Abstract
Similarities in innate immune signaling exist between mammals and the nematode Caenorhabditis elegans. Now, Ziegler et al. (2009) and Ren et al. (2009) demonstrate that a protein kinase C δ homolog in C. elegans is involved in innate immunity, providing evidence that the conservation of immune signaling networks extends further than previously thought.
Similarities exist in mammalian and invertebrate immune signaling, and common virulence factors are involved in both vertebrate and invertebrate pathogenesis (Mylonakis and Aballay, 2005). Invertebrates rely solely on an innate immune system to protect themselves from pathogens, and studies on the innate immune system of invertebrates have provided a promising start to understanding the complexity of this system in vertebrates. In particular, the ability to use Caenorhabditis elegans in high-throughput assays and in several advanced molecular studies has generated significant interest on the use of this model system for investigation of innate immunity.
The discovery of a conserved p38 MAP kinase cascade was pivotal to our understanding of C. elegans immunity (Kim et al., 2002). This was followed by studies that describe a number of other pathways that function in innate immunity in the nematode. We now know that, in addition to the p38 MAP kinase cascade, an ERK MAP kinase pathway, a DAF-2/DAF-16 insulin-like signaling pathway, and a TGF-β pathway are important components of the nematode immune signaling network. However, little is known about how the nematode senses the presence of a pathogen and the subsequent relay process culminating in p38/PMK-1 kinase activation.
In the mammalian immune signaling network, the novel isoform protein kinase C theta (PKCθ) is an integral member, linking T cell receptor stimulation with nuclear factor-κB (NF-κB) activation in lymphocytes. Studies have also demonstrated that the novel PKC isoform PKCδ is involved in immune response signaling. In the macrophage, PKCδ directly binds to the adaptor protein TIRAP/Mal, which is responsible for relaying signals sensed by two Toll-like receptors (TLRs), TLR-2 and TLR-4, ultimately resulting in activation of IKK, a kinase responsible for degradation of IκB conferring NF-κB activation (Kubo-Murai et al., 2007). Other studies have demonstrated NF-κB-dependent gene expression increases due to direct phosphorylation of IκB, either by PKCδ or by a downstream effector such as protein kinase D (PKD) (Steinberg, 2004). In addition, PKCδ is also involved in regulation of NF-κB, independently of IKK/IκB, by acting on the p38 MAP kinase, and PKCδ and the MAP kinase p38 are also involved in apoptotic signaling in some tissues (Steinberg, 2004).
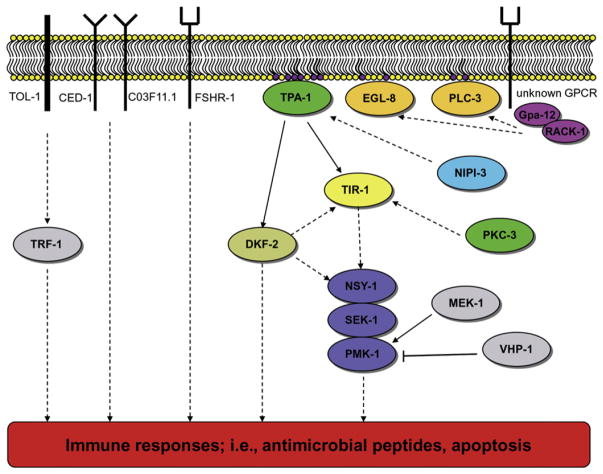
Now, two independent studies have identified the C. elegans PKCδ, TPA-1, as a key component acting before the p38 kinase cascade-mediated immune response (Figure 1) (Ren et al., 2009; Ziegler et al., 2009). Using the nematode fungal pathogen Drechmeria coniospora, Ziegler et al. (2009) identified several components contributing to the p38/PMK-1 MAP kinase-mediated immune response by monitoring the expression of the antimicrobial peptide NLP-29. Several components functioning before activation of TPA-1 were also identified including two phospholipase C proteins (EGL-8 and PLC-3) and the α and β subunits of a G protein complex. Interestingly, a second PKC (PKC-3) was also involved in nlp-29 expression in a nonredundant manner with TPA-1, and the placement of PKC-3 in the immune signaling network suggests it is also upstream of the TIP domain adaptor protein, TIR-1, as this protein is important for nlp-29 expression (Couillault et al., 2004). In an intestinal pathogen-C. elegans model, Pseudomonas aeruginosa was used to identify components acting upstream of the kinase cascade (Ren et al., 2009). The PKD DKF-2 was found to be involved in p38/PMK-1-mediated immune response to pathogens. DKF-2 is activated by phosphorylation via TPA-1. This PKD is essentially inactive when C. elegans consumes nonpathogenic bacteria, has weak activity when mildly pathogenic bacteria are consumed, and has the highest amount of catalytic activity when highly pathogenic bacteria are ingested. Thus, the intensity of intestinal DKF-2 activity not only represents the level of TPA-1 activity, but also acts as an endogenous biosensor corresponding to the degree of virulence of the pathogenic microbe. Signals transmitted by activated DKF-2 intersect with the p38/PMK-1 pathway and trigger phosphorylation and activation of PMK-1. Ren et al. (2009) find that DKF-2 also promotes immunity by a PMK-1-independent mechanism that remains to be elucidated. The immediate downstream target of DKF-2 is currently unknown, but kinase activation elicits an increase in >75 mRNAs encoding immune effectors.
Figure 1. Current Understanding of the p38/PMK-1 Kinase Cascade Innate Immunity Pathway.
An unknown GPCR responds to the presence of a pathogen activating the G protein complex, which then activates phospholipase C proteins, stimulating the synthesis of the messenger signal DAG (purple phospholipids), which then activates TPA-1 and eventually the p38/PMK MAP kinase cascade. This composite figure summarizes data regarding signaling in different tissues, and it should be noted that a number of components are absent in some tissues; e.g., DKF-2 and TOL-1 are not required in the epidermis. Solid arrows represent hypothesized direct interactions between the proteins, whereas dashed arrows indicate a suspected pathway that may contain other unknown proteins. The families of proteins are color coded as follows: G protein subunits, purple; phospholipase C, orange; protein kinase C, dark green; protein kinase D, olive green; Tribbles-like kinase, light blue; TIR domain adaptor protein, light yellow; the p38/PMK-1 MAP kinase cascade, blue; other proteins involved in innate immunity, gray. GPCR, G protein-coupled receptor.
Taken together, the PLC enzymes appear to activate TPA-1 through the messenger compound diacylglycerol (DAG), which in turn activates other signaling components in the p38 pathway. Of note is that there appears to be a divergence in this pathway, depending on the tissue involved in the infection process. In intestinal cells, DKF-2 is activated by DAG synthesis via TPA-1 (Ren et al., 2009); however, in epithelial cells, there appears to be no activation or involvement of any of the PKD proteins in C. elegans (Ziegler et al., 2009), all of which is supported by the observation that DKF-2 is expressed primarily in the intestine (Ren et al., 2009).
Ziegler et al. (2009) did not identify any corresponding G protein-coupled receptors (GPCRs), which act upon the G protein signaling complex partially composed of GPA-12 (the α subunit) and/or RACK-1 (the β subunit). However, two previously identified GPCRs, FSHR-1 and NPR-1, are known to be involved in the C. elegans immune response. FSHR-1 is an important component of innate immunity in the intestine, but the data suggests that it either functions in a parallel immune pathway or is one of at least two receptors involved in the p38-mediated immune response and must also function in an alternative immune pathway (Powell et al., 2009). The hypothesis of multiple receptors and/or G protein signaling complexes is supported by the fact that gpa-12 mutants retain a significant level of nlp-29 expression when compared to nematodes harboring a mutation in the p38 homolog, pmk-1 (Ziegler et al., 2009). As the Tribbles-like kinase NIPI-3 functions in the p38-mediated immune response by activating TPA-1 independently of the G protein GPA-12, it is a good candidate for being a component in an alternative pathway that converges at TPA-1 upstream of the MAP kinase cascade (Ziegler et al., 2009). The second GPCR, NPR-1, has been postulated to be involved in the p38-mediated immune response (Styer et al., 2008), although another study has suggested NPR-1 is involved in sensing and “avoidance” of the nematode from the pathogen (Reddy et al., 2009).
Although the C. elegans TLR, TOL-1, may be involved in the immune response in an intestinal model of Salmonella enterica infection, it is not involved in the regulation of nlp-29 expression in the epidermis (Couillault et al., 2004). Recently, homologs of CD36 and SCARF-1, two mammalian scavenger receptors, have been identified in C. elegans as CED-1 and C03F11.1, respectively. They are involved in expression of antimicrobial peptides by recognizing β-glucan in the cell wall of fungal pathogens (Means et al., 2009). Whether either of these receptors is involved in eventual activation of the p38/PMK-1 kinase cascade (or a parallel immune response pathway) remains to be determined.
In summary, understanding the immune responses of C. elegans expands our understanding of highly preserved innate immunity traits. Different C. elegans receptor(s) are involved in recognizing pathogens, and the conservation of the p38 MAP kinase cascade among evolutionarily diverse organisms demonstrates its importance in innate immunity. However, the signaling pathway ultimately leading to activation of this cascade has numerous variables. Importantly, the proteins involved in the signaling for this cascade are dependent on the C. elegans tissue type that is sensing and responding to the pathogen (Ren et al., 2009; Ziegler et al., 2009), and analysis of expression patterns of immune response genes indicates that there is a pathogen-specific profile of transcripts (Wong et al., 2007). The observed differences in immune response gene expression could be due to various receptors activating the p38/PMK-1 MAP kinase cascade in addition to parallel alternative immune pathways.
Acknowledgments
The authors wish to thank C. Rubin and J. Ewbank for comments and unpublished data. Support was provided by National Institutes of Health R01 Award AI075286 (to E.M.).
References
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, et al. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- Kubo-Murai M, Hazeki K, Sukenobu N, Yoshikawa K, Nigorikawa K, Inoue K, Yamamoto T, Matsumoto M, Seya T, Inoue N, et al. Mol Immunol. 2007;44:2257–2264. doi: 10.1016/j.molimm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Means TK, Mylonakis E, Tampakakis E, Puckett L, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, et al. J Exp Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E, Aballay A. Infect Immun. 2005;73:3833–3841. doi: 10.1128/IAI.73.7.3833-3841.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JR, Kim DH, Ausubel FM. Proc Natl Acad Sci USA. 2009;106:2782–2787. doi: 10.1073/pnas.0813048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KC, Andersen EC, Kruglyak L, Kim DH. Science. 2009;323:382–384. doi: 10.1126/science.1166527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Feng H, Fu Y, Land M, Rubin CS. Immunity. 2009;30:521–532. doi: 10.1016/j.immuni.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF. Biochem J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. Science. 2008;322:460–464. doi: 10.1126/science.1163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Bazopoulou D, Pujol N, Tavernarakis N, Ewbank J. Genome Biol. 2007;8:R194. doi: 10.1186/gb-2007-8-9-r194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K, Kurz CL, Cypowyj S, Couillault C, Pophillat M, Pujol N, Ewbank JJ. Cell Host Microbe. 2009;5:341–352. doi: 10.1016/j.chom.2009.03.006. this issue. [DOI] [PubMed] [Google Scholar]