SUMMARY
In vitro, topographical and biophysical cues arising from the extracellular matrix (ECM) direct skeletal stem cell (SSC) commitment and differentiation. However, the mechanisms by which the SSC-ECM interface is regulated and the outcome of such interactions on stem cell fate in vivo remain unknown. Here we demonstrate that conditional deletion of the membrane-anchored metalloproteinase MT1-MMP (Mmp14) in mesenchymal progenitors, but not in committed osteoblasts, redirects SSC fate decisions from osteogenesis to adipo- and chondrogenesis. By effecting ECM remodeling, MT1-MMP regulates stem cell shape, thereby activating a β1-integrin/RhoGTPase signaling cascade and triggering the nuclear localization of the transcriptional coactivators YAP and TAZ, which serve to control SSC lineage commitment. These data identify a critical MT1-MMP/integrin/YAP/TAZ axis operative in the stem cell niche that oversees SSC fate determination.
INTRODUCTION
Skeletal stem cells (SSCs; alternatively termed mesenchymal stem cells) are multipotent precursors that give rise to bone, fat, and cartilage during embryonic development and play critical roles in maintaining postnatal homeostasis (Chan et al., 2009;Méndez-Ferrer et al., 2010; Morikawa et al., 2009; Omatsu et al., 2010; Park et al., 2012; Sacchetti et al., 2007). In vivo, SSCs reside in specialized vascular or bone-associated niches where they are recruited into surrounding networks of extracellular matrix (ECM) molecules that are distinguished by their high content of type I collagen (Morikawa et al., 2009; Nilsson et al., 1998; Rowe and Weiss, 2009; Tancred et al., 2009; Tang et al., 2009). In vitro studies have highlighted the importance of SSC-ECM interactions, as lineage commitment and differentiation are sensitive to changes in matrix rigidity, topology, and dimensionality (Eyckmans et al., 2011). In these in vitro scenarios, SSC differentiation programs are analyzed under conditions in which the progenitor cell acts as a passive responder to experimental changes in ECM structure (Eyckmans et al., 2011; Huebsch et al., 2010; Kilian et al., 2010). However, whether SSCs themselves express an intrinsic ability to remodel their ECM microenvironment in order to direct cell-fate determination remains unknown.
Of the more than 20 ECM-degrading members of the matrix metalloproteinase (MMP) gene family, membrane-type 1 MMP (MT1-MMP; encoded by the gene Mmp14) serves as the dominant pericellular collagenase operative in epithelial and mesenchymal cell populations (Rowe and Weiss, 2009). To date, MT1-MMP further distinguishes itself from all other MMPs as the only family member whose global deletion in mice results in early postnatal death (Holmbeck et al., 1999; Rowe and Weiss, 2009; Zhou et al., 2000). However, the multiplicity of organ systems affected in Mmp14-targeted mice—coupled with the broad tissue distribution of the enzyme—has precluded efforts to identify the core functions of the proteinase in the in vivo setting (Rowe and Weiss, 2009). MT1-MMP knockout mice are known to display defects in bone, cartilage, and white fat depots but, in each case, altered tissue function has been postulated to arise primarily as a direct consequence of the impaired ability of osteoblasts, chondrocytes, or preadipocytes to remodel the collagen-rich pericellular milieu in which they are embedded (Chun et al., 2006; Holmbeck et al., 1999, 2003; Szabova et al., 2009; Zhou et al., 2000). Herein, using newly developed conditional knockout mice wherein Mmp14 has been inactivated in SSC populations, we alternatively describe an upstream role for MT1-MMP in controlling the ability of stem cells to undergo lineage commitment to bone, fat, or cartilage via a β1-integrin cascade that controls the transcriptional regulators Yes-association protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). These studies identify MT1-MMP as a cell-intrinsic regulator of the SSC-ECM interactions that control lineage commitment during early development.
RESULTS
Mesenchymal Progenitor/SSC-Targeted Deletion of MT1-MMP Elicits Multiple Defects in Mesenchyme-Derived Tissues
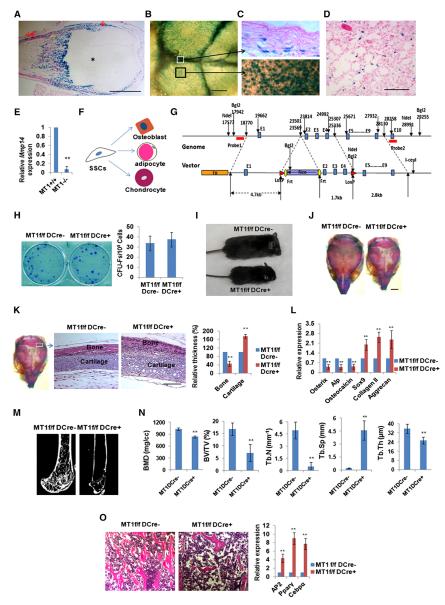
In MT1-MMP/lacZ knockin (MT1-MMP+/lacZ) mice, β-galactosidase activity is localized within tissue sites enriched in mesenchymal progenitor and SSCs such as the periosteum, perichondrium, calvaria, and bone marrow (Figures 1A–1D). As predicted, MT1-MMP is further detected in freshly isolated Sca-1+CD29+/CD45−CD11− bone-marrow-derived SSCs (Tang et al., 2009) by quantitative RT-PCR, with only background levels registering in cells isolated from Mmp14−/− mice (Figure 1E; Figure S1A available online). Furthermore, when SSCs are induced to differentiate in vitro, MT1-MMP expression is upregulated during osteogenesis, whereas commitment to either the adipogenic or chondrogenic pathways results in decreased MT1-MMP expression (Figure 1F; Figure S1B).
Figure 1. Expression and Genetic Inactivation of MT1-MMP in SSCs.
(A) LacZ expression in perichondrium (↓), periosteum (↓↓), and bone (Δ), but not cartilage (*), in 7-day-old Mmp14+/LacZ mouse femur. The scale bar represents 1 mm.
(B) LacZ expression in calvaria of a 10-day-old Mmp14+/LacZ mouse. The scale bar represents 500 μm.
(C) LacZ expression in sagittal suture mesenchymal progenitors (upper panel, section from the white boxed region in B) versus LacZ expression in mesenchymal progenitors and osteoblasts residing on the calvarial surface in 10-day-old Mmp14+/LacZ mice (black boxed region in B is enlarged). The scale bar represents 100 μm.
(D) LacZ expression in a subpopulation of bone-marrow cells in the femur of a 12-month-old Mmp14+/LacZ mouse. The scale bar represents 100 μm.
(E) Real-time PCR of Mmp14 expression in SSCs from 14-day-old Mmp14+/+ and Mmp14−/− mice (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(F) Schematic representation of the commitment of SSCs into three major lineages.
(G) Schematic of the Mmp14 genomic locus and targeting construct. After homologous recombination, the FRT-flanked Neo was inserted into the intron between exons 1 and 2, and two LoxP sites were inserted into the introns between exons 1 and 2 and exons 4 and 5.
(H) CFU-F generation from bone-marrow cells isolated from 4-week-old Mmp14f/f/Dermo1-Cre mice and control littermates (n = 6). Data are presented as mean ± SEM. Not significant, unpaired t test.
(I) Gross view of a 14-day-old Mmp14f/f/Dermo1-Cre mouse and its control littermate (Mmp14f/f).
(J) Alizarin red/Alcian blue staining of skulls isolated from 10-day-old Mmp14f/f and Mmp14f/f/Dermo1-Cre mice. The scale bar represents 2 mm.
(K) Histology of newborn Mmp14f/f and Mmp14f/f/Dermo1-Cre skulls across the lambdoid suture area (boxed area sectioned and magnified in the two panels to the right). Relative bone and cartilage thickness are shown to the right (n = 5). The scale bar represents 100 μm. Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(L) Relative mRNA expression of osteogenic and chondrogenic markers in newborn (1-day-old) calvarial RNA extracts from Mmp14f/f and Mmp14f/f/Dermo1-Cre mice (n = 6). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(M) Microcomputed tomography of the proximal femur of 12-week-old Mmp14f/f and Mmp14f/f/Dermo1-Cre mice.
(N) Bone mineral density (BMD), bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) measured by microcomputed tomography in 12-week-old Mmp14f/f and Mmp14f/f/Dermo1-Cre mice (n = 10). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(O) Histology of femur bone-marrow cavity and relative mRNA expression of adipogenic markers in bone-marrow RNA extracts from 12-week-old Mmp14f/f and Mmp14f/f/Dermo1-Cre mice (n = 6). The scale bar represents 100 μm. Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
See also Figure S1.
To assess the role of MT1-MMP in SSC commitment, a conditional Mmp14-deleted mouse model was generated by crossing Mmp14f/f mice with Dermo1-Cre-expressing mice (Figure 1G), a transgenic line wherein Cre activity is largely confined to mesenchymal progenitor cells and SSCs (Day et al., 2005; Liu et al., 2010). Neonatal Mmp14f/f/Dermo1-Cre mice appear grossly normal at birth despite confirmation that MT1-MMP has been deleted in bone-marrow-derived SSCs (Figure S1C). Further, SSC viability and number are equivalent in Mmp14f/f/Dermo1-Cre mice and Mmp14f/f mice (i.e., comparable numbers of colony-forming unit fibroblasts [CFU-Fs]; Figure 1H). Nevertheless, by postnatal days 4–5, defects in growth, both in terms of overall body size and weight, become evident, with more severe phenotypic changes displayed as the mice age (Figure 1I). By 120–150 days of age, more than 90% of Mmp14f/f/Dermo1-Cre mice (31 out of 34) die, with no mice surviving beyond 10 months.
As Mmp14f/f/Dermo1-Cre mice age, they develop a marked skeletal phenotype that includes short snouts and dome-shaped skulls (Figures 1I and 1J). Further, membranous ossification of the skull is delayed and suture closure is not observed during the life-time of the mice (Figure 1J). Cross-sections of the lambdoid suture of Mmp14f/f/Dermo1-Cre mice show delayed ossification along with thickening of the cartilaginous component relative to Mmp14f/f mice, in combination with marked decreases in mRNA levels of osteogenic markers (i.e., Osterix, alkaline phosphatase [Alp], and osteocalcin) and increased expression of chondrogenic markers (Sox9, collagen type II, and aggrecan) (Figures 1K and 1L). Defective osteogenesis is not confined to cranial tissues, as osteopenia is apparent throughout the skeleton of adult Mmp14f/f/Dermo1-Cre mice with no apparent changes in osteoclast number (Figures 1M and 1N; Figure S1D). As in the skull, defects in bone formation are also found in association with marked thickening of articular cartilage (Figure S1E). Finally, in addition to the observed changes in bone and cartilage, conditional knockout mice display significant increases in both bone-marrow adiposity and adipogenic gene expression (i.e., AP2, Pparγ, and Cebpα) (Figure 1O). By contrast, adipocytes localized to dermal fat pads that arise from distinct progenitor cell populations (Gupta et al., 2012; Tran et al., 2012) fail to generate subcutaneous fat (Figure S1F)—a phenotype similar to that described previously by our group in Mmp14−/− mice (Chun et al., 2006).
Osteoblast Progenitor Cell-Specific MT1-MMP Ablation Results in Aberrant Bone Formation with Intact Chondrogenesis and Adipogenesis
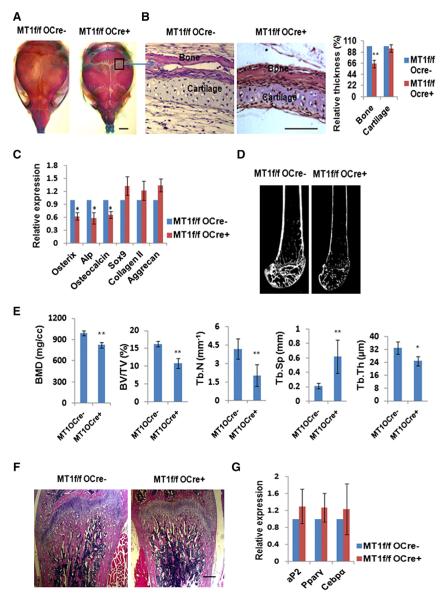
To determine whether the increase in chondrogenesis and adipogenesis observed in Mmp14f/f/Dermo1-Cre mice results from a primary ossification defect (Karsenty and Ferron, 2012), Mmp14f/f/Osterix-GFP-Cre mice were generated wherein Cre expression is confined primarily to the osteoblast progenitor lineage (Zhou et al., 2010). Mmp14f/f/Osterix-GFP-Cre mice are viable and fertile, with Mmp14 deletion confirmed in GFP-positive calvarial cells from newborn mice (Figures S2A and S2B). Conditional ablation of Mmp14 in osteoblast progenitors results in a bone phenotype with retarded membranous ossification of calvarial bones and delayed suture fusion, although milder in phenotype than that observed in Mmp14f/f/Dermo1-Cre mice (compare Figure 2A and Figure 1J). Nevertheless, cartilage deposition in newborn calvaria is comparable between Mmp14f/f/Osterix-GFP-Cre mice and Mmp14f/f mice, with no significant differences in Sox9, collagen type II, or aggrecan expression detected in calvarial extracts, despite significant decreases in Osterix, Alp, and osteocalcin expression (Figures 2B and 2C). Further, although microcomputed tomography analysis confirmed a general osteopenic phenotype in Mmp14f/f/Osterix-GFP-Cre mice (Figures 2D and 2E), neither adult femur articular cartilage nor bone-marrow adipocyte populations are expanded (Figures 2F and 2G; Figure S2C), whereas the ratio of osteoclast number to bone surface is comparable to that observed in control littermates (Figure S2D). Hence, the conditional deletion of Mmp14 in committed osteoblast progenitors decreases ossification without impacting chondrogenesis or adipogenesis—a phenotype distinct from that observed in mesenchymal progenitor/stem cell-targeted mice.
Figure 2. Osteopenia in Mice with Osteoblast Progenitor-Targeted Inactivation of MT1-MMP.
(A) Alizarin red/Alcian blue staining of skulls isolated from 10-day-old Mmp14f/f and Mmp14f/f/Osterix-GFP-Cre mice. The scale bar represents 2 mm.
(B) Histology of newborn Mmp14f/f and Mmp14f/f/Osterix-GFP-Cre mice skulls across the lambdoid suture area (boxed area in A, sectioned). Relative bone/cartilage thickness is shown to the right (n = 5). The scale bar represents 100 μm. Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(C) Relative mRNA expression of osteogenic and chondrogenic markers in 1-day-old calvarial RNA extracts from Mmp14f/f and Mmp14f/f/Dermo1-Cre mice (n = 6). Data are presented as mean ± SEM. *p < 0.05, unpaired t test.
(D) Microcomputed tomography of the proximal femur of 12-week-old Mmp14f/f and Mmp14f/f/Osterix-GFP-Cre mice.
(E) BMD, BV/TV, Tb.Th, Tb.N, and Tb.Sp were determined by microcomputed tomography in 12-week-old Mmp14f/f and Mmp14f/f/Osterix-GFP-Cre mice (n = 10). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, unpaired t test.
(F) Histology of femur from 12-week-old Mmp14f/f and Mmp14f/f/Osterix-GFP-Cre mice. The scale bar represents 1 mm.
(G) Relative mRNA expression of adipogenic markers in bone-marrow RNA extracts from 12-week-old Mmp14f/f and Mmp14f/f/Osterix-GFP-Cre mice (n = 6). Data are presented as mean ± SEM. Not significant, unpaired t test.
See also Figure S2.
MT1-MMP Regulates SSC Commitment and Differentiation
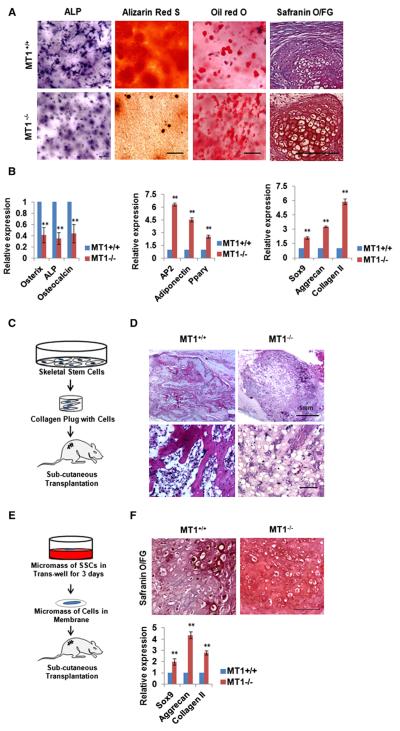
To determine whether MT1-MMP directs SSC lineage commitment in vitro, SSCs were isolated from Mmp14+/+ or Mmp14−/− mice and cultured under standard, low-density, 2D conditions in proadipogenic or -osteogenic media. In contrast to the marked defects observed in vivo, wild-type (WT) and MT1-MMP−/− cells commit to the osteogenic or adipogenic lineage in comparable fashion as determined by alkaline phosphatase or oil red O staining as well as the expression of osteogenic or adipogenic markers (Figures S3A and S3B). In vivo, however, SSCs are recruited and differentiate in close association with a 3D ECM that is rich in type I collagen, a preferred substrate for MT1-MMP (Lu et al., 2010; Maes et al., 2010; Morikawa et al., 2009). To alternatively assess the impact of MT1-MMP activity on cell-fate determination wherein 3D matrix interactions are operative, SSCs were embedded under low-density conditions in a preformed type I collagen matrix. Unlike the normal lineage commitment patterns of MT1-MMP−/− SSCs cultured in 2D collagen, null stem cells cultured under osteogenic conditions in 3D collagen hydrogels display a significant decrease in osteoblast commitment relative to MT1-MMP+/+ cells (Figures 3A and 3B). By contrast, under proadipogenic conditions in 3D culture, SSCs isolated from Mmp14−/− mice display an even stronger disposition than WT cells to commit to the adipocyte lineage (Figures 3A and 3B). Defects in MT1-MMP−/− SSC lineage commitment are not observed when cells are cultured atop (rather than embedded within) the 3D hydrogels (Figures S3C and S3D). Further, when SSCs are cultured in micromass under chondrogenic conditions, SSCs isolated from Mmp14−/− mice display a heightened chondrogenic potential relative to WT SSCs (Figures 3A and 3B). The ability of MT1-MMP to direct lineage commitment is not confined to SSCs, as calvaria-derived mesenchymal progenitors harvested from Mmp14−/− mice likewise fail to undergo normal osteogenesis whereas preferentially committing to a chondrogenic phenotype in 3D culture (Figures S3E and S3F). Finally, when MT1-MMP−/− SSCs are explanted into nude mice recipients, osteogenesis is similarly impaired, whereas adipogenic and chondrogenic potential are enhanced in vivo (Figures 3C–3F).
Figure 3. MT1-MMP Regulates SSC Commitment and Differentiation.
(A) Bone-marrow-derived SSCs were isolated from Mmp14+/+ and Mmp14−/− mice and cultured under osteogenic conditions for 7 days (alkaline phosphatase stained) or 14 days (alizarin red S stained) in 3D collagen, under adipogenic conditions in 3D collagen for 7 days (oil red O stained), or under chondrogenic conditions for 21 days (safranin O/fast green stained). The scale bars represent 100 μm.
(B) Relative mRNA expression of osteogenic, adipogenic, and chondrogenic markers from the cultures shown in (A) (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(C) Schematic of the overall experimental design for in vivo implantation.
(D) Histology of tissues isolated from mice transplanted with Mmp14+/+ or Mmp14−/− SSCs. The scale bars represent 100 μm (n = 5).
(E) Experimental design of chondrogenesis in vivo.
(F) Safranin O staining and chondrogenic marker expression levels of cartilage-like tissues isolated from mice transplanted with Mmp14+/+ or Mmp14−/− SSCs cultured as described in (E). The scale bar represents 100 μm (n = 5). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
See also Figure S3.
MT1-MMP-Dependent ECM Remodeling Controls SSC Differentiation via β1-Integrin Activation
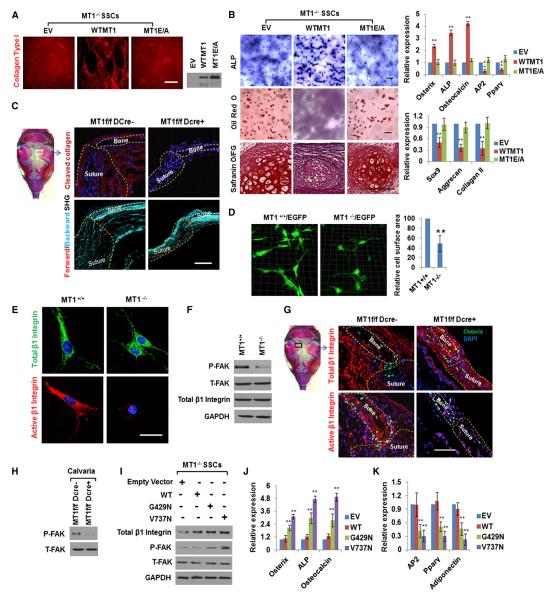
To determine whether MT1-MMP function during cell-fate specification is—or is not—dependent on its ECM remodeling activity, MT1-MMP−/− SSCs were transduced with lentiviral vectors encoding WT or a catalytically inactive MT1-MMP mutant (Shimizu-Hirota et al., 2012), and lineage commitment was assessed in vitro. Having confirmed equivalent expression of WT and mutant MT1-MMP by western blot, only WT MT1-MMP expression elicits SSC-mediated type I collagenolytic activity (Figure 4A). More importantly, reexpression of WT MT1-MMP, but not the catalytically inactive mutant, in MT1-MMP−/− SSCs increases osteogenesis in 3D culture, whereas inhibiting adipogenesis and chondrogenesis in a pattern that phenocopies the behavior of WT SSCs (Figure 4B). Consistent with these results, using a combination of (1) antibodies specific for type I collagen degradation fragments and (2) second harmonic generation (SHG) to visualize intact type I collagen networks in vivo, collagenolytic activity is readily detected within fields of collagen fibrils in Mmp14f/f calvarial bone and sutures (Figure 4C). By contrast, in Mmp14-targeted mice, collagenolytic activity is reduced to background levels amid a denser network of type I collagen fibrils (Figure 4C).
Figure 4. MT1-MMP-Dependent ECM Remodeling Regulates SSC Commitment by Activating β1-Integrin Signaling.
(A) MT1-MMP−/− SSCs were transduced with lentivirus expressing HA-tagged WT MT1-MMP (WTMT1), an MT1-MMP mutant (MT1E/A), or an empty vector (EV). Collagen degradation results are shown in the left-hand panel (collagen colored red). The scale bar represents 200 μm. The expression of the HA-tagged MT1-MMP proteins is shown in the right-hand panel.
(B) ALP and oil red O staining of SSCs from (A) cultured under osteogenic conditions in 3D collagen for 14 days or following safranin O staining of SSCs cultured under micromass chondrogenic conditions for 21 days. Relative mRNA expression of markers is shown in the right-hand panels (n = 3). The scale bars represent 100 μm. Data are presented as mean ± SEM. **p < 0.01, one-way ANOVA.
(C) Collagen cleavage in newborn (1-day-old) calvarial bone and suture. The scale bar represents 50 μm. In the lower panels, collagen deposition in newborn calvarial bone and suture is shown as detected by confocal imaging of SHG signals (forward scattering, red; backward scattering, blue). The scale bar represents 50 μm.
(D) Three-dimensional reconstruction and relative cell-surface area of SSCs isolated from Mmp14+/+/EGFP or Mmp14−/−/EGFP mice and cultured in 3D collagen. Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(E) Immunofluorescence of total (green) and active (red) β1-integrin in SSCs isolated from Mmp14+/+ or Mmp14−/− mice and cultured in 3D collagen. The scale bar represents 15 μm.
(F) Western blot of P-FAK, T-FAK, and total β1-integrin in SSCs isolated from Mmp14+/+ or Mmp14−/− mice and cultured in 3D collagen for 2 days.
(G) Immunofluorescence of total and active β1-integrin in newborn calvarial cells of Mmp14f/f or Mmp14f/f/Dermo1-Cre mice. The scale bar represents 50 μm.
(H) Western blot of P-FAK and T-FAK in calvarial lysates isolated from newborn Mmp14f/f or Mmp14f/f/Dermo1-Cre mice.
(I) MT1-MMP−/− SSCs transduced with an empty vector lentivirus or viruses expressing either the human WT or mutant β1-integrins (G429N or V737N) were cultured in 3D collagen gels for 2 days and probed with anti-β1-integrin, P-FAK, and T-FAK antibodies.
(J) Relative mRNA expression of osteogenic markers in the lentiviral-transduced cells from (I) cultured in 3D collagen for 7 days under osteogenic conditions (n = 3). Data are presented as mean ± SEM. **p < 0.01, one-way ANOVA.
(K) Relative mRNA expression of adipogenic markers in SSCs shown in (I) cultured under adipogenic conditions in 3D collagen for 7 days (n = 3). Data are presented as mean ± SEM. **p < 0.01, one-way ANOVA.
See also Figure S4.
Cell adhesion to the ECM triggers cell spreading and shape changes that are required for β1-integrin activation, a critical step in the SSC differentiation program (Eyckmans et al., 2011). As assessed by imaging of SSCs from GFP transgenic mice in 3D culture, MT1-MMP+/+/GFP cells assume a spread, fibroblast-like, spindle morphology, whereas MT1-MMP−/−/GFP SSCs, by virtue of their inability to remodel the surrounding collagen matrix, are confined to more constrained shapes with a 50% decrease in relative cell-surface area (Figure 4D). Likewise, in GFP-expressing transgenic mouse tissues, MT1-MMP−/− mesenchymal progenitors and osteoblasts, as well as fibroblast-like cells in the bone-marrow compartment, display smaller cell bodies with a 2-fold mean decrease in cell-surface area relative to that found in Mmp14+/+/GFP mice (Figure S4A). As SSC-collagen interactions are mediated largely by β1-integrins (Popov et al., 2011), the total versus active levels of β1-integrin were assessed with conformation-specific antibodies in MT1-MMP+/+ and MT1-MMP−/− SSCs in 3D culture as well as in vivo (Rantala et al., 2011). Immunostaining of in vitro cultures reveals that the pools of both total and active β1-integrins are distributed throughout the cell bodies of MT1-MMP+/+ SSCs, but in MT1-MMP−/− SSCs only low levels of active β1-integrin are detected, with the bulk of the total β1-integrin content confined to the perinuclear region (Figure 4E). Consistent with the impaired activation status of β1-integrins in MT1-MMP−/− SSCs, FAK phosphorylation at Y397 (P-FAK; a downstream target of activated β1-integrins; Eyckmans et al., 2011; Paszek et al., 2005) is down-regulated in MT1-MMP null SSCs cultured in 3D collagen gels, despite comparable levels of total FAK (T-FAK) or β1-integrin (Figure 4F). Similarly, in Mmp14f/f mice, active β1-integrin is readily detected in osteoblast-related cells surrounding bone as well as in mesenchymal progenitor/SSCs (Figure 4G). However, although the total number of Osterix-positive cells is decreased in the calvaria of Mmp14f/f/Dermo1-Cre mice, active β1-integrin staining is even more dramatically reduced in tandem, with an almost complete loss of P-FAK in calvarial extracts (Figures 4G and 4H).
Highlighting a dominant role for β1-integrin signaling in regulating SSC differentiation in 3D culture, when β1-integrin activity is blocked with neutralizing antibodies, the osteogenic potential of WT SSCs is inhibited, whereas adipogenesis and chondrogenesis are enhanced (Figures S4B and S4C). Given these results, we sought to determine whether integrin activation alone could bypass the 3D-specific requirement for MT1-MMP in the SSC differentiation program. As such, MT1-MMP−/− SSCs were transduced with either WT β1-integrin or a series of activated β1-integrin mutants, and the cells were cultured in 3D collagen. Importantly, expression of either a constitutively active β1-integrin mutant (G429N) or a V737N β1-integrin mutant that supports integrin clustering (Paszek et al., 2005), but not the WT integrin, successfully triggers FAK activation in MT1-MMP−/− SSCs while rescuing osteogenic potential and inhibiting adipogenic activity (Figures 4I–4K); although both β1-integrin mutants are able to rescue the MT1-MMP null phenotype, the V737N self-clustering variant is more active. Hence, the forced activation of β1-integrin in MT1-MMP−/− SSCs bypasses the 3D-specific defects observed in lineage commitment and differentiation.
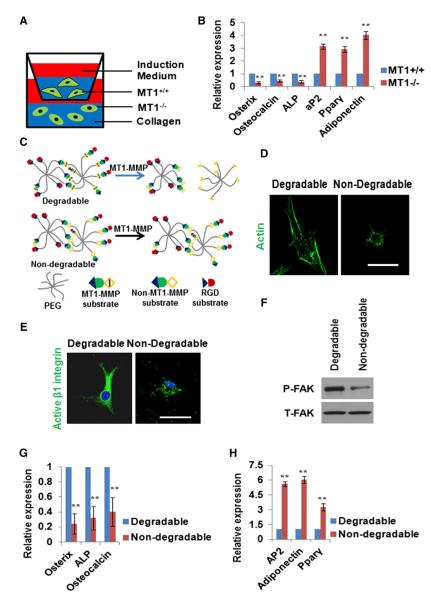
Consistent with the unimpaired ability of MT1-MMP−/− SSCs to commit to the osteoblast and adipocyte lineages in 2D culture (Figures S3A and S3B), MT1-MMP null SSCs activate β1-integrin and FAK in a fashion indistinguishable from that observed in MT1-MMP+/+ SSCs under planar culture conditions (Figures S4D and S4E). Although these results rule out any requirement for MT1-MMP-dependent proteolysis of cell-associated or -secreted products in regulating SSC commitment in 2D culture, MT1-MMP-derived proteolytic products conceivably control stem cell fate, but only in 3D culture. As such, MT1-MMP+/+ and MT1-MMP−/− SSCs were embedded in 3D collagen gels and cocultured together in a dual osteogenic and adipogenic induction medium (Figure 5A). Under these conditions, however, WT cells are unable to rescue the MT1-MMP null phenotype (Figure 5B). Alternatively, if MT1-MMP activity is restricted to the control of 3D cell shape, then MT1-MMP+/+ SSCs should recapitulate the MT1-MMP null phenotype if embedded in matrices whose structure is resistant to MT1-MMP-dependent hydrolysis. Hence, WT SSCs were cultured in synthetic poly(ethylene glycol) hydrogels that incorporate MT1-MMP-sensitive or -insensitive peptide cross-bridges (Ehrbar et al., 2011) (Figure 5C). As expected, whereas MT1-MMP+/+ SSCs are able to alter cell shape and activate β1-integrin as well as FAK in MT1-MMP-hydrolyzable hydrogels, the WT cells remain “locked” in a spherical shape in the MT1-MMP-resistant matrix, and the integrin/FAK axis is not engaged (Figures 5D–5F). Further, when WT SSCs are cultured in MT1-MMP-sensitive hydrogels in the combined presence of osteogenic and adipogenic factors, the cells preferentially commit to osteoblastogenesis (Figures 5G and 5H). Conversely, in MT1-MMP-resistant hydrogels, WT SSCs that are prevented from undergoing shape change alternatively commit to adipogenesis (Figures 5G and 5H). In alginate gels, an MMP-insensitive, polysaccharide matrix, MT1-MMP+/+ or MT1-MMP−/− SSCs costimulated with osteogenic and adipogenic factors, or chondrogenic factors alone, retain spherical shapes and display equivalent osteogenic, adipogenic, and chondrogenic potential (Figure S5). Taken together, these results support a model wherein MT1-MMP-dependent proteolysis of the pericellular ECM drives the cell-shape changes necessary for β1-integrin activation.
Figure 5. β1-Integrin Activation and Three-Dimensional Cell-Shape Change.
(A) Schematic of MT1-MMP+/+ and MT1-MMP−/− SSCs in 3D collagen coculture for 7 days under dual osteogenic/adipogenic induction.
(B) Osteogenic and adipogenic markers quantified by real-time PCR in MT1-MMP+/+ and MT1-MMP−/− SSCs cocultured as described in (A) (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(C) Schematic of PEG-based hydrogel. MT1-MMP-sensitive or -insensitive peptides were crosslinked to the PEG backbone with a cell-adhesive RGD peptide integrated into the hydrogel.
(D) Phalloidin staining of MT1-MMP wild-type SSCs cultured in MT1-MMP-degradable or -nondegradable hydrogels for 15 days. The scale bar represents 10 μm.
(E) Immunofluorescence of active β1-integrin in SSCs cultured in 3D PEG-based hydrogel for 15 days. The scale bar represents 15 μm.
(F) Western blot of P-FAK and T-FAK in SSCs cultured in 3D PEG-based hydrogel for 15 days.
(G) Relative mRNA expression of osteogenic markers in MT1-MMP+/+ SSCs cultured in 3D hydrogels (MMP-degradable or -nondegradable) for 15 days followed by a 7 day period under dual osteogenic/adipogenic induction (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(H) Relative mRNA expression of adipogenic markers in MT1-MMP+/+ SSCs cultured in 3D hydrogels (MMP-degradable or -nondegradable) for 15 days followed by a 7 day period under dual induction conditions (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
See also Figure S5.
ECM Remodeling and the RhoGTPase/ROCK-Dependent Control of Pericellular Rigidity
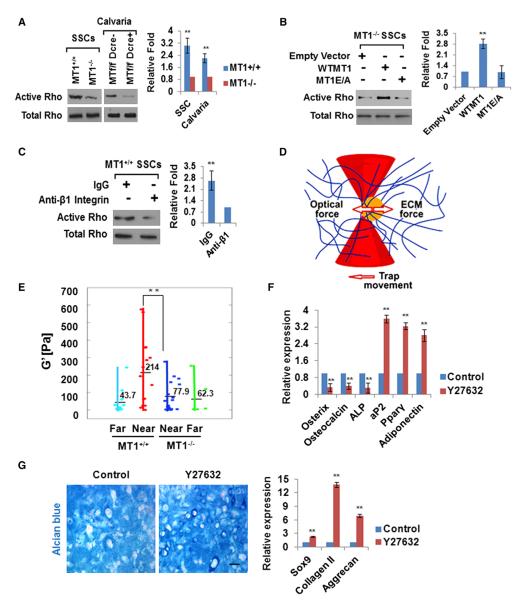
In 2D culture, cell-shape changes associated with β1-integrin activation trigger RhoGTPase-dependent cytoskeletal reorganization (Eyckmans et al., 2011). Consistent with an MT1-MMP-dependent requirement for β1-integrin activation in 3D collagen hydrogels, MT1-MMP−/− SSCs express decreased Rho-GTP levels relative to WT cells (Figure 6A). In vivo—as in 3D culture—decreased RhoGTPase activity is similarly found in calvarial extracts isolated from MT1-MMPf/f/Dermo1-Cre mice relative to MT1-MMPf/f mice (Figure 6A). Furthermore, the 3D-specific defects in RhoGTPase activation observed in MT1-MMP null SSCs can be rescued by expressing WT MT1-MMP but not the catalytically inactive protease (Figure 6B). A direct link between β1-integrin activation and the induction of Rho-GTPase activity is confirmed by culturing MT1-MMP+/+ SSCs in 3D collagen gels in the presence of neutralizing anti-β1-integrin antibody wherein RhoGTPase activation is inhibited (Figure 6C). Conversely, defects in RhoGTPase activation as well as F-actin organization observed in MT1-MMP−/− SSCs in 3D culture are rescued fully by expressing either the G429N or V737N β1-integrin mutants in the null cells without affecting cell morphology or size (Figures S6A–S6D).
Figure 6. ECM Remodeling Regulates RhoGTPase Signaling, Pericellular Rigidity, and SSC Commitment.
(A) Active and total RhoGTPase levels were determined in SSCs isolated from Mmp14+/+ or Mmp14−/− mice and cultured in 3D collagen for 2 days as well as in newborn calvarial lysates isolated from Mmp14f/f or Mmp14f/f/Dermo1-Cre mice. Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(B) Active and total RhoGTPase levels were determined in SSCs isolated from Mmp14−/− mice that were transduced with empty vector or lentiviral constructs expressing WT MT1-MMP (WTMT1) or mutant MT1-MMP (MT1E/A) and cultured in 3D collagen for 2 days. Data are presented as mean ± SEM. **p < 0.01, one-way ANOVA.
(C) Active and total RhoGTPase levels in SSCs isolated from Mmp14+/+ mice and cultured in 3D collagen with rat IgG or anti-β1-integrin antibody for 2 days. Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(D) Schematic of local collagen rigidity assayed by microrheology. Beads are trapped and oscillated in a laser trap where bead position is detected by a quadrant photodiode. The interaction between the elastic force from the matrix and the optical force from the laser is used to determine the elastic modulus (G’).
(E) G’ values near (<5 μm) and distant (>100 μm) from MT1-MMP+/+ and MT1-MMP−/− SSCs cultured in 3D collagen are shown. Each point represents the average G’ (across frequencies) for each bead. Data are presented as mean ± SEM. **p < 0.01, one-way ANOVA.
(F) Relative mRNA expression of osteogenic and adipogenic markers in MT1-MMP+/+ SSCs cultured in 3D collagen and treated with or without 10 μM Y27632 for 7 days under osteogenic or adipogenic conditions, respectively (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(G) Alcian blue staining of MT1-MMP+/+ SSCs cultured in 3D collagen and treated with or without 10 μM Y27632 for 21 days under chondrogenic conditions. The scale bar represents 100 μm. Relative mRNA expression of chondrogenic markers is shown in the right-hand panel (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
See also Figure S6.
RhoGTPase-dependent ROCK activation upregulates cytoskeletal tension by triggering the assembly of actomyosin networks (Eyckmans et al., 2011). To determine whether MT1-MMP controls the magnitude of the traction forces exerted by sscs in collagen gels, fibril stiffness in 3d cultures was assessed by laser tweezer-based active microrheology (Kotlarchyk et al., 2011) (Figure 6D). Whereas MT1-MMP+/+ SSCs exert traction on the surrounding 3D collagen matrix that increases pericellular rigidity to levels 4-fold greater than baseline, MMP14−/− SSCs display a complete defect in their ability to increase local rigidity (Figure 6E; Figure S6E). The critical importance of these tractional forces on the SSC differentiation program is confirmed by the ability of the ROCK inhibitor Y27632 to reduce cytoskeletal tension in MT1-MMP+/+ SSCs (Figure S6F), and recapitulate the phenotype of MT1-MMP−/− SSCs, namely an inability to fully commit to osteogenesis while promoting adipogenesis as well as chondrogenesis in 3D culture (Figures 6F and 6G). Thus, MT1-MMP-dependent remodeling of the ECM triggers β1-integrin signaling and RhoGTPase activation, leading to the ROCK-mediated increases in pericellular rigidity that control SSC commitment.
MT1-MMP-Mediated ECM Remodeling Triggers YAP/TAZ-Dependent SSC Commitment
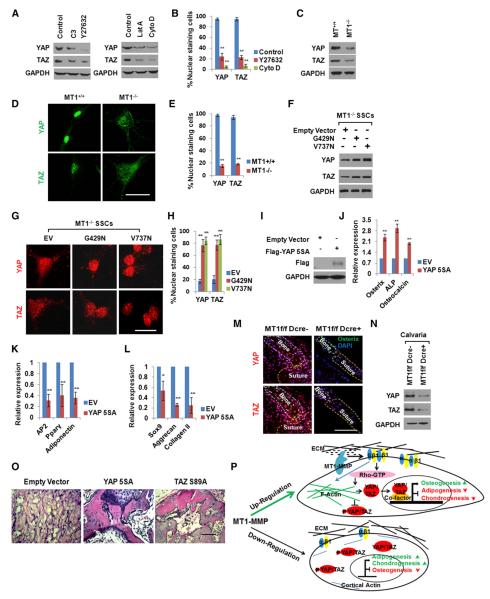
Recent studies link ROCK, stress fiber assembly, and cytoskeletal force generation to the activation of YAP and TAZ nuclear signaling networks (Dupont et al., 2011; Sansores-Garcia et al., 2011; Wada et al., 2011). As such, we sought to determine whether YAP/TAZ expression and intracellular localization in SSCs are regulated by MT1-MMP in a 3D-specific fashion. Preliminary studies performed in 3D collagen gels demonstrate that (1) inhibiting either RhoGTPase or ROCK with C3 toxin or Y27632, respectively, or (2) blocking F-actin assembly with latrunculin A or cytochalasin D decreases the expression and nuclear localization of YAP/TAZ (Figures 7A and 7B). Given that YAP/TAZ protein levels and nuclear localization are regulated by Rho/ROCK activity, and the 3D-specific ability of MT1-MMP to trigger Rho/ROCK signaling, the status of the transcriptional coactivators was examined in WT and MT1-MMP−/− SSCs. Relative to WT SSCs, 3D-embedded, MT1-MMP−/− SSCs express only low levels of YAP/TAZ protein, with the bulk of their content confined to the cytosolic compartment (Figures 7C–7E). Interestingly, MT1-MMP−/− SSCs express YAP and TAZ mRNAs at WT levels (Figure S7A), but preferentially accumulate phosphorylated forms of YAP that are normally sequestered in the cytosol and/or targeted for proteosomal degradation (similar increases in phospho-YAP are found when WT SSCs are cultured in the presence of C3 toxin or Y27632) (Figure S7B) (Zhao et al., 2012). Consistent with the ability of constitutively active β1-integrins to rescue RhoGTPase activation and F-actin assembly in MT1-MMP−/− SSCs, transducing these cells with the β1-integrin mutant expression vectors also increases YAP/TAZ levels and directs their localization to the nuclear compartment (Figures 7F–7H). By contrast, in 2D culture, where MT1-MMP does not play a required role in directing cell-shape changes, YAP/TAZ protein expression, phosphorylation, and nuclear localization are regulated comparably in MT1-MMP−/− and MT1-MMP-transduced null cells (Figures S7C–S7E).
Figure 7. MT1-MMP-Dependent YAP/TAZ Activation Triggers SSC Commitment.
(A) YAP and TAZ expression levels in MT1-MMP+/+ SSCs embedded in 3D collagen gels for 2 days and treated with Rho inhibitor C3 (3 μg/ml), ROCK inhibitor Y27632 (10 μM), latrunculin A (Lat A; 0.5 μM), or cytochalasin D (CytoD; 1 μM).
(B) Quantification of nuclear-stained cells from (A) (n = 3). Data are presented as mean ± SEM. **p < 0.01, one-way ANOVA.
(C) YAP and TAZ expression levels in MT1-MMP+/+ or MT1-MMP−/− SSCs embedded in 3D collagen gels for 2 days.
(D) YAP and TAZ localization in MT1-MMP+/+ or MT1-MMP−/− SSCs embedded in 3D collagen gels for 2 days. The scale bar represents 20 μm.
(E) Quantification of nuclear-stained cells from (D) (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(F) YAP and TAZ expression levels in MT1-MMP−/− SSCs infected with an empty vector or lentiviral constructs expressing G429N or V737N β1-integrin mutants and cultured in 3D collagen for 2 days.
(G) YAP and TAZ localization in cells from (F) embedded in 3D collagen gels for 2 days. The scale bar represents 20 μm.
(H) Quantification of nuclear-stained cells from (G) (n = 3). Data are presented as mean ± SEM. **p < 0.01, one-way ANOVA.
(I) Expression of YAP-5SA probed with an anti-Flag antibody in MT1-MMP−/− SSCs infected with a lentiviral empty vector or virus expressing Flag-tagged YAP-5SA.
(J) Osteogenic marker expression in MT1-MMP−/− SSCs infected with a lentiviral control vector or virus expressing Flag-tagged YAP-5SA, embedded in 3D collagen, and cultured for 7 days under osteogenic conditions (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(K) Adipogenic marker expression in MT1-MMP−/− SSCs infected with a lentiviral control vector or virus expressing Flag-tagged YAP-5SA, embedded in 3D collagen, and cultured for 7 days under adipogenic conditions (n = 3). Data are presented as mean ± SEM. **p < 0.01, unpaired t test.
(L) Chondrogenic marker expression in MT1-MMP−/− SSCs infected with a lentiviral control vector or a virus expressing Flag-tagged YAP-5SA and cultured in 3D collagen for 14 days under chondrogenic conditions (n = 3). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, unpaired t test.
(M) YAP and TAZ localization in newborn calvaria of Mmp14f/f or Mmp14f/f/Dermo1-Cre mice. The scale bar represents 50 μm.
(N) YAP and TAZ protein levels in newborn Mmp14f/f or Mmp14f/f/Dermo1-Cre calvarial lysates.
(O) Histology of tissue isolated from mice transplanted with MT1-MMP−/− SSCs transduced with a lentiviral control vector or viruses expressing either Flag-tagged YAP-5SA or TAZ-S89A (n = 5). The scale bar represents 100 μm.
(P) Schematic model of MT1-MMP-dependent control of SSC lineage commitment.
See also Figure S7.
Finally, to determine whether YAP/TAZ expression/nuclear localization is sufficient to control SSC commitment in 3D culture independently of MT1-MMP activity, MT1-MMP−/− SSCs were transduced with constitutively active forms of YAP (i.e., 5SA-YAP) or TAZ (TAZ-S89A) and differentiation was induced (Dupont et al., 2011; Hong et al., 2005). Remarkably, following 5SA-YAP or TAZ-S89A transduction, 3D-specific lineage commitment defects in MT1-MMP-deleted SSCs are rescued with marked increases in osteogenesis, complementing decreases in adipogenesis and chondrogenesis to WT levels without affecting SSC morphology (Figures 7I–7L; Figures S7F–S7I). Given the dominant role of the YAP/TAZ axis in regulating SSC differentiation in vitro, the status of the transcriptional activators was next assessed in vivo. As predicted, whereas calvaria from Mmp14f/f mice display high expression levels of the YAP/TAZ proteins coupled with strong nuclear staining, extracts prepared from Mmp14f/f/Dermo1-Cre mice exhibit a striking decrease in YAP and TAZ by both western blot and immunohistochemistry (Figures 7M and 7N). Importantly, the defective osteogenic potential of MT1-MMP−/− SSCs can likewise be rescued in vivo following transduction either with 5SA-YAP or TAZ-S89A and subcutaneous transplantation into nude mice recipients (Figure 7O). Taken together, these data identify MT1-MMP as a 3D-specific in vivo regulator of the YAP/TAZ axis responsible for controlling SSC commitment and differentiation.
DISCUSSION
Herein, we demonstrate that MT1-MMP directs SSC lineage commitment and differentiation by controlling a 3D-specific ECM remodeling program that triggers β1-integrin activation by modulating cell-shape changes and initiating a downstream RhoGTPase-actomyosin signaling cascade that drives the nuclear localization of the YAP/TAZ cotranscriptional activators (Figure 7P). Both in vitro and in vivo, MT1-MMP-deleted SSCs display profound defects in osteogenesis that are coupled with compensatory increases in adipogenic and chondrogenic potentials. Earlier studies proposed that the loss of MT1-MMP proteolytic activity in terminally differentiated osteoblasts or chondrocytes affects skeletal development directly as a function of impaired collagen remodeling or FGFR2 signaling (Chan et al., 2012; Holmbeck et al., 1999, 2003; Zhou et al., 2000). However, our studies describe a more upstream impact on the overall SSC lineage commitment program itself. Although Dermo1-Cre expression extends beyond SSCs to include a number of mesenchymal tissues (Tran et al., 2010), each of the phenotypic defects we observed in osteoblastogenesis, chondrogenesis, and adipogenesis in the targeted mice is duplicated by MT1-MMP−/− SSCs cultured in vitro as well as following their in vivo transplantation into WT recipients. Furthermore, when MT1-MMP is alternatively targeted in committed osteoblasts/osteocytes using Osterix-Cre transgenic mice, skeletal defects are confined to the osteoblast lineage alone. Although Osterix-Cre can also be expressed in (pre)hypertrophic chondrocytes (Zhou et al., 2010), cartilage remodeling is unaffected in these mice—a finding consistent with our inability to detect MT1-MMP expression in cartilaginous tissues and the inability of MT1-MMP knockout transgenic mice engineered to overexpress MT1-MMP in chondrocytes to resorb the expanded cartilage network (Szabova et al., 2009).
In considering the potential mechanisms by which MT1-MMP regulates SSC function, the expanse of the enzyme’s substrate repertoire—from ECM macromolecules and cell-surface molecules to chemokines and growth factors (Jin et al., 2011; Nishida et al., 2012; Rowe and Weiss, 2009)—raised a number of possibilities. However, in contradistinction to their in vivo phenotype, MT1-MMP null SSCs cultured in vitro under standard, 2D conditions maintain a completely normal potential for lineage commitment and differentiation. Hence, MT1-MMP-dependent hydrolysis of intracellular, cell-surface, or secreted macromolecules does not play an obligatory role in controlling SSC lineage decisions. By contrast, only when SSCs are embedded in 3D type I collagen hydrogels designed to recapitulate an in vivo-like microenvironment do the MT1-MMP-deleted cells display alterations in lineage commitment similar to those observed in MT1-MMP-targeted mice in vivo. Although 3D type I collagen matrices do not necessarily reflect all in vivo environments, we note that SSCs express multiple collagen-binding integrins as well as type I collagen (Popov et al., 2011; Rodríguez et al., 2000). Further, SSCs are found either embedded within—or recruited to—type I collagen-rich tissues in vivo (Maes et al., 2010; Morikawa et al., 2009; Nilsson et al., 1998; Tancred et al., 2009; Tang et al., 2009). Nevertheless, MT1-MMP-ECM interactions are not limited to type I collagen alone, and SSCs can also be found within type II/III collagen-rich as well as fibronectin-replete tissues that are also sensitive to MT1-MMP-dependent hydrolysis (Sabeh et al., 2010; Shi and Sottile, 2011). Last, although MT1-MMP can activate downstream MMPs (i.e., MMP-2, MMP-8, or MMP-13), mice harboring global knockouts of these proteinases display only subtle and transient defects in skeletal development relative to those described herein (Krane and Inada, 2008).
Insights gleaned from 2D and 3D culture systems have led to the construction of molecular models whereby cell-shape-dependent integrin-ligand engagement elicits a signaling cascade that induces Rho/ROCK activation, stress fiber formation, focal adhesion site maturation, and increased cytoskeletal tension (Eyckmans et al., 2011). Although MT1-MMP-dependent cell-shape changes cannot be decoupled from MT1-MMP-catalyzed increases in pericellular ECM rigidity, each of these variables is known to play critical roles in SSC commitment programs (Eyckmans et al., 2011; Tee et al., 2011). Conversely, in the absence of MT1-MMP—or under conditions in which MT1-MMP is downregulated (e.g., during adipogenesis or chondrogenesis)—SSCs confine themselves to a smaller surface area/volume, decrease integrin activation as well as Rho-linked signaling cascades, and alternatively commit to adipogenic or chondrogenic pathways. It is important to stress that MT1-MMP null SSCs can be redirected away from adipogenesis or chondrogenesis toward osteogenesis in 3D culture by expressing β1-integrin mutants that trigger downstream signaling cascades independently of any requirement for pericellular collagenolysis. Nevertheless, despite the ability of forced β1-integrin signaling to circumvent the requirement for MT1-MMP in vitro, the proteinase appears to play a required role in directing SSC commitment in vivo and, in its absence, alternate mechanisms for bypassing ECM remodeling are unable to optimally control SSC commitment.
In probing for final effector pathways capable of transducing MT1-MMP-initiated signaling cascades into SSC lineage commitment decisions, earlier studies identified the transcriptional coactivator TAZ as a key regulator of SSC-osteoblast differentiation under 2D culture conditions (Hong et al., 2005). TAZ, by virtue of its ability to interact with RNX2 and PPARγ, directs transcriptional enhancement of RNX2 activity, thereby stimulating osteoblastogenesis, whereas repressing PPARγ functions critical to adipocyte formation (Hong et al., 2005). Conversely, in the absence of TAZ, SSC commitment to osteoblast formation is prevented whereas promoting adipogenesis (Hong et al., 2005). More recently, the cotranscriptional activities of TAZ as well as YAP have been shown to fall under the regulation of F-actin assembly, which in turn serves to promote YAP/TAZ protein stability and nuclear localization (Dupont et al., 2011; Sansores-Garcia et al., 2011; Wada et al., 2011; Zhao et al., 2012). Currently, requirements for ROCK-mediated, F-actin-dependent cytoskeleton tension or regulation of the upstream Hippo pathway, particularly the Lats1/2 kinases, in the YAP/TAZ activation network remain controversial (Zhao et al., 2012). Nevertheless, efforts to dissect the functional impact of YAP/TAZ on cell behavior or differentiation in mammalian models have been confined to 2D culture systems where requirements for ECM remodeling have been bypassed. Alternatively, using the 3D ECM as a more physiologic template for developing insights into cell behavior in the in vivo setting, we now identify MT1-MMP as an upstream and necessary activating cofactor in regulating the YAP/TAZ-dependent control of SSC lineage commitment.
In the bone-marrow niche and bone matrix in vivo, SSCs display pericyte-like characteristics where they reside in juxtaposition to endothelial cells, osteoblasts, macrophages, and hematopoietic stem cells (Méndez-Ferrer et al., 2010; Morikawa et al., 2009; Omatsu et al., 2010; Park et al., 2012; Sacchetti et al., 2007). Whereas our studies have focused on SSC-fate decisions limited to the selection of osteoblastogenic, adipogenic, or chondrogenic differentiation programs, recent studies have identified additional roles for bone-marrow SSCs, adipocytes, and osteoblasts in controlling bone repair, hematopoiesis, lymphocyte commitment, and differentiation as well as blood cell mobilization (Maes et al., 2010; Naveiras et al., 2009; Park et al., 2012; Raaijmakers et al., 2010; Shi et al., 2011; Zhu et al., 2007). These complex intercellular interactions are further complemented by new roles for MT1-MMP in controlling macrophage gene expression by proteinase-dependent as well as -independent mechanisms (Ehninger and Trumpp, 2011; Sakamoto and Seiki, 2010; Shimizu-Hirota et al., 2012). Together, these findings support a model wherein MT1-MMP—a proteinase whose expression in humans is potentially regulated by polymorphisms, mutations, and diverse skeletal-associated factors ranging from estrogens to fluid sheer stress (Chun et al., 2010;Evans et al., 2012; Kang et al., 2011; Liao et al., 2004)—serves as a key regulator of the SSC niche.
EXPERIMENTAL PROCEDURES
Mice
To generate the Mmp14Lox strain, a targeted ES cell line (Figure 1G) was injected into 129SV blastocysts with chimeric offspring mated to generate the Mmp14Lox/− allele. Mmp14Lox/− mice were mated to C57BL/6 mice for at least six generations, and Mmp14Lox/− mice were then bred to generate Mmp14Lox/Lox mice. MT1-MMP+/LacZ mice in a C57BL/6 background were generated as described (Yana et al., 2007). Dermo1-Cre knockin mice (Yu et al., 2003), Osterix-GFP-Cre transgenic mice (Rodda and McMahon, 2006), and CByJ.B6-Tg (CAG-EGFP)1Osb/J mice were obtained from the Jackson Laboratory. Mmp14+/− mice (Swiss Black background), obtained from the National Institutes of Health (Holmbeck et al., 1999), were mated to produce Mmp14−/− and Mmp14+/+ mice. Nu/Nu mice were bought from Charles River Laboratories. All mouse work was performed with the approval of the Institutional Animal Care and Use Committee of the University of Michigan.
Cell Culture
Bone-marrow cells were isolated from mice and cultured in DMEM supplemented with 10% fetal bovine serum (FBS). The adherent colonies were sorted by flow cytometry with antibodies to Sca-1, CD29, CD45, and CD11b as described (Tang et al., 2009). The sorted cells (termed SSCs) were cultured and used within five passages (Tang et al., 2009). For 3D culture, 5 × 105 cells/ml were embedded in 2.16 mg/ml rat-tail-derived type I collagen gels (Sabeh et al., 2009) and cultured in Transwell dishes with a 0.4 μm pore size. For PEG-based hydrogel cultures, 2 × 105 cells/ml were mixed with hydrogel solution and cultured according to the manufacturer’s instructions (QGel SA). RGD peptide-modified alginate gels were prepared as described (Yu et al., 2009). In brief, 2 × 105 cells/ml (for osteogenesis and adipogenesis) or 2 × 106 cells/ml (for chondrogenesis) were suspended in the peptide-modified alginate gel solution and a CaCl2 solution was added to induce solidification. For 2D culture, SSCs were cultured at low density atop tissue-culture plastic or glass slides in six-well dishes (1 × 104 cells/9.4 cm2).
Differentiation of SSCs In Vitro
SSCs were induced with osteogenic medium (50 μM ascorbic acid, 10 nM dexamethasone, 10 mM β-glycerolphosphate, and 2% FBS in low-glucose DMEM) or adipogenic medium (1 μM dexamethasone, 50 μM indomethacin, 500 nM 3-isobutyl-1-methylxanthine, 5 μg/ml insulin, and 2% FBS in lowglucose DMEM) (Anjos-Afonso and Bonnet, 2008). For dual induction, equal volumes of osteogenic and adipogenic media were added to the SSC cultures (McBeath et al., 2004). For chondrogenesis, SSC micromass cultures were carried out as described (Murdoch et al., 2007) or with cells embedded in either 3D collagen or alginate. SSCs were induced with a StemPro Chondrogenesis Differentiation Kit (Invitrogen). The differentiated cells were stained for alkaline phosphatase activity, alizarin red S for detecting calcium deposits, oil red O for detecting lipid deposits, or Alcian blue (glycosaminoglycans) and safranin O (cartilage mucopolysaccharides)/fast green for monitoring chondrogenesis.
Vector Construction and Gene Expression
Where indicated, MT1-MMP−/− SSCs were transduced with either full-length human MT1-MMP, a catalytically inactive MT1-MMP mutant harboring an E-A240 substitution in the catalytic domain (Shimizu-Hirota et al., 2012), a constitutively active β1-integrin mutant (G429N), a β1-integrin clustering mutant (V737N) (Paszek et al., 2005), constitutively active 5SA-YAP1 (Zhao et al., 2012), or constitutively active TAZ-S89A (Hong et al., 2005). All constructs were cloned into the pLentilox-IRS-GFP lentiviral vector, and transduced SSCs were sorted by flow cytometry. Expression of exogenous proteins was confirmed by western blot.
Statistics
Statistical analysis was performed with the Student’s t test or by two-way ANOVA. *p < 0.05, **p < 0.01. All experiments were repeated three or more times.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant CA07169915 to S.J.W. Work performed in this study was also supported by Michigan Diabetes Research and Training Center Cell and Molecular Biology Core NIH grant P60 DK020572. We thank Suneel Apte for helpful comments.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2013.04.011.
REFERENCES
- Anjos-Afonso F, Bonnet D. Isolation, culture, and differentiation potential of mouse marrow stromal cells. Curr. Protoc. Stem Cell. Biol. 2008;7:2B.3.1–2B.3.11. doi: 10.1002/9780470151808.sc02b03s7. [DOI] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KM, Wong HL, Jin G, Liu B, Cao R, Cao Y, Lehti K, Tryggvason K, Zhou Z. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev. Cell. 2012;22:1176–1190. doi: 10.1016/j.devcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Chun TH, Inoue M, Morisaki H, Yamanaka I, Miyamoto Y, Okamura T, Sato-Kusubata K, Weiss SJ. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 2010;59:2484–2494. doi: 10.2337/db10-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrbar M, Sala A, Lienemann P, Ranga A, Mosiewicz K, Bittermann A, Rizzi SC, Weber FE, Lutolf MP. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J. 2011;100:284–293. doi: 10.1016/j.bpj.2010.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BR, Mosig RA, Lobl M, Martignetti CR, Camacho C, Grum-Tokars V, Glucksman MJ, Martignetti JA. Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am. J. Hum. Genet. 2012;91:572–576. doi: 10.1016/j.ajhg.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker’s guide to mechanobiology. Dev. Cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, Spiegelman BM. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J. Cell Biol. 2003;163:661–671. doi: 10.1083/jcb.200307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Zhang F, Chan KM, Xavier Wong HL, Liu B, Cheah KS, Liu X, Mauch C, Liu D, Zhou Z. MT1-MMP cleaves Dll1 to negatively regulate Notch signalling to maintain normal B-cell development. EMBO J. 2011;30:2281–2293. doi: 10.1038/emboj.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Kwak HI, Kaunas R, Bayless KJ. Fluid shear stress and sphingosine 1-phosphate activate calpain to promote membrane type 1 matrix metalloproteinase (MT1-MMP) membrane translocation and endothelial invasion into three-dimensional collagen matrices. J. Biol. Chem. 2011;286:42017–42026. doi: 10.1074/jbc.M111.290841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarchyk MA, Shreim SG, Alvarez-Elizondo MB, Estrada LC, Singh R, Valdevit L, Kniazeva E, Gratton E, Putnam AJ, Botvinick EL. Concentration independent modulation of local micromechanics in a fibrin gel. PLoS One. 2011;6:e20201. doi: 10.1371/journal.pone.0020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane SM, Inada M. Matrix metalloproteinases and bone. Bone. 2008;43:7–18. doi: 10.1016/j.bone.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Liao EY, Liao HJ, Guo LJ, Zhou HD, Wu XP, Dai RC, Luo XH. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is down-regulated in estrogen-deficient rat osteoblast in vivo. J. Endocrinol. Invest. 2004;27:1–5. doi: 10.1007/BF03350902. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Fatahi R, Kronenberg M, Kalajzic I, Rowe D, Li Y, Maye P. Isolation of murine bone marrow derived mesenchymal stem cells using Twist2 Cre transgenic mice. Bone. 2010;47:916–925. doi: 10.1016/j.bone.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Li XY, Hu Y, Rowe RG, Weiss SJ. MT1-MMP controls human mesenchymal stem cell trafficking and differentiation. Blood. 2010;115:221–229. doi: 10.1182/blood-2009-06-228494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch AD, Grady LM, Ablett MP, Katopodi T, Meadows RS, Hardingham TE. Chondrogenic differentiation of human bone marrow stem cells in Transwell cultures: generation of scaffold-free cartilage. Stem Cells. 2007;25:2786–2796. doi: 10.1634/stemcells.2007-0374. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J. Histochem. Cytochem. 1998;46:371–377. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- Nishida C, Kusubata K, Tashiro Y, Gritli I, Sato A, Ohki-Koizumi M, Morita Y, Nagano M, Sakamoto T, Koshikawa N, et al. MT1-MMP plays a critical role in hematopoiesis by regulating HIF-mediated chemokine/cytokine gene transcription within niche cells. Blood. 2012;119:5405–5416. doi: 10.1182/blood-2011-11-390849. [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, Schieker M, Docheva D. Integrins α2β1 and α11β1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala JK, Pouwels J, Pellinen T, Veltel S, Laasola P, Mattila E, Potter CS, Duffy T, Sundberg JP, Kallioniemi O, et al. SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat. Cell Biol. 2011;13:1315–1324. doi: 10.1038/ncb2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Rodríguez JP, Montecinos L, Ríos S, Reyes P, Martínez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J. Cell. Biochem. 2000;79:557–565. doi: 10.1002/1097-4644(20001215)79:4<557::aid-jcb40>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu. Rev. Cell Dev. Biol. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J. Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Fox D, Weiss SJ. Membrane-type I matrix metalloproteinase-dependent regulation of rheumatoid arthritis synoviocyte function. J. Immunol. 2010;184:6396–6406. doi: 10.4049/jimmunol.0904068. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Seiki M. A membrane protease regulates energy production in macrophages by activating hypoxia-inducible factor-1 via a non-proteolytic mechanism. J. Biol. Chem. 2010;285:29951–29964. doi: 10.1074/jbc.M110.132704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Sottile J. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J. Cell Sci. 2011;124:4039–4050. doi: 10.1242/jcs.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating Toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Hirota R, Xiong W, Baxter BT, Kunkel SL, Maillard I, Chen XW, Sabeh F, Liu R, Li XY, Weiss SJ. MT1-MMP regulates the PI3Kδ·Mi-2/NuRD-dependent control of macrophage immune function. Genes Dev. 2012;26:395–413. doi: 10.1101/gad.178749.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabova L, Yamada SS, Wimer H, Chrysovergis K, Ingvarsen S, Behrendt N, Engelholm LH, Holmbeck K. MT1-MMP and type II collagen specify skeletal stem cells and their bone and cartilage progeny. J. Bone Miner. Res. 2009;24:1905–1916. doi: 10.1359/JBMR.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancred TM, Belch AR, Reiman T, Pilarski LM, Kirshner J. Altered expression of fibronectin and collagens I and IV in multiple myeloma and monoclonal gammopathy of undetermined significance. J. Histochem. Cytochem. 2009;57:239–247. doi: 10.1369/jhc.2008.952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee SY, Fu J, Chen CS, Janmey PA. Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J. 2011;100:L25–L27. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Jarrell A, Zentner GE, Welsh A, Brownell I, Scacheri PC, Atit R. Role of canonical Wnt signaling/β-catenin via Dermo1 in cranial dermal cell development. Development. 2010;137:3973–3984. doi: 10.1242/dev.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi S, Aoki T, Sato H, et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J. Cell Sci. 2007;120:1607–1614. doi: 10.1242/jcs.000679. [DOI] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Yu J, Gu Y, Du KT, Mihardja S, Sievers RE, Lee RJ. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009;30:751–756. doi: 10.1016/j.biomaterials.2008.09.059. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhang Z, Feng JQ, Dusevich VM, Sinha K, Zhang H, Darnay BG, de Crombrugghe B. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc. Natl. Acad. Sci. USA. 2010;107:12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.