Abstract
We had demonstrated that Bcl-2 and Bcl-2Δ21, a C-terminal truncated Bcl-2 sequence, inactivate SERCA1 in isolated sarcoplasmic reticulum (SR), accompanied by a translocation from caveolae-related domains of the SR. Here, we present evidence for the interaction of Bcl-2 with SERCA2b in C2C12 myoblast and HEK293 cells. Bcl-2 and SERCA2b co-immunoprecipitated from lysate and microsomal fractions of Bcl-2-overexpressing cells. However, Bcl-2 overexpression resulted only in a slight translocation from the CRD and no significant SERCA inactivation. In isolated HEK293 cell microsomes, incubation with Bcl-2Δ21 afforded SERCA2b inactivation and some translocation. HSP70, HSP90, HSP27, and alpha-crystallin attenuated Bcl-2Δ21-dependent SERCA2b inactivation. An in vitro mechanistic study with the SERCA1 isoform shows that HSP70 (i) protects SERCA1 from the inactivation by Bcl-2Δ21, (ii) inhibits SERCA1 translocation from CRD fractions, and (iii) prevents the Bcl-2Δ21-dependent loss of FITC labeling. Our data demonstrate that the mechanism of SERCA inactivation by Bcl-2 established in vitro for the SERCA1 isoform can be extended to the main housekeeping SERCA2b isoform, and that functional interactions of SERCA2b and Bcl-2 in the cell may be modulated by HSP70 and other chaperone and stress-regulated proteins.
INTRODUCTION
Muscular dystrophies as well as age-associated muscle loss and atrophy are often linked to a pro-apoptotic cell phenotype, characterized by elevated cytosolic and mitochondrial Ca2+ concentrations [1–3]. Though several apoptotic signaling pathways are implicated in muscle cell loss, a mitochondria-mediated Ca2+-dependent pathway seems most relevant to age-associated sarcopenia [4,5]. In addition to triggering mitochondria-mediated apoptosis, Ca2+ serves as an important second messenger controlling a wide range of cellular functions through activation, modulation and termination of cellular processes [6–9], and, specifically for muscle tissue, myofibril contraction/relaxation. Such a variety of functions for a single agent cannot be explained without a high degree of compartmentalization of the respective processes. Indeed, skeletal and heart muscle contain two distinct pools of subsarcolemmal and intermyofibrillar mitochondria, which exhibit different physiological properties and different susceptibility towards pro-apoptotic stimuli [10]. The ER/SR, a major compartment for intracellular calcium storage, is also highly heterogeneous within the cell, and different transport proteins are involved in Ca2+ handling in different parts of the muscle cell. The “housekeeping” Ca2+ pump loading the ER integrated with the subsarcolemmal mitochondria is SERCA2b (and, potentially, SERCA3 in some types of cells), and the store-operated Ca2+ release is initiated by IP3 production and activation of the IP3R [6,11]. This machinery is involved in Ca2+ signaling and apoptosis. In addition, the ER performs protein folding and quality control through Ca2+-dependent regulation of chaperon-associated folding of newly synthesized proteins. In contrast, the terminal cisterns of the SR juxtaposed to myofibril contractile protein complexes contain SERCA1 (fast-twitch skeletal muscle) or SERCA2a (myocardium, slow-twitch skeletal muscle) where the main Ca2+ release channel is RyR integrated with excitation-contraction coupling proteins [12].
Ca2+ translocation from the ER into the mitochondria can activate Ca2+-dependent caspases to execute apoptosis, and Ca2+-dependent dehydrogenases involved in ATP-production [13,14]. The release of Ca2+ from the ER by the IP3R was suggested as the main mechanism responsible for apoptosis in models of mitochondrial Ca2+ overload [14,15], and regulation of IP3R function by IP3R-binding proteins was proposed [15]. The crosstalk between the ER and the mitochondria relies on close protein-mediated contacts between these organelles and the existence of a low-affinity Ca2+-uniporter in the outer mitochondrial membrane (MCU) [8,13]. In the ER, calcium depletion causes ER stress characterized by the accumulation of misfolded proteins that would otherwise be exported to the cytosol for degradation through the ubiquitin-dependent proteasome [16,17]. ER stress activates the unfolded protein response (UPR) and, depending on its extent, triggers apoptotic cell death, PARP-dependent necrosis, or an autophagy survival response [18,19].
A large body of evidence indicates that Ca2+ homeostasis is modulated by Bcl-2 family proteins [20–23] that may be involved in the control of mitochondria-mediated apoptosis, UPR, ER stress-mediated apoptosis, autophagy and starvation-induced apoptosis [24]. The balance between anti- and pro-apoptotic proteins of the Bcl-2 family at the ER membrane was implicated in the ER Ca2+ load regulation [20–25]. Bcl-2 represents an anti-apoptotic member of the Bcl-2 family of proteins. It was initially suggested that the anti-apototic function of Bcl-2 is related to its localization to the mitochondria and heterodimerization with other, pro-apoptotic, Bcl-2 family members [23,26]. However, there is also growing evidence for Bcl-2 localization to the ER [20], and direct Bcl-2-dependent inhibition of apoptosis and autophagy through modulation of the ER Ca2+ level [21,23,28]. These effects of Bcl-2 may result from (i) leakage of Ca2+ from the ER through pores formed by Bcl-2 [21,23], (ii) a direct interaction of Bcl-2 with the IP3R, mediating Ca2+ release from the ER [18,29], and/or (iii) the interaction with other proteins involved in Ca2+ homeostasis. A physical interaction between Bcl-2 and the IP3R1 isoform was demonstrated, which affects IP3R1 phosphorylation to control the rate of Ca2+ release from the ER [29]. However, it appears that the IP3R3 isoform is predominantly responsible for the transport of Ca2+ to the mitochondria, and phosphorylation of IP3R3 had no effect on Ca2+ homeostasis [25].
Recently we reported that the interaction of Bcl-2 with SERCA1 in vitro can result in partial inactivation of the Ca2+–pump [30,31], a feature, which may lead to a reduction in the luminal Ca2+ load. This effect was specific to SERCA, since Bcl-2 did not inhibit other types of Ca2+ transporters, such as the plasma membrane Ca-ATPase (PMCA). These data suggest that in the cell Bcl-2 may also regulate the luminal Ca2+ load of the ER through inhibition of other SERCA isoforms, representing an additional anti-apoptotic mechanism for Bcl-2. Both full length Bcl-2 and Bcl-2Δ21, a truncated Bcl-2 isoform, lacking the hydrophobic transmembrane domain, interact with and inhibit the Ca-ATPase activity of SERCA1. This inhibition is associated with a partial unfolding of the protein [30], and with displacement of SERCA from specific SR membrane domains, which we referred to as caveolae-related domains (CRD), based on their characteristic migration during sucrose density gradient fractionation [31]. Importantly, this mechanism would only require a local and partial inactivation of SERCA below a specific threshold, which ensures reduced Ca2+ uptake into the ER/SR, but avoids ER stress and UPR-related apoptosis.
The present study further explores the interaction of Bcl-2 and with SERCA1 and with SERCA2, and addresses the effect of Bcl-2 overexpression on SERCA2b in the C2C12 mouse myoblast cell line (HEK293 cells were implicated in some experiments as a non-muscle cell model). The non-differentiated C2C12 myoblasts were chosen since they do not express SERCA1 (unless differentiation is initiated) and can be easily transfected with vectors regulating Bcl-2 expression. Here, we demonstrate that after overexpression of Bcl-2 in C2C12 myoblast cells, Bcl-2 can be detected in microsomal fractions, and a direct interaction of Bcl-2 with SERCA2 is indicated by immunoprecipitation (IP) from the microsomal fractions. However, unlike in the in vitro experiments, only a slight translocation of SERCA2 from the CRD is detected consistent with no significant change in the ER-specific 45Ca2+-uptake. We further demonstrate that HSP70 can interact with SERCA2b and Bcl-2 in microsomes isolated from C2C12 and HEK293 cells, and hypothesize that the chaperone family of proteins may protect SERCA2b from Bcl-2-dependent inactivation and translocation from the CRD. We performed a mechanistic study on the interaction of Bcl-2, SERCA1, and HSP70 in isolated skeletal muscle SR in vitro, and provide direct evidence that Bcl-2-dependent inhibition of SERCA1 activity is diminished by HSP70. The protection afforded by HSP70 correlates with (i) the levels of SERCA1 translocated from the CRD, (ii) an alteration of its nucleotide-binding domain, assessed through FITC labeling, and (iii) inhibition of Ca-ATPase activity and 45Ca2+ uptake. Similar results on the protection by HSP70 and other heat shock proteins against Bcl-2-mediated inhibition of Ca2+ uptake were obtained also in microsomes isolated from HEK293 cells. Our data confirm the hypothesis that SERCA2b may be a target for Bcl-2 in vivo, but that such interaction may be modulated by chaperones and other stress-regulated proteins.
EXPERIMENTAL
Materials
The chemiluminescence detection kit for western blotting (WB) was obtained from Amersham Biosciences (Piscataway, NJ, USA). The protease inhibitor mixtures (Complete, Mini) were from Roche Applied Science (Indianapolis, IN, USA). Recombinant rat HSP70 (SPP-758), Mouse anti-HSP70 monoclonal antibody (SPA-810), and rabbit anti-HSP70 polyclonal antibody (SPA-812) were from Stressgen Biotechnologies (Ann Arbor, MI, USA). HSP90β, HSP27, and α-crystallin were purchased from Enzo Life Sciences (Farmingdale, NY). GroEL was a gift of Dr. M. Fisher (University of Kansas Medical Center). FITC and the anti-fluorescein/Oregon Green-conjugated monoclonal antibody 4-4-20 were from Molecular Probes (Eugene, OR, USA). 45Ca2+ was obtained from Amersham Biosciences (Piscataway, NJ, USA). Mouse monoclonal anti-Bcl-2 antibodies (sc-7382) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-HSP70 antibodies (SPA-812), and mouse monoclonal anti-SERCA2 antibodies (MA3-910) were from Thermo-Fisher (Rockford, IL, USA). Anti-SERCA1 (MA3-912) mouse monoclonal antibodies were obtained from Affinity Bioreagents (Golden, CO, USA). Secondary HRP-conjugated anti-rabbit antibodies were from Sigma (St. Louis, MO, USA), and HRP-conjugated anti-mouse antibodies were obtained from Pierce Biotechnology (Rockford, IL, USA).
Cell Culture and Transfections
C2C12 mouse myoblast and HEK293 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA), supplemented with 10% FBS and penicillin/streptomycin (Invitrogen), at 37°C and 5% CO2 in a humidified chamber. Bcl-2 chimera plasmid DNAs were purified on Qiagen columns and transfections of the cells were performed using the Lipofectamine 2000 reagent according to the manufacturer's instructions (Invitrogen). The best result for overexpression was attained after 24 h of transfection. The expression of HSP70 was induced by heat stress; for this purpose C2C12 myoblast cells were incubated at 42°C for 1 h and transferred to recover from heat stress at 37°C for the next 18 h. For WB analysis, cells were washed twice with PBS and solubilized in lysis buffer (20 mM Tris, pH 8, 150 mM NaCl, 1% Triton X-100, 10 mM orthovanadate, 10 mM EDTA, 2 mM PMSF, 1 mM DTT, 1 × protease inhibitor mixture from Roche Applied Science, Indianapolis, IN, USA) and then briefly sonicated, and incubated at 4°C for 30 min. Afterwards, the cell lysate was centrifuged at 12,000 × g for 10 min at 4°C to remove cellular debris. Protein concentration of each freshly prepared cell lysate was determined with Coomassie Blue Plus protein reagent (Pierce, Rockford, IL, USA). After mixing with 4× SDS loading buffer, whole cell extracts were resolved by Bio-RAD 4–20% gradient gels using tris/glycine running buffer, followed by electroblotting onto PVDF membranes (Millipore, Billerica, MA, USA) for WB analyses. For IP, cells were rinsed twice with PBS, solubilized in the buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA with freshly added 1 mM PMSF, 1 mM DTT, 1× protease inhibitor mixture (Roche Applied Science) and lysed for 30 min at 4°C. The cell lysate was centrifuged at 12,000 × g for 10 min at 4°C to remove cellular debris.
Bcl-2Δ21 Expression and Purification
Plasmids encoding the GST-Bcl-2Δ21 fusion protein were constructed by subcloning human Bcl-2Δ21cDNA into the pGEX3T vector (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Bcl-2Δ21 was produced in Escherichia coli DH1 as the host strain. A 10-ml overnight culture was used to inject 1 liter of LB medium, which was further incubated at 37°C until an A600 value of 0.4 was achieved. The cells were induced with 0.1 mM IPTG and incubated at 30°C for an additional 6 h before harvesting by centrifugation. Then, cells were re-suspended in 10 ml of STE solution containing 1% Triton X-100 (w/v) and protease inhibitors, and disrupted by sonication. The resulting lysate was centrifuged at 12,000 × g for 15 min at 4°C to pellet cellular debris. Glutathione-agarose beads were added to the supernatant and incubated at 4°C with gentle rotation for 4 h. The beads were washed twice with 50 ml of ice-cold PBS without Triton X-100. After incubation with thrombin for 1 h at room temperature, cleaved proteins were eluted with 0.5 ml of STE buffer. To 0.5 ml elution fractions 20 µl of prewashed thrombin-binding beads (Sigma, St. Louis, MO, USA) were added, followed by incubation for 30 min at 4°C, and removal of the beads by centrifugation. Purified proteins were characterized by SDS-PAGE in 4–20% tris-glycine gels (Invitrogen, Carlsbad, CA) followed by Coomassie Colloidal Blue staining (Pierce, Rockford, IL).
Isolation of sarcoplasmic reticulum
Native SR vesicles (light fraction) were prepared from hind limb skeletal muscle (fast-twitch fibers) of 5–6 months old F1 hybrid Fisher 344×Brown Norway rats as described earlier [32]. Briefly, muscle (usually 20–30 g) was homogenized in a Waring blender for 1 min. at maximal speed at 4°C in 3 volumes of buffer containing 0.1 M KCl, 0.1 mM EDTA and 20 mM MOPS (pH 7.4). The homogenate was centrifuged at 5,000 × g for 20 min to remove cell debris, the pellet washed again under the same conditions, and the pooled supernatants centrifuged at 11,800 × g for 20 min to pellet the mitochondria. To dissolve myosin, the supernatant was filtered through 6 layers of cheesecloth and solid KCl was added to make a final concentration of 0.6 M. After 20 min incubation, the SR was pelleted at 23,500 × g for 1 h. The supernatant was decanted and the pellets were re-suspended in a medium containing 0.3 M sucrose and 20 mM MOPS (pH 7.0), and centrifuged for 30 min at 100,000 × g. SR vesicles were re-suspended in a small volume of medium consisting of 0.3 M sucrose and 20 mM MOPS (pH 7.0) using a Dounce homogenizer, aliquoted, quickly frozen in liquid nitrogen, and stored at −70°C. Protein concentration was measured by a micro-assay with Coomassie Plus protein reagent (Pierce, Rockford, IL, USA). The fraction of SERCA1 in the SR, determined by densitometry of Coomassie Blue stained gels, was ca. 40% relative to total protein.
Preparation of CRD
For the isolation of CRD, SR or C2C12 myoblast cells were lysed in 2 ml ice-cold 0.5 M sodium carbonate buffer (pH 11) using 20 strokes of a Dounce glass homogenizer followed by sonication. The homogenate was adjusted to 45% (w/v) sucrose by the addition of 90% sucrose in a buffer, containing 25 mM MES (pH 6.5), 150 mM NaCl, 5 mM EDTA, and 1 mM PMSF, and placed in the bottom of an ultracentrifuge tube. A discontinuous sucrose gradient was performed by overlaying this solution with 4 ml of 38% sucrose and 3 ml of 5% sucrose (both sucrose solutions prepared in the buffer containing 0.25 M sodium carbonate). The tube was then centrifuged at 4°C for 16–18 h at 130,000 × g in an SW41 rotor using an L90K ultracentrifuge (Beckman Coulter Inc., Fullerton, CA). The first 2 ml-fraction from the top of the gradient was discarded and normally 9 fractions (for some experiments only 4 enlarged fractions) were manually collected from the top of the gradient to the bottom. To determine the distribution of CRD-associated proteins within the gradient, each fraction was analyzed by SDS-PAGE, followed by WB analysis with appropriate antibodies. An aliquot of each fraction was taken for labeling with FITC.
Cell fractionation
Cell fractionation was achieved by differential centrifugation as described previously [31]. Cells were washed twice with PBS buffer, harvested in PBS and centrifuged at 1000 × g for 3 min. Subsequently, cells were swollen in hypotonic solution of 10 mM Tris-HCl (pH 7.5) containing 0.5 mM MgCl2 for 10 min on ice followed by a homogenization with 30 strokes in a glass Dounce homogenizer. The homogenate was diluted with an equal volume of a solution of 0.5 mM sucrose, 6 mM 2-mercaptoethanol, 40 µM CaCl2, 300 mM KCl, 10 mM Tris-HCl (pH 7.5) and the suspension was centrifuged at 1,500 × g for 5 min to remove nuclei. To pellet mitochondria, the suspension was centrifuged at 10,000 × g for 20 min and the supernatant was adjusted to 0.6 M KCl by the addition of 0.9 ml 2.5 M KCl solution and centrifuged at 100,000 × g for 1 h to sediment the microsomal fraction. The pellets of mitochondria and microsomal fractions were washed in a solution containing 0.25 M sucrose, 0.15 M KCl, 3 mM 2-mercaptoethanol, 20 µM CaCl2, 10 mM tris-HCl (pH 7.5) and concentrated by respective centrifugations.
Incubation of SR with Bcl-2 and HSP70 for SERCA activity assay
For incubations with Bcl-2Δ21 and HSP70, the concentrated SR suspension (≥ 20 mg SR protein/ml) was diluted to achieve a final concentration of 0.5–1 mg SR protein/ml in STE buffer with a minimal contribution of the SR storage buffer to the incubation medium (less than 5%). SR was incubated with recombinant Bcl-2Δ21 in the STE buffer in plastic tubes without agitation at 37°C in a dry thermostat. 1 mM PMSF was added to prevent proteolytic degradation of the proteins [30]. Total, Ca2+-dependent and basal ATPase activities of SERCA in the SR were determined at 25°C by colorimetric assay of inorganic phosphate (Pi) in the presence or absence of the calcium ionophore, A23187, as outlined in our previous paper [30]. In all our experiments, the measured Ca2+-dependent ATP hydrolysis was attributed to SERCA because the Ca2+-dependent Ca-ATPase activity was completely inhibited by the addition of 20 µM thapsigargin (Sigma, St. Louis, MO, USA).
FITC Binding Capacity of SERCA1
SR (2 µg) was incubated at 37°C for different times in the absence or in the presence of Bcl-2Δ21 and/or HSP70, and subsequently added to 100 µl of FITC labeling buffer, containing 50 mM Tris-HCl, pH 8.8, 250 mM sucrose, 0.1 mM CaCl2, 5 mM MgCl2, 20 µM FITC and 1 mM PMSF. After incubation at 25°C for 60 min in the dark, 4 × sample buffer was added to stop the reaction, the solution boiled for 1 min, fractionated by SDS-PAGE, and analyzed by WB using the anti-fluorescein/Oregon Green antibody to detect FITC-labeled SERCA1.
SERCA activity by 45Ca2+ uptake
SERCA activity was measured by 45Ca2+ uptake based on published method [33]. SR or microsomal fractions from C2C12 and HEK293 cells (4 µg protein) were incubated at 37°C for different times in the absence or presence of indicated concentrations of Bcl-2Δ21 and heat shock proteins in a buffer containing 50 mM MOPS (pH 7.0), 0.12 M KCl, 10 mM MgCl2, and aliquots of buffers used for storage of recombinant proteins (the respective controls also contained those storage buffers without proteins). To determine SERCA-dependent Ca2+ uptake, one from paired samples was treated with 10 µM thapsigargin. 45Ca2+ uptake buffer (Tris-HCl 30 mM, pH 7.0, KCl 100 mM, MgCl 2. 6 mM, EGTA 0.15 mM, CaCl2 200 µM, oxalate 10 mM) was mixed with 1 µCi 45Ca2+ and 5 mM ATP at 37°C. The reaction was started by the addition of protein. Aliquots of this mixture (5 µg of protein in 100 µL), in triplicates, were filtered through Millipore Membrane filters at 30, 60 and 90 minutes after incubation. The filters were rinsed 2 times with 2.5 mL washing buffer (PBS, pH 7.0, containing 2 mM EGTA), dried, and subjected to counting by a scintillation detector.
Solution-binding assay and co-immunoprecipitation
To monitor the association between SERCA1, Bcl-2Δ21 and HSP70, 2 µg of SR in lysis buffer containing 10 mM Tris-HCl (pH 7.4), 10 mM EDTA, 1% Nonindet P-40, 1 mM PMSF, and protease inhibitors (Roche Diagnostics, Indianapolis, IN) were incubated with 2 µg GST-Bcl-2Δ21 in the absence or presence of different amounts of HSP70 for 1 h at 4°C. Following the incubation with 10 µL (packed volume) of glutathione–sepharose beads for 1 h, the beads were separated by centrifugation at 10,000 × g, and washed six times with the lysis buffer. Proteins were separated by SDS-PAGE and analyzed by WB. For the IP of HSP70 and Bcl-2Δ21, 2 µg of HSP70 and different amounts of Bcl-2Δ21 were solubilized in the above buffer in the presence of anti-Bcl-2 antibodies (1 µg) and incubated for 1 h at 4°C. Pre-washed protein A-agarose (30 µL) was then added to the samples, and after 1 h incubation, protein A-agarose together with the immuno-complex was obtained by centrifugation and re-suspended in a sample buffer. For IP from cultured cells, cell lysate or isolated microsomes were pre-cleared with IgG and protein A-agarose beads for 30 min at 4°C. Then 1 µg of antibody was added for 1 h at 4°C followed by the addition of protein A-agarose (30 µL) for 1 h at 4°C. Protein A-agarose with the immuno-complex was obtained by centrifugation, re-suspended in SB, and separated by SDS-PAGE to analyze by WB.
SDS-PAGE and WB analysis
Proteins were separated on precast Bio-Rad 4–20% gradient gels using Tris-glycine running and sample buffers (Invitrogen, Carlsbad, CA, USA). The gels were then either stained for proteins by Coomassie Blue or electro-blotted onto a 0.45 µm PVDF membrane (Millipore, Billerica, MA, USA) prior to WB analysis. The spots were visualized with the ECL or ECL-Plus detection kit (Amersham Biosciences, Piscataway, NJ, USA) according to the manufacturer’s procedure.
LC-MS/MS analysis of protein in-gel digests
After SDS-PAGE separation, protein bands were excised from the gel, extensively washed, reductively alkylated and in-gel digested with trypsin, and resulting peptide samples introduced into an LTQ–FT hybrid linear quadrupole ion trap Fourier transform ion cyclotron resonance (FT–ICR) mass spectrometer (ThermoFinnigan, Bremen, Germany) via capillary liquid chromatography, analogous to a procedure published earlier [30]. For protein identification, raw experimental files were processed by TurboSequest search using BioWorks 3.2 (ThermoFinnigan). The resulting MS/MS peak lists in .dta format were combined within each experiment using an in-house written Perl script and submitted for peptide/protein identification to the Mascot 2.2 database-searching program (Matrix Science, London, UK) using the most recent Swiss–Prot or IPI databases with a fragment ion mass tolerance of 0.20 amu and a parent ion tolerance of 10 ppm. Trypsin specificity and up to two missed cleavage sites were used in the search parameters. Carboxymethylation of cysteine residues was considered as a fixed modification. Sequest and Mascot results were imported into Scaffold 3.0 software (Proteome Software, Portland, OR, USA; a free viewer is available at http://www.proteomesoftware.com) for analyzing with the X!Tandem search algorithm and statistical validation of peptide/protein identities. Peptide and protein identifications were accepted if they could be established at greater than 95% probability.
RESULTS
Overexpression of Bcl-2 in C2C12 cells
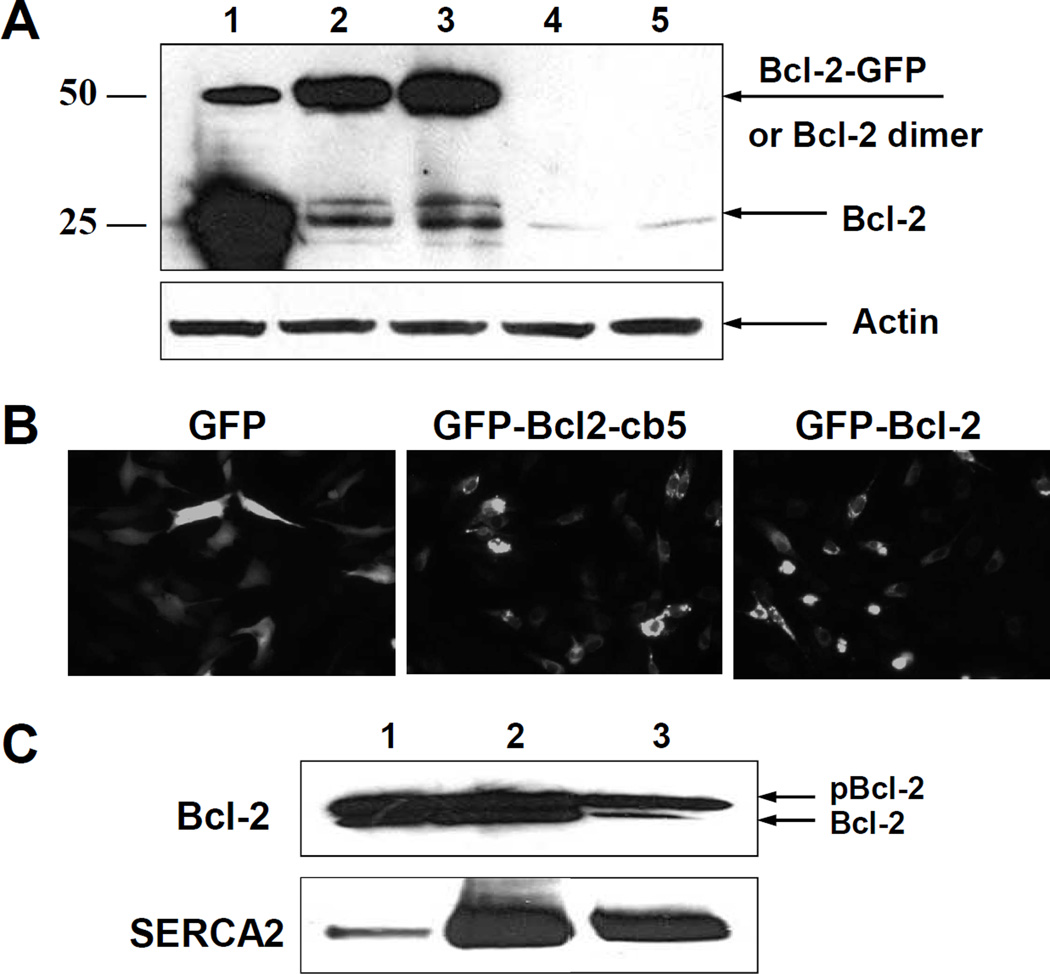
To determine whether Bcl-2 may affect SERCA activity within a cell, we overexpressed Bcl-2 in C2C12 myoblasts. Proliferating C2C12 cells contain small endogenous levels of Bcl-2 detectable by WB (Figure 1A, lane 5). We transfected cells with cDNA encoding different chimeras of Bcl-2, containing either GFP for visualization of transfection efficiency and subcellular localization (GFP-Bcl-2), a sequence for specific targeting of Bcl-2 expression to the ER from cytochrome b5 (cb5), i.e. Bcl-2-cb5, or both GFP and cb5, i.e. GFP-Bcl-2-cb5. Figure 1A shows the levels of Bcl-2 in C2C12 myoblasts after 24 h of transfection with cDNA encoding Bcl-2-cb5 (lane 1), GFP-Bcl-2 (lane 2), GFP-Bcl-2-cb5 (lane 3) or GFP alone (lane 4). All Bcl-2-containing cDNAs significantly increased the levels of Bcl-2 in comparison with an empty vector (lane 5). Figure 1A demonstrates that the expression level of Bcl-2-cb5 is higher compared to the levels of GFP-Bcl-2 and GFP-Bcl-2-cb5 (compare lanes 1, 2 and 3). Bcl-2-cb5 overexpression results in a slight electrophoretic mobility shift relative to Bcl-2 (see also Figure 3A, below), in agreement with the additional presence of the cb5 target sequence.
Figure 1. Expression of Bcl-2 in C2C12 myoblast cells.
A – C2C12 myoblast cells were harvested after 24 h of transfection with different Bcl-2 DNA chimeras and the level of Bcl-2 protein expression was analyzed by WB: 1- Bcl-2-cb5; 2 – GFP-Bcl-2; 3 – GFP-Bcl-2-cb5; 4 – GFP; 5 – empty vector. B – Fluorescent microscopy images of the C2C12 cells after overexpression of GFP (left), GFP-Bcl-2-cb5 (middle), and GFP-Bcl-2 (right). C – C2C12 myoblast cells were transfected with Bcl-2 DNA, and after 24 h, fractionated by differential centrifugation and analyzed by WB for Bcl-2 and SERCA2 in: (1) whole cell lysate, (2) mitochondria, and (3) microsomal fraction.
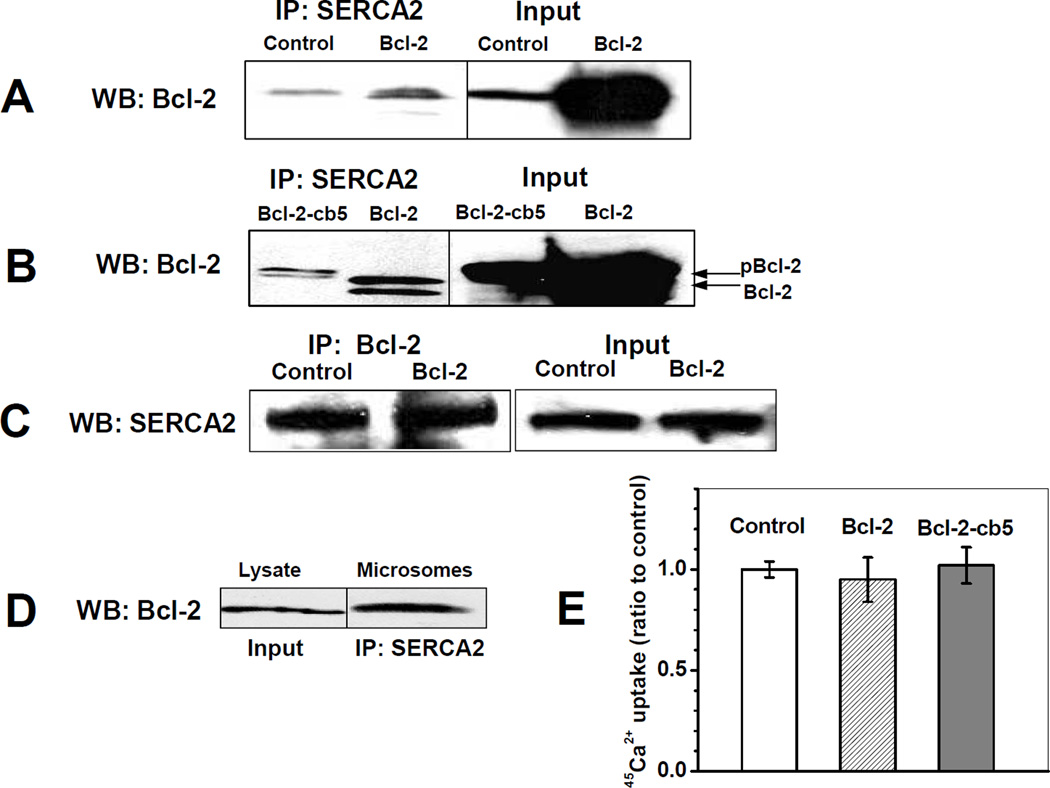
Figure 3. Interactions of SERCA2b and Bcl-2 in C2C12 myoblast cells.
C2C12 myoblasts were transfected with empty vector (control), or with Bcl-2 and Bcl-cb5 DNA followed by IP at 24 h after transfection: A,B - IP with anti-SERCA2 antibody and WB analysis for Bcl-2; C - IP with anti-Bcl-2 antibody and WB analysis for SERCA2; D – WB analysis for Bcl-2 in total lysate (1) and in the microsomal fraction of non-transfected C2C12 cells after IP with anti-SERCA2 antibodies (2). In A–D, protein bands were obtained in the same blots after which lanes were merged for comparison. Input data are shown for source samples prior to IP. E – Effect of Bcl-2 overexpression on thapsigargin-inhibitable 45Ca2+ uptake by C2C12 microsomes. For Bcl-2 overexpressing cells, average and experimental error are calculated from the data of 4 independent experiments.
Bcl-2 subcellular localization was controlled by fluorescence microscopy of GFP for GFP-Bcl-2 and GFP-Bcl-2-cb5 constructs in comparison with GFP alone. Figure 1B demonstrates that expression of GFP alone results in a distribution across the entire cell body, which is quite different from GFP-Bcl-2 and GFP-Bcl-2-cb5. Overexpression of the Bcl-2-containing constructs is observed primarily in the perinuclear region for both chimeras, irrespective of the presence of the ER-specific targeting sequence of cb5 (Figure 1B). The perinuclear region corresponds to the ER and mitochondria. To directly confirm Bcl-2 localization to the ER, C2C12 myoblast cells were transfected with Bcl-2, followed by subcellular fractionation after 24 h of transfection and WB analysis. Figure 1C shows that overexpression of Bcl-2 in C2C12 myoblast cells resulted in high levels of Bcl-2 protein in both mitochondrial and microsomal fractions, where at least two bands of the protein were detected, which likely correspond to non-phosphorylated (lower) and phosphorylated (upper band) forms of Bcl-2 [27,34]. Of note, the major band of Bcl-2 in microsomes (Figure 1C, lane 3) corresponds to the phosphorylated form of the protein, whereas mitochondria (Figure 1C, lane 2) contained nearly equal amounts of both the non-phosphorylated and phosphorylated Bcl-2. Microsomal and mitochondrial fractions were enriched with SERCA2 as compared to cell lysate (compare lanes 1 and 3 in the bottom panel of Figure 1C); the presence of SERCA2b in the mitochondrial fraction is likely due to contamination with mitochondria-associated membranes (MAM) of the ER. However, only the microsomal fraction showed tapsigargin-dependent 45Ca2+ uptake (data not presented), suggesting that SERCA2b in the mitochondrial fraction is present in a non-functional form, perhaps in leaky membrane vesicles contaminating the mitochondrial fraction.
Effect of Bcl-2 overexpression on SERCA2b localization to the ER CRD in C2C12 myoblasts
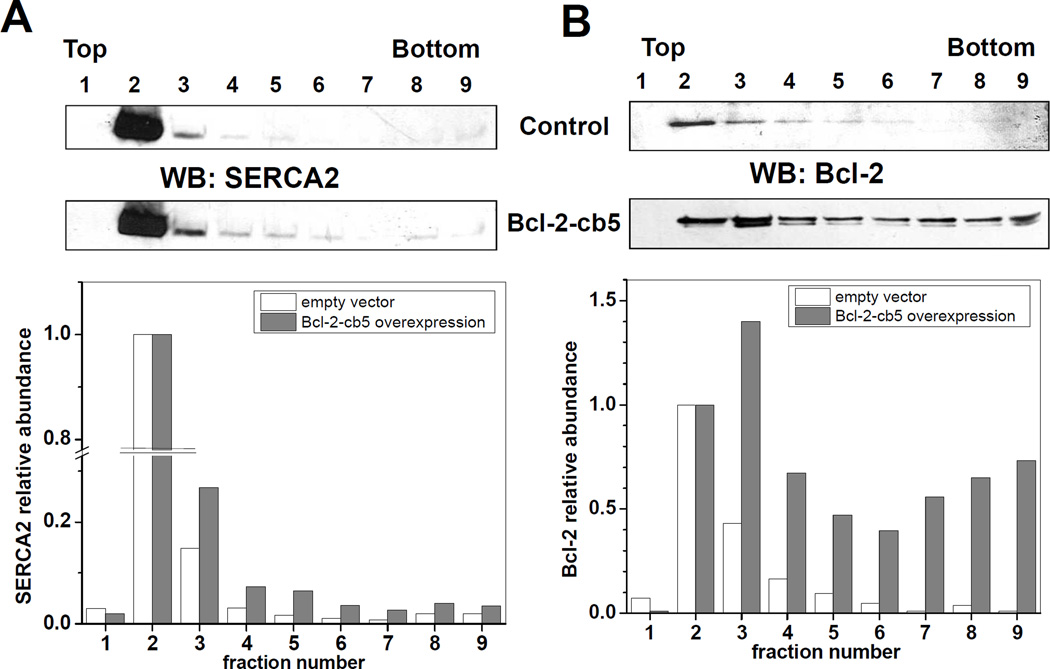
To assess whether Bcl-2 in C2C12 myoblast cells causes translocation of SERCA2b from CRD similar to those detected in in vitro experiments with SERCA1, we analyzed the distribution of SERCA2b from C2C12 myoblast cells in sucrose density gradient fractions after 24 h post-transfection with Bcl-2-cb5. Figure 2A shows the WB analysis of SERCA2 distribution in the fractions of control (empty vector) and Bcl-2-cb5 overexpressing C2C12 cells. SERCA2b localized predominantly to fraction 2 (CRD) with minor distribution into higher density fractions (control; Figure 2A). Bcl-2-cb5 overexpression caused a slight SERCA2b translocation from the CRD fraction to higher density fractions (3 to 6) of the gradient (Bcl-2-cb5; Figure 2A). WB analysis of Bcl-2 distribution demonstrates that in control cells Bcl-2 is mainly co-localized with SERCA2b, predominantly in fraction 2 (control; Figure 2B), whereas in cells overexpressing Bcl-2-cb5 the fusion protein is detected mainly in fraction 3, and also in the bottom fractions of the gradient (Bcl-2-cb5; Figure 2B). Similar results were obtained also for the overexpression of Bcl-2, GFP-Bcl-2, and GFP-Bcl-2-cb5 (data not shown); for all Bcl-2-containing constructs the effect on SERCA2b translocation in C2C12 myoblast cells was small compared to our in vitro experiments with purified rat skeletal muscle SR exposed to Bcl-2Δ21 [30,31].
Figure 2. Effect of Bcl2-cb5 overexpression on sucrose density gradient fractionation of C2C12 myoblast cells.
C2C12 myoblast cells were transfected with either empty vector (control) or Bcl2-cb5 DNA followed by sucrose density gradient separation after 24 h post-transfection, as described under “Experimental Procedures”. Fractions were collected from the top of the tube and analyzed by WB with anti-SERCA2 (A) and anti-Bcl-2 (B) antibodies. Fraction numbers are indicated at respective lanes. WB densitometry data normalized to the content of protein in fraction 2 are shown below respective blots.
Interaction of SERCA2b and Bcl-2 in C2C12 myoblasts
Because Bcl-2 is partially localized to the microsomal fractions of C2C12 cells, and partially co-localized with SERCA2b in CRD fractions of sucrose density gradient separation, we assessed the interaction of SERCA2b with both endogenous and overexpressed Bcl-2 in C2C12 myoblasts by co-IP. Overexpression of Bcl-2 increased the amounts of Bcl-2 co-immunoprecipitated with SERCA2b compared to controls (Figure 3A). Unlike endogenous Bcl-2, co-immunoprecipitated Bcl-2 after overexpression was detected in both the phosphorylated and the non-phosphorylated forms (Figures 3A and 3B), in agreement with data presented in Figure 1C. Our results show that the co-IP of Bcl-2 with SERCA2b was similar for C2C12 myoblasts overexpressing Bcl-2 and Bcl-2-cb5, irrespective of the presence of the ER-targeting sequence cb5 (Figure 3B). Figure 3C (upper panel) shows that overexpression of Bcl-2 in C2C12 myoblast cells did not significantly change the absolute levels of SERCA2b; the same results were obtained for the overexpression of GFP-Bcl-2 and GFP-Bcl-2-cb5 (data not shown). We also used an anti-Bcl-2 antibody to co-immunoprecipitate SERCA2b in C2C12 myoblasts, and detected co-IP of SERCA2b in both control and Bcl-2-overexpressing cells (Figure 3C, bottom panel). The lack of a significant difference between the amounts of co-immunoprecipitated SERCA2b for control and Bcl-2 overexpressing cells is probably due to a limited capacity of the antibodies used in this experiment. This set of data demonstrates that in C2C12 myoblast lysate Bcl-2 interacts with SERCA2b. To test whether this interaction occurs at the level of the ER, we performed cell fractionation by differential centrifugation. The data presented in Figure 3D confirm that Bcl-2 and SERCA2b co-immunoprecipitate from the microsomal fraction of C2C12 myoblast cells (even without Bcl-2 overexpression) suggesting a physiological relevance of this protein-protein interaction. However, we failed to detect any statistically significant changes of thapsigargin-inhibitable 45Ca2+ uptake in microsomes isolated from C2C12 myoblasts after 24 h of overexpression of Bcl-2 relative to controls (Figure 3E). Plausible reasons for this lack of effect may be that (i) in the cells, binding of overexpressed Bcl-2 to SERCA2b may occur in a non-inactivating manner, or (ii) other constituents of C2C12 cells may modulate the interaction of SERCA2b with Bcl-2 thereby protecting SERCA2b from Bcl-2-mediated conformational change and loss of activity, or reversing SERCA2b inactivation.
Interaction of SERCA2b with Bcl-2 in microsomes isolated from HEK293 cells and effect of heat shock proteins
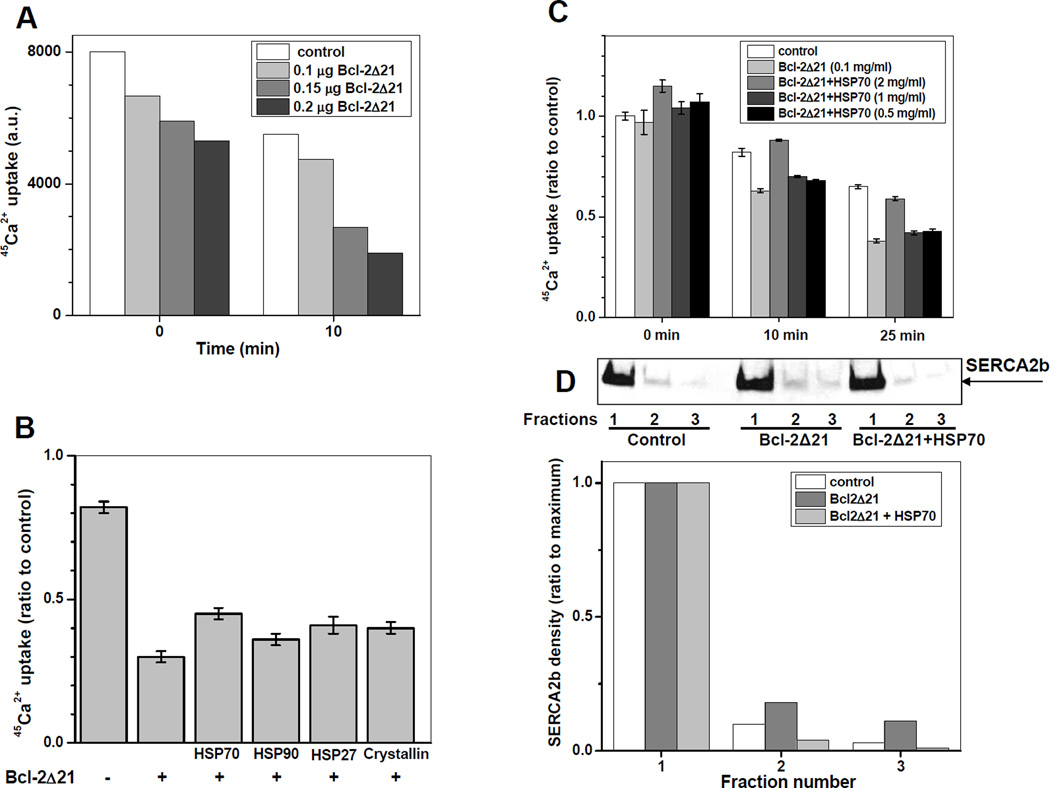
To ensure that the lack of effect of Bcl-2 overexpression on SERCA2b activity in C2C12 myoblasts is not muscle cell-type-specific, we studied the interaction of Bcl-2 with SERCA2b in human embryonic kidney (HEK293) cells, which do not express muscle-specific SERCA isoforms. Figure 4A shows that in HEK293 cells SERCA2b co-immunoprecipitates with Bcl-2 from microsomes. Nevertheless, we did not detect any significant difference between 45Ca2+ uptake by microsomes isolated from Bcl-2 overexpressing and sham-transfected cells (Figure 4B and 4C), consistent with our results from C2C12 cells. Importantly, the Ca2+ transporting activity of SERCA2b in HEK293 cell microsomes exhibited a fast decay (by ca. 40%) during the first 20 minutes of incubation at 37°C, which can be attributed to “thermal inactivation” described earlier for SERCA1 [35] and observed in our experiments with SR (see, e.g., Figure 8A below), though at significantly slower rates. Despite thermal inactivation, the in vitro incubation of HEK293 cell microsomes with recombinant Bcl-2 (Figure 5A) resulted in a significant Bcl-2Δ21 concentration-dependent inhibition of Ca2+ uptake by microsomes isolated from HEK293 cells (Figure 5A), consistent with a Bcl-2-dependent inactivation of SERCA2b. Using these conditions we further tested the hypothesis that other proteins may modulate the inhibitory effect of Bcl-2 on SERCA2b. LC-MS/MS analysis of proteins co-immunoprecipitated with Bcl-2 and SERCA2b from C2C12 or HEK293 cell lysates and microsomes identified HSP70, HSP90, as well as GRP78, and ERdj5, suggesting that SERCA2b and/or Bcl-2 interact with multiple proteins. In these experiments, protein identification was based on the presence of at least 2 and up to 20 unique peptides with >95% probability, from the sequences of proteins digested and extracted from gel slices of appropriate molecular weight. HSP70 (and GroEL, the bacterial analog to the mitochondrial heat shock protein 60) can protect SERCA1 [35,36] and SERCA2a [37] against thermal inactivation, suggesting that they may also protect SERCA2b against Bcl-2-dependent inactivation. Four representative cytoplasmic/ER residing heat shock proteins (HSP70, HSP90, HSP27, and α-crystallin) were tested in vitro with regard to their capacity at inhibiting Bcl-2Δ21-dependent inactivation of SERCA2b in microsomes of HEK293 cells (Figure 5B). All of the added heat shock proteins demonstrated a measurable extent of protection against Bcl-2Δ21-dependent inactivation. Note, that the first bar in Figure 5B represents the control experiment, displaying 45Ca2+-uptake after heat inactivation in the absence of Bcl-2Δ21. Among the proteins tested, HSP70 afforded the highest protection of SERCA2b; this effect of HSP70 is concentration-dependent (Figure 5C). For short incubation times, a protection against thermal inactivation of SERCA2b by 2 mg/ml HSP70 was also observed (Figure 5C). Under these conditions, we were able to detect a slight translocation of SERCA2b from the CRD fractions of sucrose gradient density separation after incubation with Bcl-2Δ21, and its reversal by HSP70 (Figure 5D). For densitometry analysis on a single gel, sucrose density gradient centrifugation was first run separately for controls, microsomes exposed to Bcl-2Δ21, and microsomes exposed to Bcl-2Δ21 and HSP70, and for each sample nine fractions were collected. Then, these nine fractions were combined into 3 × 3 fractions, so that a total three fractions resulted for each experimental sample, which were then run on a single gel, as shown in Figure 5D.
Figure 4. Interaction of Bcl-2 with SERCA2b in HEK293 cell microsomes.
A – WB analysis for SERCA2b immunoprecipitated from HEK293 cell microsomes using anti-Bcl-2 antibodies (1) or protein A beads only (2); the two bands represent SERCA2b monomer and aggregate. B – WB analysis for Bcl-2 in microsomes of HEK293 cells transfected with empty vector (1) or Bcl-2 (2). C – Lack of the effect of Bcl-2 overexpression on Ca2+ uptake by microsomes isolated from HEK293 cells.
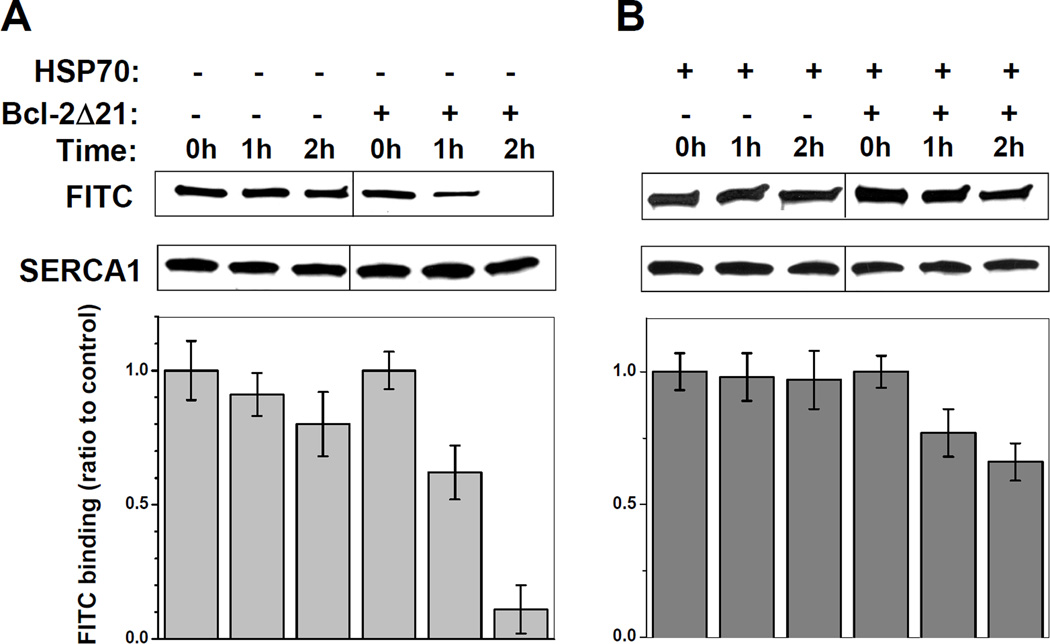
Figure 8. Effect of HSP70 on FITC binding capacity of SERCA1 in the presence of Bcl-2Δ21.
SR (0.5 mg/ml protein) was incubated at 37°C in STE buffer containing 1 mM PMSF in the absence or presence of Bcl-2Δ21 (at a molar ratio [Bcl-2Δ21]/[SERCA1]=2) (A) and 0.25 mg/ml HSP70 (B). The aliquots were taken at 0, 1, and 2 h of incubation, labeled with FITC, and analyzed by WB as described under “Experimental Procedures”. Data are expressed relative to control SR samples without incubation.
Figure 5. Heat shock protein attenuation of SERCA2b inactivation by Bcl-2Δ21 in microsomes isolated from HEK293 cells.
A – Time- and concentration-dependence for SERCA2b inhibition by Bcl-2Δ21 (protein amounts are indicated in the insert) in microsomes isolated from HEK293 cells (4 µg protein in 10µL sample). B – Effect of heat shock proteins (2 µg each) on the inactivation by 0.2 µg of Bcl-2Δ21 of Ca2+ uptake by microsomes (4 µg protein) isolated from HEK293 cells (co-incubated for 10 min at 37°C). C - Time- and HSP70 concentration-dependence for protection against Bcl-2Δ21-depedent inactivation of SERCA2b. Protein concentrations are shown in the insert. D – WB densitometry analysis of SERCA2b distribution in sucrose density gradient separation without or with Bcl-2Δ21 and HSP70. For densitometry analysis on a single gel, sucrose density gradient centrifugation was first run separately for controls, microsomes exposed to Bcl-2Δ21, and microsomes exposed to Bcl-2Δ21 and HSP70, and for each sample nine fractions were collected. Then, these nine fractions were combined into 3 × 3 fractions, so that a total three fractions resulted for each experimental sample, which were then run on a single gel. Data for each sample are normalized to the amounts of SERCA2b in the first pooled (CRD) fraction.
Direct interaction of HSP70 with SERCA2b in C2C12 myoblasts
The interaction of HSP70 with SERCA2a expressed in HEK293 cells based on co-IP has been reported [37] suggesting that these two proteins interact in vivo. Accordingly, LC-MS/MS analysis of proteins co-immunoprecipitated with SERCA2b from the microsomal fraction of C2C12 myoblast cells revealed that after in-gel digestion of a 70-kDa protein band at least 3 peptides from the sequence of HSP70 were identified at a 95% confidence level (MS/MS data not shown).
Effect of HSP70 and GroEL on Bcl-2Δ21-dependent inactivation of SERCA1 in vitro
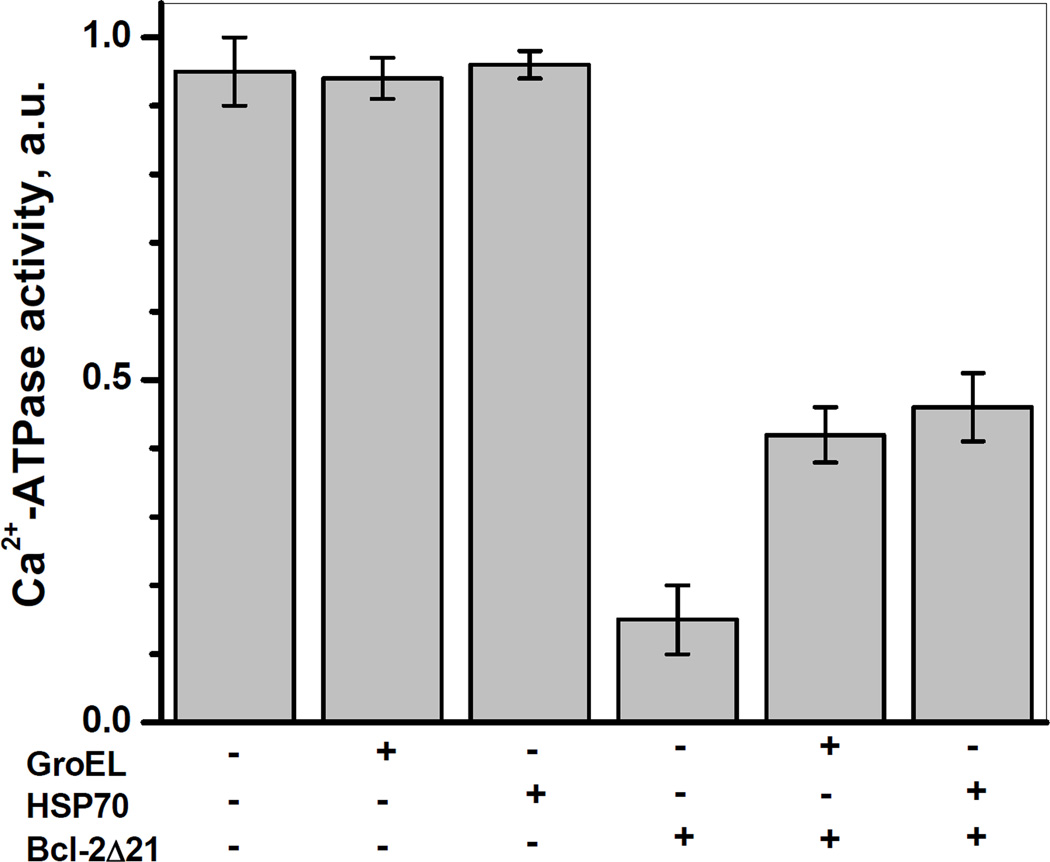
Figure 6 demonstrates that incubation of the SR with HSP70 or GroEL per se did not significantly change the Ca-ATPase activity of SERCA1. However, the loss of more than 80% of SERCA1 activity in vitro, observed in the presence of Bcl-2Δ21 at a ratio of [Bcl-2Δ21]/[SERCA1]=2 after 2 h of incubation at 37°C, was partially prevented (by ca. 27±4 or 31±5%, respectively) by the presence of HSP70 or GroEL at 1:1 molar ratios of chaperone to SERCA1. These protective effects of quite different chaperone proteins support our hypothesis that other chaperone protein family members may also protect SERCA from Bcl-2-dependent inactivation.
Figure 6. HSP70 and GroEL attenuate Bcl-2Δ21-dependent inhibition of Ca2+-ATPase activity of SERCA1.
SR (0.5 mg/ml protein) was incubated in STE buffer containing 1 mM PMSF at 37°C in the absence or presence of Bcl-2Δ21 (at a molar ratio of [Bcl-2Δ21]/[SERCA]=2), HSP70 or GroEL (0.25 mg/ml each). The aliquots were analyzed for Ca2+-ATPase activity after 2 h of incubation as described under “Experimental Procedures”.
Effect of HSP70 on SERCA localization to the CRD
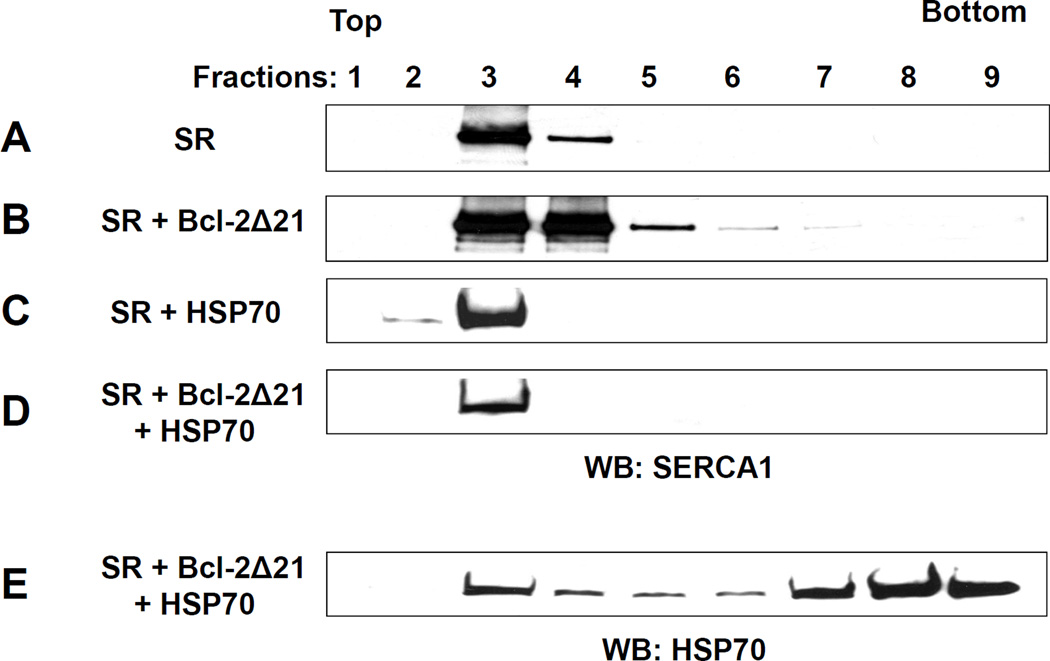
We tested whether HSP70 can prevent the partial displacement of SERCA1 from the CRD fraction induced by Bcl-2 in vitro. SR (0.5 mg/ml protein) was incubated for 2 h at 37°C with Bcl-2Δ21 at a molar ratio of [Bcl-2Δ21]/[SERCA1]=2 in the absence or presence of 0.25 mg/ml HSP70 followed by sucrose density gradient fractionation (Figure 7). Incubation of SR with HSP70 did not significantly change SERCA1 localization to the CRD in the sucrose density gradient fractions (Figure 7A and 7C). Incubation with Bcl-2Δ21 leads to SERCA1 translocation (Figure 7B), while co-incubation of HSP70 with SR and Bcl-2Δ21 prevents SERCA1 from the Bcl-2Δ21-induced displacement from the CRD (Figure 7D). Figure 7E demonstrates that HSP70 is distributed over the entire array of sucrose density gradient fractions, a feature which was also observed for Bcl-2 after overexpression in C2C12 cells (Figure 2B).
Figure 7. Effect of HSP70 on sucrose gradient fractionation of skeletal muscle SR.
SR (20 µg of total protein) was incubated at 37°C in STE buffer containing 1 mM PMSF for 1 h (A) without additions, (B) with Bcl-2Δ21 at a molar ratio [Bcl-2Δ21]/[SERCA1] =2, (C) in the presence of 10 µg of HSP70, and (D and E) in the presence of 10 µg of HSP70 and Bcl-2Δ21 at a molar ratio [Bcl-2Δ21]/[SERCA1] =2. After sucrose density gradient separation, fractions were collected from the top of the gradient, separated by SDS-PAGE, and analyzed by WB with anti-SERCA1 (A–D) or anti-HSP70 (E) antibody. Fraction numbers are indicated at respective lanes.
FITC-Binding capacity of SERCA1 in the presence of Bcl-2Δ21 and HSP70
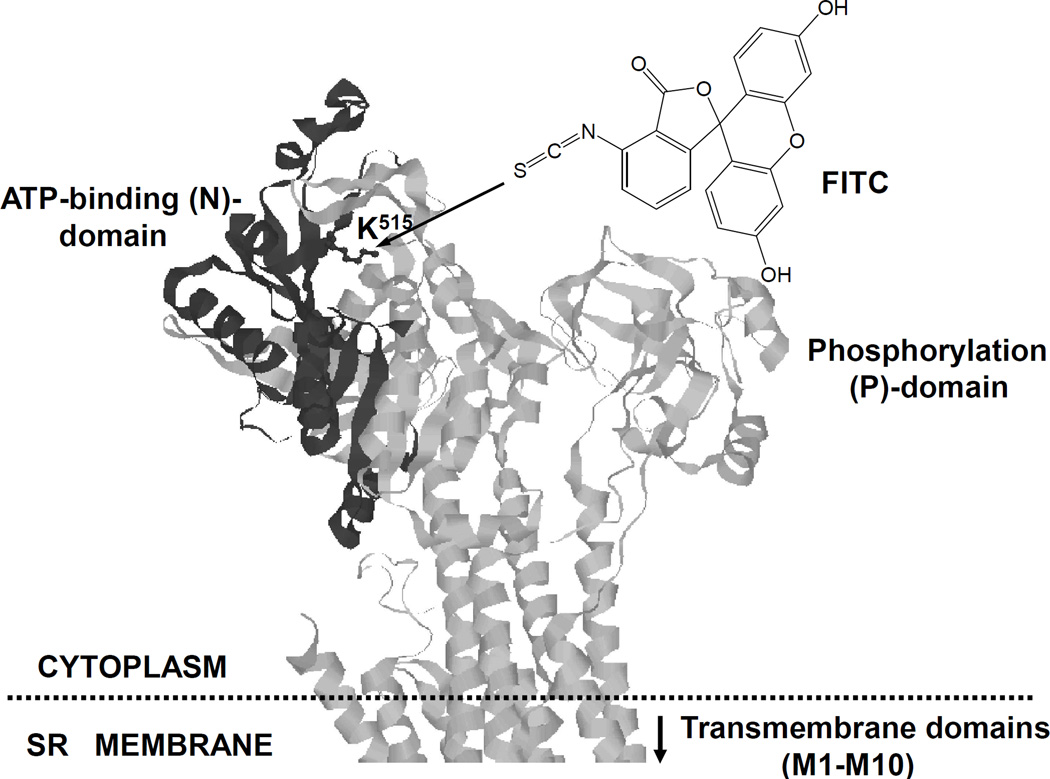
To assess the structural integrity and conformational transitions of SERCA1 in the presence of Bcl-2 and HSP70 in vitro, we used FITC labeling. The FITC binding capacity of SERCA1 characterizes the structural integrity of the nucleotide-binding domain (NBD) (Scheme 1), where the FITC-binding residue, Lys515, is located close to the ATP-binding site [38]. The FITC binding capacity of SERCA1 was analyzed by WB, and quantified by densitometry, where FITC binding of SERCA1 was normalized to the actual amount of SERCA1 (Figure 8). The incubation of 0.5 mg/ml SR alone for 2 h shows a slight time-dependent decrease in the FITC-binding capacity (Figure 8A), which is in accord with published data on the thermal inactivation of SERCA1 [35]. In the absence of HSP70, the incubation of SERCA1 with Bcl-2Δ21 at a molar ratio of [Bcl-2Δ21]/[SERCA1]=2 for 1 and 2 h at 37°C resulted in a ca. 40 and 90% inhibition of FITC labeling, respectively (Figure 8A). However, the addition of 0.25 mg/ml HSP70 significantly attenuated the Bcl-2Δ21-dependent loss of SERCA1 FITC labeling (Figure 8B). This result suggests that HSP70 protects the NBD of SERCA1 from Bcl-2Δ21-dependent destabilization.
Scheme 1. 3-D presentation of SERCA1 structure showing Lys515 residue as a target for FITC labeling.
The nucleotide binding domain (NBD) in the cytoplasmic part of SERCA1 is highlighted.
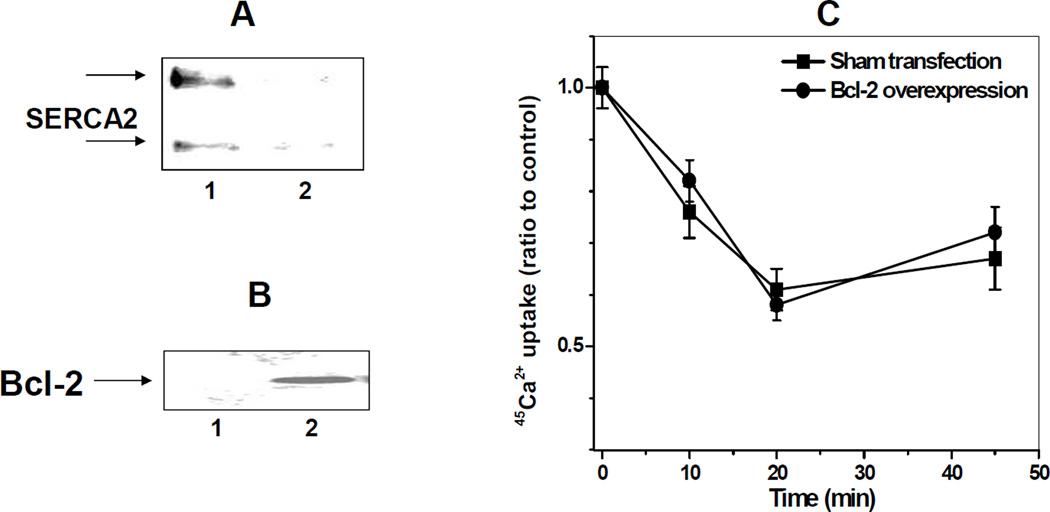
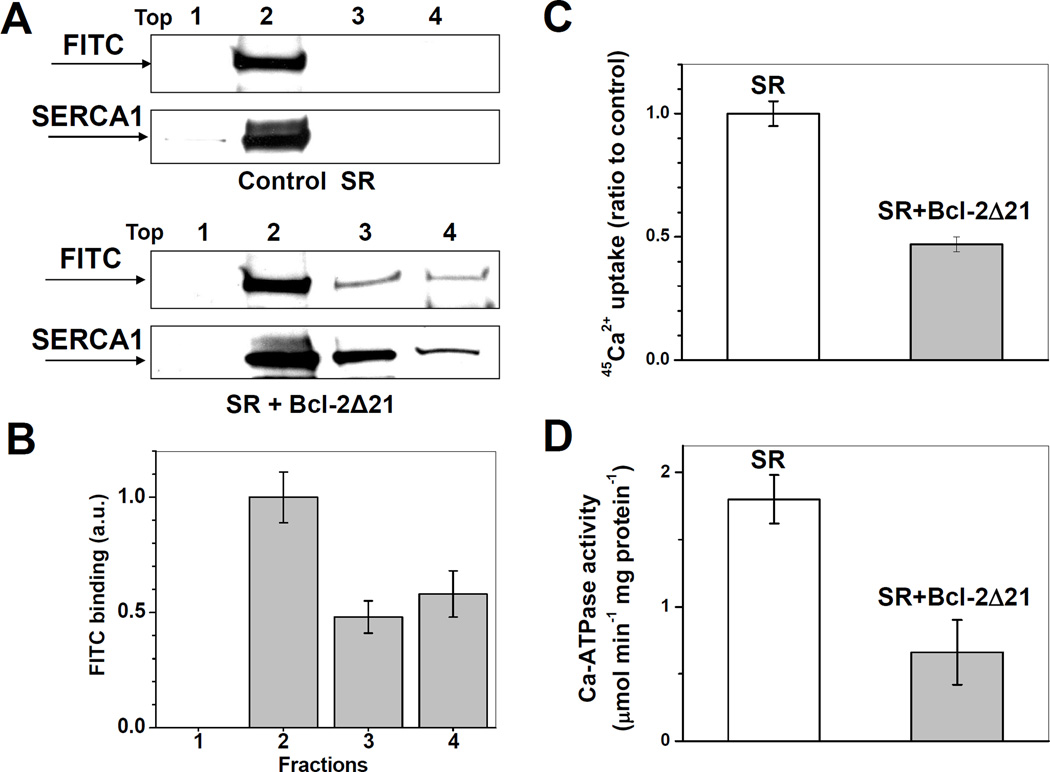
FITC labeling permits to corroborate shifts in sucrose density gradient fractionation with structural changes of SERCA1 displaced from higher-ordered membrane domains such as CRD. After incubation of the SR at 37°C for 1 h in the absence or presence of Bcl-2Δ21 at a molar ratio of [Bcl-2Δ21]/[SERCA1]=2, samples were fractionated by sucrose density gradient centrifugation (Figure 9A; here, only 4 pooled fractions were collected for the convenience of quantitative analysis), the respective fractions labeled with FITC, and analyzed by WB with anti-SERCA1 and anti-fluorescein antibodies. Consistent with our previous data [31], the co-incubation of SR with Bcl-2Δ21 induced inhibition of SERCA1 Ca2+-transporting (Figure 8C) and Ca2+-ATPase (Figure 9D) activity, measured in samples before sucrose density gradient fractionation, and induced a partial displacement of SERCA1 from the CRD (fraction 2) to fractions of the higher density (fractions 3 and 4) (Figure 9A). FITC-labeling of SERCA1 was maximal in fraction 2 (CRD) and significantly less in fractions 3 and 4 (Figure 9B), analyzed by densitometry of Western blots in Figure 9A, where FITC labeling is normalized to the content of SERCA1. These data demonstrate that the translocated protein displays structural modifications of the NBD, consistent with our earlier data, where Bcl-2Δ21-dependent conformational changes of SERCA1 were detected by labeling of solvent-accessible Cys residues [30]. This effect of Bcl-2Δ21 is accompanied by a significant decrease in SR 45Ca2+ uptake, which is completely attributable to SERCA1-specific Ca-ATPase activity based on the effect of a specific inhibitor, thapsigargin. These results confirm our previous observations and provide complimentary evidence for (i) structural changes of SERCA1 accompanying the loss of function resulting from the interaction with Bcl-2Δ21, and (ii) potential protection of SERCA integrity and function through interaction with a chaperone protein family member, HSP70.
Figure 9. Bcl-2Δ21-dependent inhibition of SERCA1 Ca2+-transporting activity and FITC-binding capacity in skeletal muscle SR.
SR (0.5 mg/ml protein) was incubated in STE buffer at 37°C in the presence of 1 mM PMSF without or with Bcl-2Δ21 at a molar ratio [Bcl-2Δ21]/[SERCA1]=2. A - Aliquots were taken at 0 (control) or 1 h incubation, followed by sucrose density gradient separation. The aliquots of each fraction were taken for FITC labeling and analyzed by WB with anti-fluorescein and anti-SERCA1 antibody. Fraction numbers are indicated at respective lanes. B – Densitometry quantification of data from panel A, in the presence of Bcl-2, where FITC binding capacity is normalized by SERCA1 content in the respective fractions and expressed relative to the maximal FITC binding capacity in the fraction 2 (CRD). C and D - Aliquots taken after 1 h of incubation were assayed, respectively, for tapsigargin-inhibitable ATP-dependent 45Ca2+ uptake and for Ca-ATPase activity, as described in “Experimental Procedures”.
Associations of SERCA1 with Bcl-2Δ21 and HSP70
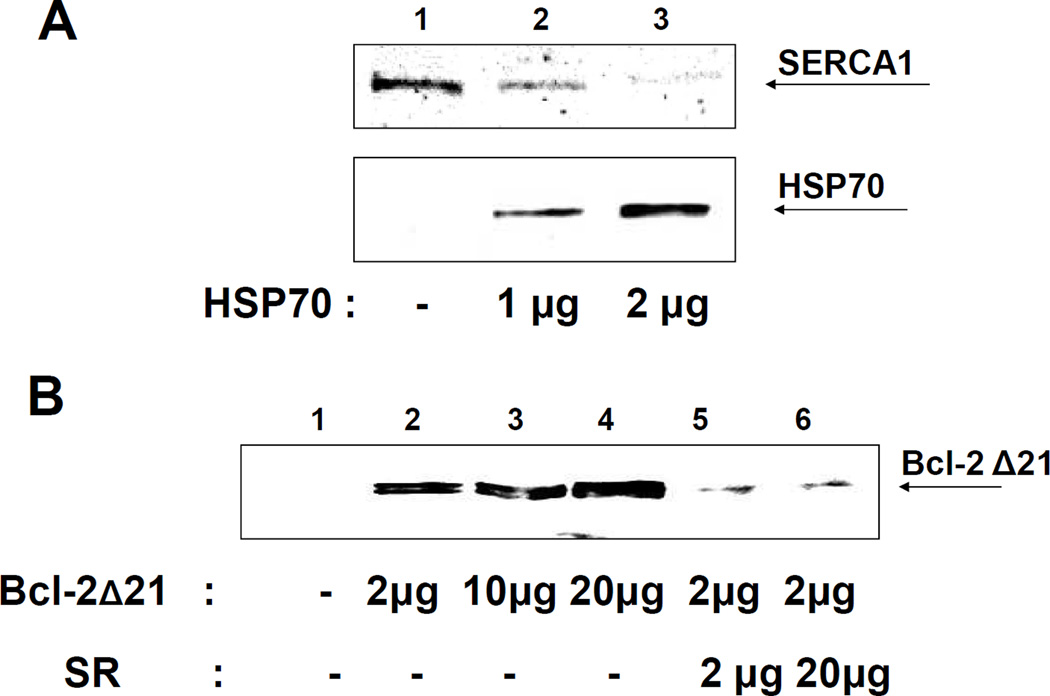
HSP70 may protect SERCA against Bcl-2-dependent inhibition and destabilization through association with either or both proteins. We analyzed the effect of HSP70 on the association of SERCA1 with GST-Bcl-2Δ21 using a GST-binding assay. SR (2 µg of total protein, containing ca. 0.8 µg SERCA) was incubated with GST-Bcl-2Δ21 (2 µg) in the absence or in the presence of HSP70. Subsequently, protein complexes were purified with GSH-agarose beads, and analyzed by WB for SERCA1 and HSP70 proteins. Figure 10A shows that the addition of 1 µg of HSP70 to the incubations results in a ca. 50 % decrease of the amount of SERCA1 associated with GST-Bcl-2Δ21 (compare lanes 1 and 2 in Figure 10A, upper panel), while 2 µg of HSP70 almost completely blocked the association of Bcl-2Δ21 and SERCA1 (Figure 10A, lane 3). WB analysis for HSP70 shows that this effect is accompanied by an increasing direct association of HSP70 with Bcl-2Δ21-GST (Figure 10A, lanes 2 and 3 in the bottom panel) suggesting that HSP70 competitively blocks the Bcl-2Δ21-SERCA1 association through direct interaction with GST-Bcl-2Δ21.
Figure 10. Competition of HSP70 and Bcl-2Δ21 for SERCA1 binding.
A – GST-Binding assay for SERCA1 and Bcl-2Δ21. Rat skeletal muscle SR (2 µg protein) was incubated with 2 µg of GST-Bcl-2Δ21 in the absence or presence of different amounts of HSP70, followed by the pull-down with GST-agarose beads, SDS-page separation of bound proteins, and WB analysis with anti-SERCA1 and anti-HSP70 antibodies. B - Co-IP of Bcl-2Δ21 with HSP70 used as bait. HSP70 (2 µg) was incubated with different amounts of Bcl-2Δ21 (0–20 µg) in the absence or presence of SR (2 and 20 µg of total protein) prior to the pull-down with anti-HSP70 protein antibodies bound to protein G-agarose beads, followed by SDS-PAGE separation of proteins, and WB analysis with anti-Bcl-2 antibodies.
We then examined co-IP of Bcl-2Δ21 with HSP70, using an anti-HSP70 antibody as an anchor. Figure 10B shows that 2 µg of HSP70 can co-immunoprecipitate approximately 2 µg of Bcl-2Δ21 (a slight increase in association at 20 µg of Bcl-2 is likely due to non-specific binding), suggesting a saturation of HSP70 with Bcl-2Δ21 at a molar ratio of HSP70 to Bcl-2Δ21 of about 1:3. However, the addition of 2 µg SR (at a molar ratio of [HSP70]:[SERCA1]:[Bcl-2Δ21] ≈ 2:1:6) almost completely abolished the HSP70-Bcl-2Δ21complex (Figure 10B, lane 5), suggesting that binding of HSP70 with Bcl-2Δ21 is weaker then the associations of HSP70 and/or of Bcl-2Δ21 with SERCA1 in the SR.
DISCUSSION
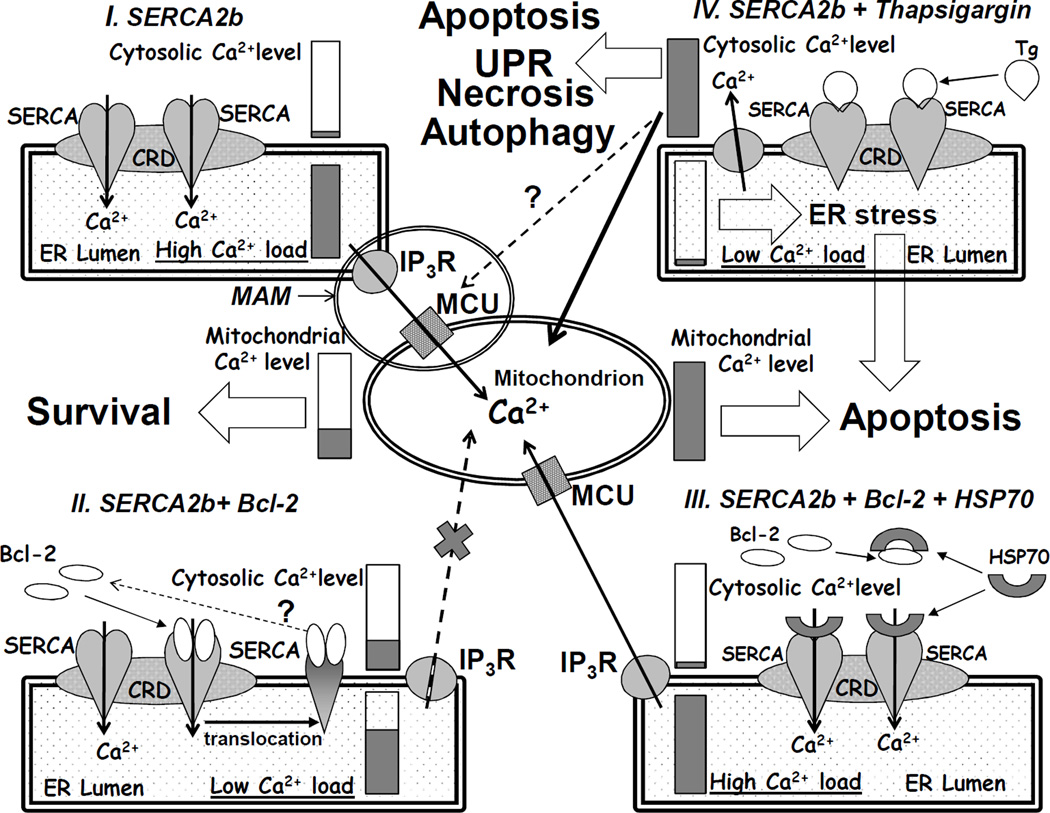
Recently, the physiological importance of cross-talk between ER and the mitochondria for Ca2+ homeostasis was supported by data on stabilization of IP3R by mitochondrial chaperones [39,40]. It is well established that Bcl-2 regulates Ca2+-homeostasis at the level of the ER and interaction of Bcl-2 with the IP3R may represent a mechanism, which contributes to the anti-apoptotic effect of Bcl-2 [20–25]. In addition, there is growing evidence for a role of SERCA in apoptosis [6,41–43]. Some proteins can interact with and modulate SERCA activity. For example, sarcolipin in skeletal muscle and phospholamban together with its binding partner HAX-1 in cardiac muscle were identified as physiological regulators of SERCA1 and SERCA2a isoforms, respectively [44,45]. Oxidative stress can induce structural alterations of SERCA and change its activity [35,37,46]. Specifically, the activity of SERCA can be regulated through S-glutathiolation and oxidative/nitrosative modification of specific cysteine residues, and by tyrosine nitration [33,46]. HSP70, a chaperon protein of the HSP70 family, which is partially co-localized to the ER and up-regulated under conditions of oxidative stress, may play a protective role in models of stroke and myocardial ischemia [47–49], and prevents SERCA from thermal inactivation [35,37]. We recently reported that Bcl-2 and Bcl-2Δ21 inhibit SERCA1 in skeletal muscle SR in vitro, accompanied by a translocation of SERCA1 from the SR CRD [23,24]. However, it seems unlikely that skeletal or cardiac muscle-specific SERCA isoforms, which are highly specialized to provide excitation-contraction coupling through repetitive changes in Ca2+ levels next to contractile protein machinery, could be involved in the regulation of apoptosis in respective cells. For non-muscle cells, omnipresent SERCA2b (and, in some cases, SERCA3) represent appropriate targets. However, even in muscle cells SERCA2b is also present in low amounts (relative to SERCA1 or SERCA2a) and could contribute to apoptotic regulation. The question is how Bcl-2 would selectively interact with a specific SERCA isoform in excess of another one. This visible paradox may be resolved assuming that in both muscle and non-muscle cells local Ca2+ levels are involved in multiple cellular functions and responses, so that in addition to SERCA isoform specificity, a spatial and structural diversity of the ER and other cellular organelles is required. In particular, for apoptotic regulation through ER Ca2+ levels a synapse-like contact between the ER and the mitochondria has been suggested [8,13]. Morphologically, they are referred to as mitochondria-associated membranes (MAM; see Scheme 2, panel I) and implicate at least two additional Ca2+ transporting proteins, IP3R and MCU, which work in local co-operation with SERCA2b. Obviously, even in non-muscle cells, only a fraction of SERCA2b is involved in such structures and represents a subject for apoptotic regulation.
Scheme 2. The interplay between SERCA, Bcl-2, and HSP70, which can regulate apoptosis, URL, and autophagy responses through the control of ER Ca2+ levels: comparison to thapsigargin-induced apoptosis.
In the present work, the interaction of Bcl-2 and SERCA2b was examined in C2C12 myoblast cells overexpressing Bcl-2. C2C12 myoblasts express the SERCA2b isoform, whereas terminally differentiated, post-mitotic myotubes predominantly express the fast-twitch skeletal muscle-specific SERCA1 isoform, lose the ability for proliferation, and do not express significant amounts of Bcl-2 (data not presented; see also [50]). Importantly, the overexpression of Bcl-2 in C2C12 myoblasts did not change the expression levels of SERCA2b (Figure 3B) in contrast to data obtained, e.g., in an epithelial breast cell line [41], suggesting that the C2C12 cell model is suitable for studies on protein-protein interactions not altered through transcription. A direct interaction of Bcl-2 and SERCA2b in C2C12 myoblast cells was confirmed by co-IP (Figure 3); we also observed a slight translocation of SERCA2b from CRD to higher density fractions (Figure 2). However, the modest translocation of SERCA2b from CRD in C2C12 cells overexpressing Bcl-2 is not accompanied by SERCA inhibition in microsomes (measured prior to CRD separation). It is possible that in intact cells, the binding of Bcl-2 to SERCA2b is modulated by chaperones or, at least, proceeds in a non-inactivating manner. In contrast, the exposure of SERCA2b in isolated microsomes to Bcl-2Δ21 results in inactivation, and translocation from the CRD, consistent with our data on SERCA1 in isolated SR. A cartoon summarizing the effect of Bcl-2 on SERCA2b, together with potential consequences for luminal and cytosolic levels of Ca2+ is displayed in Scheme 2, panel II. Importantly, the interaction of Bcl-2 with both SERCA1 and SERCA2b can be modified in vitro by HSP70 and other representative chaperones (Figures 5B and 6). Using FITC labeling of Lys515 (Scheme 1), we were able to monitor the conformational integrity of the SERCA1 NBD in different sucrose density gradient fractions. Although the procedure of sucrose density gradient fractionation by itself causes some conformational changes of SERCA1, we observed that relative FITC-labeling of SERCA1 within fractions 3 and 4 (non-CRD), is lower than for fraction 2 (CRD) reflecting structural changes of SERCA1 induced by Bcl-2. HSP70 protected the SERCA1 NBD against Bcl-2-dependent conformational transition (Figure 8), and translocation from the SR CRD (Figure 7D). HSP70 partially co-localized with SERCA1 in the CRD fractions (Figure 7E), and competed with Bcl-2 for the association with SERCA1 in the SR. Similar, though less significant, effects of HSP70 were detected in experiments with microsomes isolated from HEK293 cells expressing SERCA2b (Figure 5D). In the latter case, experiments with FITC-labeling were non-conclusive due to the relatively low abundance of SERCA2b and the presence of other proteins reactive with FITC. A hypothetical scheme displaying the interplay between SERCA, Bcl-2, and HSP70 is shown in Scheme 2, panel III. We note that the bacterial analog of mitochondrial HSP60, GroEL, has a similar effect on SERCA1 in vitro, while HSP90, HSP27, and α-crystallin partially protected SERCA2b in isolated HEK293 cell microsomes, suggesting that SERCA protection is not restricted to a single heat shock protein isoform.
To date, a number of reports demonstrate a role of the HSP70 family of proteins in prevention of stress-induced apoptosis [47–49] and the apoptosis of cancer cells [51–54], although the actual mechanisms of HSP70 protection against apoptosis are not clear. Eight isoforms of the HSP70 family proteins differ from each other by expression levels and cellular localization, and are involved in different intracellular activities from protein folding to signaling. Among six cytosolic and nuclear HSP70 isoforms, the expression of HSP70-1a, -1b (HSP72) and HSP70-6 is regulated by stress-inducible transcription factors, resulting in the protection against ischemia, heat and oxidative stress [47–49]. Hsc70 (HSP70-8), HSP70-1t and HSP70-2 are constitutively expressed isoforms of the HSP70 family with housekeeping chaperone functions essential for cell survival [54,55]. The chaperon activity of HSP70 family proteins assists in the folding of newly synthesized proteins, in the transport of proteins across membranes to various organelles, in the UPR by refolding of misfolded and aggregated proteins [54–56], and in chaperon-mediated autophagy [54,57,58]. Although Ca2+ is a well-known regulator of cell metabolism, and apoptosis in particular, very little is known about a possible interaction of HSP70 with proteins involved in Ca2+ homeostasis. It is known that HSP70 directly associates with Bcl-2 family proteins, particularly with Bax [59,60] and Bcl-2 [60], and also with SERCA1 and SERCA2a [35,37]. Here, we demonstrate HSP70 attenuation of Bcl-2-dependent SERCA inhibition in vitro. The results of our in vitro experiments suggest that the interaction of SERCA with Bcl-2 and HSP70 occurs within the CRD domains of the SR (Figure 7). Both Bcl-2 and HSP70 display anti-apoptotic activity yet exert opposite effects on SERCA activity and conformation. These opposite effects are not inconsistent with the anti-apoptotic functions: in vitro, Bcl-2Δ21 and Bcl-2 [30] cause significant to complete inhibition of SERCA1 and SERCA2b (Figures 5A and 6), depending on the molar ratios of Bcl-2Δ21/SERCA and the time of incubation. A complete inactivation of SERCA, such as induced by a specific inhibitor, thapsigargin, causes apoptosis [61] though via a different mechanism (Scheme 2, panel IV). Here, a primary increase in cytosolic Ca2+ concentrations triggers delayed secondary Ca2+ store-operated responses (reviewed in [61]); a permeability of inactivated SERCA2b Ca2+ channels may also contribute [62,63]. In contrast, a partial inhibition of SERCA2b, resulting in reduced luminal Ca2+ levels [21,24], may be anti-apoptotic. Hence, specifically with regard to the modulation of SERCA activity, HSP70 (and other chaperones) may be responsible for attenuating the inhibitory effect of Bcl-2 so that Bcl-2-dependent SERCA inactivation does not become pro-apoptotic (Scheme 2, panel III).
In fact, stabilization of SERCA by HSP70 in complex with Bcl-2 may be similar to stabilization of HIF-1a in a complex with Bcl-2 and HSP90 described elsewhere [64]. Possibly, HSP70 maintains SERCA in an active state in a tertiary complex of SERCA-Bcl-2-HSP70, but it cannot be excluded that some other chaperones of the ER/mitochondria network may modulate the SERCA-Bcl-2 interaction to mediate Ca2+ transfer from ER to the mitochondria. HSP70 can regulate apoptosis through multiple mechanisms by interaction with different signaling and apoptosis-regulating proteins. Our data present a new possible mechanism, where HSP70 may modulate the interaction of Bcl-2 with SERCA and maintain SERCA in an active state that may be essential for the regulation of Ca2+-dependent apoptosis, UPR response and authophagy [16–19,65]. Future studies should be designed to address the role of SERCA-Bcl-2-HSP70 interactions in vivo in different types of pathological conditions.
ACKNOWLEDGEMENTS
This research was supported by grants from the American Heart Association (O355555Z) and the NIH (PO1AG12993). We thank Drs. T. Williams and N. Galeva (MS and APL Labs, University of Kansas) for mass spectrometry support and Dr. D. Hui for the help with the cloning of Bcl-2 constructs.
Abbreviations
- Bcl-2
b-cell lymphoma 2 protein
- Bcl-2Δ21
human recombinant Bcl-2 with the 21 C-terminal amino acids deleted
- CRD
caveolae-related domains
- DMEM
Dulbecco modified Eagle medium
- DTT
dithiothreitol
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- HIF
hypoxia-inducible factor
- HRP
horseradish peroxidase
- HSP
heat shock protein
- IP
immunoprecipitation
- IP3
inositol-3-phosphate
- IP3R
inositol-3-phosphate receptor
- IPTG
isopropyl-D-thiogalactopyranoside
- LB
Luria-Bertani medium
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MAM
mitochondria-associated membranes of ER
- MCU
mitochondrial Ca2+ uniporter
- NBD
nucleotide-binding domain
- PARP
poly(ADP)-ribose polymerase
- SDS-PAGE
sodium dodecyl sulfate -polyacrylamide gel electrophoresis
- PP
protein phosphatase
- SERCA
sarco(endo)plasmic reticulum Ca-ATPase
- SB
sample buffer
- SR
sarcoplasmic reticulum
- STE
Tris-buffered saline solution
- TM
transmembrane domain
- WB
Western blot
- UPR
unfolded protein response
REFERENCES
- 1.Basset O, Boittin F-X, Cognard C, Constantin B, Ruegg UT. Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem. J. 2006;395:267–276. doi: 10.1042/BJ20051265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirks-Naylor AJ, Lennon-Edwards S. Cellular and molecular mechanisms of apoptosis in age-related muscle atrophy. Curr. Aging Sci. 2011 Apr. [Epub ahead of print] [PubMed] [Google Scholar]
- 3.Marzetti E, Privitera G, Simili V, Wohlgemuth SE, Aulisa L, Pahor M, Leeuwenburgh C. Multiple pathways to the same end: mechanisms of myonuclear apoptosis in sarcopenia of aging. Scientific World Journal. 2010;10:340–349. doi: 10.1100/tsw.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzetti E, Hwang JCY, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Biochim. Biophys. Acta. 2010;1800:235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeuwenburgh C. Role of apoptosis in sarcopenia. J. Gerontol. Med. Sci. 2003;58A:999–1001. doi: 10.1093/gerona/58.11.m999. [DOI] [PubMed] [Google Scholar]
- 6.Demaurex N, Distelhorst C. Cell biology. Apoptosis - the calcium connection. Science. 2003;300:67–69. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- 7.Hajnóczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 8.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao J, Huang X, Feit-Leithman RA, Lee Neve R, Snider W, Dartt DA, Chen DF. Bcl-2 enhances Ca2+ signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J. 2005;24:1068–1078. doi: 10.1038/sj.emboj.7600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am. J. Physiol. Cell Physiol. 2005;289:C994–C1001. doi: 10.1152/ajpcell.00031.2005. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell. Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulhunty AF. Excitation-contraction coupling from the 1950s into the new millennium. Clin. Exp. Pharmacol. Physiol. 2006;33:763–772. doi: 10.1111/j.1440-1681.2006.04441.x. [DOI] [PubMed] [Google Scholar]
- 13.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim. Biophys. Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Patterson RL, Boehning D, Snyder SH. Inositol-1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 16.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 17.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 18.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 20.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 21.Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oakes SA, Lin SS, Bassik MC. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr. Mol. Med. 2006;6:99–109. doi: 10.2174/156652406775574587. [DOI] [PubMed] [Google Scholar]
- 23.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez D, Rojas-Rivera D, Hetz C. Integrating stress signals at the endoplasmic reticulum: The Bcl-2 protein family rheostat. Biochim. Biophys. Acta. 2010 Nov. doi: 10.1016/j.bbamcr.2010.11.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Rojas-Rivera D, Caballero B, Zamorano S, Lisbona F, Hetz C. Alternative functions of the BCL-2 protein family at the endoplasmic reticulum. Adv. Exp. Med. Biol. 2010;687:33–47. doi: 10.1007/978-1-4419-6706-0_2. [DOI] [PubMed] [Google Scholar]
- 26.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer S. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. J. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 27.Lin SS, Bassik MC, Suh H, Nishino M, Arroyo JD, Hahn WC, Korsmeyer SJ, Roberts TM. PP2A regulates BCL-2 phosphorylation and proteasome-mediated degradation at the endoplasmic reticulum. J. Biol. Chem. 2006;281:23003–23012. doi: 10.1074/jbc.M602648200. [DOI] [PubMed] [Google Scholar]
- 28.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schöneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) Biochem. J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dremina ES, Sharov VS, Schöneich C. Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation. Biochemistry. 2006;45:175–184. doi: 10.1021/bi050800s. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez JL, Rosemblatt M, Hidalgo C. Highly purified sarcoplasmic reticulum vesicles are devoid of Ca2+-independent ('basal') ATPase activity. Biochim. Biophys. Acta. 1980;599:552–568. doi: 10.1016/0005-2736(80)90199-6. [DOI] [PubMed] [Google Scholar]
- 33.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 34.Zhu W, Cowie A, Wasfy GW, Penn LZ, Leber B, Andrews DW. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J. 1996;15:4130–4141. [PMC free article] [PubMed] [Google Scholar]
- 35.Tupling AR, Gramolini AO, Duhamel TA, Kondo H, Asahi M, Tsuchiya SC, Borrelli MJ, Lepock JR, Otsu K, Hori M, MacLennan DH, Green HJ. HSP70 binds to the fast-twitch skeletal muscle sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA1a) and prevents thermal inactivation. J. Biol. Chem. 2004;279:52382–52399. doi: 10.1074/jbc.M409336200. [DOI] [PubMed] [Google Scholar]
- 36.Javed MU, Michelangeli F, Lund PA. GroEL protects the sarcoplasmic reticulum Ca(++)-dependent ATPase from inactivation in vitro. Biochem. Mol. Biol. Int. 1999;47:631–638. doi: 10.1080/15216549900201683. [DOI] [PubMed] [Google Scholar]
- 37.Fu MH, Tupling AR. Protective effects of Hsp70 on the structure and function of SERCA2a expressed in HEK-293 cells during heat stress. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1175–H1183. doi: 10.1152/ajpheart.01276.2008. [DOI] [PubMed] [Google Scholar]
- 38.Pick U, Karlish SJ. Indications for an oligomeric structure and for conformational changes in sarcoplasmic reticulum Ca2+-ATPase labeled selectively with fluorescein. Biochim. Biophys. Acta. 1980;626:255–261. doi: 10.1016/0005-2795(80)90216-0. [DOI] [PubMed] [Google Scholar]
- 39.Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J. Biol. Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- 40.Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo TH, Kim HR, Zhu L, Yu Y, Lin HM, Tsang W. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene. 1998;17:1903–1910. doi: 10.1038/sj.onc.1202110. [DOI] [PubMed] [Google Scholar]
- 42.Lee DI, Sumbilla C, Lee M, Natesavelalar C, Klein MG, Ross DD, Inesi G, Hussain A. Mechanisms of resistance and adaptation to thapsigargin in androgen-independent prostate cancer PC3 and DU145 cells. Arch. Biochem. Biophys. 2007;464:19–27. doi: 10.1016/j.abb.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad S, Ahmad A, Dremina ES, Sharov VS, Guo X, Jones TN, Loader JE, Tatreau JR, Perraud AL, Schöneich C, Randell SH, White CW. Bcl-2 suppresses sarcoplasmic/ endoplasmic reticulum Ca2+-ATPase expression in cystic fibrosis airways: role in oxidant-mediated cell death. Am. J. Respir. Crit. Care Med. 2009;179:816–826. doi: 10.1164/rccm.200807-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLennan DH, Asahi M, Tupling AR. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann. N Y Acad. Sci. 2003;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- 45.Vafiadaki E, Arvanitis DA, Pagakis SN, Papalouka V, Sanoudou D, Kontrogianni-Konstantopoulos A, Kranias EG. The anti-apoptotic protein HAX-1 interacts with SERCA2 and regulates its protein levels to promote cell survival. Mol. Biol. Cell. 2009;20:306–318. doi: 10.1091/mbc.E08-06-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Csordás G, Hajnóczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim. Biophys. Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plumier JC, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell. Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radford NB, Fina M, Benjamin IJ, Moreadith RW, Graves KH, Zhao P, Gavva S, Wiethoff A, Sherry AD, Malloy CR, Williams RS. Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc. Natl. Acad. Sci. USA. 1996;93:2339–2342. doi: 10.1073/pnas.93.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong KY, Lai CC, Lille S, Chang C, Su CY. Stable overexpression of the constitutive form of heat shock protein 70 confers oxidative protection. J. Mol. Cell Cardiol. 1998;30:599–608. doi: 10.1006/jmcc.1997.0623. [DOI] [PubMed] [Google Scholar]
- 50.Dominov JA, Dunn JJ, Miller JB. Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J. Cell Biol. 1998;142:537–544. doi: 10.1083/jcb.142.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J. Biol. Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- 52.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends. Biochem. Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Daugaard M, Kirkegaard-Sørensen T, Ostenfeld MS, Aaboe M, Høyer-Hansen M, Orntoft TF, Rohde M, Jäättelä M. Lens epithelium-derived growth factor is an Hsp70-2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 2007;67:2559–2267. doi: 10.1158/0008-5472.CAN-06-4121. [DOI] [PubMed] [Google Scholar]
- 54.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol. 2010;8:e1000410. doi: 10.1371/journal.pbio.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Q, She H, Gearing M, Colla E, Lee M, Shacka JJ, Mao Z. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science. 2009;323:124–127. doi: 10.1126/science.1166088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kettern N, Dreiseidler M, Tawo R, Höhfeld J. Chaperone-assisted degradation: multiple paths to destruction. Biol. Chem. 2010;391:481–489. doi: 10.1515/BC.2010.058. [DOI] [PubMed] [Google Scholar]
- 59.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J. Biol. Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 60.Gotoh T, Terada K, Oyadomari S, Mori M. Hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- 61.Denmeade SR, Isaac JT. The SERCA pump as a therapeutic target. Cancer Biol. Ther. 2005;4:14–22. doi: 10.4161/cbt.4.1.1505. [DOI] [PubMed] [Google Scholar]
- 62.Macdonald WA, Stephenson DG. Effect of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J. Physiol. 2001;532:499–588. doi: 10.1111/j.1469-7793.2001.0499f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J. Physiol. 2009;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trisciuoglio D, Gabellini C, Desideri M, Ziparo E, Zupi G, Del Bufalo D. Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90. PLoS One. 2010;5:e11772. doi: 10.1371/journal.pone.0011772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Kettern N, Dreiseidler M, Tawo R, Höhfeld J. Chaperone-assisted degradation: multiple paths to destruction. Biol. Chem. 2010;391:481–489. doi: 10.1515/BC.2010.058. [DOI] [PubMed] [Google Scholar]