Abstract
Background
Phototherapy is one of the most efficacious treatment options for psoriasis. New, emerging studies are beginning to define the biological mechanisms by which phototherapy improves psoriasis.
Methods
To provide an overview of the mechanisms thought to be responsible for the therapeutic effects of phototherapy, a review was performed on all relevant published studies in the Medline database from January 1st, 1985 to August 15th, 2011.
Findings
Four categories of action were proposed in the literature to describe the effects of phototherapy in psoriasis: 1) alteration of cytokine profile, 2) induction of apoptosis, 3) promotion of immunosuppression, and 4) all other mechanisms.
Conclusions
Phototherapy acts through a combination of pathways to confer therapeutic benefits in psoriasis, and these different modalities may help explain its particular usefulness in treating this cutaneous disease.
Introduction
Phototherapy has long been used for the treatment of skin conditions. In ancient times, Egyptians were known to use sunlight to treat a variety of skin ailments, while other early civilizations including the Romans and Greeks also used sunlight for therapeutic purposes.1 More sophisticated uses of phototherapy, specifically for the treatment of psoriasis, have only occurred more recently—with significant developments taking place starting in the early 20th century. In 1925, Dr. William Goeckerman described the benefits of treating psoriasis using ultraviolet rays in combination with crude coal tar.2 In the 1950s, Dr. John Ingram developed a treatment regimen using ultraviolet B (UVB) radiation in conjunction with coal tar and anthralin paste.3 In the 1970s, broadband UVB was discovered to be effective in clearing mild forms of psoriasis when given in doses,4 while ultraviolet A (UVA) irradiation in combination with either oral5, 6 or topical application7 of psoralen, was found to be effective in treating psoriasis. In the 1980s, a more defined wavelength of UVB was discovered by researchers to be particularly effective in treating psoriasis—and was subsequently referred to as narrowband UVB (nbUVB).8
Phototherapy is now one of the most common treatment options for psoriasis—with nbUVB and psoralen ultraviolet A (PUVA) as the most widely used applications.9, 10 Clinical studies have also demonstrated the efficacy of phototherapy as one of the most effective treatment options, especially for patients with widespread disease who have moderate to severe psoriasis.11 Although phototherapy is now recognized as one of the efficacious treatment options for psoriasis, the biological mechanisms by which phototherapy improves psoriasis are only now becoming understood. In this review, we provide an overview of several mechanisms thought to be responsible for the therapeutic effects of phototherapy in psoriasis.
Methods
A review of studies that investigated the mechanisms of action of phototherapy in psoriasis was performed. Eligibility criteria included all studies published in English in the Medline database from January 1, 1985 to August 15th, 2011. Two investigators independently completed the screening process to minimize the risk of bias, and disagreements were resolved by mutual consensus. The inclusion criteria included all original peer-reviewed articles, including all relevant human and non-human in vitro and in vivo studies. Articles were found using the following MESH terms: “Phototherapy” AND “Psoriasis.” Keyword searches were also used as a secondary search strategy to ensure all relevant articles were included. After completing the literature search, the key findings of each study were extracted and were broadly categorized.
Results
Four categories of action were proposed in the literature to describe the effects of phototherapy on psoriasis: 1) alteration of cytokine profile, 2) induction of apoptosis, 3) promotion of immunosuppression, and 4) all other mechanisms.
Alteration of Cytokine Profile
Helper T cells guide the host immune response by differentiating into distinct effector subsets, which result in alternative patterns of cytokine expression. The three main T helper subsets in humans are Th1, Th2, and Th17. Psoriasis is thought to be a Th1/Th17 mediated inflammatory process driven by over-expression of Th1 and Th17-associated cytokines—leading to keratinocyte hyperproliferation and inflammation (Figure 1). As such, psoriatic lesions are characterized by an increase in Th1 and Th17 cytokines, and a relative decrease in Th2 cytokines compared to normal skin.12, 13 Several studies have described how phototherapy reverses the cytokine profile typically seen in psoriasis, by shifting the immune response towards the counter-regulatory Th2 axis and away from the Th1/Th17 inflammatory axis.
Figure 1.
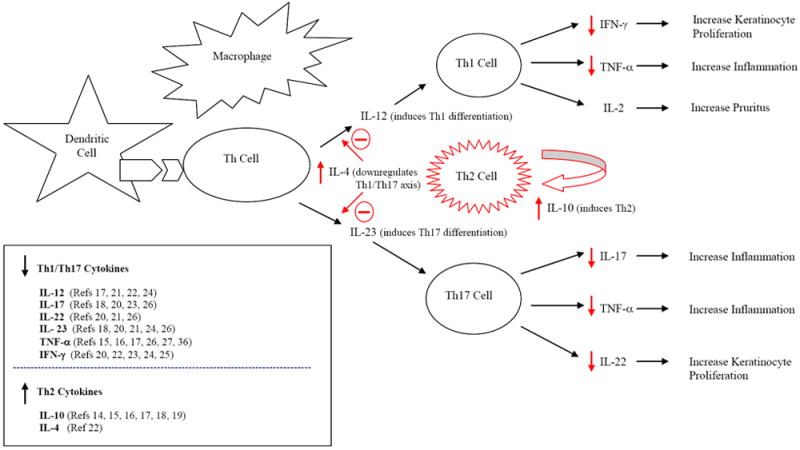
Immunopathogenesis of psoriasis and the impact of phototherapy on altering cytokine profile. In psoriasis, antigen presenting cells (dendritic and macrophage cells) activate naïve helper T (Th) cells and induce their differentiation into Th1 and Th17 cells, leading to the release of Th1 and Th17 cytokines that promote inflammation and epidermal hyperplasia. Phototherapy has been shown to down-regulate the Th1/Th17 pro-inflammatory axis and up-regulate the counter-regulatory Th2 pathway, leading to clinical improvement. Effects of phototherapy shown in red.
Several early studies described an up-regulation of Th2 cytokines in response to phototherapy. UVB-irradiated keratinocyte cultures from nine healthy subjects led to enhanced transcription and expression of IL-1014—a key immunomodulatory cytokine in the Th2 pathway. Increased IL-10 levels were also observed in the skin of 71 healthy patients exposed to three minimal erythema doses (MED) of solar-simulated UV-radiation, most notably at 15-24 hours post-irradiation,15 while another study found significant increases in IL-10 concentration in UVB irradiated skin of 25 healthy patients after three MED, with maximal values at approximately the same time point.16 The administration of recombinant IL-10 into psoriatic plaques resulted in increased Th2 cytokine expression of IL-4, IL-5, and IL-10, and decreased Th1 cytokine expression of IL-12 and TNF-alpha.17 Subsequent studies have reported similar patterns of upregulation of IL-10 expression in response to UV exposure—including increased expression of IL-10 in both the epidermis and dermis of psoriasis patients who were exposed to controlled natural sunlight,18 and greater levels of IL-10 expression in circulating T-cells in four psoriasis subjects exposed to nbUVB.19
Several studies have also consistently found evidence of down-regulation of the Th1/Th17 pro-inflammatory pathways in psoriasis with phototherapy. One group found that natural sun exposure led to a decrease in Th17 inflammatory cytokine expression, specifically IL-17 and IL-23, in the psoriatic lesions of three out of four patients investigated in the study.18 nbUVB treatment suppressed Th1/Th17 cytokines in fourteen patients with moderate-to-severe psoriasis, with decreased levels of IL-12, IL-17, IL-20, IL22, and IL-23 observed—but only in psoriatic plaques that showed clinical improvement with phototherapy.20 Similarly, chronic plaque-type psoriasis lesions that were responsive to nbUVB treatment showed decreased expression of IFN-γ inducers in the Th1/Th17 pathways, specifically IL-12, IL-18, and IL-23.21 One group of researchers reported that nbUVB irradiated psoriasis lesions had significantly decreased levels of mRNA expression of IL-12 and IFN-γ, while IL-4 expression—a Th2 cytokine that suppresses the Th1/Th17 axis—was discovered to be significantly upregulated.22 A recent study employing gene expression profiling revealed that the Th17 and IFN signaling pathways are inhibited in human psoriatic skin irradiated with nbUVB.23 Researchers also found decreased expression of Th1/Th17 inflammatory cytokines, specifically IFN-γ, IL-12, and IL-23, in both the epidermis and dermis in the psoriatic lesions of fifteen patients who underwent twenty sessions of PUVA therapy.24 Similar findings with PUVA therapy were also reported in a study that used a mouse model to show evidence of IL-23/Th17 axis suppression, including results that indicated a down-regulation of IFN-γ expression by 10-fold after eight sessions of photochemotherapy.25
While most studies have found localized changes in cytokine expression within the psoriatic lesions, two studies have found systemic changes in cytokine expression that reflected a down-regulation of the Th1/Th17 axis. In one case-control study, thirty-four psoriasis patients treated with either PUVA or nbUVB had significantly lower plasma levels of TNF-α and IL-23 at end of the third weeks of treatment, along with decreased serum levels of IL-22 and IL-17 at the completion of six weeks of therapy.26 Another study found a decreased number of regulatory CD4+CD25+ T cells, as well as lowered levels of TNF-α in the serum of 12 patients with severe psoriasis after one-month of PUVA therapy—compared to before treatment.27
Induction of Apoptosis
Apoptosis is the process of programmed cell death that can be induced by a variety of external stimuli including hypoxia, infection, heat, and radiation exposure. Several factors can also trigger the process of organized cell death—injury to cellular membranes, damage to intracellular organelles, and activation of tumor suppressor genes. Characteristic morphological changes in apoptosis include cell shrinkage, membrane blebbing, and chromosomal DNA fragmentation.28
Several studies have implicated apoptosis as a key process by which phototherapy is effective in the treatment of psoriasis. Interestingly, the induction of apoptosis appears to occur in several different skin cell types. One study found that UVB therapy led to selective apoptosis and profound depletion of T lymphocytes in epidermal psoriatic tissue, but minimally in the dermis. In this study, keratinocytes also underwent apoptosis, but only in response to high levels of UVB. The authors concluded that the deletion of activated lymphocyte clones were responsible for producing sustained therapeutic improvements typically seen with phototherapy.29 Similarly, nbUVB was found to lead to the apoptosis of T lymphocytes from the epidermis and dermis of psoriatic lesions, both in vivo and in vitro.30 Enhanced keratinocyte apoptosis was observed in thirty psoriasis patients on PUVA therapy after six weeks of treatment.31 Similarly, increased levels of keratinocyte apoptosis and significant expression of epidermal p53, a tumor suppressor protein, were seen in the non-lesional skin of five psoriasis patients who underwent nbUVB irradiation.32 That both T cells and keratinocytes may be targets of apoptosis was confirmed in a study which showed that the percentage distribution of the two cell types undergoing UVB-induced apoptosis was approximately the same as their relative proportions in epidermis.33 Although Langerhans cells (LCs) were found to be depleted in the epidermis following UVB irradiation, this may reflect migration of LCs from skin to subcutaneous lymph node, although apoptosis likely played a small role as well.34 In sum, evidence from the literature indicates that phototherapy induces apoptosis of a variety of relevant cells in psoriasis, including epidermal T cells, dermal T cells, keratinocytes, and to a lesser degree, Langerhans cells (Figure 2).
Figure 2.
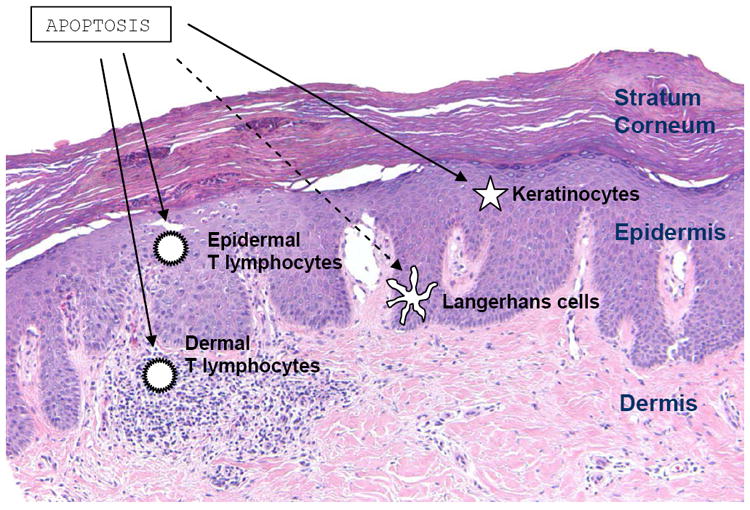
Cellular targets of phototherapy-induced apoptosis in the treatment of psoriasis. Phototherapy is believed to induce programmed cell death in T lymphocytes in both the epidermal and dermal psoriatic tissues. Apoptosis of keratinocytes is also thought to occur in lesional and nonlesional epidermis, while Langerhans cell depletion from apoptosis is believed to play a less significant role. Photo courtesy of Beth Ruben, M.D., Departments of Dermatology and Pathology, University of California, San Francisco.
The specific mechanisms by which phototherapy induces apoptosis have been described in a number of studies. UVB-mediated apoptosis in human epithelial cells is thought to occur through DNA damage—particularly through the formation of pyrimidine dimers, and through injury to the cellular membrane—resulting in death receptor activation and the triggering of the apoptosis cascade.35 More specifically, UV light is thought to induce clustering and internalization of TNF, IL-1, and EGF receptors on cell surfaces,36 and activate CD95 surface molecules,37 both of which trigger pathways that lead to programmed cell death. UV radiation is also believed to induce apoptosis by creating reactive oxygen species that damage cellular, mitochondrial, and nuclear membranes—and by directly damaging DNA.38-40 Furthermore, UVB-irradiation can induce apoptosis of intraepidermal T cells, by increasing Fas-ligand expression on the surface of keratinocytes, which then bind to infiltrated T cells in the epidermis to trigger apoptosis.41, 42
Promotion of Immunosuppression
In addition to its role in triggering apoptosis in psoriasis lesions and down-regulating the Th1/Th17 inflammatory pathways, phototherapy has been suggested in a number of studies to induce other changes that reflect immunosuppression.
UV-induced immunosuppression of epidermal Langerhans cells (LCs) was described in several studies to help explain the therapeutic effects of phototherapy. In one study, researchers found a decrease in LCs in non-lesional epidermal tissue in five psoriasis patients who were exposed to nbUVB.32 Similarly, decreased LC density was observed in healthy human skin after exposure to either UV solar simulated radiation, UVA radiation alone, or UVA + UVB.43 Decreased density of LCs in lesional epidermis was also observed in response to natural sun exposure.18 These findings were confirmed by researchers who found significant reductions in LCs in the epidermis, and significant increases in LCs in the draining lymph nodes, in mice irradiated with chronic solar simulated radiation.44 In vitro studies demonstrated that low-dose UVB irradiation of healthy human skin resulted in decreased dendritic cell expression of B7 co-stimulatory signals, which normally bind to CD28 and CTLA-4 on T lymphocytes.45
Besides inducing the migration of Langerhans cells out of the skin and immunosuppression of Langerhans cells, phototherapy has been implicated in causing other immunomodulatory effects. A team of Japanese researchers discovered evidence of decreased mast cell degranulation and histamine release from UVB and PUVA irradiated skin in animal models 46-48—findings that may be associated with the reduction of erythema and pruritus in psoriatic lesions.49 A separate study also reported evidence of diminished contact hypersensitivity in UVB irradiated skin in an animal model,50 while some studies,51-53 but not all,54 have implicated the accumulation of cis-urocanic acid, formed in the epidermis by UV irradiation, as a mediator of contact hypersensitivity suppression. Furthermore, researchers found increased recruitment of FOXP3+ cells, which are regulatory T cells that suppress the activation of the immune system, in the dermal compartment of human psoriatic skin irradiated with natural sunlight.18
Other Mechanisms
Additional hypotheses have been raised in the literature to explain the therapeutic benefits of phototherapy in the treatment of psoriasis. Cell-cycle arrest has been attributed to the therapeutic properties of phototherapy. In one case-control study comparing 25 psoriasis patients to 10 healthy controls, lower levels of p53—a cell-cycle suppressor protein, and higher levels of cyclin D1—a cell-cycle promoter protein, were found in skin biopsies of psoriatic lesions. Normalization of both p53 and cyclin D1 occurred after psoriatic skin was exposed to either nbUVB or PUVA therapy.55 These findings are similar to other studies which found decreased expression of cyclin D1 after UV irradiation of murine macrophage56 and human epidermoid carcinoma cells.57
Another mechanism proposed to describe the therapeutic action of phototherapy in psoriasis is the inhibition of dsRNA activity. Psoriatic lesions have increased double-stranded RNA (dsRNA) receptor level and activity, which is thought to play a role in the innate immune system. Normalization of dsRNA occurred with exposure to nbUVB irradiation through the inhibition of both mRNA expression and dsRNA receptor synthesis.58
Furthermore, phototherapy is also thought to alter gene expression in psoriatic skin beyond the mechanisms discussed in the first three broad categories of action. One group found that the insulin-like growth factor-binding protein 7 (IGFBP7) gene is under expressed in psoriatic epidermis, but that nbUVB led to significant increased levels of expression. The authors hypothesized that IGFBP7 may be an anti-proliferative protein that controls cell migration, angiogenesis, and inflammation.59
Discussion
A comprehensive review of the literature suggests that phototherapy acts through a combination of pathways to confer therapeutic benefits in psoriasis. A number of mechanisms of have been implicated, including alterations in cytokine expression that suppress the Th1/Th17 inflammatory axis while up-regulating the Th2 pathway, induction of apoptosis of various cutaneous cells, and immunosuppression of Langerhans cells and other key cells that play a significant role in the inflammatory process of psoriasis. These different modalities suggest that phototherapy treatment for psoriasis is likely intricate and multi-faceted—and reflects the complex nature of psoriasis pathophysiology.
While the mechanisms described in this manuscript have been classified into four broad categories of action, some of these mechanisms defined in the literature may have overlapping features. For example, apoptosis of Th1 and Th17 helper T cells may lead to a decrease in Th1 and Th17 associated cytokines, and apoptosis may also be considered a form of immunosuppression. As such, these classifications should be used with flexibility, as these categories have shared overlapping characteristics.
Furthermore, it remains unclear as to which processes described in the literature play a more significant role in the therapeutic benefits of phototherapy, or which processes occur first. Several different modalities may be responsible for creating synergistic effects. Future studies will be needed to clarify which modes of action play a larger role in the treatment of psoriasis, and more precisely, how these mechanisms are interrelated.
Acknowledgments
Funding Sources: None.
Footnotes
Conflict of Interest Disclosure: None.
Prior Presentation: None.
References
- 1.McDonagh AF. Phototherapy: from ancient Egypt to the new millennium. J Perinatol. 2001;21(Suppl 1):S7–S12. doi: 10.1038/sj.jp.7210625. [DOI] [PubMed] [Google Scholar]
- 2.Perry HO, Soderstrom CW, Schulze RW. The Goeckerman treatment of psoriasis. Arch Dermatol. 1968;98:178–82. [PubMed] [Google Scholar]
- 3.Ingram JT. The approach to psoriasis. Br Med J. 1953;2:591–4. doi: 10.1136/bmj.2.4836.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359–62. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- 5.Melski JW, Tanenbaum L, Parrish JA, Fitzpatrick TB, Bleich HL. Oral methoxsalen photochemotherapy for the treatment of psoriasis: a cooperative clinical trial. J Invest Dermatol. 1977;68:328–35. doi: 10.1111/1523-1747.ep12496022. [DOI] [PubMed] [Google Scholar]
- 6.Wolff KW, Fitzpatrick TB, Parrish JA, Gschnait F, Gilchrest B, Honigsmann H, et al. Photochemotherapy for psoriasis with orally administered methoxsalen. Arch Dermatol. 1976;112:943–50. [PubMed] [Google Scholar]
- 7.Walter JF, Voorhees JJ. Psoriasis improved by psoralen plus black light. Acta Derm Venereol. 1973;53:469–72. [PubMed] [Google Scholar]
- 8.Fischer T, Alsins J. Treatment of psoriasis with trioxsalen baths and dysprosium lamps. Acta Derm Venereol. 1976;56:383–90. [PubMed] [Google Scholar]
- 9.Duarte I, Cunha JA, Bedrikow RB, Lazzarini R. What is the most common phototherapy prescription for psoriasis: NB-UVB or PUVA? Prescription behavior. An Bras Dermatol. 2009;84:244–8. doi: 10.1590/s0365-05962009000300005. [DOI] [PubMed] [Google Scholar]
- 10.Koo JY. Current consensus and update on psoriasis therapy: a perspective from the U.S. J Dermatol. 1999;26:723–33. doi: 10.1111/j.1346-8138.1999.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 11.Lapolla W, Yentzer BA, Bagel J, Halvorson CR, Feldman SR. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936–49. doi: 10.1016/j.jaad.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–9. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 13.Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–5. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 14.Enk CD, Sredni D, Blauvelt A, Katz SI. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;154:4851–6. [PubMed] [Google Scholar]
- 15.Barr RM, Walker SL, Tsang W, Harrison GI, Ettehadi P, Greaves MW, et al. Suppressed alloantigen presentation, increased TNF-alpha, IL-1, IL-1Ra, IL-10, and modulation of TNF-R in UV-irradiated human skin. J Invest Dermatol. 1999;112:692–8. doi: 10.1046/j.1523-1747.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 16.Skov L, Hansen H, Allen M, Villadsen L, Norval M, Barker JN, et al. Contrasting effects of ultraviolet A1 and ultraviolet B exposure on the induction of tumour necrosis factor-alpha in human skin. Br J Dermatol. 1998;138:216–20. doi: 10.1046/j.1365-2133.1998.02063.x. [DOI] [PubMed] [Google Scholar]
- 17.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–94. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soyland E, Heier I, Rodriguez-Gallego C, Mollnes TE, Johansen FE, Holven KB, et al. Sun exposure induces rapid immunological changes in skin and peripheral blood in patients with psoriasis. Br J Dermatol. 2011;164:344–55. doi: 10.1111/j.1365-2133.2010.10149.x. [DOI] [PubMed] [Google Scholar]
- 19.Sigmundsdottir H, Johnston A, Gudjonsson JE, Valdimarsson H. Narrowband-UVB irradiation decreases the production of pro-inflammatory cytokines by stimulated T cells. Arch Dermatol Res. 2005;297:39–42. doi: 10.1007/s00403-005-0565-9. [DOI] [PubMed] [Google Scholar]
- 20.Johnson-Huang LM, Suarez-Farinas M, Sullivan-Whalen M, Gilleaudeau P, Krueger JG, Lowes MA. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J Invest Dermatol. 2010;130:2654–63. doi: 10.1038/jid.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piskin G, Tursen U, Sylva-Steenland RM, Bos JD, Teunissen MB. Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers -- IL-12, IL-18 and IL-23. Exp Dermatol. 2004;13:764–72. doi: 10.1111/j.0906-6705.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 22.Walters IB, Ozawa M, Cardinale I, Gilleaudeau P, Trepicchio WL, Bliss J, et al. Narrowband (312-nm) UV-B suppresses interferon gamma and interleukin (IL) 12 and increases IL-4 transcripts: differential regulation of cytokines at the single-cell level. Arch Dermatol. 2003;139:155–61. doi: 10.1001/archderm.139.2.155. [DOI] [PubMed] [Google Scholar]
- 23.Racz E, Prens EP, Kurek D, Kant M, de Ridder D, Mourits S, et al. Effective treatment of psoriasis with narrow-band UVB phototherapy is linked to suppression of the IFN and Th17 pathways. J Invest Dermatol. 2011;131:1547–58. doi: 10.1038/jid.2011.53. [DOI] [PubMed] [Google Scholar]
- 24.Ravic-Nikolic A, Radosavljevic G, Jovanovic I, Zdravkovic N, Mitrovic S, Pavlovic S, et al. Systemic photochemotherapy decreases the expression of IFN-gamma, IL-12p40 and IL-23p19 in psoriatic plaques. Eur J Dermatol. 2011;21:53–7. doi: 10.1684/ejd.2010.1199. [DOI] [PubMed] [Google Scholar]
- 25.Singh TP, Schon MP, Wallbrecht K, Michaelis K, Rinner B, Mayer G, et al. 8-methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+ regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder. J Immunol. 2010;184:7257–67. doi: 10.4049/jimmunol.0903719. [DOI] [PubMed] [Google Scholar]
- 26.Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumour necrosis factor-alpha levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol. 2010;163:1282–90. doi: 10.1111/j.1365-2133.2010.09992.x. [DOI] [PubMed] [Google Scholar]
- 27.Rotsztejn H, Zalewska A, Trznadel-Budzko E, Lewkowicz P, Banasik M, Tchorzewski H, et al. Influence of systemic photochemotherapy on regulatory T cells and selected cytokine production in psoriatic patients: a pilot study. Med Sci Monit. 2005;11:CR594–8. [PubMed] [Google Scholar]
- 28.Lippens S, Hoste E, Vandenabeele P, Agostinis P, Declercq W. Cell death in the skin. Apoptosis. 2009;14:549–69. doi: 10.1007/s10495-009-0324-z. [DOI] [PubMed] [Google Scholar]
- 29.Krueger JG, Wolfe JT, Nabeya RT, Vallat VP, Gilleaudeau P, Heftler NS, et al. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J Exp Med. 1995;182:2057–68. doi: 10.1084/jem.182.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozawa M, Ferenczi K, Kikuchi T, Cardinale I, Austin LM, Coven TR, et al. 312-nanometer ultraviolet B light (narrow-band UVB) induces apoptosis of T cells within psoriatic lesions. J Exp Med. 1999;189:711–8. doi: 10.1084/jem.189.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceovifá R, Pasifá A, Lipozencifá J, Jakifá-Razumovifá J, Szirovicza L, Kostovifá K. Antiproliferative, antiangiogenic and apoptotic effect of photochemotherapy (PUVA) in psoriasis patients. Coll Antropol. 2007;31:551–6. [PubMed] [Google Scholar]
- 32.DeSilva B, McKenzie RC, Hunter JA, Norval M. Local effects of TL01 phototherapy in psoriasis. Photodermatol Photoimmunol Photomed. 2008;24:268–9. doi: 10.1111/j.1600-0781.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- 33.Weatherhead SC, Farr PM, Jamieson D, Hallinan JS, Lloyd JJ, Wipat A, et al. Keratinocyte Apoptosis in Epidermal Remodeling and Clearance of Psoriasis Induced by UV Radiation. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolgen W, Both H, van Weelden H, Guikers KL, Bruijnzeel-Koomen CA, Knol EF, et al. Epidermal langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: apoptosis versus migration. J Invest Dermatol. 2002;118:812–7. doi: 10.1046/j.1523-1747.2002.01742.x. [DOI] [PubMed] [Google Scholar]
- 35.Kulms D, Pppelmann B, Yarosh D, Luger TA, Krutmann J, Schwarz T. Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci U S A. 1999;96:7974–9. doi: 10.1073/pnas.96.14.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–7. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 37.Aragane Y, Kulms D, Metze D, Wilkes G, Popelmann B, Luger TA, et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140:171–82. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godar DE. UVA1 radiation triggers two different final apoptotic pathways. J Invest Dermatol. 1999;112:3–12. doi: 10.1046/j.1523-1747.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 39.Kulms D, Zeise E, Popelmann B, Schwarz T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene. 2002;21:5844–51. doi: 10.1038/sj.onc.1205743. [DOI] [PubMed] [Google Scholar]
- 40.Morita A, Werfel T, Stege H, Ahrens C, Karmann K, Grewe M, et al. Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. J Exp Med. 1997;186:1763–8. doi: 10.1084/jem.186.10.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leverkus M, Yaar M, Gilchrest BA. Fas/Fas ligand interaction contributes to UV-induced apoptosis in human keratinocytes. Exp Cell Res. 1997;232:255–62. doi: 10.1006/excr.1997.3514. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez-Steil C, Wrone-Smith T, Sun X, Krueger JG, Coven T, Nickoloff BJ. Sunlight-induced basal cell carcinoma tumor cells and ultraviolet-B-irradiated psoriatic plaques express Fas ligand (CD95L) J Clin Invest. 1998;101:33–9. doi: 10.1172/JCI1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seite S, Zucchi H, Moyal D, Tison S, Compan D, Christiaens F, et al. Alterations in human epidermal Langerhans cells by ultraviolet radiation: quantitative and morphological study. Br J Dermatol. 2003;148:291–9. doi: 10.1046/j.1365-2133.2003.05112.x. [DOI] [PubMed] [Google Scholar]
- 44.McLoone P, Woods GM, Norval M. Decrease in langerhans cells and increase in lymph node dendritic cells following chronic exposure of mice to suberythemal doses of solar simulated radiation. Photochem Photobiol. 2005;81:1168–73. doi: 10.1562/2005-04-10-RA-484. [DOI] [PubMed] [Google Scholar]
- 45.Weiss JM, Renkl AC, Denfeld RW, de Roche R, Spitzlei M, Schopf E, et al. Low-dose UVB radiation perturbs the functional expression of B7.1 and B7.2 co-stimulatory molecules on human Langerhans cells. Eur J Immunol. 1995;25:2858–62. doi: 10.1002/eji.1830251022. [DOI] [PubMed] [Google Scholar]
- 46.Danno K, Toda K, Horio T. The effect of 8-methoxypsoralen plus long-wave ultraviolet (PUVA) radiation on mast cells: PUVA suppresses degranulation of mouse skin mast cells induced by compound 48/80 or concanavalin A. J Invest Dermatol. 1985;85:110–4. doi: 10.1111/1523-1747.ep12276497. [DOI] [PubMed] [Google Scholar]
- 47.Danno K, Toda K, Horio T. Ultraviolet-B radiation suppresses mast cell degranulation induced by compound 48/80. J Invest Dermatol. 1986;87:775–8. doi: 10.1111/1523-1747.ep12458843. [DOI] [PubMed] [Google Scholar]
- 48.Toda K, Danno K, Tachibana T, Horio T. Effect of 8-methoxypsoralen plus long-wave ultraviolet (PUVA) radiation on mast cells. II. In vitro PUVA inhibits degranulation of rat peritoneal mast cells induced by compound 48/80. J Invest Dermatol. 1986;87:113–6. doi: 10.1111/1523-1747.ep12523611. [DOI] [PubMed] [Google Scholar]
- 49.Horio T. Indications and action mechanisms of phototherapy. J Dermatol Sci. 2000;23(Suppl 1):S17–21. doi: 10.1016/s0923-1811(99)00069-9. [DOI] [PubMed] [Google Scholar]
- 50.Miyauchi H, Horio T. Ultraviolet B-induced local immunosuppression of contact hypersensitivity is modulated by ultraviolet irradiation and hapten application. J Invest Dermatol. 1995;104:364–9. doi: 10.1111/1523-1747.ep12665832. [DOI] [PubMed] [Google Scholar]
- 51.Beissert S, Mohammad T, Torri H, Lonati A, Yan Z, Morrison H, et al. Regulation of tumor antigen presentation by urocanic acid. J Immunol. 1997;159:92–6. [PubMed] [Google Scholar]
- 52.Gibbs NK, Norval M, Traynor NJ, Crosby JC, Lowe G, Johnson BE. Comparative potency of broad-band and narrow-band phototherapy sources to induce edema, sunburn cells and urocanic acid photoisomerization in hairless mouse skin. Photochem Photobiol. 1993;58:643–7. doi: 10.1111/j.1751-1097.1993.tb04946.x. [DOI] [PubMed] [Google Scholar]
- 53.Gilmour JW, Vestey JP, Norval M. The effect of UV therapy on immune function in patients with psoriasis. Br J Dermatol. 1993;129:28–38. doi: 10.1111/j.1365-2133.1993.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 54.Guckian M, Jones CD, Vestey JP, Cooper EJ, Dawe R, Gibbs NK, et al. Immunomodulation at the initiation of phototherapy and photochemotherapy. Photodermatol Photoimmunol Photomed. 1995;11:163–9. doi: 10.1111/j.1600-0781.1995.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 55.Abou EL-Ela M, Nagui N, Mahgoub D, El-Eishi N, Fawzy M, El-Tawdy A, et al. Expression of cyclin D1 and p16 in psoriasis before and after phototherapy. Clin Exp Dermatol. 2010;35:781–5. doi: 10.1111/j.1365-2230.2009.03774.x. [DOI] [PubMed] [Google Scholar]
- 56.Miyakawa Y, Matsushime H. Rapid downregulation of cyclin D1 mRNA and protein levels by ultraviolet irradiation in murine macrophage cells. Biochem Biophys Res Commun. 2001;284:71–6. doi: 10.1006/bbrc.2001.4950. [DOI] [PubMed] [Google Scholar]
- 57.Kim AL, Athar M, Bickers DR, Gautier J. Ultraviolet-B-induced G1 arrest is mediated by downregulation of cyclin-dependent kinase 4 in transformed keratinocytes lacking functional p53. J Invest Dermatol. 2002;118:818–24. doi: 10.1046/j.1523-1747.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- 58.Racz E, Prens EP, Kant M, Florencia E, Jaspers NG, Laman JD, et al. Narrowband ultraviolet B inhibits innate cytosolic double-stranded RNA receptors in psoriatic skin and keratinocytes. Br J Dermatol. 2011;164:838–47. doi: 10.1111/j.1365-2133.2010.10169.x. [DOI] [PubMed] [Google Scholar]
- 59.Hochberg M, Zeligson S, Amariglio N, Rechavi G, Ingber A, Enk CD. Genomic-scale analysis of psoriatic skin reveals differentially expressed insulin-like growth factor-binding protein-7 after phototherapy. Br J Dermatol. 2007;156:289–300. doi: 10.1111/j.1365-2133.2006.07628.x. [DOI] [PubMed] [Google Scholar]


