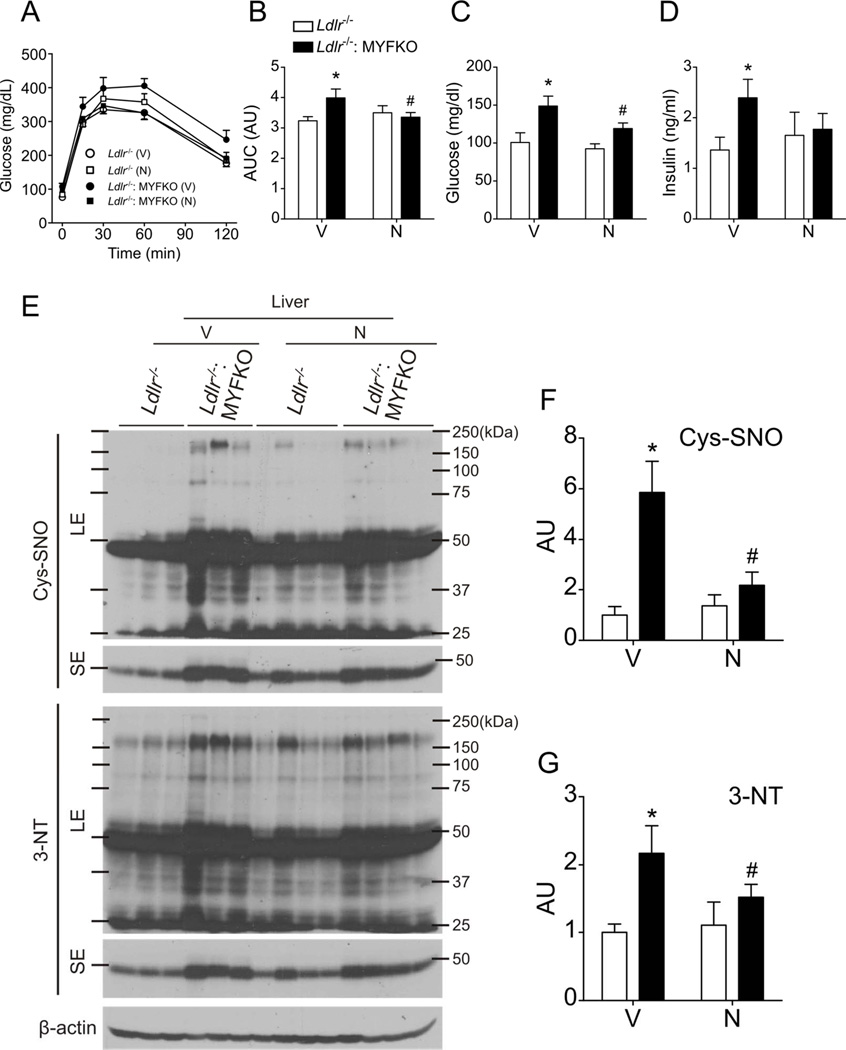
To test the causative role of oxidative stress and NO-mediated post-translational protein modifications in glucose metabolism and atherosclerosis, we treated WTD-fed mice with the antioxidant, NAC. NAC relieves oxidative stress in hematopoietic cells of mice with Mx1-Cre-mediated triple FoxO ablation 24. NAC can also lower Cys-SNO levels by displacing NO from cysteine-NO bonds 37. Seven weeks of oral NAC treatment prevented the increase of WBC and monocytes in Ldlr−/−: MYFKO mice that was seen in vehicle-treated mice (Figure 7A–E). Fourteen weeks of NAC treatment reduced WTD-induced atherosclerosis and macrophage accumulation (Figure 7F–7I), restored glucose tolerance, and decreased fasting glucose and insulin levels in WTD-fed Ldlr−/−: MYFKO mice (Figure 8A–8D). These changes were reflected in reduced hepatic Cys-SNO and 3-NT levels (Figure 8E–8G). The data suggest that oxidative stress and NO-mediated protein nitrosylation/nitration play a role in the development of atherosclerosis and hepatic insulin signaling abnormalities in Ldlr−/−: MYFKO mice.
Figure 7. Blood cell counts and atherosclerosis following NAC treatment.
(A) WBC, and (B and C) percentage and (D and E) absolute number of CD45+ CD115– Ly6Chi (neutrophils) and CD45+ CD115+ cells (monocytes) of 13-week-old WTD-fed Ldlr−/− and Ldlr−/−: MYFKO mice treated with Vehicle (V) or N-acetyl-cysteine (N) for 7 weeks (n=4–5). (F) Representative en face Oil Red-O staining and (G) quantification of total, arch, and descending thoracic aortic lesion area of 20-week-old WTD-fed Ldlr−/− and Ldlr−/−: MYFKO mice treated with Vehicle (V) or N-acetyl-cysteine (N) for 14 weeks (n=6–9). (H) Representative pictures and (I) quantification of aortic root lesion area and Mac3-positive areas (n=7–8). * p <0.05, ** p < 0.01 vs. Ldlr−/−. # p < 0.05 vs. vehicle-treated Ldlr−/−: MYFKO, respectively.
Figure 8. Metabolic parameters and protein nitrosylation/nitration following NAC treatment.
Intraperitoneal glucose tolerance tests and (B) area under the curve (AUC) of 18-week-old WTD-fed Ldlr−/− and Ldlr−/−: MYFKO mice treated with Vehicle (V) or N-acetyl-cysteine (N) for 12 weeks after an 18-hr fast (n=6–9). Serum (C) glucose and (D) insulin levels in 20-week-old mice fed WTD and fasted for 16 hr (n=6–9). (E) Representative immunoblots and quantification of (F) Cys-SNO and (G) 3-NT in liver from 20-week-old mice after 14 weeks on WTD and treatment with Vehicle (V) or N-acetyl-L-cysteine (N) (n=3–4). SE: short exposure, LE: long exposure. *, # p < 0.05 vs. vehicle-treated Ldlr−/−, or Ldlr−/−: MYFKO mice, respectively.
DISCUSSION
In the present work, we studied the role of myeloid FoxO in the pathogenesis of atherosclerosis. As FoxO proteins are negative regulators of insulin action 38, and given our findings in other conditional FoxO knockouts 12, 29, 39 as well as in the converse model of InsR ablation 40, we expected to find a protective role of this targeted mutation against atherosclerosis. Instead–and consistent with another study on InsR myeloid-specific knockout16–we find that MYFKO mice develop more severe atherosclerosis in the Ldlr knockout background. We have identified two mechanisms to account for this outcome: (i) a marked expansion of neutrophils and monocytes, likely caused by increased proliferation of GMP; and (ii) increased oxidative stress and iNOS-derived NO production in macrophages. We also detected a mild impairment of hepatic insulin sensitivity that might contribute to the phenotype.
The induction of GMP proliferation and neutrophil/monocyte number by FoxO ablation is probably due to inhibition of cell-cycle arrest, and decreased apoptosis. In addition, increased oxidative stress, as seen in mice with FoxO ablation, enhances short-term hematopoietic stem cell proliferation 24 and could thus explain the reversal of monocytosis in NAC-treated Ldlr−/−: MYFKO mice.
Correlation studies suggest that neutrophilia and monocytosis are linked with atherosclerosis in humans as well as in experimental animals. We have reported that deletion of the transporters Abca1 and Abcg1, which promote cholesterol efflux to apoA-1 or HDL, worsens atherosclerosis in mice by increasing neutrophil and monocyte number 18. Unlike the triple Lys-M-cre Foxo knockout, the pan-BM Abca1/g1 knockout affects proliferation of hematopoietic stem cells 18, 21,22. Thus, while the mechanisms of myeloid cell expansion in these two models are different, both point to a pathophysiologic link between common correlates of cardiovascular disease (HDL-cholesterol and insulin, respectively), white cell counts, and macrophage content of atherosclerotic lesions, suggesting a new therapeutic approach to cardiovascular disease aimed at reversing these abnormalities of stem/progenitor cell proliferation. Interestingly, in humans metabolic syndrome is associated with increased monocyte and neutrophil levels 41, which in turn are linked to increased CHD 17. Our data indicate that hyperinsulinemia–a common correlate of insulin resistance–increases FoxO phosphorylation and nuclear exclusion in macrophages, establishing a potential mechanism whereby insulin resistance increases CMP/GMP proliferation and myelogenesis.
Interestingly, Foxo4−/− mice in the Apoe−/− background display deterioration of atherosclerosis with increased macrophage content of lesions that can be transferred by Foxo4−/− bone marrow and this is associated with increased IL-6 and ROS production in cultured Foxo4−/− macrophages 42. These findings are consistent with the decreased atherosclerosis in Apoe−/− mice lacking InsR in myeloid cells, in which FoxO is expected to be constitutively active in macrophages 43.
The three FoxO isoforms function in a redundant manner to suppress proliferation or promote apoptosis 19, 25, 39, 44, including in BM 24. We have shown additive effects of FoxO1 and FoxO3a knockdowns on free-cholesterol-induced apoptosis in macrophages15. Consistently, we did not find evidence of increased predisposition to atherosclerosis in single Foxo1 knockouts in the present study, even though FoxO1 is the most abundant isoform in macrophages. This finding allays fears that FoxO1-specific inhibitors–which are being developed as insulin sensitizers 45–might increase CV risk. In addition, the extent of leukocytosis in MYFKO mice on an Ldlr-competent background is modest compared to Ldlr−/−: MYFKO mice. These observations suggest that homozygous loss of all three Foxo alleles, as well as Ldlr or Apoe ablation, are required to affect granulocytes, monocytes and their progenitors, and contribute to the development of atherosclerosis.
The decline of Sod, catalase, and Gadd45 levels in FoxO-deficient GMP and macrophages is consistent with the role of FoxO in the antioxidant response 32, and is further corroborated by the beneficial effect of antioxidant treatment on metabolism and atherosclerosis in Ldlr−/−: MYFKO mice. These data suggest that increased oxidative stress is pathogenic in atherosclerosis development in Ldlr−/−: MYFKO mice.
Epidemiological studies consistently show a positive correlation between leukocytosis and coronary artery disease 17. Neutrophils and monocytes play important roles in atherosclerosis. Neutrophils are the first cell type to home in to vascular endothelial cells during atherogenesis, triggering inflammatory signals that promote intimal recruitment of monocytes, namely the inflammatory Ly-6Chi subset, which increases steeply in atherosclerotic mice 27, 46.
These cells, once they enter the lesion, differentiate into macrophages, leading to foam cell formation 5 and progress the disease.
The GMP lineage-restricted proliferation of myeloid cells in Ldlr−/−: MYFKO mice is probably due to the fact that LysM-cre is hardly expressed in hematopoietic stem cells or CMP 47. That the increased GMP proliferation observed did not result in increased numbers of GMP is likely due to their accelerated turnover, consistent with the observation that FoxO-deficient BM has enhanced short-term and deficient long-term repopulating ability 24.
The increase in lesion macrophages seen in MYFKO mice is likely secondary to combined effects of FoxO ablation to decrease apoptosis and cell cycle arrest. The former could depend on decreased activation of FasL, the ligand for the Fas-dependent cell death pathway, or pro-apoptotic Bim. In addition, FoxO can promote cell cycle arrest 38 and FoxO ablation can increase macrophage survival 15.
Interestingly, we find a reduction of cell cycle genes (Cdkn1b, 2a, 2b and p53) that is consistent with increased atherosclerosis seen when Cdkn1b or p53 are ablated in BM cells 48–50, as well as with the proposed role of Cdkn2a as a modifier of atherosclerosis susceptibility 51.
The role of macrophage apoptosis in atherosclerosis is context-dependent, as apoptosis is thought to suppress plaque progression in early stages and promote plaque necrosis in advanced stages 52. However, animal studies suggest that macrophage apoptosis is a negative regulator of plaque growth even after long-term (10 to 15 weeks) cholesterol-rich diet 49, 53–56, which is equivalent in duration to the present study. Therefore, decrease of apoptosis could also partly contribute to the progression of atherosclerosis in Ldlr−/−: MYFKO mice.
We also find increased iNOS-dependent NO production. However, we don’t know if this is secondary to iNOS induction or to altered macrophage composition, with increased numbers of Ly6Chi monocytes. iNOS-derived NO and ROS worsen atherogenesis, and genetic ablation of iNOS in WTD-fed Apoe−/− mice decrease atherosclerosis 57. In addition, focal ROS and peroxynitrite at the vascular wall can trigger endothelial dysfunction and smooth muscle cell migration, leading to atherosclerosis 52. Therefore, increase of iNOS-derived NO and ROS in MYFKO macrophages can promote atherosclerosis.
Cys-SNO and 3-NT are biomarkers of nitrosative and nitrative stress and are associated with insulin resistance 34. In the present study, we observed an increase of Cys-SNO and 3-NT in WTD-fed Ldlr−/−: MYFKO mice, but not in SD- or HFD-fed MYFKO mice. It’s possible that it is secondary to increased NO generated by liver macrophages through iNOS. The fact that it’s only observed in WTD-fed Ldlr−/−: MYFKO mice, is consistent with the finding that iNOS-deficiency in myeloid cells does not prevent insulin resistance in SD- nor HFD-fed mice 58. These observations suggest that “metabolic stress” caused by Ldlr−/− background and/or WTD (lipotoxicity, inflammation, or metabolic inflexibility) is necessary for iNOS-derived NO production in macrophages and liver protein nitrosylation/nitration. Indeed, our in vitro experiments showed no difference in basal NO production in macrophages from WT and MYFKO mice. Thus, it appears that multiple pathophysiologic triggers are required (hypernsulinemia, dyslipidemia) to induce insulin resistance via nitrosylation/nitration.
In conclusion, our study demonstrates that FoxO in myeloid cells plays a significant role in contributing to increased risks of atherosclerosis and glucose metabolism, through neutrophilia/monocytosis, oxidative stress, and iNOS-derived NO overproduction. Our study suggests that reversing these abnormalities benefits diabetes and its macrovascular complications.
Supplementary Material
Novelty and Significance.
What Is Known?
Insulin/Foxo signaling plays a central role in macrophage function.
Myeloid-specific ablation of insulin receptor attenuates atherosclerosis in hypercholesterolemic mice.
Constitutive activation of FoxO by deacetylation suppresses inflammatory responses in macrophages.
What New Information Does This Article Contribute?
Myeloid-specific FoxO knockout causes neutrophilia and monocytosis, accelerating atherosclerosis in hypercholesterolemic mice.
Myeloid-specific FoxO knockout increases oxidative stress and inducible nitric oxide synthase (iNOS) in macrophages.
Antioxidant therapy alleviates atherosclerosis in myeloid-specific FoxO knockout mice.
FoxO in vascular endothelial cells plays a key role in promoting atherosclerosis in hypercholesterolemic mice by suppressing endothelial nitric oxide synthase (eNOS) and enhancing inflammatory responses. However, the role of FoxO in myeloid cells in atherosclerosis remains unknown. We created myeloid-specific FoxO-knockout (MYFKO) mice to examine the role of FoxO in the development of atherosclerosis. MYFKO mice on the LDL receptor null background (Ldlr−/−: MYFKO mice) display neutrophilia, monocytosis, and develop more atherosclerosis than Ldlr−/− controls. Ldlr−/−: MYFKO mice also show increased proliferation of granulocyte-macrophage progenitors in bone marrow. Inducible nitric oxide synthase (iNOS)-dependent nitric oxide production and oxidative stress are increased in macrophages from MYFKO mice. These events were reversed by antioxidant therapy, resulting in decreased atherosclerosis. An important implication of this study is that constitutive inactivation of FoxO in myeloid cells, as induced by chronic hyperinsulinemia in type 2 diabetes, can contribute to the development of atherosclerosis.
ACKNOWLEDGMENTS
We thank R. DePinho for providing mice. We thank members of the Accili laboratory for constructive advice and discussion of the data.
SOURCES OF FUNDING
K. T. was the supported by a postdoctoral fellowship for research abroad from the Japan Society for the Promotion of Science. M.W. is supported by The Netherlands Organization of Scientific Research (NWO VENI 916.11.072). A.J.M. was supported by a post-doctoral fellowship from the American Heart Association (AHA; 12POST11890019). This work was supported by National Institutes of Health grants HL87123, DK58282 and DK63608 (Columbia University Diabetes Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.National institute of diabetes and digestive and kidney diseases. National diabetes statistics fact sheet: General information and national estimates on diabetes in the united states. 2005 [Google Scholar]
- 2.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 5.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S, Liang CP, Westerterp M, Senokuchi T, Welch CL, Wang Q, Matsumoto M, Accili D, Tall AR. Hepatic insulin signaling regulates vldl secretion and atherogenesis in mice. J Clin Invest. 2009;119:1029–1041. doi: 10.1172/JCI36523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SH, Ginsberg HN. Increased very low density lipoprotein (vldl) secretion, hepatic steatosis, and insulin resistance. Trends in endocrinology and metabolism: TEM. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai D, Chen C, Han S, Ganda A, Murphy AJ, Haeusler R, Thorp E, Accili D, Horton JD, Tall AR. Regulation of hepatic ldl receptors by mtorc1 and pcsk9 in mice. J Clin Invest. 2012;122:1262–1270. doi: 10.1172/JCI61919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell. Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein e null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, Depinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. Foxos integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabas I, Tall A, Accili D. The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circ Res. 2010;106:58–67. doi: 10.1161/CIRCRESAHA.109.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases er stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell metabolism. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Senokuchi T, Liang CP, Seimon TA, Han S, Matsumoto M, Banks AS, Paik JH, DePinho RA, Accili D, Tabas I, Tall AR. Forkhead transcription factors (foxos) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. Diabetes. 2008;57:2967–2976. doi: 10.2337/db08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteine-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baetta R, Corsini A. Role of polymorphonuclear neutrophils in atherosclerosis: Current state and future perspectives. Atherosclerosis. 2010;210:1–13. doi: 10.1016/j.atherosclerosis.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. Foxos are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuchiya K, Banks AS, Liang CP, Tabas I, Tall AR, Accili D. Homozygosity for an allele encoding deacetylated foxo1 protects macrophages from cholesterol-induced inflammation without increasing apoptosis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2920–2928. doi: 10.1161/ATVBAHA.110.219477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 24.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. Foxos are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of foxp3+ regulatory t cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 26.Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-specific effects of pecam-1 on atherosclerosis in ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1996–2002. doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor foxo1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez E, Flier E, Molle D, Accili D, McGraw TE. Hyperinsulinemia leads to uncoupled insulin regulation of the glut4 glucose transporter and the foxo1 transcription factor. Proc Natl Acad Sci U S A. 2011;108:10162–10167. doi: 10.1073/pnas.1019268108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. Foxo transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 33.Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, Bluher M, Kolanus W, Kahn CR, Bruning JC. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS genetics. 2010;6:e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 35.Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: Potential role of tyrosine nitration of insulin signaling proteins. Diabetes. 2010;59:861–871. doi: 10.2337/db09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinozaki S, Choi CS, Shimizu N, Yamada M, Kim M, Zhang T, Shiota G, Dong HH, Kim YB, Kaneki M. Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice. J Biol Chem. 2011;286:34959–34975. doi: 10.1074/jbc.M110.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scharfstein JS, Keaney JF, Jr, Slivka A, Welch GN, Vita JA, Stamler JS, Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994;94:1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Accili D, Arden KC. Foxos at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 39.Haeusler RA, Kaestner KH, Accili D. Foxos function synergistically to promote glucose production. J Biol Chem. 2010;285:35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases er stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu M, Zhang QJ, Wang L, Li H, Liu ZP. Foxo4 inhibits atherosclerosis through its function in bone marrow derived cells. Atherosclerosis. 2011;219:492–498. doi: 10.1016/j.atherosclerosis.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteine-deficient mice against atherosclerosis. Cell. Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka J, Qiang L, Banks AS, Welch CL, Matsumoto M, Kitamura T, Ido-Kitamura Y, DePinho RA, Accili D. Foxo1 links hyperglycemia to ldl oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes. 2009;58:2344–2354. doi: 10.2337/db09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka H, Nagashima T, Shimaya A, Urano Y, Shimokawa T, Shibasaki M. Effects of the novel foxo1 inhibitor as1708727 on plasma glucose and triglyceride levels in diabetic db/db mice. Eur J Pharmacol. 2010;645:185–191. doi: 10.1016/j.ejphar.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye M, Iwasaki H, Laiosa CV, Stadtfeld M, Xie H, Heck S, Clausen B, Akashi K, Graf T. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 2003;19:689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 48.Guevara NV, Kim HS, Antonova EI, Chan L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med. 1999;5:335–339. doi: 10.1038/6585. [DOI] [PubMed] [Google Scholar]
- 49.van Vlijmen BJ, Gerritsen G, Franken AL, Boesten LS, Kockx MM, Gijbels MJ, Vierboom MP, van Eck M, van De Water B, van Berkel TJ, Havekes LM. Macrophage p53 deficiency leads to enhanced atherosclerosis in apoe*3-leiden transgenic mice. Circ Res. 2001;88:780–786. doi: 10.1161/hh0801.089261. [DOI] [PubMed] [Google Scholar]
- 50.Diez-Juan A, Perez P, Aracil M, Sancho D, Bernad A, Sanchez-Madrid F, Andres V. Selective inactivation of p27(kip1) in hematopoietic progenitor cells increases neointimal macrophage proliferation and accelerates atherosclerosis. Blood. 2004;103:158–161. doi: 10.1182/blood-2003-07-2319. [DOI] [PubMed] [Google Scholar]
- 51.Kuo CL, Murphy AJ, Sayers S, Li R, Yvan-Charvet L, Davis JZ, Krishnamurthy J, Liu Y, Puig O, Sharpless NE, Tall AR, Welch CL. Cdkn2a is an atherosclerosis modifier locus that regulates monocyte/macrophage proliferation. Arterioscler Thromb Vasc Biol. 2011;31:2483–2492. doi: 10.1161/ATVBAHA.111.234492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: The importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada S, Ding Y, Tanimoto A, Wang KY, Guo X, Li Z, Tasaki T, Nabesima A, Murata Y, Shimajiri S, Kohno K, Ichijo H, Sasaguri Y. Apoptosis signal-regulating kinase 1 deficiency accelerates hyperlipidemia-induced atheromatous plaques via suppression of macrophage apoptosis. Arterioscler Thromb Vasc Biol. 2011;31:1555–1564. doi: 10.1161/ATVBAHA.111.227140. [DOI] [PubMed] [Google Scholar]
- 55.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor aim/spalpha/api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Bolick DT, Skaflen MD, Johnson LE, Kwon SC, Howatt D, Daugherty A, Ravichandran KS, Hedrick CC. G2a deficiency in mice promotes macrophage activation and atherosclerosis. Circ Res. 2009;104:318–327. doi: 10.1161/CIRCRESAHA.108.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Deficiency of inducible no synthase reduces advanced but not early atherosclerosis in apolipoprotein e-deficient mice. Life Sci. 2006;79:525–531. doi: 10.1016/j.lfs.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 58.Lu M, Li P, Pferdekamper J, Fan W, Saberi M, Schenk S, Olefsky JM. Inducible nitric oxide synthase deficiency in myeloid cells does not prevent diet-induced insulin resistance. Mol Endocrinol. 2010;24:1413–1422. doi: 10.1210/me.2009-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.