Abstract
The G-protein-coupled receptor molecules and downstream effectors that are used by taste buds to detect sweet, bitter, and savory tastes are also utilized by chemoresponsive cells of the airways to detect irritants. Here, we describe the different cell types in the airways that utilize taste-receptor signaling to trigger protective epithelial and neural responses to potentially dangerous toxins and bacterial infection.
The respiratory tract and the digestive tract face a similar dilemma: they need to allow into the body essential substances, while at the same time guarding against the intake of toxins or infectious agents. To accomplish this end, both organ systems employ a similar strategy of maintaining chemosensors at the intake points for each system, with the possibility of evoking protective reflexes upon detection of a potential toxin (23, 63). In the case of the digestive tract, the protective reflex triggered by oral chemoreceptors is gagging, choking, or even vomiting in response to aversive tastes, whereas in the respiratory system the reflex can be sneezing, coughing, or apnea. In both systems, activation of the chemodetectors also can evoke changes in local epithelial characteristics or local autonomic reflexes, e.g., salivation, secretion, or changes in ciliary function or motility.
For the digestive tract, the chemosensors monitoring intake include taste buds, which are specialized endorgans of 50–100 cells designed to distinguish appetitive from potentially toxic compounds. Each cell of a taste bud (taste cell) is molecularly differentiated to respond to one of the five main taste qualities: salty, sour, bitter, sweet, and umami (the savory taste of glutamate and other amino acids). Transduction of the former two qualities, salty and sour, relies on ion channels or conductances, whereas transduction of the latter three qualities depends on G-protein-coupled receptors (GPCRs) and their attendant downstream signaling pathways (12). The sensation of bitter, which evokes an innate aversive reaction to many noxious substances, depends on members of the Tas2R family, of which ∼25–35 generate functional T2R receptors in placental mammals (15, 17). Although these taste-related GPCRs were first identified by their robust expression in taste epithelia, subsequent analysis reveals the presence of these putative taste receptors in diverse organ systems of the body including especially the hollow organs of the digestive and respiratory systems (2, 6, 19, 30, 38, 40, 48, 61, 72, 75).
In the digestive system, the taste receptors can signal the presence of either appetitive (carbohydrates or amino acids detected by T1R heterodimers) or aversive (toxins) substances, detected by T2R family receptors. Activation of the T1R-expressing cells can evoke appropriate digestive reflexes such as release of GLP or other digestive enzymes (6). Conversely, activation of T2R-expressing cells can provoke reflex flushing of the lower gut (35). In the airways, T2R expression predominates (75), and the reflexes initiated by chemical stimulation appear to be mostly protective (19, 78).
In this review, we focus on the utilization of the taste receptor cascade by elements of the respiratory system in the generation of protective, so-called “chemofensor” (or chemesthetic) reflexes (23). In the respiratory system, these reflexes include changes in respiration (e.g., apnea), changes in epithelial function, and perhaps even alterations in airway patency. Thus activation of the chemofensor system of the airways activates intraepithelial signaling as well as engages central pattern generator networks for respiratory control. For a review of how taste signaling is utilized in the gut, the reader should refer to other recent papers (6, 20).
Canonical Taste Signaling Cascade
For the taste qualities of sweet, umami, and bitter, taste receptor cells employ a panel of G-protein-coupled taste receptors (GPCRs) coupled to a common downstream signaling cascade involving PLCβ2, IP3R3, and TrpM5 (see FIGURE 1) (13, 38, 53, 87). Specificity of the system is due to differential expression of particular GPCRs. The sensations of sweet and umami, which signal nutrients, rely on heterodimers of the Tas1R family, which form, respectively, T1R2/T1R3 (sweet) and T1R1/T1R3 (umami) heterodimers. Detection of bitter is mediated by a family of T2R receptors, with each taste cell expressing several of the family members (9, 10, 51). Heterologous expression studies show that many members of the T2R family respond to a limited molecular range of compounds, although some T2R receptors are more broadly responsive (51). Since each bitter-responsive taste cell expresses multiple T2R members, the responses of taste cells can be quite broad, albeit not universal for all bitter-tasting compounds (9).
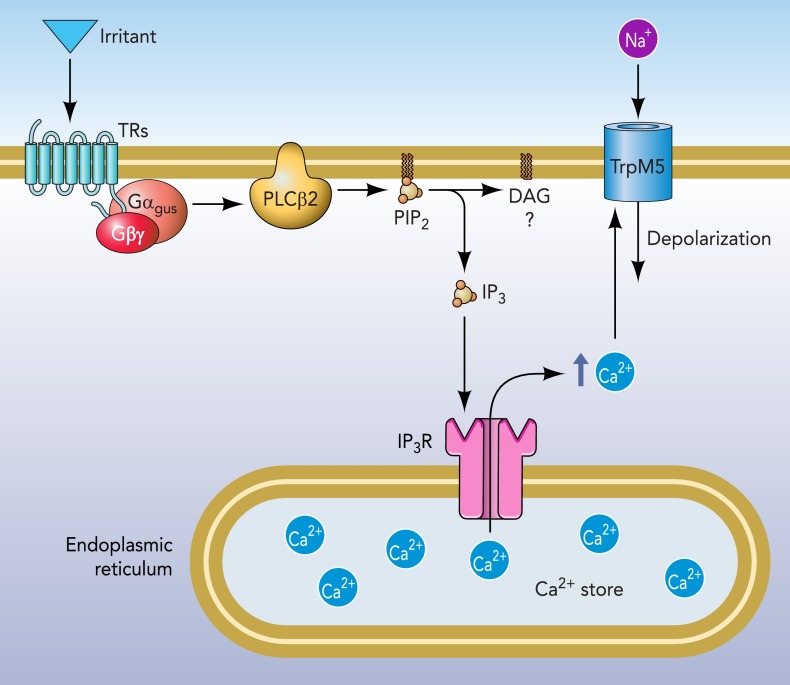
FIGURE 1.
Crucial elements of the taste transduction cascade for umami, sweet, and bitter qualities
Taste receptor (TR) molecules (either T1R or T2R family members) are coupled to G proteins such as α-gustducin. Activation of the receptor by a tastant results in dissociation of the G protein βγ-subunits, which activates PLCβ2 to liberate IP3 from the membrane. The IP3 then acts on IP3R3 on the endoplasmic reticulum to release Ca2+ from stores. The increase in intracellular Ca2+ activates the TrpM5 ion channel to permit influx of Na+, which depolarizes the cell. The combination of depolarization and increased intracellular Ca2+ effects release of neurotransmitter. In taste buds, the crucial transmitter is ATP released in a nonvesicular fashion through gated hemichannels (32, 60). In epithelial chemoresponsive cells including SCCs, transmitter release is likely to involve acetylcholine released by a vesicular mechanism.
Nonetheless, expression of the canonical downstream taste signaling cascade elements of PLCβ2, IP3R3, and TrpM5 is not limited to taste buds. Diverse cell populations in both the digestive organs and in the respiratory tract express these elements associated with chemotransduction (3, 4, 13, 27, 36, 38, 55, 75).
The first component of the taste transduction cascade to be identified outside of the taste epithelium was the taste-associated G-protein α-gustducin (31). In taste buds, expression of α-gustducin is strongly associated with expression of the T2R receptors and less so with the T1R family of receptors (8, 37, 52, 69, 73, 76, 80, 83). Similarly, outside of the taste epithelium, α-gustducin expression is strongly associated with expression of T2R family receptors (25, 34, 61, 75, 78) but is also associated with T1R expression in various chemoreceptors of the digestive tract (6, 33).
Canonical Taste Signaling in the Airways
The presence of α-gustducin immunoreactivity in the airways was first reported in a subset of bipolar cells in the vomeronasal neuroepithelium and in scattered cells of the nasal respiratory epithelium (85). Subsequently, Finger et al. (19) showed that similar α-gustducin-expressing epithelial cells, called therein solitary chemoreceptor cells (SCCs), were heavily innervated by peptidergic fibers of the trigeminal nerve, presumably polymodal nociceptors. Similarly, α-gustducin-immunoreactive epithelial cells, called solitary chemosensory cells (SCCs), were also described in laryngeal and tracheal respiratory epithelium of mouse, rat, and cow (50, 64, 79).
Although α-gustducin is a useful marker for many chemoresponsive epithelial cells, this G-protein is not present in all cells that express other elements of the canonical taste signaling cascade. Many more cells express TrpM5 than α-gustducin, although a substantial overlap exists between these populations (42–44, 63, 75). The function of the TrpM5-expressing epithelial cells that do not also express other elements of the T2R-signaling cascade remains unresolved.
The clearest example of a cell type that expresses TrpM5 but lacks many other elements of the taste transduction cascade is the olfactory receptor neuron (27, 36, 43). Olfactory receptor neurons use a cAMP transduction cascade that is entirely different than taste cells (66), yet a subset of olfactory receptor neurons express TrpM5 (43). How TrpM5 relates to the canonical olfactory transduction cascade is problematic, but its expression in olfactory receptor neurons is labile, being dependent on basal activity levels and other factors (57).
In addition to olfactory receptor neurons, at least three distinct cell types within the airways express TrpM5 and other elements of the taste transduction cascade. These are 1) microvillous cells of the olfactory epithelium, 2) solitary chemosensory cells of the nasal respiratory epithelium, and 3) brush cells in the trachea. In addition, ciliated epithelial cells and smooth muscle cells of the airways express T2R (bitter) receptors in humans (16, 68). In the next section, we discuss the possible functional role of taste-related signaling in these various tissues. We (75) as well as other investigators (63) have applied a common name to these different cell types, i.e., calling them all “solitary chemosensory cells.” We now prefer reserving this term for cells of the nasal respiratory epithelium, which are heavily innervated and which, therefore, evoke a sensation (likely pain or irritation since they are innervated by polymodal nociceptors). An alternative collective for these chemoreponsive cells of the airways could be “diffuse chemosensory system” (63) or “chemofensor” complex (23), but each of these terms is not yet sharply defined. Features shared by these chemoresponsive airway cells include expression of TrpM5 and other elements of the canonical taste transduction cascade, a cholinergic phenotype (40, 56), and responsiveness to an array of noxious substances (19, 25, 42, 44, 56, 78). How they differ is in terms of overall morphology and relationship to sensory nerves, as shown in FIGURE 2 (19, 40, 42).
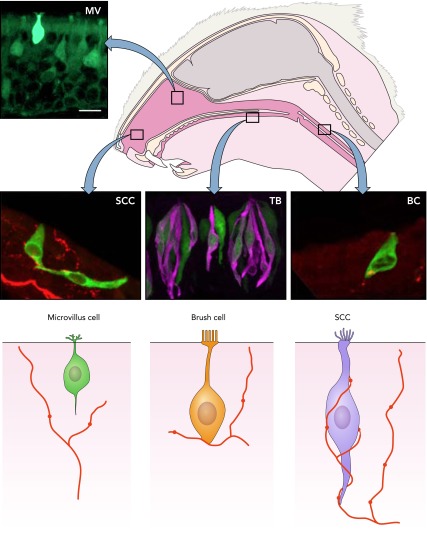
FIGURE 2.
Chemoresponsive cells that express elements of the taste transduction cascade are present throughout the respiratory system as well as in taste buds (TB)
Despite similarities in molecular characteristics, these chemoresponsive cells are not identical. We distinguish at least three types of chemoresponsive cells within the respiratory system: 1) solitary chemosensory cells (SCCs) distributed within the anterior nasal epithelium, 2) microvillous cells (MV) within the olfactory epithelium, and 3) brush cells (BC) in the trachea. Each of these cell types has a distinctive morphology and different relationships to the afferent nerve fibers in the vicinity. Bottom: semischematic diagrams of the three TrpM5+ cell types, showing differences in morphology and relationship to local nerve fibers.
Microvillous Cells of the Olfactory Epithelium
The major cell types comprising the olfactory epithelium are olfactory receptor neurons (ORNs) and sustentacular (supporting) cells. The sustentacular cells are relatively small cells with somata situated at the top of the epithelium. The cell bodies of olfactory receptor neurons lie deeper in the epithelium but extend an apical process, which reaches above the sustentacular cells, where it swells into an olfactory knob extending numerous long immotile cilia. A less common cell type of the olfactory epithelium is the microvillous cell. These cells have somewhat diverse appearances with cell bodies situated within or just beneath the layer of sustentacular cells but still above the majority of the olfactory receptor neurons (see FIGURE 2). Microvillous cells extend an apical process to the surface of the epithelium, and the detailed morphology of this apical process varies slightly according to cell type (27). In all cases, the apical process has microvilli and expresses espin, the cytoskeletal protein that characterizes many sensory cells (56, 67). These microvillous cells of the main olfactory epithelium are not heavily innervated, although occasional nerve fibers may make en passant contact with the basal portion of these cells (27, 42). Although the microvillous cells of the main olfactory epithelium have been reported to lack expression of α-gustducin, PLCβ2, and some other elements of the taste transduction cascade, we find evidence for these under appropriate fixation conditions (28).
The microvillous cells of the main olfactory epithelium respond to numerous irritants as well as to most classical odorants when present at sufficiently high concentrations (25, 44). Upon activation, the microvillous cells release acetylcholine, which acts on muscarinic receptors expressed by elements of the surrounding epithelium (56). The acetylcholine released into the epithelium will also likely activate nicotinic receptors on the nearby nerve fibers, although no specific synaptic structures are reported. The net effect of the acetylcholine released into the surrounding epithelium is unknown, although it does cause significant increases in cytosolic Ca2+ in nearby sustentacular cells via release from intracellular stores (56). How this may affect the overall functionality of the epithelium is unclear, although sustentacular cells have been implicated in regulation of the perireceptor milieu for the adjacent olfactory receptor neurons (22, 74). A subset of olfactory receptor neurons themselves are modulated by the released acetylcholine, which seems to decrease the overall responsiveness of the ORNs to the cAMP-mediated signaling cascade utilized for olfactory transduction. Taken together, these results suggest that the microvillous cells of the main olfactory epithelium may serve as governors to decrease the responsiveness of nearby ORNs at high levels of odorants.
Solitary Chemosensory Cells
Solitary chemosensory cells (SCCs) were first described in various fishes by Mary Whitear in the 1970s (81). The SCCs are columnar epithelial cells scattered within the epidermis of all anamniote aquatic vertebrates. SCCs are topped by a tuft of wavy microvilli and form prominent synapses with nerve fibers at their base. Since SCCs occur on the body surface as well as in the nasal cavity and respiratory passageways (5, 29), they are innervated by a variety of nerves including spinal, trigeminal, and perhaps vagus nerves. Based purely on morphological critera, Dr. Whitear concluded that SCCs are a chemosensory cell. We suggest that these characteristics be used as defining features for SCCs in all vertebrates: an isolated elongate cell in the epithelium, with a wavy microvillous apex, and heavily innervated by local nerve fibers.
Using these criteria, an apparently identical cell type can be identified within the nasal passages of amniotes including reptiles (26) and mammals (19, 65, 79). The SCC in mammalian nasal cavities has all the characteristics of the SCC as defined for fishes but differs from other chemoresponsive cells (microvillous cells and brush cells) in several ways (see FIGURE 2). First, SCCs have what appear to be flexible microvilli, whereas brush cells are defined by a characteristic tuft of stiff apical microvilli, which give the cell its name (40, 45). Second, brush cells, like microvillous cells, are sparsely innervated (40) often by en passant contacts, whereas nasal SCCs are intimately entwined with nerve processes with which they repeatedly synapse (19).
The SCCs of the nasal cavity in mammals express all of the elements of the canonical taste transduction cascade from receptors to TrpM5 (19, 36, 50, 75, 78, 79). Furthermore, these cells show a cholinergic phenotype similar to other chemoreceptor cells of the respiratory passageways (40, 56).
Functional studies show that the nasal SCCs respond to a variety of compounds including classical bitter substances (e.g., denatonium) (25, 78), strong odorants (44), and bacterial signaling molecules (acyl-homoserine lactones) (78). Furthermore, activation of these cells results in downstream activation of trigeminal afferents, presumably the peptidergic polymodal nociceptors that heavily innervate the nasal mucosa (19, 24). Activation of these nasal trigeminal nociceptors evokes apnea via a central brain stem reflex (19, 58, 78, 82), and for certain classes of compounds, the apneic reflex depends on the integrity of SCC signaling (19, 78).
These same trigeminal nociceptors not only send impulses centripetally but also collateralize extensively within and beneath the nasal mucosa. The trigeminal polymodal nociceptors themselves express the bioactive peptides substance P and CGRP, as well as chemosensitive Trp channels including TrpV1, TrpM8, and TrpA1 (21). Thus activation of these fibers, whether directly via chemical activation of Trp channels or indirectly via SCCs, results in release of peptide mediators into the surrounding epithelium and onto nearby blood vessels, causing neurogenic inflammation (46, 47).
We have been able to dissociate the two modes of activation of the trigeminal nociceptors by comparing wild-type mice with those with genetic deletion of either α-gustducin or the downstream trasnduction channel TrpM5 present in the SCCs but not in the nerve fibers (78) (see FIGURE 3). The irritation produced by T2R ligands such as denatonium or acyl-homoserine lactones depends on the integrity of the T2R signaling system in the SCCs, whereas activation by capsaicin (which directly activates the TrpV1 channel on the nerve fibers) does not (see FIGURE 4). But activation of the peptidergic nerve fibers by either mechanism results in similar downstream release of peptides, which evoke plasma leakage from nearby blood vessels (77) (FIGURE 4).
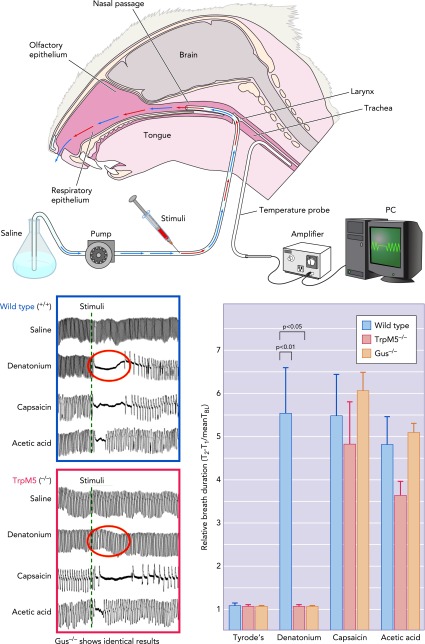
FIGURE 3.
Diagram of respiratory assay (modified Alarie test)
Following a tracheotomy, the anesthetized mouse was permitted to breathe freely through the lower end of the trachea; respiration was monitored by thermocouple. A constant flow of saline was injected into the upper end of the trachea and allowed to pass freely through the nasal passages exiting the nostril. Alterations in respiration reflected detection of irritants injected into the constant retronasal flow, as shown in the respiratory records shown in the graphs at bottom left. In TrpM5 (or α-gustducin)-KO mice, the irritant effect of the bitter-tasting substance denatonium was absent, although other irritants, which directly activate nerve fibers, remain effective at altering the respiratory rate (78). The bar graph at bottom right shows that depression of respiration by denatonium requires functionality of the taste signaling cascade, whereas capsaicin and acetic acid, which directly activate the nerve fibers, are not dependent on taste cascade signaling.
FIGURE 4.
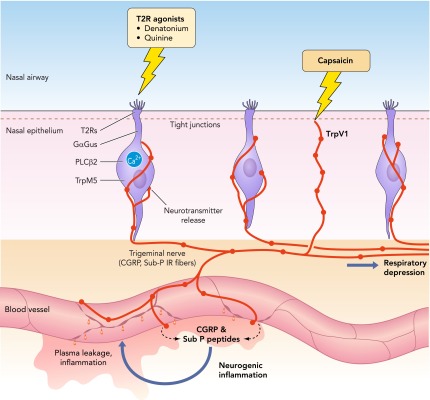
Inhaled irritants can activate either the nerve fibers directly or the SCCs, which synapse onto the nerve fibers
These nerve fibers are polymodal nociceptors that release the peptides substance P and CGRP onto peripheral blood vessels. The vessels respond by dilation and opening of endothelial junctional complexes to produce a local neurogenic inflammation. We assay for this inflammation by measuring plasma leakage from the vessels into the surrounding nasal epithelium.
Tracheal Brush Cells
Brush cells, characterized by a tuft of long, stiff apical microvilli, were first identified as a unique cell type in 1956 (59). The first suggestion that brush cells in the trachea might be chemoreceptive came from Luciano (45), a hypothesis finally confirmed in 2011 (40). Although many brush cells do make contact with nerve fibers (40, 45), others do not. In contrast, the nasal SCCs are intimately entwined by nerve fibers (FIGURE 2). Thus two features distinguish nasal SCCs from tracheal brush cells: apical ultrastructure and relationship to nerve fibers. In other respects, the cells are quite similar, displaying the canonical taste transduction cascade, including T2R receptors, a cholinergic phenotype, and activation by bitter-tasting ligands (40, 44, 75, 78, 79). Tracheal brush cells also are distinct from neuroendocrine cells, which, like brush cells, are specialized epithelial cells scattered within the tracheal epithelium (40, 50, 71).
Tracheal brush cells express multiple T2R (bitter taste) receptors and respond to corresponding bitter-tasting ligands, denatonium, and cycloheximide (40, 75). Like nasal SCCs, tracheal brush cells initiate a respiratory reflex response (40) in keeping with the contacts between brush cells and vagal afferent fibers. A likely mechanism underlying this is that the brush cells release acetylcholine into the surrounding tissue and onto nearby nerve fibers, which have nicotinic cholinergic receptors. The acetylcholine thereby depolarizes the nerve fiber, which not only conveys this information to the CNS for respiratory control but also would likely release peptides and other inflammatory mediators into the surrounding tissues (40), resulting in neurogenic inflammation. Thus, in the trachea as in the nasal cavity, the chemosensory cells may evoke both an integrative reflex via the brain stem and a local tissue response to activation.
Taste Receptors in the Absence of Canonical Taste Transduction
Two independent groups have reported the presence of TR-family receptors in the airways but in the absence of the canonical downstream signaling cascade associated with taste. The two locations where taste receptors appear to act in a cell-autonomous fashion without the full panoply of canonical downstream elements are in ciliated epithelial cells of the lung and in bronchiolar smooth muscle.
The ciliated epithelial cells of human lower airways express numerous T2Rs, α-gustducin, and PLCβ2 but lack other downstream signal components of the canonical taste transduction cascade (68). Application of bitter-tasting substances to cultures of ciliated airway epithelia increases the intracellular Ca2+ levels in the cells and thereby increases the ciliary beat frequency of the cell (68). The ciliated epithelial cells are not innervated, and no sensory function is ascribed to the transduction of bitter-tasting ligands by the motile cilia in this system.
A second noncanonical signaling system associated with expression of the T2R receptors is in airway smooth muscle (16). In that system, activation of the T2Rs triggers a PLC-mediated increase in intracellular Ca2+, which acts on local big potassium (BKCa) channels to hyperpolarize the muscle cell, resulting in muscle relaxation. Additional mechanisms may be in play, however (54, 86), since blockade of the BKCa channels does not completely abolish the relaxation induced by bitter-tasting ligands (1).
A cell-autonomous action for taste receptor signaling is not confined to the airways; diverse cells in the gastrointestinal system express taste receptors and related downstream effectors (31, 33, 34, 39, 41, 48, 62). For example, taste receptor signaling in pancreatic β-cells of the pancreas triggers insulin release in response to sweeteners including fructose (14, 41). But this response is independent of neural activation, and hence the β-cell should not be considered chemosensory but rather chemoresponsive.
Diversity of Taste Receptors
In contrast to the chemoresponsive cells of the gastrointestinal system, which may express either T1R or T2R receptors, the taste-receptor-expressing cells of the airways largely express members of the T2R family of receptors. In the taste system, T2R receptors respond to bitter-tasting ligands with various degrees of specificity (7, 51). In rats, we have probed for eight different T2R family members, and all eight were detected by PCR in tissue from trachea, only seven in bronchi, and only two in the lungs (75). The full expression profile of taste-related GPCRs in airway tissues remains to be determined. The expression of different T2R family members differs along the length of the digestive tract (84), and a similar situation may obtain in the airways as well.
In the taste system, the T2R receptors respond to diverse bitter-tasting compounds (51), often hallmarks of toxic plant compounds. Similarly, in the airways, the T2R-expressing cells respond to many bitter-tasting substances and may trigger protective reflexes in response to inhalation of toxic dusts or aerosols. In addition, the SCCs of the nasal cavity (and perhaps other T2R-expressing chemoresponsive cells of the airways) respond to bacterially produced metabolites (56) and signaling molecules including the acyl-homoserine lactones used as quorum-sensing molecules by Pseudomonas aeruginosa bacteria (78). Detection of such microbial-produced molecules may provide the system with a means for responding to proliferating or invasive microbial populations before they cause tissue damage. For example, Pseudomonas aeruginosa quietly inhabits many of the airway mucosal surfaces at low population levels (18) but may become pathogenic at higher population densities when the bacteria transform to form a biofilm and aggressively attack the underlying epithelium. The bacteria themselves monitor their population through the agency of acyl-homoserine lactone (AHL) quorum-sensing molecules (70). When the concentration of the AHLs gets sufficiently high [roughly 100 μM (11)], the bacteria alter their behavior from commensal to pathogenic (70). When concentrations of AHLs reach ∼50 μM, nasal SCCs begin to respond (78) and will induce neurogenic inflammatory changes in the epithelium, thereby recruiting elements of the immune system to the affected area. Thus SCCs generate not only a neural signal in response to irritants but also induce an adaptive local inflammatory response (FIGURE 4), which will tend to ameliorate the effects of a growing bacterial population.
More enigmatic is the expression of T1R family receptors in the respiratory epithelium. The T1R family of receptors in taste buds form heterodimers, which respond to sweet (T1R2 + T1R3) or umami (savory) (T1R1 + T1R3). In the taste system, these are both appetitive qualities that will tend to induce more consumption rather than avoidance of the ligand-bearing material. So what the function of T1R receptors in the nasal cavity may be is a mystery. Since the T1R-expressing SCC and brush cells appear identical to those expressing T2R family members, activation of these chemosensors will likely evoke sensations and responses similar to those generated by activation of T2R-expressing cells in the airways, i.e., irritation and protective reflexes. It is, however, hard to imagine what might be the natural ligands for such chemosensors. One possibility might be that T1R3-expressing cells of the airways are involved in regulation of glucose clearance from the mucus layer (49), but further research is necessary before this is certain. Nonetheless, the expression of T1R family members by airway epithelial cells indicates that the molecular identity of the receptor is not indicative of hedonic function, i.e., excess sugar in airway mucus is not perceived as a positive quality, although it presumably will activate the same class of molecular receptor as do sweet substances applied to the tongue. This is another example of the Müller's law of specific nerve energies.1
Conclusions
The canonical taste transduction cascade is utilized by many cells of the body, including epithelial cells of the airways, to detect and respond to substances taken into the body. Whereas in the taste system members of the T2R family of receptors generate a sensation of bitter, in the airways, the same receptors will give rise to a sensation of irritation. In the upper respiratory tract, activation of the chemoresponsive cells will generate both protective respiratory responses and a local neurogenic inflammation. Thus the chemoresponsive cells of the respiratory system can be viewed as an array of chemoresponsive cells that mediate reflexive actions that protect the organism, i.e., a chemofensor network (23) helping the body eliminate or avoid toxins and infectious agents.
Footnotes
This work was supported by National Institute on Deafness and Other Comunication Disorders Grants P30 DC-04657, RO1 DC-009820 (to T. E. Finger), and RO3 DC-012413 (M. Tizzano).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: M.T. and T.E.F. conception and design of research; M.T. and T.E.F. performed experiments; M.T. and T.E.F. analyzed data; M.T. and T.E.F. interpreted results of experiments; M.T. and T.E.F. prepared figures; M.T. and T.E.F. drafted manuscript; M.T. and T.E.F. edited and revised manuscript; M.T. and T.E.F. approved final version of manuscript.
1A common question is why, if bitter-tasting molecules such as denatonium activate a T2R receptor in nasal SCCs, is it not perceived as the taste of bitter rather than as irritation. The answer lies in the “Law of Specific Nerve Energies” enunciated by Johannes Peter Müller in about 1835. This law states that sensations evoked by activation of a nerve are related to the nature of the nerve and not to the physical energy of the stimulus, e.g., pressure on the eye produces the sensation of light, although no light energy (photons) is involved. The identity of a receptor (whether molecular or cellular) is not the determining factor for perception; it is the identity of the nerve that is crucial. Since the SCCs synapse onto trigeminal pain fibers and not onto taste fibers, activation of the SCCs is interpreted by the brain as a pain signal (irritation), not a taste signal.
References
- 1. An SS, Robinett KS, Deshpande DA, Wang WC, Liggett SB. Reply to: Activation of BK channels may not be required for bitter tastant-induced bronchodilation. Nat Med 18: 650–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Behrens M, Meyerhof W. Oral and extraoral bitter taste receptors. Results Probl Cell Differ 52: 87–99, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bezencon C, Furholz A, Raymond F, Mansourian R, Metairon S, Le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol 509: 514–525, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32: 41–49, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Braun CB, Northcutt RG. Cutaneous exteroreceptors and their innervation in hagfishes. In: The Biology of Hagfishes, edited by Jørgenson JM, et al. London: Chapman and Hall, 1998, p. 512–532 [Google Scholar]
- 6. Breer H, Eberle J, Frick C, Haid D, Widmayer P. Gastrointestinal chemosensation: chemosensory cells in the alimentary tract. Histochem Cell Biol 138: 13–24, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Brockhoff A, Behrens M, Massarotti A, Appendino G, Meyerhof W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J Agric Food Chem 55: 6236–6243, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci 23: 9947–9952, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science 291: 1557–1560, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell 100: 703–711, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Charlton TS, de Nys R, Netting A, Kumar N, Hentzer M, Givskov M, Kjelleberg S. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2: 530–541, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol 190: 285–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci 2: 6, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci USA 107: 5208–5213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conte C, Ebeling M, Marcuz A, Nef P, Andres-Barquin PJ. Evolutionary relationships of the Tas2r receptor gene families in mouse and human. Physiol Genomics 14: 73–82, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong D, Jones G, Zhang S. Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evol Biol 9: 12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, Martinez FJ, Huffnagle GB. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLos One 6: e16384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA 100: 8981–8986, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finger TE, Kinnamon SC. Taste isn't just for taste buds anymore. F1000 Biol Rep 3: 20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerhold KA, Bautista DM. Molecular and cellular mechanisms of trigeminal chemosensation. Ann NY Acad Sci 1170: 184–189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Getchell TV, Margolis FL, Getchell ML. Perireceptor and receptor events in vertebrate olfaction. Prog Neurobiol 23: 317–345, 1984 [DOI] [PubMed] [Google Scholar]
- 23. Green BG. Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem Senses 37: 201–206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gulbransen B, Silver W, Finger TE. Solitary chemoreceptor cell survival is independent of intact trigeminal innervation. J Comp Neurol 508: 62–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol 99: 2929–2937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen A. Olfactory and solitary chemosensory cells: two different chemosensory systems in the nasal cavity of the American alligator, Alligator mississippiensis. BMC Neurosci 8: 64, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansen A, Finger TE. Is TrpM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci 9: 115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hansen A, Tizzano M. TrpM5-expressing superficial epithelial cells in the main olfactory epithelium of mice. Chem Senses 36: A78–A79, 2011 [Google Scholar]
- 29. Hansen A, Zippel HP, Sorensen PW, Caprio J. Ultrastructure of the olfactory epithelium in intact, axotomized, and bulbectomized goldfish, Carassius auratus. Microsc Res Tech 45: 325–338, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res 339: 493–504, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 93: 6631–6634, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104: 15069–15074, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA 108: 2094–2099, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signaling induces ABCB1 through a mechanism involving CCK. Biochem J 438: 33–37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8: 49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 312: 500–506, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Kinnamon SC. Taste receptor signalling: from tongues to lungs. Acta Physiol (Oxf) 204: 158–168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 90: 822S–825S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schutz B, Kotlikoff M, Ibanez-Tallon I, Kummer W. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA 108: 9478–9483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 109: 524–532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin W, Ezekwe EA, Jr, Zhao Z, Liman ER, Restrepo D. TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci 9: 114, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci USA 104: 2471–2476, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol 99: 1451–1460, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Luciano L, Reale E, Ruska H. Über eine chemorezeptive Sinneszelle in der Trachea der Ratte. Z Zellforsch 85: 350–375, 1968 [PubMed] [Google Scholar]
- 46. Lundberg JM, Brodin E, Hua X, Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand 120: 217–227, 1984 [DOI] [PubMed] [Google Scholar]
- 47. Lundberg JM, Martling CR, Saria A. Substance P and capsaicin-induced contraction of human bronchi. Acta Physiol Scand 119: 49–53, 1983 [DOI] [PubMed] [Google Scholar]
- 48. Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Merigo F, Benati D, Cristofoletti M, Amaru F, Osculati F, Sbarbati A. Glucose transporter/T1R3-expressing cells in rat tracheal epithelium. J Anat 221: 138–150, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A. Alpha-gustducin immunoreactivity in the airways. Cell Tissue Res 319: 211–219, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35: 157–170, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Ming D, Ninomiya Y, Margolskee RF. Blocking taste receptor activation of gustducin inhibits gustatory responses to bitter compounds. Proc Natl Acad Sci USA 96: 9903–9908, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses 26: 259–265, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Morice AH, Bennett RT, Chaudhry MA, Cowen ME, Griffin SC, Loubani M. Effect of bitter tastants on human bronchi. Nat Med 17: 775, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Nakamura E, Hasumura M, San Gabriel A, Uneyama H, Torii K. New frontiers in gut nutrient sensor research: luminal glutamate-sensing cells in rat gastric mucosa. J Pharm Sci 112: 13–18, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Ogura T, Szebenyi SA, Krosnowski K, Sathyanesan A, Jackson J, Lin W. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J Neurophysiol 106: 1274–1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oshimoto A, Wakabayashi Y, Restrepo D. Trpm5 expression is activity dependent in mouse odorant receptor neurons. Soc Neurosci: 71 1274–7, 2010 [Google Scholar]
- 58. Panneton WM, Gan Q, Sun DW. Persistence of the nasotrigeminal reflex after pontomedullary transection. Respir Physiol Neurobiol 180: 230–236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat 44: 345–412, 1956 [DOI] [PubMed] [Google Scholar]
- 60. Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 26: 657–667, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 291: G171–G177, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291: G792–G802, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Sbarbati A, Bramanti P, Benati D, Merigo F. The diffuse chemosensory system: exploring the iceberg toward the definition of functional roles. Prog Neurobiol 91: 77–89, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Osculati F. Laryngeal chemosensory clusters. Chem Senses 29: 683–692, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Sbarbati A, Osculati F. Solitary chemosensory cells in mammals? Cells Tissues Organs 175: 51–55, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev 78: 429–466, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Sekerkova G, Zheng L, Loomis PA, Changyaleket B, Whitlon DS, Mugnaini E, Bartles JR. Espins are multifunctional actin cytoskeletal regulatory proteins in the microvilli of chemosensory and mechanosensory cells. J Neurosci 24: 5445–5456, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shindo Y, Miura H, Carninci P, Kawai J, Hayashizaki Y, Ninomiya Y, Hino A, Kanda T, Kusakabe Y. G alpha14 is a candidate mediator of sweet/umami signal transduction in the posterior region of the mouse tongue. Biochem Biophys Res Commun 376: 504–508, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Smith RS, Harris SG, Phipps R, Iglewski B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol 184: 1132–1139, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sorokin SP, Hoyt RF, Jr, Shaffer MJ. Ontogeny of neuroepithelial bodies: correlations with mitogenesis and innervation. Microsc Res Tech 37: 43–61, 1997 [DOI] [PubMed] [Google Scholar]
- 72. Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obesity 15: 73–78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and gustducin in palatal taste buds of mice. Chem Senses 32: 255–262, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Strotmann J, Breer H. Internalization of odorant-binding proteins into the mouse olfactory epithelium. Histochem Cell Biol 136: 357–369, 2011 [DOI] [PubMed] [Google Scholar]
- 75. Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 11: 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tizzano M, Dvoryanchikov G, Barrows JK, Kim S, Chaudhari N, Finger TE. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neurosci 9: 110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tizzano M, Finger TE. Nasal epithelium inflammation: involvement of solitary chemosensory cells after short term presentation of the irritating compound denatonium benzoate. Chem Senses. In press [Google Scholar]
- 78. Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA 107: 3210–3215, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tizzano M, Merigo F, Sbarbati A. Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J Anat 209: 333–337, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ueda T, Ugawa S, Yamamura H, Imaizumi Y, Shimada S. Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J Neurosci 23: 7376–7380, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Whitear M. Solitary Chemoreceptor Cells, in Chemoreception in Fishes, edited by Hara TJ. London: Elsivier, 1992, p. 1037376–125 [Google Scholar]
- 82. Widdecombe J. Respiratory Reflexes, in Air Pollution and Health, edited by Holgate ST, et al. London: Academic, 1999, p. 3257376–340 [Google Scholar]
- 83. Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature 381: 796–800, 1996 [DOI] [PubMed] [Google Scholar]
- 84. Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99: 2392–2397, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zancanaro C, Caretta CM, Merigo F, Cavaggioni A, Osculati F. Alpha-gustducin expression in the vomeronasal organ of the mouse. Eur J Neurosci 11: 4473–4475, 1999 [DOI] [PubMed] [Google Scholar]
- 86. Zhang CH, Chen C, Lifshitz LM, Fogarty KE, Zhu MS, ZhuGe R. Activation of BK channels may not be required for bitter tastant-induced bronchodilation. Nat Med 18: 648–50; author reply 650–1, 2012 [DOI] [PubMed] [Google Scholar]
- 87. Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003 [DOI] [PubMed] [Google Scholar]