Abstract
Objective
An adaptation to chronic total parenteral nutrition (TPN; 75% of non protein calories as glucose) is the liver becomes a major consumer of glucose with lactate release as a by-product. The liver is able to further increase liver glucose uptake when a small dose of fructose is acutely infused via the portal system. Glucagon, commonly elevated during inflammatory stress, is a potent inhibitor of glucose uptake by the liver during TPN. The aim was to determine if chronic fructose infusion could overcome the glucagon-mediated decrease in hepatic glucose uptake.
Material/methods
Studies were performed in conscious insulin-treated chronically catheterized pancreatectomized dogs that adapted to TPN for 33 h. They were then assigned to one of 4 groups: TPN (C), TPN + fructose (4.4 μmol·kg−1·min−1, F), TPN+ glucagon (0.2 pmol·kg−1·min−1, GGN), or a TPN + fructose and glucagon (F+GGN) for an additional 63h (33–96h). Insulin, fructose and glucagon were infused into the portal vein. During that period all animals received a fixed insulin infusion 0.4mU· kg−1·min−1 (33–96h) and the glucose infusion rates were adjusted to maintain euglycemia (6.6 mM).
Results
Chronic fructose infusion was unable to further enhance net hepatic glucose uptake (NHGU; μmol·kg−1·min−1) (31.1±2.8 vs. 36.1±5.0; C vs. F) nor was it able to overcome glucagon-mediated decrease in NHGU (10.0±4.4 vs. 12.2±3.9; GGN vs. F+GGN).
Conclusion
In summary, chronic fructose infusion cannot augment liver glucose uptake during TPN nor can it overcome the inhibitory effects of glucagon.
Keywords: liver, fructose, glucagon, lactate
Introduction
Individuals in stressed states such as sepsis, trauma, and burns often are unable to receive nutritional support via the enteral route; total parenteral nutrition (TPN) is an important intervention required to meet caloric needs [1–3]. Unfortunately hyperglycemia is a common complication. It is controversial if tight glucose control improved outcomes including mortality [1, 2, 4, 5].
The liver plays a central in the regulation of glucose homeostasis especially during chronic TPN, TPN augments its capacity to remove glucose and metabolize it to lactate [6, 7]. This response, though present, is attenuated in the presence of an underlying stress such as infection [8]. Hospitalized patients receiving carbohydrate rich TPN experience hyperglycemia [9]. The inability of the liver to switch from a glucose producing organ to a glucose consuming organ in response to TPN contributes to the hyperglycemia [10]. Thus approaches which can sustain or augment hepatic glucose utilization may limit stress-induced hyperglycemia in nutritionally supported patients.
Fructose is a potent stimulator of liver glucose uptake. It can augment phosphorylation, glycogen synthetic and glycolytic capacity of the liver by the activation of glucokinase (GK) and 6-phosphofructo-1-kinase. Fructose 1-phosphate generated by hepatic metabolism of fructose activates GK by inhibiting the binding of glucokinase to glucokinase regulatory protein (GKRP). The binding of GK to 6-phosphofructo-2-kinase (PFK-2)/fructose-2,6-bisphosphatase facilitates the activation of 6-phosphofructo-1-kinase [11, 12]. While high fructose diets can induce insulin resistance [13, 14], when small amounts of fructose (7.5g) are added to a 75g oral glucose load, glucose tolerance is improved [15]. Interestingly this percentage is similar to the fructose consumption (10–15% of daily carbohydrate intake) in the American diet [16]. Fructose has been used as a non glucose source for carbohydrate in nutritionally supported patients[17]. Infusions of small amounts of fructose in vivo in the fasted state enhanced net hepatic glucose uptake (NHGU) in a dose-dependent manner [18]. This increase does not persist when fructose is chronically infused possibly because of compensatory decreases in insulin secretion. Thus, chronic fructose infusion may in the absence of pancreatic adaptations augment liver glucose uptake in the TPN adapted animal.
Glucagon is a potent inhibitor of the TPN-mediated increase in NHGU[19]. It is increased during infection and contributes to the infection-induced impairment in NHGU and to the associated hyperglycemia[20]. Glucagon is an inhibitor of hepatic glycolysis and of both glucokinase and 6-phosphofructo-1-kinase. The aim was to assess whether chronic infusion of fructose can augment liver glucose uptake and reverse the glucagon-mediated decrease in NHGU and hepatic glycolysis in a setting where pancreatic compensation cannot occur. The chronically catheterized conscious dog model in which the pancreas is removed and insulin is replaced allowed us to examine the chronic interaction of fructose and glucagon in a setting of a fixed insulin and glucose environment during TPN.
Methods
Animal Preparation
Male and female non-pregnant mongrel dogs were fed standard Kal-Kan meat (Vernon, CA) and Purina Lab Canine Diet (Purina Mills, St. Louis, MO) once daily and had free access to water. Dogs were housed in a facility that met Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. All protocols were approved by the Vanderbilt University Medical Center Animal Care Committee. Prior to surgery and before the initiation of continuous insulin administration and parenteral nutritional support, animals was determined to be healthy if they had a good appetite (i.e., consumed at least 75% of the daily ration), normal stools, a hematocrit >35%, and a leukocyte count <18,000mm−3. Animals with symptoms of liver disease, infection, or have inconsistent eating habits were excluded from the study or data analysis.
Experiment Preparation
A laparotomy was performed using sterile techniques with general anesthesia. During the laparotomy, the pancreas was removed and blood sampling catheters were placed in the portal and left common hepatic veins. Infusion catheters were placed in the splenic vein for insulin and/or glucagon administration and in the inferior vena cava (IVC) for delivery of nutritional support. Flow probes (Transonic Systems, Ithaca, NY) were placed in the left common iliac vein with the tip positioned distal to the anastomosis with the IVC and in the abdominal aorta via the right external iliac artery [19, 21].
After removal of the pancreas dogs were treated with subcutaneous injections of regular (~11 U/day; Eli Lilly, Indianapolis, IN) and NPH (~18 U/day; intermediate-acting insulin) insulin daily. The doses were adjusted to maintain near normoglycemia (glucose concentration was checked twice daily). The insulin injections were stopped when TPN was initiated. A variable intraportal insulin infusion was then given to maintain normoglycemia. Pancrease ®MT10 (Ortho-McNeil Pharm. Inc., Raritan, NJ) was added to the diet to facilitate digestion after the pancreas was removed.
Nutritional Support (TPN)
Free catheter ends were exteriorized from the subcutaneous pocket under local anesthesia (2% Lidocaine; Abbott, North Chicago, IL) after allowing ≥14 days for recovery from surgery. TPN was infused into one of the IVC catheters with an ambulatory infusion pump (Dakmed, Buffalo, NY). Insulin or glucagon was infused into the catheter in the splenic vein by means of an infusion pump (Walkmed-350; Mckinley, Lakewood, CO). All dogs wore a jacket (Alice King Chatham, Los Angeles, CA) with two large pockets to hold the TPN containing bag and pumps. Once initiated TPN was the sole exogenous caloric source for 4 days. The TPN was designed to be isocaloric based on predicted resting energy expenditure. In addition to saline, potassium, phosphates, and a multivitamin supplement, the TPN contained glucose (50% Dextrose; provided 75% of nonprotein calories), 20% Intralipid® (Baxter Healthcare, Deerfield, IL; provided 25% of the non protein calories) and Travasol® to supply basal nitrogen requirements.
Experimental Design
All dogs received continuous TPN for 4 days. The bag was changed once daily. The animals were completely adapted to TPN after 48 hours and liver glucose uptake remains constant thereafter. A variable intraportal infusion of insulin was given to maintain euglycemia (~6.6 mM; 120 mg/dl) for the first 48 hours and was thereafter, held constant at 0.4mU·kg−1·min−1 for the duration of the study. Animals were then assigned to one of 4 groups (n=6–8/group): control (C), fructose infusion (4.4 μmol· kg−1·min−1;F), basal infusion of glucagon (0.2pmol·kg−1·min−1; GGN) or combined glucagon and fructose infusion (F+GGN) and were infused for an additional 48h. In a separate group of studies (n=5) fructose, xylitol (4.2–5.2 μmol·kg−1·min−1) and glucagon were infused. These latter studies were discontinued because of liver complications. Insulin, fructose, xylitol and glucagon were infused into the portal vein.
Experimental Protocol
After 48 hours of receiving the assigned infusions hepatic and non hepatic metabolism was assessed. On the morning of the study, all free ends of the catheters and flow probes(Transonic Systems, Ithaca, NY) were exteriorized under local anesthesia. For the duration of the study, the dog was placed in a Pavlov harness. A primed (10 μCi) continuous (0.1 μCi/min) infusion of [3-3H] glucose were infused into the IVC for the duration of the study. After a 120 min equilibration period blood samples were taken every 30 min between 120–240 min. Blood samples were taken from the artery, portal vein, hepatic vein, and iliac vein. Throughout the study period all nutritional support and hormone infusion were continued. At the conclusion of the study period, animals were sacrificed with a lethal dose of pentobarbital sodium. Tissue samples were then taken from each lobe of liver along with a skeletal muscle sample. All tissue samples were immediately frozen with a Wollenberger clamp precooled in liquid nitrogen and stored at −70°C.
Calculations
Net hepatic substrate uptake was calculated using the formula [(Fa × A) + (Fp × P) − H] × HBF, where A, P and H represent blood substrate concentrations in the femoral artery, portal vein and hepatic veins, respectively. Fa and Fp are the fractional contributions of the hepatic artery and the portal veins, respectively, to the total hepatic blood flow (HBF). Blood lactate, alanine, glycerol and β-hydroxybutyrate and liver glycogen content and glucokinase activity were assessed as described previously [22–24]. Hepatic glucose production was calculated as the difference between the unidirectional hepatic glucose uptake (HGU) and net hepatic glucose uptake (NHGU), where HGU is the ratio of hepatic [3H] glucose uptake and the corresponding [3H] glucose specific activity. Net non-hepatic glucose uptake was calculated as the difference between exogenous glucose infusion rate (GIR) and NHGU. Hepatic glycogen cycling was calculated as the total tracer incorporation of [3H] glucose into liver glycogen (dpm) divided by the product of the plasma glucose specific activity (dpm/mg), body weight and time.
Statistics
All data were expressed as means ± SEM. Statistical analysis was performed using mixed effects model for repeated measures. Topelitz covariance was used in the model. Pair wise comparisons were not made unless the overall model for each variable tested met the significance level of p<0.05. All data are reported as an average of 5 points during the sampling period. SPSS version 17.0 was used for all statistical analysis.
Results
General characteristics and Hormones
Body weight, liver weight, and blood flow (hepatic artery, portal vein, and iliac artery) were measured in control (C), fructose (F), glucagon (GGN), and fructose + glucagon (F+GGN) groups and are shown in Table 1. Blood flow rates were not different between groups. Arterial plasma insulin and cortisol concentrations were similar across groups and did not change significantly over time (Table 1). The glucagon concentration was increased in the glucagon-infused groups.
Table 1.
Body and liver weights, basal hemodynamics and hormone concentration in chronically catheterized conscious dogs receiving TPN (C), TPN and fructose (F), TPN and glucagon (GGN), or TPN and both F and GGN (F+GGN).
| C | F | GGN | F+GGN | |
|---|---|---|---|---|
| Body weight, kg | 21±2.4 | 21±0.8 | 21±1.3 | 21±2.4 |
| Liver weight, g | 1037±122 | 1112±112 | 794±260 | 980±213 |
| Hepatic arterial blood flow ml·kg−1·min−1 | 5±1 | 6±1 | 6±1 | 5 ±1 |
| Portal vein blood flow ml·kg−1·min−1 | 24±3 | 21±2 | 19±2 | 22±2 |
| Iliac artery blood flow ml·kg−1·min−1 | 6.4±0.5 | 5.6±0.8 | 7.2±1.2 | 6.0±1.0 |
| Arterial plasma insulin μU/ml | 9.8±2.1 | 11.1±1.8 | 9.4±1.4 | 7.1±0.7 |
| Arterial plasma cortisol μg/dl | 3.4±0.5 | 3.5±0.3 | 3.6±0.4 | 3.9±0.3 |
| Arterial plasma glucagon pg/ml | 24±5 | 21±3 | 39±3a | 33±4a |
p < 0.05 vs C. Data are expressed as mean ± SEM
Hepatic glucose metabolism
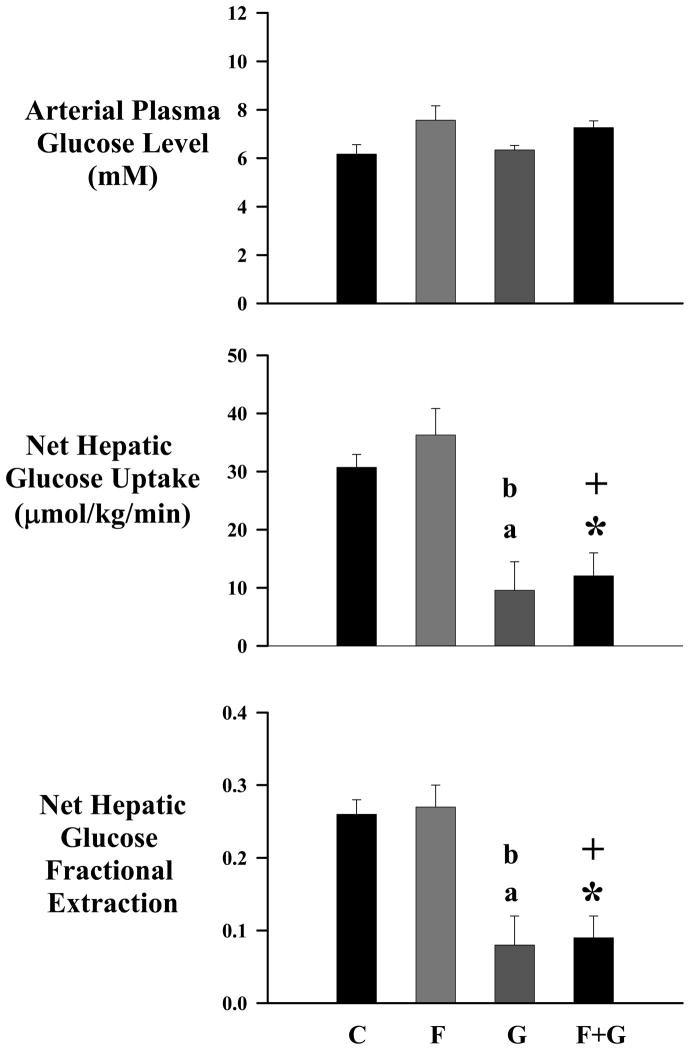
Arterial plasma glucose concentration (Fig 1) was clamped at similar concentrations in all four groups. Net hepatic glucose uptake (NHGU) was not further increased by chronic fructose infusion (C vs. F). Chronic glucagon infusion impaired NHGU and this decrease was not reversed by chronic fructose infusion (Fig 1).
Figure 1.
Arterial plasma glucose concentration, net hepatic glucose uptake and net hepatic glucose fractional extraction in chronically catheterized conscious dogs receiving TPN (C), TPN and fructose (F), TPN and glucagon (GGN), or TPN and both F and GGN (F+GGN). NHGU (F+GGN vs C * p<0.005, F+GGN vs F + p<0.005; G vs C a p<0.005, GGN vs F b p<0.005). Glucose fractional extraction (F+GGN vs C * p<0.005, F+GGN vs F + p<0.005; GGN vs C a p<0.005, GGN vs F b p<0.005) Data are expressed as mean±SEM.
Though hepatic glucose production did not statistically differ among the four groups, unidirectional hepatic uptake (HGU) was significantly different among C, and F compared to F+GGN, p<0.001 (Table 2). Fructose had no effect on HGU or NHGU in the presence of glucagon when compared to glucagon alone (Fig 1). Glucagon infusion decreased hepatic glycogen content and liver mass. Fructose enhanced glycogen deposition, but was unable to overcome the effects of glucagon when given in combination (Table 2). Hepatic glycogen cycling was increased by fructose infusion (3.8±0.5 vs. 8.3±0.5 mg·kg−1·min−1; C vs. F; p<0.05) and infusion of glucagon decreased hepatic glycogen cycling and attenuated the fructose-mediated increase in hepatic glycogen cycling (0.5±0.5 vs. 1.6 ±0.5 μmolg·kg−1·min−1; GGN vs. F+GGN).
Table 2.
Tracer determined hepatic glucose flux and hepatic glycogen content and glucokinase activity (GK) in chronically catheterized conscious dogs receiving TPN (C), TPN and fructose (F), TPN and glucagon (GGN), or TPN and both F and GGN (F+GGN).
| C | F | GGN | F+GGN | |
|---|---|---|---|---|
| Unidirectional hepatic glucose uptake, μmol·kg−1·min−1 | 32.2±2.8 | 43.9±3.9 | 17.8±3.9 | 16.1±3.3a |
| Hepatic glucose production, μmol·kg−1·min−1 | 2.2±1.7 | 7.2±3.9 | 7.8±3.3 | 4.4±1.1 |
| Glycogen, μmol/g liver | 589±44 | 722±33 | 344±67 | 539±72 |
| GK (μU/mg protein) | 11.5±1.5 | 6.4±0.9 | 9.7±1.1 | 17.6±5.3 |
C vs. F+GGN, F vs. F+GGN p<.001. Data are expressed as mean ± SEM
Metabolic substrate kinetics
Arterial lactate levels did not differ among the four groups (Table 3). Net hepatic lactate output in the control and fructose alone groups was similar. However glucagon decreased net hepatic lactate release by up to 40% in the glucagon groups compared to control and fructose alone groups. While β-OH-butyrate and glycerol kinetics were not altered by glucagon or fructose, non-esterified fatty acid levels and net hepatic uptake of non-esterified fatty acids increased in the glucagon treated animals. Glucagon infusion increased net hepatic fractional extraction of alanine and decreased arterial concentration of alanine. Interestingly chronic fructose infusion attenuated the glucagon-mediated increase in net hepatic alanine uptake and fractional extraction.
Table 3.
Arterial substrate concentrations, net hepatic substrate uptake, and net hepatic substrate fractional extraction (FE) in chronically catheterized conscious dogs receiving TPN (C), TPN and fructose (F), TPN and glucagon (GGN), or TPN and both F and GGN (F+GGN).
| C | F | GGN | F+GGN | |
|---|---|---|---|---|
| Lactate Concentration | 1199±163 | 1725±295 | 1398±411 | 1651±333 |
| Lactate Uptake | −38±2 | −37±4 | −20±7* | −16±4* |
| Alanine Concentration | 915±121 | 1040±182a | 545±101* | 675±93 |
| Alanine Uptake | 0.90±0.57 | 1.19±0.81 | 2.21±0.25* | 1.62±0.33 |
| Alanine FE | 0.03±0.02 | 0.05±0.03a | 0.20±0.07* | 0.09±0.01* |
| Glycerol Concentration | 62±4 | 52±7 | 61±11 | 61±8 |
| Glycerol Uptake | 1.02±0.10 | 1.03±0.15 | 1.01±0.24 | 1.16±0.19 |
| Glycerol FE | 0.61±0.05 | 0.67±0.02 | 0.70±0.04 | 0.70±0.03 |
| β-OH-butyrate Concentration | 25±2 | 26±2 | 26±3 | 22±5 |
| β-OH-butyrate Uptake | −0.27±0.08 | −0.20±0.1 | −0.51±0.05 | −0.40±0.10 |
| Non-esterified fatty acid Concentration | 220±34 | 202±15a | 396±35 | 294±46 |
| Non-esterified fatty acid Uptake | 0.14±0.19 | 0.37±0.31 | 1.39±0.56* | 0.98±0.31* |
| Non-esterified fatty acid FE | 0.03±0.06 | 0.06±0.05a | 0.20±0.12* | 0.16±0.02* |
Data are expressed as mean ± SEM. FE, fractional hepatic extraction;. Concentrations are in μM; uptake rates are in μmol·kg−1·min−1;
p < 0.05 vs. C;
p < 0.05 vs. GGN.
Nonhepatic glucose metabolism
Non-hepatic glucose uptake tended to increase with fructose alone, but decreased in the presence of glucagon (21.2±2.1, 24.6±3.6, 15.9±3.7 and 20.5±4.0 μmol·kg−1·min−1; C, F, GGN, F+GGN; p<0.05 C vs. GGN). This decrease was absent when fructose and glucagon were combined.
Discussion
Hyperglycemia is a major problem in stressed patients receiving TPN. The objective was to determine if the addition of small quantities of fructose known to acutely augment hepatic glucose phosphorylation capacity to the TPN infusate could ameliorate the glucagon-mediated decrease in hepatic glucose disposal. Using the chronically catheterized pancreatectomized conscious dog model that was insulin-treated we examined the chronic interaction of glucagon and fructose in modulating liver glucose uptake in the absence of compensatory changes in insulin secretion. Chronic infusion of TPN augmented NHGU and very low doses of glucagon impaired this response [6, 19]. The infusion of fructose was unable to attenuate this decrease. Thus, using a low dose of fructose to augment hepatic glucose phosphorylation capacity alone may not be an effective target to improve hepatic glucose utilization in stressed patients receiving TPN.
Chronic fructose infusion did not further enhance NHGU in TPN adapted animals even when pancreatic compensation was prevented. In the complete absence of insulin, fructose at low concentrations, translocates and activates glucokinase [12, 25]. Having complete control of the pancreatic hormones allowed us to have a clearer understanding of the observed interaction of glucagon and fructose. As expected TPN markedly increased NHGU [26]. In infected animals in which we did not prevent compensatory changes in insulin and glucagon chronic fructose infusion did not augment NHGU[27]. In the present study changes in insulin and glucagon were prevented and fructose was equally ineffective. If chronic infusion of fructose was equally effective in augmenting NHGU as an acute infusion of fructose, NHGU should have increased by ~17 μmol·kg−1·min−1[28, 29]. The failure of chronic fructose infusion is unlikely to be due to a failure of fructose to sustain the translocation of glucokinase or a compensatory fall in total glucokinase activity. As acute discontinuation of a chronic fructose infusion markedly impaired NHGU [30]. Total hepatic glucokinase activity tended to decrease and high doses fructose can induce insulin resistance[31]. However given that fructose infusion increased hepatic glycogen cycling and content, a compensatory decrease in total glucokinase is unlikely to explain the failure of chronic fructose to augment NHGU. Fructose may be unable to chronically sustain an increase in NHGU, if glucokinase is not the prime determinant of NHGU in adapted settings. In contrast to acute regulation of glucose disposal where glycogen synthesis is a major metabolic fate, in chronic nutritional support hepatic lactate release and glucose oxidation are the main metabolic fates. Thus, for chronic fructose to sustain an increase in NHGU hepatic glycolytic capacity would have to increase in parallel with any increase in glucose phosphorylation capacity [29, 32]. While fructose via it activation of 6-phosphofructose-1-kinase can augment glycolysis [11], it is possible that glycolysis was already maximally activated by the TPN.
Chronic fructose infusion was also unable to reverse the inhibition of NHGU by chronic glucagon infusion. As we had seen previously chronic infusion of glucagon decreased liver glucose uptake and lactate release during TPN [19]. The failure of fructose to augment NHGU in the absence of glucagon infusion cannot be explained by the possibility that NHGU was already maximally activated as NHGU can increase further[20]. The glucagon-mediated decrease in NHGU was not accompanied by a decrease in glucokinase. Interestingly fructose infusion was able to augment total glucokinase activity in the liver and was able to modestly increase glycogen cycling even in the presence of glucagon. Thus stress hormones like glucagon probably exert their greatest effect on liver glucose uptake at a site distal to glucokinase and 6-phosphofructo-1-kinase. We tried to activate pyruvate kinase by combining fructose and xylitol infusion in a group of (n=5) studies[33]. However liver dysfunction developed as evidenced by an increase in plasma bilirubin. The protocol was discontinued. A recent report indicates a similar complication with xylitol in dogs[34].
Chronic fructose infusion impaired glucagon-stimulated net hepatic alanine uptake. Glucagon is a potent stimulator of net hepatic alanine fractional extraction [35, 36]. Interestingly in the absence of glucagon infusion fructose increased net hepatic alanine fractional extraction but attenuated the glucagon-mediated increase. The mechanism for the decrease in net hepatic alanine fractional extraction is unclear.
Fructose did not impair glucagon-stimulated liver NEFA uptake. NEFA is the major fuel oxidized by the liver and can modulate the suppression of glucose production by insulin. Our recent work indicates NEFAs interact with glucagon to impair NHGU during TPN[21]. In the present study, NEFA uptake and fractional extraction by the liver were increased, despite only modest changes in circulating NEFA concentrations.
In summary, while net hepatic glucose uptake is enhanced when fructose is acutely infused, chronic fructose infusion is unable to augment NHGU even if pancreatic compensation is prevented. Moreover fructose is equally ineffective when liver glucose uptake is impaired by the chronic administration of glucagon. Thus, the beneficial effects of fructose (or its target glucokinase) on hepatic glucose uptake are short lived and would not likely be of therapeutic use in the long-term treatment of stress induced hyperglycemia encountered in the nutritionally supported setting.
Acknowledgments
We gratefully acknowledge the technical support provided by Amy Nunnally, Jaime Adcock, Eric Nass, Doss Neal, Ben Farmer, the Diabetes Research and Training Center Hormone and Analytical Services Core and Metabolic Physiology Shared Resource Core in these studies.
Funding: These studies were funded from the following sources NIH DK43748 (PI: Owen McGuinness), NIH DK20593 (Diabetes and Research and Training Center) and American Diabetes Association (PI: Owen McGuinness)
Abbreviations
- TPN
total parenteral nutrition
- GK
glucokinase
- GKRP
glucokinase regulatory protein
- NHGU
net hepatic glucose uptake
- IVC
inferior vena cava
- NEFA
non-esterified fatty acids
- HBF
hepatic blood flow
- GIR
glucose infusion rate
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose
Author contributions: Drs Johnson, Chen and McGuinness helped in the study design, study execution, data collection, analysis and interpretation and manuscript writing. Phil Williams, Tammy Santomango and D. Brooks Lacy helped conduct the study and collect data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Berghe G, Wilmer A, Hermans G, et al. Intensive Insulin Therapy in the Medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 2.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 3.Robinson LE, van Soeren MH. Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues. 2004;15:45–62. doi: 10.1097/00044067-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 4.The NICE-SUGAR Study Investigators. Intensive versus Conventional Glucose Control in Critically Ill Patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanyam A. Intensive Glycemic Control in the Intensive Care Unit: Promises and Pitfalls. J Clin Endocrinol Metab. 2009;94:416–417. doi: 10.1210/jc.2008-2620. [DOI] [PubMed] [Google Scholar]
- 6.Chen S-S, Torres-Sanchez CJ, Hosein N, et al. Time course of the hepatic adaptation to TPN: interaction with glycogen depletion. Am J Physiol. 2005;288:E163–170. doi: 10.1152/ajpendo.00192.2004. [DOI] [PubMed] [Google Scholar]
- 7.McGuinness OP. Defective glucose homeostasis during infection. Annu Rev Nutr. 2005;25:9–35. doi: 10.1146/annurev.nutr.24.012003.132159. [DOI] [PubMed] [Google Scholar]
- 8.McGuinness OP, Donmoyer CM, Ejiofor J, et al. Hepatic and Muscle Glucose Metabolism During Total Parenteral Nutrition: Impact Of Infection. Am JPhysiol. 1998;275:E763–E769. doi: 10.1152/ajpendo.1998.275.5.E763. [DOI] [PubMed] [Google Scholar]
- 9.Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:665–79. doi: 10.1016/j.beem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Basu R, Basu A, Johnson CM, et al. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes. 2004;53:2042–50. doi: 10.2337/diabetes.53.8.2042. [DOI] [PubMed] [Google Scholar]
- 11.Smith WE, Langer S, Wu C, et al. Molecular Coordination of Hepatic Glucose Metabolism by the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase: Glucokinase Complex. Mol Endocrinol. 2007;21:1478–1487. doi: 10.1210/me.2006-0356. [DOI] [PubMed] [Google Scholar]
- 12.Van Schaftingen E, Detheux M, Da Cunha MV. Short-term control of glucokinase activity: a role of a regulatory protein. FASEB J. 1994;8:414–419. doi: 10.1096/fasebj.8.6.8168691. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y, Wang D, Topczewski F, et al. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem. 2007;18:1–9. doi: 10.1016/j.jnutbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Bizeau ME, Pagliassotti MJ. Hepatic adaptations to sucrose and fructose. Metabolism. 2005;54:1189–201. doi: 10.1016/j.metabol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Moore MC, Cherrington AD, Mann SL, et al. Acute Fructose Administration Decreases the Glycemic Response to an Oral Glucose Tolerance Test in Normal Adults. J Clin Endocrinol Metab. 2000;85:4515–4519. doi: 10.1210/jcem.85.12.7053. [DOI] [PubMed] [Google Scholar]
- 16.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 17.Valero MA, Leon-Sanz M, Escobar I, et al. Evaluation of nonglucose carbohydrates in parenteral nutrition for diabetic patients. Eur J Clin Nutr. 2001;55:1111–1116. doi: 10.1038/sj.ejcn.1601274. [DOI] [PubMed] [Google Scholar]
- 18.McGuinness OP, Cherrington AD. Effects of fructose on hepatic glucose metabolism. Curr Opin Clin Nutr Metab Care. 2003;6:441–448. doi: 10.1097/01.mco.0000078990.96795.cd. [DOI] [PubMed] [Google Scholar]
- 19.Chen S-S, Zhang Y, Santomango TS, et al. Glucagon chronically impairs hepatic and muscle glucose disposal. Am J Physiol. 2007;292:E928–935. doi: 10.1152/ajpendo.00063.2006. [DOI] [PubMed] [Google Scholar]
- 20.Chen SS, Donmoyer CM, Pearson DA, et al. Impact of infection on glucose-dependent liver glucose uptake during TPN: interaction with insulin. Am J Physiol. 2004;286:E286–95. doi: 10.1152/ajpendo.00286.2003. [DOI] [PubMed] [Google Scholar]
- 21.Chen SS, Santomango TS, Williams PE, et al. Glucagon-mediated impairments in hepatic and peripheral tissue nutrient disposal are not aggravated by increased lipid availability. Am J Physiol. 2009;296:E1172–8. doi: 10.1152/ajpendo.90821.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd B, Burrin J, Smythe P, et al. Enzymatic fluorometric continuous flow assays for blood glucose, lactate, pyruvate, alanine, glycerol and 3-hydroxybutyrate. Clin Chem. 1978;24:1724–1729. [PubMed] [Google Scholar]
- 23.Barzilai N, Rossetti L. Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem. 1993;268:25019–25025. [PubMed] [Google Scholar]
- 24.Chan TM, Exton JH. A method for the determination of glycogen content and radioactivity in small quantities of tissues or isolated hepatocytes. Anal Biochem. 1976;71:96–105. doi: 10.1016/0003-2697(76)90014-2. [DOI] [PubMed] [Google Scholar]
- 25.Agius L. Control of glucokinase translocation in rat hepatocytes by sorbitol and the cytosolic redox state. Biochem J. 1994;298:237–243. doi: 10.1042/bj2980237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen SS, Donmoyer C, Zhang Y, et al. Impact of enteral and parenteral nutrition on hepatic and muscle glucose metabolism. JPEN. 2000;24:255–260. doi: 10.1177/0148607100024005255. [DOI] [PubMed] [Google Scholar]
- 27.Donmoyer CM, Lacy DB, Zhang Y, et al. Impact of chronic fructose infusion on hepatic metabolism during TPN administration. Am J Physiol. 2002;283:E1151–E1158. doi: 10.1152/ajpendo.00223.2001. [DOI] [PubMed] [Google Scholar]
- 28.Donmoyer CM, Ejiofor J, Lacy DB, et al. Fructose augments infection-impaired net hepatic glucose uptake during TPN administration. Am J Physiol. 2001;280:E703–E711. doi: 10.1152/ajpendo.2001.280.5.E703. [DOI] [PubMed] [Google Scholar]
- 29.Shiota M, Galassetti P, Monahan M, et al. Small amounts of fructose markedly augment net hepatic glucose uptake in the conscious dog. Diabetes. 1998;47:867–873. doi: 10.2337/diabetes.47.6.867. [DOI] [PubMed] [Google Scholar]
- 30.Dunaway GA, Jr, Weber G. Rat liver phosphofructokinase isozymes. Arch Biochem Biophys. 1974;162:620–628. doi: 10.1016/0003-9861(74)90224-0. [DOI] [PubMed] [Google Scholar]
- 31.Dirlewanger M, Schneiter P, Jequier E, et al. Effects of fructose on hepatic glucose metabolism in humans. Am J Physiol. 2000;279:E907–E911. doi: 10.1152/ajpendo.2000.279.4.E907. [DOI] [PubMed] [Google Scholar]
- 32.Petersen KF, Laurent D, Yu C, et al. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50:1263–1268. doi: 10.2337/diabetes.50.6.1263. [DOI] [PubMed] [Google Scholar]
- 33.Doiron B, Cuif M-H, Chen R, et al. Transcriptional Glucose Signaling through The Glucose Response Element Is Mediated by the Pentose Phosphate Pathway. J Biol Chem. 1996;271:5321–5324. doi: 10.1074/jbc.271.10.5321. [DOI] [PubMed] [Google Scholar]
- 34.Campbell A, Bates N. Xylitol toxicity in dogs. Vet Rec. 2008;162:254. doi: 10.1136/vr.162.8.254-c. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson RW, Steiner KE, Davis MA, et al. Similar dose responsiveness of hepatic glycogenolysis and gluconeogenesis to glucagon in vivo. Diabetes. 1987;36:382–389. doi: 10.2337/diab.36.3.382. [DOI] [PubMed] [Google Scholar]
- 36.Kilberg MS, Stevens BR, Novak DA. Recent advances in mammalian amino acid transport. Annu Rev Nutr. 1993;13:137–165. doi: 10.1146/annurev.nu.13.070193.001033. [DOI] [PubMed] [Google Scholar]