Abstract
γ-Aminobutyric acid (GABA) is likely expressed in horizontal cells of all species, although conflicting physiological findings have led to considerable controversy regarding its role as a transmitter in the outer retina. This study has evaluated key components of the GABA system in the outer retina of guinea pig, an emerging retinal model system. The presence of GABA, its rate-limiting synthetic enzyme glutamic acid decarboxylase (GAD65 and GAD67 isoforms), the plasma membrane GABA transporters (GAT-1 and GAT-3), and the vesicular GABA transporter (VGAT) was evaluated by using immunohistochemistry with well-characterized antibodies. The presence of GAD65 mRNA was also evaluated by using laser capture microdissection and reverse transcriptase-polymerase chain reaction. Specific GABA, GAD65, and VGAT immunostaining was localized to horizontal cell bodies, as well as to their processes and tips in the outer plexiform layer. Furthermore, immunostaining of retinal whole mounts and acutely dissociated retinas showed GAD65 and VGAT immunoreactivity in both A-type and B-type horizontal cells. However, these cells did not contain GAD67, GAT-1, or GAT-3 immunoreactivity. GAD65 mRNA was detected in horizontal cells, and sequencing of the amplified GAD65 fragment showed approximately 85% identity with other mammalian GAD65 mRNAs. These studies demonstrate the presence of GABA, GAD65, and VGAT in horizontal cells of the guinea pig retina, and support the idea that GABA is synthesized from GAD65, taken up into synaptic vesicles by VGAT, and likely released by a vesicular mechanism from horizontal cells.
Keywords: GABA, GAD, GAT, VGAT, VIAAT, horizontal cells, retina, visual system
In the mammalian retina, horizontal cells likely synthesize γ-aminobutryic acid (GABA), although there are inconsistent and conflicting findings regarding both their expression of GABA and its synthetic enzyme, L-glutamic acid decarboxylase (GAD), in different species. Most studies describe GABA- and GAD-immunoreactive horizontal cells in the cat, rabbit, and monkey retina (Mosinger et al., 1986; Osborne et al., 1986; Agardh et al., 1987; Mosinger and Yazulla, 1987; Chun and Wässle, 1989; Pourcho and Owczarzak, 1989; Wässle and Chun, 1989; Vardi et al., 1994; Johnson and Vardi, 1998; Marc et al., 1998). In contrast, in the adult guinea pig retina, it is reported that GABA-containing horizontal cells are absent (Agardh et al., 1986; Osborne et al., 1986; Loeliger and Rees, 2005). There has also been a failure to consistently demonstrate either GABA or GAD immunoreactivity in horizontal cells of both adult mouse and rat retinas. In these species, GABA and GAD immunoreactivities are transiently expressed by horizontal cells during the first several weeks of postnatal retinal development, and immunoreactivity is consistently reported to be absent in adult retinas (Vaughn et al., 1981; Lin et al., 1983; Schnitzer and Rusoff, 1984; Brandon, 1985; Mosinger et al., 1986; Osborne et al., 1986; Versaux-Botteri et al., 1989; Yamasaki et al., 1999; Fletcher and Kalloniatis, 1997; Yamasaki et al., 1999; Dkhissi et al., 2001).
Unlike what is reported for GABA and GAD immunostaining, the vesicular GABA/glycine transporter (VGAT), which mediates the uptake and storage of GABA and/or glycine in synaptic vesicles (for reviews, see Liu and Edwards, 1997; Gasnier, 2004), is reliably expressed in mammalian horizontal cells (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Johnson et al., 2003; Guo et al., 2009). VGAT immunoreactivity is mainly in horizontal cell processes and their endings located within the photoreceptor synaptic triad (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Johnson et al., 2003; Guo et al., 2009), suggesting that GABA accumulates into vesicles of horizontal cell endings. Earlier studies showing small, clear-core vesicles in horizontal cell processes and endings are also congruent with the idea that there are synaptic vesicles in these endings (Dowling and Boycott, 1966; Dowling et al., 1966; Gray and Pease, 1971; Fisher and Boycott, 1974; Linberg and Fisher, 1988; Spiwoks-Becker et al., 2001).
GABA plasma membrane transporters (GATs) mediate high-affinity GABA uptake from the extracellular space and likely terminate GABA's synaptic action in the central nervous system (CNS) (for reviews, see Chen et al., 2004; Conti et al., 2004; Kanner, 2006). In addition to a role in the clearance of GABA, GATs may also release GABA by a reverse transport mechanism (O'Malley et al., 1992; Dalby, 2003; Richerson and Wu, 2003; Wu et al., 2007). A GAT reportedly mediates GABA release from retinal horizontal cells in fish and amphibians (Schwartz, 1982; Yazulla and Kleinschmidt, 1982, 1983; Schwartz, 1987, 2002; Attwell et al., 1993). However, in the mammalian retina, there is doubt regarding the hypothesis that GABA is released from horizontal cells by a plasma membrane transporter, because currently known GAT isoforms (GAT-1, GAT-2, and GAT-3) are not expressed by horizontal cells (Brecha and Weigmann, 1994; Durkin et al., 1995; Honda et al., 1995; Johnson et al., 1996; Hu et al., 1999; Casini et al., 2006; Guo et al., 2009). Furthermore, mammalian horizontal cells do not accumulate GABA and GABA analogs, as would be expected if a functional GAT were expressed by these cells (Neal and Iversen, 1972; Bruun and Ehinger, 1974; Marshall and Voaden, 1975; Cunningham et al., 1981; Agardh and Ehinger, 1982, 1983; Blanks and Roffler-Tarlov, 1982; Wässle and Chun, 1989).
The aim of the present study was to systematically evaluate the cellular expression of several key components of the GABA system in the outer retina of the guinea pig. These studies found that GABA, GAD65, and VGAT are strongly expressed in both A- and B-type horizontal cells. Together, the localization of the GAD65 and VGAT immunoreactivities to horizontal cell processes and endings suggests the local synthesis and accumulation of GABA into synaptic vesicles, and the likely release of GABA from these cells by a vesicular mechanism.
MATERIALS AND METHODS
Animals
Hartley guinea pigs (Charles River, Wilmington, MA) of either sex at 5–8 weeks age were used for these studies. Guinea pigs were maintained on a 12-hour light/12-hour dark cycle. Animal housing and all experiments were performed in accordance with the guidelines and policies for the welfare of experimental animals issued by the UCLA Animal Research Committee, and U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Guinea pigs were euthanized with 1–3% isoflurane (Hospira, Lake Forest, IL) before dissection of the eye.
For vertical retinal sections, the eyes were enucleated, the anterior chamber and lens were removed, and the eyecups were immersion-fixed in 4% (w/v) paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) for 15–60 minutes at room temperature. Following fixation, the eyes were stored in 30% sucrose for 48 hours at 4°C, briefly washed in 0.1 M PB, embedded in OCT compound (Sakura Finetek, Torrance, CA), and rapidly frozen with dry ice. The eyecup was cut vertically into 12-μm-thick sections with a Leica CM3005 cryostat (Leica Microsystems, Bannockburn, IL), and the retinal sections were mounted onto gelatin-coated slides, air-dried, and stored at −20°C. Every 10th section was collected from superior retina to inferior retina. For horizontal sections, the retinas were dissected and immersion-fixed in 4% PFA in 0.1 M PB for 1 hour at 4°C and were transferred to 30% sucrose in 0.1 M PB for 48 hours at 4°C. Retinal whole mounts were then frozen flat on an ice block and cut at 35 lm parallel to the retinal layers by using an AO sliding microtome (model 860, American Optical, Buffalo, NY).
Acute dissociation of retinal cells
The retina was dissected from the eyecup in Hanks' balanced salt solution (HBSS, pH 7.4), cut into pieces, and incubated in Ca2+- and Mg2+-free HBSS containing papain (40–45 U/ml, pH 7.4, Worthington, Lakewood, NJ) for 40 minutes at 37°C. The pieces of retina were transferred to medium containing Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA), 10% fetal bovine serum (Invitrogen), 1X penicillin-streptomycin-glutamine (Invitrogen), and DNase I (100 U/ml, pH 7.4, Worthington), and gently triturated by a glass pipette with the opening pore narrowed by flame to obtain suspensions of isolated cells. Isolated cells were seeded into 12-well cell culture plates onto coverslips coated with concanavalin A (1 mg/ml, Sigma-Aldrich, St. Louis, MO). The cells were allowed to settle for 1–3 hours at 37°C to adhere to the coverslips. The cells were then fixed with 4% PFA for 10 minutes at room temperature and subsequently used for immunocytochemistry.
Antibodies
Primary antibodies were as follows: rabbit polyclonal antibody to GABA (gift of Dr. David Pow, University of Queensland, Australia), mouse monoclonal antibodies to GAD65 (MAB531R, clone GAD-6, Millipore, Temecula, CA), GAD67 (MAB5406, clone 1G10.2, Millipore), and VGAT (131 011; Synaptic Systems, Göttingen, Germany) and affinity-purified rabbit polyclonal antibodies directed to the C-terminus of GAT-1 (346O) and GAT-3 (374D [Johnson et al., 1996]). A mouse monoclonal antibody to the calcium-activated, phospholipid-dependent protein kinase C (K01197M, clone MC5; Biodesign International, Saco, ME) was used to identify rod bipolar cells in mammalian retina (Ghosh et al., 2001). A mouse monoclonal and a rabbit polyclonal antibody to calbindin D-28k (calbindin) were used to identify horizontal cells. Calbindin D-28k is robustly expressed in axonless A-type and axon-bearing B-type horizontal cells in different mammalian retinas (Röhrenbeck et al., 1987; Celio, 1990; Peichl and González-Soriano, 1994; Massey and Mills, 1996; Johnson and Vardi, 1998; Haverkamp and Wässle, 2000; Raven and Reese, 2002; Hirano et al., 2005; Loeliger and Rees, 2005; Deng et al., 2006; Gargini et al., 2007; Hirano et al., 2007).
Antibody characterization
All of the antibodies used in this study have been previously characterized in mammalian retina as summarized below. Additional information is provided in Table 1.
TABLE 1.
Antibodies Used in This Study1
| Antibody | Host | Antigen | Company | Cat. no. | Dilution |
|---|---|---|---|---|---|
| GABA | Rabbit polyclonal | GABA conjugated to a carrier protein thyroglobulin using paraformaldehyde | Gift of Dr. David Pow, University of Queensland, Australia | N/A | 1:1,000 |
| GAD65 | Mouse monoclonal | Purified rat brain GAD, clone-6 | Millipore, Temecula, CA | MAB351R | 1:1,000 |
| GAD67 | Mouse monoclonal | A recombinant human GAD67-his tag containing the his tag and the unique N terminal region of GAD67 (GAD67 4–101: STPSSSATSSNAGADPNTTNLRPTTYDTWCGVAHGCTRKLGLKICGFLQRTNSLEEKSRLVSAFKERQSSKNLLSCENSDRDARFRRTETDFSNLFAR. Information provided by Millipore technical support) | Millipore | MAB5406 | 1:800 |
| VGAT | Mouse monoclonal | Synthetic peptide AEPPVEGDIHYQR (VGAT75-87 in rat) coupled to KLH | Synaptic Systems, Göttingen, Germany | 131 011 | 1:500 |
| Calbindin | Mouse monoclonal | Bovine kidney calbindin-D | Sigma-Aldrich, St. Louis, MO | C9848 | 1:2,500 |
| Calbindin | Rabbit polyclonal | Recombinant rat calbindin D-28k | Swant, Bellinzona, Switzerland | CB38a | 1:8,000 |
| GAT-1 | Rabbit polyclonal | C-terminus of rat GAT-1 (aa 588–599) coupled to KLH | Millipore | AB1570 | 1:200 |
| GAT-3 | Rabbit polyclonal | C-terminus of rat GAT-3 (aa 607–627) coupled to KLH. | Millipore | AB1574 | 1:250 |
| PKC | Mouse monoclonal | PKC from bovine brain, clone MC5 | Meridian Life Science, Saco, ME | K01107M | 1:200 |
Information supplied by the vendor unless otherwise shown. Abbreviations: GABA, γ-aminobutyric acid; GAD, glutamic acid decarboxylase; GAT, GABA transporter; KLH, keyhole limpet hemocyanin; PKC, protein kinase C; VGAT, vesicular GABA transporter.
Mouse monoclonal anti-calbindin D-28K antibody (C9848, clone CB-955; Sigma-Aldrich): This antibody recognizes calbindin D-28K (calbindin) in Western blots of human striatal neurons (Benchoua et al., 2006) and in tissues from other species, including guinea pig (Sigma-Aldrich data sheet). This antibody does not react with other related proteins, including calbindin D-9K, calretinin, and parvalbumin in Western blot (Sigma-Aldrich data sheet). This antibody has been used to immunostain horizontal cells in mouse, rat, ferret, rabbit, and pig retinas (Rossi et al., 2003; Renteria et al., 2005; Deng et al., 2006; Lee et al., 2006; Zhang et al., 2006; Gargini et al., 2007; Hirano et al., 2007; Damiani et al., 2008; Ettaiche et al., 2009; Kyhn et al., 2009).
Rabbit polyclonal anti-calbindin D-28K antibody (CB38a; Swant, Bellinzona, Switzerland): This antibody detects a single band of the expected molecular size (~27–28 kDa) for calbindin in Western blots of brain lysates from multiple species, including rat and guinea pig (Katsetos et al., 1994; Veenstra et al., 1997; Swant datasheet). This antibody also detects a band of the expected molecular size (~29–30 kDa) of calretinin at 10-fold higher dilutions used to detect calbindin (Swant datasheet). This antibody shows specific calbindin immunostaining in the cerebellum that matches previous findings (Garcia-Segura et al., 1984; Celio, 1990). This antibody has been used to immunostain horizontal cells in various mammalian retinas, including the retinas of guinea pig (Loeliger and Rees, 2005), rat (Gaillard et al., 2008), and mouse (Haverkamp and Wässle, 2000; Loeliger and Rees, 2005; Gaillard et al., 2008).
Rabbit polyclonal anti-GABA antibody: This rabbit polyclonal antibody (GABA) was made to a GABA-porcine thyroglobulin conjugate that was coupled by 4% paraformaldehyde and co-adsorbed with colloidal gold particles (Pow and Crook, 1993). This antibody detects GABA-PFA-bovine serum albumin (BSA) conjugates and does not cross-react with glutamine-, aspartate-, glycine-, and glutamate-PFA-BSA conjugates in immunoblotting assays (Pow and Crook, 1993). Immunohistochemistry experiments show that preadsorption of this antibody with the GABA-PFA-BSA conjugate eliminated specific GABA immunostaining in rabbit retina and cat hypothalamus (Pow and Crook, 1993; Pow et al., 1995). Preadsorption of this antibody with GABA-BSA conjugate eliminated specific GABA immunostaining in guinea pig retina (Fig. 1). This antibody has been used to immunostain retinal cells in different mammalian retinas, including rabbit, rat, mouse, and guinea pig (Pow and Crook, 1993; Pow et al., 1995; Haverkamp and Wässle, 2000; Sullivan et al., 2003; Catalani et al., 2004; Loeliger and Rees, 2005). The overall immunostaining pattern is similar to the GABA immunostaining patterns observed by using other GABA antibodies (Chun and Wässle, 1989; Pourcho and Owczarzak, 1989; Wässle and Chun, 1989; Johnson and Vardi, 1998; Haverkamp and Wässle, 2000).
Mouse monoclonal anti-GAD65 antibody (MAB351R, clone GAD-6; Millipore): This mouse monoclonal antibody (lgG2a isotype) is derived from a hybridoma produced from a mouse immunized by immunoaffinity-purified rat brain GAD from a GAD65 affinity column (Gottlieb et al., 1986; Chang and Gottlieb, 1988). This antibody detects a single band of the expected molecular size (~59 kDa) of GAD65 (Erlander et al., 1991) in Western blots of unfractionated homogenates of whole rat brain (Chang and Gottlieb, 1988). This antibody does not cross-react with GAD67 in Western blots of rat brain homogenates (Chang and Gottlieb, 1988). In addition, a single band corresponding to GAD65 in Western blots was absent in homogenates of brain from GAD65 knockout mice (Yamamoto et al., 2004). This antibody has been used to immunostain amacrine and horizontal cells in different mammalian retinas, including mouse, rat, rabbit, cat, and monkey retinas (Johnson and Vardi, 1998; Haverkamp and Wässle, 2000; Lee et al., 2006; Ding and Weinberg, 2007).
Mouse monoclonal anti-GAD67 antibody (MAB5406, clone 1G10.2; Millipore): This antibody recognizes a single band (~67 kDa) at the expected molecular size of GAD67 (Erlander et al., 1991) in a Western blot of rat cortex, and it does not cross-react with the GAD65 isoform in a Western blot of rat cortex (Fong et al., 2005). The GAD67 signal is reduced in a Western blot of the striatum of a heterozygous GAD67 knockout mouse compared with a wild-type mouse (Heusner et al., 2008). GAD67 immunostaining is also reduced in the central nervous system of this GAD67 mutant compared with wild-type mice (Heusner et al., 2008). The immunostaining pattern in the retina and brain (Erlander et al., 1991; Fong et al., 2005; Heusner et al., 2008) using this antibody is similar to the pattern observed by using other polyclonal GAD67 antibodies (Esclapez et al., 1994; Vardi and Auerbach, 1995; Haverkamp and Wässle, 2000; Fong et al., 2005).
Rabbit polyclonal anti-GAT-1 antibody (AB1570, Millipore; Johnson et al., 1996): This antibody recognizes a single band of the expected molecular size (~67 kDa) of GAT-1 (Guastella et al., 1990) in Western blots of mouse brain and retina lysates (Guo et al., 2009). Preadsorption of this antibody with the rat GAT-1588–599 peptide that was used for immunization at concentrations of 10−5 to 10−7 M eliminated GAT-1 immunostaining in mouse, rat, and monkey retina and brain sections (Ikegaki et al., 1994; Honda et al., 1995; Johnson et al., 1996; Conti et al., 1998; Casini et al., 2006; Guo et al., 2009). GAT-1 immunostaining in rat retinal sections was not affected by preadsorption of the GAT-1 antibody with the C-terminus GAT-2594–602 (RLTELESNC) or GAT-3607–627 (CEAKVKGDGTISAITEKETHF) peptides at concentrations of 10−5 to 10−7 M (Johnson et al., 1996). This antibody has been used to immunostain amacrine and Müller cells in the mouse and rat retina (Johnson et al., 1996; Guo et al., 2009). The immunostaining pattern of this antibody matches the GAT-1 immunostaining pattern obtained by using another GAT-1 antibody in the rat retina (Honda et al., 1995).
Rabbit polyclonal anti-GAT-3 antibody (AB1574, Millipore; Johnson et al., 1996): This antibody recognized a single band of the expected molecular size (~71 kDa) of GAT-3 (Lopez-Corcuera et al., 1992) in Western blots of mouse brain and retina lysates (Guo et al., 2009). Preadsorption of this antibody with the rat GAT-3607–627 peptide that was used for immunization at concentrations of 10−5 to 10−7 M eliminated GAT-3 immunostaining in mouse, rat, and monkey retina and brain sections (Johnson et al., 1996; Minelli et al., 1996; Guo et al., 2009). Furthermore, the GAT-3 immunostaining in rat retinal sections was not affected by preadsorption of the GAT-3 antibody with the C-terminus GAT-1588–599 or GAT-2594–602 peptides at concentrations of 10−5 to 10−7 M (Johnson et al., 1996). This antibody has been used to immunostain amacrine and Müller cells in the rat and mouse retina (Johnson et al., 1996; Guo et al., 2009). The immunostaining pattern of this antibody matches the GAT-3 immunostaining pattern obtained by using another GAT-3 antibody in rat retina (Honda et al., 1995).
Mouse monoclonal anti-VGAT antibody (131 011, clone 117G4, Synaptic Systems): This mouse monoclonal antibody (IgG3 isotype) recognizes a single band of the expected molecular size (~57 kDa) of VGAT in Western blots of mouse brain and retina lysates (McIntire et al., 1997; Sagné et al., 1997; Guo et al., 2009). Preadsorption of this antibody with the VGAT N-terminus peptide, VGAT75-87 (AEPPVEGDIHYQR), that was used for immunization, eliminated the VGAT signal in Western blots of a rat brain homogenate (Synaptic Systems, data sheet). This antibody has been used to immunostain amacrine and horizontal cells in mouse retina (Guo et al., 2009). Preadsorption of this antibody with the VGAT N-terminus peptide, VGAT75-87, at concentrations of 10−5 to 10−7 M eliminated VGAT immunostaining in mouse retina sections (data not shown; Guo et al., 2009). The immunostaining pattern of this antibody matches the VGAT immunostaining pattern obtained by using other VGAT antibodies in the rat and mouse retina (Cueva et al., 2002; Jellali et al., 2002; Johnson et al., 2003).
Mouse monoclonal anti-PKC (K01197M, clone MC5; Meridian Life Science, Saco, ME): PKC is a well-characterized marker for rod bipolar cells in retina of many species (Haverkamp and Wässle, 2000; Ghosh et al., 2001). This monoclonal antibody (clone MC5) was raised against PKC (Mr 79–80 kDa) purified from bovine brain. The PKC antibody recognized the purified PKC protein, as well as an 80-kDa band from whole-cell extracts of rat glioma and murine NIH3T3 cell lines on Western blots, and specifically immunoprecipitated PKC from cell lysates of 328 glioma and SVK 14 cell lines. The epitope was mapped by enzyme-linked immunosorbent assay (ELISA) by using a peptide corresponding to PKC304–309 of bovine brain PKC and shorter fragments thereof to the hinge region (PKC304–309: KFEKAK), close to or at the trypsin cleavage site of PKC (Young, 1988). Binding of the antibody was completely blocked by the full-length peptide but not blocked by a unrelated peptide (Young, 1988). This antibody reacts with PKC-α/β-1/β-2 isoforms (Meridian Life Science datasheet).
Figure 1.
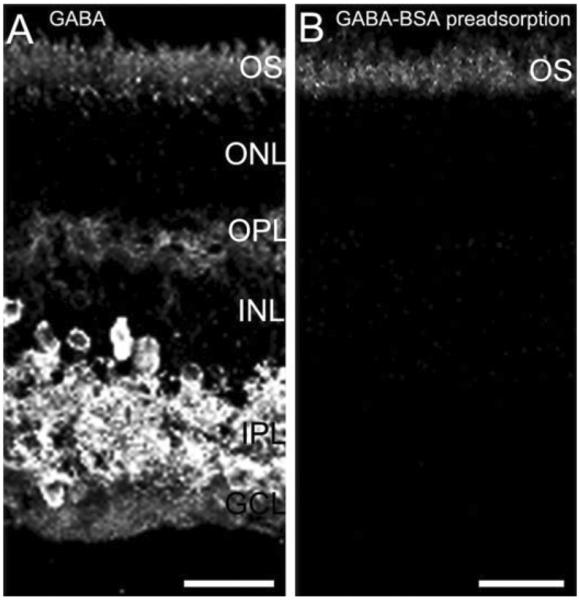
Specific GABA immunostaining is in the guinea pig retina. GABA immunostaining is absent following preadsorption of the GABA antibody with a GABA-BSA conjugate. Guinea pig retinal vertical sections were immunostained with GABA antibody (A) or GABA antibody preadsorbed with GABA-BSA conjugate (B). A: Robust GABA immunostaining is distributed to amacrine cell and displaced amacrine cell somata, as well as their processes in the IPL in the inner retina, and weak GABA immunostaining is in the outer retina in horizontal cell bodies and processes. B: Retina vertical section immunostained with GABA antibody preadsorbed by GABA-BSA conjugate. GABA immunoreactivity in the outer and inner retina was eliminated by GABA-BSA preadsorption; the outer segments (OS) of photoreceptors showed nonspecific staining that was not blocked by GABA-BSA conjugates. Confocal images were scanned at 1-lm intervals, and six optical sections were obtained and compressed for viewing. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 20 μm in A,B.
Western blotting
Western blotting with the VGAT antibody was carried out to establish the presence of VGAT in the guinea pig retina, and to further characterize the specificity of this antibody as described in Guo et al. (2009). In brief, the retina and brain were homogenized, and 10–20 μg of total protein from brain and retina homogenates were run on a 4–20% polyacrylamide gel. Membranes were probed with the antibody against VGAT (1:1,500) diluted in blocking buffer containing 25 mM Tris·Cl (pH 7.5), 150 mM NaCl, 0.1 mM phenylmethanesulphonylfluoride (PMSF), 2 mM EDTA, 5% nonfat dry milk, and 0.05% Tween-20; then labeled bands were visualized by using horseradish peroxidase (HRP)-conjugated, goat anti-mouse secondary antibody (Pierce, Rockford, IL) diluted in blocking buffer, and detected with enhanced chemiluminescence on Kodak BioMax light film.
Immunohistochemistry
Immunostaining was carried out with the indirect immunofluorescence method (Hirano et al., 2007) by using retinal sections from central and peripheral retina, whole mounts, and acutely dissociated cells. Retinal sections were incubated in 10% normal goat serum (NGS), 1% BSA, 0.5% Triton X-100, and 0.05% sodium azide in 0.1 M phosphate-buffered saline (PBS), pH 7.4, for 1 hour at room temperature. The blocking solution was replaced by the primary antibody diluted in 3% NGS, 1% BSA, 0.5% Triton X-100, and 0.05% sodium azide in PBS and incubated overnight at 4°C in a humidified chamber. Following three washes of 0.1 M PB for 10 minutes each, the sections were incubated for 30–60 minutes at room temperature in secondary antibodies conjugated to either Alexa 488 or Alexa 568 at a dilution of 1:1,000 (Invitrogen), diluted in the same solution as the primary antibody, except that sodium azide was omitted. Following washes in 0.1 M PB, the sections were air-dried, and mounted in Aqua Poly/Mount (Polysciences, Warrington, PA).
Horizontal retinal sections were incubated free floating with primary antibodies for 7 days and secondary antibodies for 3 days at 4°C on a shaker. The primary antibody/antigen complex was detected by using the appropriate secondary antibodies conjugated to either Alexa 488 or Alexa 568 at a dilution of 1:1,000 (Invitrogen). Following washes in 0.1 M PB, the sections were mounted in Aqua Poly/Mount.
Acutely dissociated retinal cultures were incubated in primary and secondary antibodies for overnight and 1 hour, respectively, in 12-well cell culture plates. Antibody dilutions were the same as used for the vertical sections. The washing steps were performed by briefly dipping coverslips into 0.1 M PB for three times, and coverslips were air-dried and mounted on gelatin-coated slides.
For immunohistochemistry controls, all antibodies were tested on guinea pig retina as single-labeling experiments at least three times to confirm specificity and optimize concentration prior to performing any double-labeling experiments. Preadsorption experiments using GABA-BSA eliminated the specific immunostaining GABA antibody (Fig. 1). For double-labeling controls, one of the two primary antibodies used for double labeling was omitted during the primary incubation step. In this case, only the immunostaining by the remaining primary antibody was detected.
Confocal imaging
Images of retinal sections, whole mounts, and acutely dissociated retinal cells were acquired by using a Zeiss Laser Scanning Microscope 510 META (Carl Zeiss Microimaging, Thornwood, NY). Samples were imaged with either a Plan Neofluar 40× 1.3 NA oil objective, or a C-Apochromat 40× 1.2 NA water objective. To identify fluorescent signals, different lasers were used for excitation: for Alexa 488, the 488-nm argon laser line was used, and for Alexa 568, the 543-nm HeNe laser line was used. During acquisition of signals from double-labeled specimens, the scans were collected sequentially to prevent spectral bleed-through. Specific laser excitation and filters were used to achieve proper separation of signals (for single labeling with Alexa 488: 505–530 BP; Alexa 568: 560 LP; for double labeling with Alexa 488 and Alexa 568, 505–530 BP and 560–LP were used, respectively). All images were acquired at a resolution of 2,048 × 2,048 as 12-bit signals. To increase the signal-to-noise, images were averaged online (e.g., n = 4), and the scan speed and photomultiplier detector gain were decreased. Most confocal images were acquired at an approximate optical thickness of 1.0 μm, usually 0.7–1.0 Airy unit. For projections, typically 5–6 optical sections, but as many as 12, as noted, were acquired and compressed for viewing. Digital confocal images were saved as Zeiss .LSM files, and final publication quality images were exported in the .TIFF format by using Zeiss LSM 510 Meta software version 3.2 (Carl Zeiss Microimaging). All images were processed, adjusted for brightness and contrast, and resized to 300 dpi by using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Laser capture microdissection (LCM) of horizontal cells
The eyecups were cryoprotected in 30% sucrose at 4°C for 4 hours, embedded into OCT, and fresh frozen with dry ice. Vertical retinal sections were made at 10 μm by using a Leica CM3005 cryostat, mounted onto polyethylene-naphthalate (PEN) membrane-based slides (Leica Microsystems), and stored at −80°C. Sections were fixed with ice-cold 70% ethanol for 1 minute, serially dehydrated by dipping into 75%, 95%, and 100% ethanol for 1 minute each, and air-dried for 10–30 minutes inside a laminar flow hood. Horizontal cells were identified in the vertical section viewed with differential interference contrast (DIC) optics in the Leica AS LMD system (Leica Microsystems) based on their cell morphology, size, and the localization of the cell bodies close to the outer plexiform layer (OPL). A circular boundary was defined within the contour of the cell body for laser capture of a single horizontal cell in a retinal section, to ensure minimal contamination from surrounding area. Horizontal cells were collected by using the Leica AS LMD system following the manufacturer's operation manual. The following settings of the Leica AS LMD system were employed: laser beam intensity = 11, aperture = 9–11, apertureDiff = 45, bridge = medium.
Degenerate RT-PCR
Forty horizontal cells were collected by using LCM, and total RNA was isolated by using the Absolutely RNA nanoprep kit (Stratagene, La Jolla, CA). RNA preparations were reverse-transcribed by using oligo-dT primers and the SuperScript III First-Strand Synthesis System (Invitrogen), according to the manufacturer's instructions. Platinum PCR SuperMix (Invitrogen) was used for polymerase chain reaction (PCR) reactions. Degenerate primers complementary to highly conserved regions of mouse GAD65 were used with the first-strand cDNA as template to amplify a 166-bp DNA product. The conserved domains of the GAD65 protein was screened by using National Center for Biotechnology Information (NCBI) protein blast; the designed primers recognize the mRNA sequence corresponding to the middle of the pyridoxal-dependent decarboxylase conserved domain of the GAD65 protein: the sense primer (5′-TAYAARATHTGGATGCA-3′) and the antisense primer (5′-CATCATYTTRTGNGGRTTCC-3′). For sequencing purposes, T7 and T3 sequences were tagged to the 5′ ends of the sense and antisense primers, respectively. PCR was performed by using degenerate primers at a concentration of 12 μM and 40 cycles of the following amplification profile: template denaturation and enzyme activation for 1 minute at 94°C, denaturation for 30 second at 94°C, annealing for 30 seconds at 55°C, extension for 45 seconds at 72°C. DNA amplification products (amplicons) were separated on 1.8% agarose gels, extracted by using QIAEX II Gel Extraction Kit (QIAGEN, Valencia, CA) and sent for sequencing at the UCLA Genotyping and Sequencing Core (http://www.genoseq.ucla.edu/action/view/Main_Page).
RESULTS
Horizontal cells were identified on the basis of their soma size, position in the distal inner nuclear layer (INL), and distribution of their processes in the OPL. Guinea pig horizontal cells also contain calbindin immunoreactivity (Hamano et al., 1990; Peichl and González-Soriano, 1994; Loeliger and Rees, 2005). The present study included an analysis of both central and peripheral retinal regions.
GABA immunoreactivity
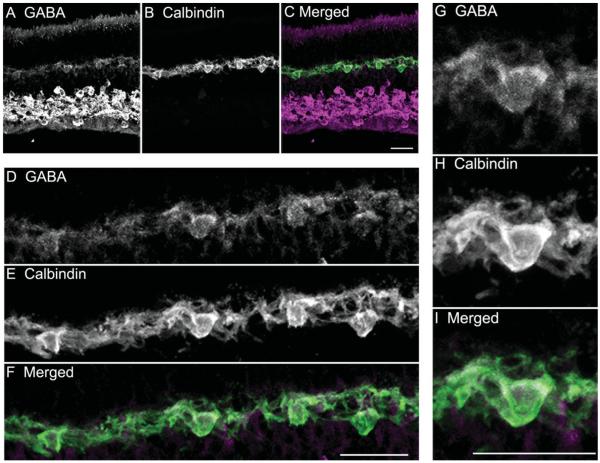
Specific GABA immunostaining was in both central and peripheral retina, and was localized to amacrine and horizontal cells (Fig. 2). In the inner retina, strong GABA immunostaining was found in many amacrine cell somata, in displaced amacrine cell somata, and in their processes distributed to all laminae of the inner plexiform layer (IPL; Fig. 2A). In the outer retina, GABA immunostaining was strongest in horizontal cell somata, and immunostaining was weaker in horizontal cell processes and endings (Fig. 2D & G). All calbindin-immunoreactive horizontal cell bodies contained GABA immunoreactivity, implying that both A- and B-type horizontal cells have GABA.
Figure 2.
GABA immunoreactivity is localized to horizontal cell bodies and their processes. A vertical section through the guinea pig retina was double labeled with antibodies to GABA and calbindin-28K (calbindin). A: Weak GABA immunostaining is in the outer retina, and robust GABA immunostaining is distributed to amacrine cell and displaced amacrine cell somata, and their processes in the IPL in the inner retina. B: Strong calbindin immunoreactivity occurred in horizontal cell somata and processes in the outer retina. C: Merged image shows the co-localization of GABA and calbindin immunoreactivities in the outer retina. D–F: Enlarged images of the OPL show the co-localization of GABA and calbindin immunoreactivities. D: GABA immunoreactivity in horizontal cell somata and processes. E: Calbindin immunoreactivity in horizontal cell somata and processes in the OPL. F: Merged image. Co-localization of GABA and calbindin immunoreactivity is in horizontal cell somata and processes. G–I: Enlarged images showing distribution of GABA and calbindin immunoreactivities in a horizontal cell. G: GABA immunoreactivity. H: Calbindin immunoreactivity is in the horizontal cell body, processes, and endings. I: Merged image show that the GABA immunoreactivity is in the horizontal cell body and processes. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C), F (applies to D–F), and I (applies to G–I).
Previous findings in adult guinea pig retina reported little or no GABA immunoreactivity in horizontal cells (Agardh et al., 1986; Osborne et al., 1986; Loeliger and Rees, 2005). We found that fixation duration markedly influenced the levels of GABA immunostaining in the outer retina. Short periods of fixation from 15 to 30 minutes gave better immunostaining of horizontal cells and their processes; the GABA immunolabeling was generally strong and specific. However, longer fixation periods (≥60 minutes) resulted in GABA immunostaining at levels at or just above background, and often immunoreactivity was undetectable (data not shown).
GAD65 and GAD67 immunoreactivity
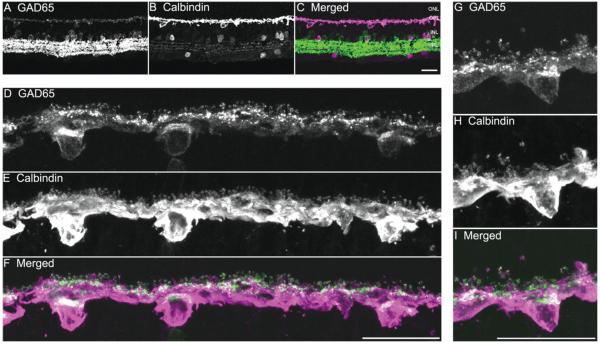
GAD is the key synthetic enzyme that converts glutamic acid into GABA. Both GAD isoforms, GAD65 and GAD67, are found in horizontal cells of some mammalian retinas (Agardh et al., 1987; Vardi et al., 1994; Johnson and Vardi, 1998; Calaza et al., 2006). In guinea pig retina, GAD65 immunoreactivity was in amacrine and displaced amacrine cell bodies, and in their processes distributed in the IPL (Kao and Sterling, 2006), as well as horizontal cell bodies and their processes and endings (Fig. 3). GAD65, and calbindin immunoreactivity was co-localized in horizontal cell bodies and processes in vertical sections (Fig. 3F,I). The intensity of GAD65 immunostaining in horizontal somata was weaker than in processes and terminals. Interestingly, there was a polarization of the distribution of the GAD65 immunostaining in horizontal cell somata: high levels of GAD65 immunoreactivity were concentrated in the somatic region adjacent to the nucleus closest to the OPL (Fig. 3D,G). Strong GAD65 immunostaining was also observed along the processes and at their endings with a punctate staining pattern (Fig. 3G).
Figure 3.
GAD65 immunoreactivity is localized to horizontal cells. A vertical section through a guinea pig retina was double labeled with antibodies to GAD65 and calbindin. A: Weak GAD65 immunostaining is present in the cell bodies and processes in the OPL; strong GAD65 immunoreactivity is distributed to amacrine cell somata and their processes in the IPL. B: Calbindin is expressed in horizontal cell somata and processes in the outer retina, and some amacrine and ganglion cell somata and their processes in the inner retina. C: Merged image shows the co-localization of GAD65 and calbindin immunoreactivities in the outer retina. D–F: Enlarged images of the OPL show the distribution of GAD65 and calbindin immunoreactivities. D: GAD65-immunoreactive cell bodies, processes, and tips in the OPL. E: Calbindin-immunoreactive horizontal cell bodies, processes, and tips. F: Merged image shows the co-expression of GAD65 and calbindin immunoreactivities in the OPL, indicating that GAD65 immunoreactivity is localized to horizontal cell bodies, processes, and tips. G–I: Enlarged images show distribution of GAD65 and calbindin immunoreactivities in a horizontal cell. G: GAD65 immunoreactivity. H: Calbindin immunoreactivity is in horizontal cell body, processes, and tips. I: Merged image shows that GAD65 immunoreactivity is concentrated in a subcellular region of the soma close to the OPL, and in processes and terminals. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C), F (applies to D–F), and I (applies to G–I).
Similar to GABA immunostaining, GAD65 immunoreactivity was in all the horizontal cells that were calbindin immunoreactive in vertical sections (Fig. 3F & I), suggesting that GAD65 immunoreactivity was in both A- and B-type horizontal cells. However, double labeling for calbindin and GAD65 in horizontal sections revealed that about 80% (six fields [225 × 225 μm] from five horizontally sectioned retinas) of the calbindin-expressing horizontal cells contain GAD65 immunoreactivity (Fig. 4). The white dots in Figure 4 indicate the position of somata containing both GAD65 and calbindin immunoreactivity. There were fewer GAD65-immunoreactive horizontal cell bodies in the horizontal sections; the lack of GAD65 immunostaining is likely due to technical issues including the plane of section through the horizontal cell bodies, tissue fixation, and incomplete GAD65 antibody penetration of the tissue.
Figure 4.
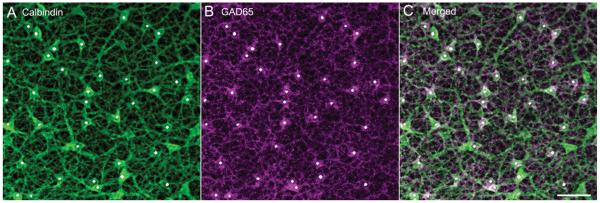
Calbindin and GAD65 double staining of a horizontal section through the outer retina showing the presence of calbindin-immunostained horizontal cells containing GAD65 immunoreactivity. Horizontal cells that contain calbindin and GAD65 are marked by a white dot. A: Horizontal cell somata and processes containing calbindin immunoreactivity. B: Horizontal cell somata and processes containing GAD65 immunoreactivity. C: Merged image shows that most calbindin-immunostained horizontal cells contained GAD65 immunoreactivity. Confocal images were scanned at 1-μm intervals, and 12 optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C).
GAD65 immunoreactivity was also in cells in acutely dissociated preparations from adult retina. Horizontal cells were identified on the basis of the co-localization of GAD65 and calbindin immunoreactivity in large somata having the appearance of horizontal cells (Fig. 5). Putative A-type horizontal cells were identified on the basis of a symmetrical distribution of dendrites and an absence of an axon-like process (Fig. 5A–D). Putative B-type horizontal cells were identified on the basis of multiple fine-caliber dendrites (Fig. 5E–H). Some of these cells had a thicker, axon-like process characteristic of guinea pig axons (Peichl and González-Soriano, 1994) that often ended with finer branches (Fig. 5E–H). In some cases, this putative axon and terminal system was absent, likely lost during the dissociation procedure.
Figure 5.
GAD65 and calbindin double staining of acutely dissociated horizontal cells. A–D: Putative axonless A-type horizontal cell double labeled with GAD65 and calbindin antibodies. A: GAD65 immunoreactivity is localized throughout the horizontal cell cytoplasm. B: Calbindin immunoreactivity is in horizontal cell body, processes, and tips. C: Merged image shows co-localization of GAD65 and calbindin immunoreactivities. D: DIC image of the field of view. E–H: Putative axon-bearing B-type horizontal cell double labeled with GAD65 and calbindin antibodies. E: GAD65 immunoreactivity is localized throughout the horizontal cell. F: Calbindin immunoreactivity is in horizontal cell body, processes, and tips. G: Merged image shows co-localization of GAD65 and calbindin immunoreactivities. Arrowhead indicates an axon-like process. H: DIC image of the field of view. Confocal images were scanned at 1-μm intervals, and five optical sections were obtained and compressed for viewing. Scale bar = 10 μm in A–H.
GAD65 immunoreactivity was distributed to the putative A- and B-type horizontal cells in all cellular compartments, including their soma, processes, and endings, in the dissociated preparations (Fig. 5). Immunostaining was absent in the nucleus. The distribution of GAD65 immunoreactivity in dissociated horizontal cells differed from the asymmetric distribution of GAD65 immunoreactivity observed in horizontal cells from retinal sections and whole mounts.
GAD65 mRNA in horizontal cells
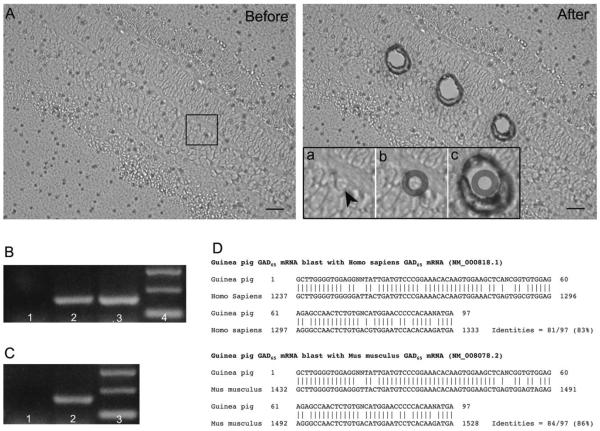
Degenerate oligonucleotide primers were designed to highly conserved sequences of the known GAD65 proteins to screen for GAD65 expression in guinea pig horizontal cells. A 166-bp PCR fragment was amplified from first-strand cDNA synthesized from guinea pig brain (Fig. 6B, lane 2), retina (Fig. 6B, lane 3), and 40 horizontal cells (Fig. 6C, lane 2) collected by LCM (Fig. 6A). Both DNA strands of the PCR products from retina were sequenced at least twice; sequencing results indicated that it had about 86% identity (84/97) to mouse (Mus musculus) GAD65 mRNA, and about 83% identity (81/97) to Homo sapiens GAD65 mRNA sequence (Fig. 6D sequence overlay). As expected, the deduced amino acid sequence appears to be conserved between guinea pig and mouse, with most of the nucleotide differences occurring in the third position of the codon.
Figure 6.
Laser capture microdissection and degenerate RT-PCR. A: Images of a vertical section through an adult guinea pig retina before (left) and after (right) laser capture microdissection. Inset (a–c) shows the steps of a horizontal cell being laser captured. a: The horizontal cell body was identified based on its morphology and its location next to the OPL (arrowhead). b: A circular boundary was defined around the cell contour for laser microdissection, as indicated by the dark gray circle. c: After laser microdissection. B: Degenerate RT-PCR amplified a 166-bp product (including 40 bp from tagged T3 and T7 sequences) from oligo(dT)-primed cDNA synthesized from both adult guinea pig brain and retina total RNA. Lanes: 1. Negative control in which reverse-transcribed cDNA was omitted. 2. PCR product amplified from brain cDNA. 3. PCR product amplified from retina cDNA. 4: 100-bp DNA ladder. C: Degenerate RT-PCR using cDNA synthesized from total RNA prepared from 40 laser-capture-microdissected horizontal cells amplified a 166-bp product. Lanes: 1. Negative control in which reverse-transcribed cDNA was omitted. 2. PCR product amplified from horizontal cell cDNA. 3. 100-bp DNA ladder. D: Nucleotide sequence alignment between the amplified guinea pig GAD65 mRNA sequence and that of human (Homo sapiens, top) and mouse (Mus musculus, bottom). Scale bar = 20 μm in A (applies to A,B).
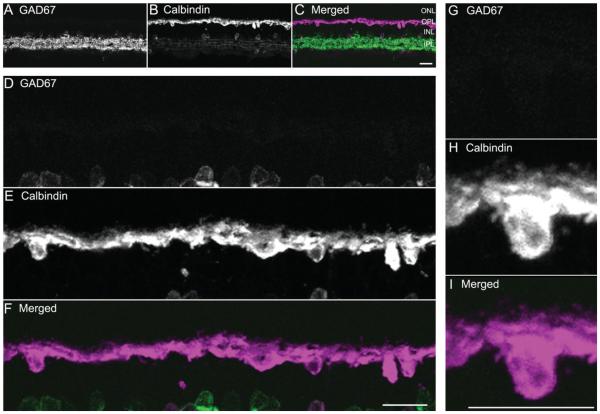
Strong GAD67 immunostaining was found in many amacrine cells, displaced amacrine cells, and their processes in the IPL (Fig. 7A). In contrast, GAD67 immunostaining was absent from the outer retina (Fig. 7A,D), indicating that guinea pig horizontal cells do not contain GAD67. To test whether low levels of GAD67 immunoreactivity were present in horizontal cells, some sections were immunostained with high concentrations (up to 1:100) of GAD67 antibodies; however, no specific immunostaining was observed in the OPL.
Figure 7.
GAD67 immunoreactivity was not present in horizontal cell bodies or processes. A vertical section through a guinea pig retina was double labeled with antibodies to GAD67 and calbindin. A: GAD67 is distributed to amacrine cell somata and their processes in the IPL. No GAD67 immunoreactivity was observed in the OPL. B: Calbindin is expressed in horizontal cell somata and processes in the outer retina, and some amacrine and ganglion cell somata and their processes in the inner retina. C: Merged image shows the localization of GAD67 and calbindin immunoreactivity in amacrine cell bodies. D–F: Enlarged images of the outer retina show GAD67 and calbindin immunoreactivities in the OPL. D: No GAD67 immunoreactivity is observed in the OPL. E: Calbindin-immunoreactive horizontal cell bodies and processes. F: Merged image shows no co-localization. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C), F (applies to D–F), and I (applies to G–I).
VGAT immunoreactivity
VGAT accumulates GABA into synaptic vesicles (Chaudhry et al., 1998; Dumoulin et al., 1999a; Takamori et al., 2000). In the retina, VGAT is prominently expressed in horizontal cell endings in several mammalian species (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Johnson et al., 2003; Guo et al., 2009).
VGAT immunoreactivity was detected in extracts of the guinea pig retina and brain by Western blotting (Fig. 8). A single band of Mr ~57 kDa was detected for guinea pig retina and brain. This is the predicted molecular weight of VGAT in mouse and rat brain and retina (McIntire et al., 1997; Sagné et al., 1997; Chaudhry et al., 1998; Takamori et al., 2000; Bragina et al., 2007; Guo et al., 2009). These studies also indicated the specificity of the VGAT antibody in guinea pig tissues, because it detected a single band in these preparations.
Figure 8.
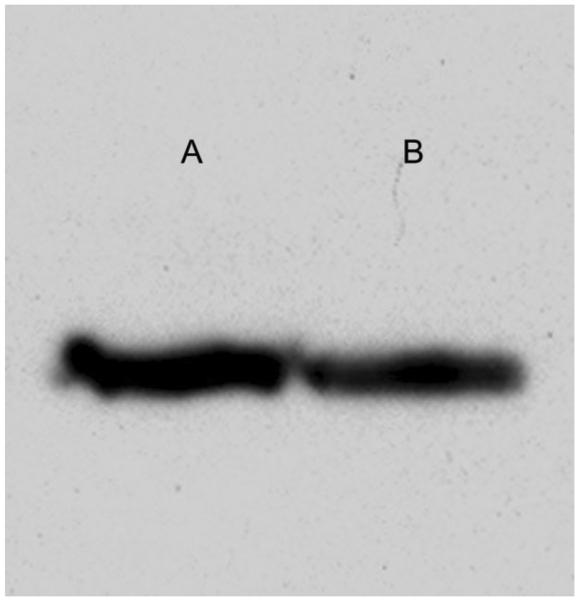
Immunoblot of VGAT in adult guinea pig retina (lane A) and brain (lane B) homogenates. The VGAT (Synaptic Systems #131 011, 1:1,500) monoclonal antibody detected a single band at Mr ~57 kDa in both retina and brain. Retina and brain total protein: 8–10 μg per lane.
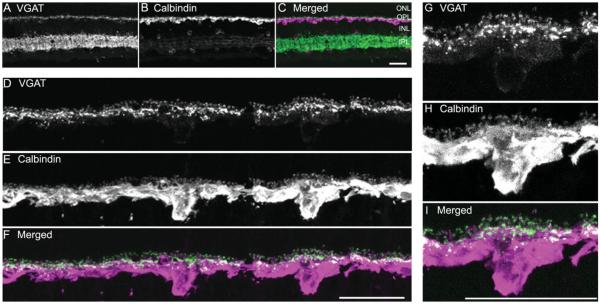
VGAT immunostaining was expressed by both amacrine and horizontal cells in the guinea pig retina (Fig. 9). Many, but not all, amacrine and displaced amacrine cells were immunolabeled for VGAT. Amacrine and displaced amacrine cell somata were characterized by weak immunostaining of their cytoplasm. In contrast, very strong VGAT-immunostained processes were distributed to all laminae of the IPL (Fig. 9A). Very weak VGAT immunoreactivity was also in horizontal cell somata (Fig. 9D). In the outer retina, VGAT immunoreactivity was principally distributed to horizontal cell processes and endings, with the strongest immunostaining in the tips producing a punctate staining pattern (Fig. 9D,G).
Figure 9.
VGAT immunoreactivity is localized to horizontal cell bodies and processes. A vertical section through an adult guinea pig retina was double immunostained with antibodies to VGAT and calbindin. A: VGAT immunoreactivity is present in the cell bodies and processes in the OPL; VGAT immunoreactivity is distributed to amacrine cell and displaced amacrine cell somata and their processes in the IPL. B: Calbindin is expressed by horizontal cell somata and processes in the outer retina, and some amacrine and ganglion cell somata and their processes in the inner retina. C: Merged image shows the localization of VGAT and calbindin immunoreactivities in the retina. D–F: Enlarged images of the OPL show the co-localization of VGAT and calbindin immunoreactivities in horizontal cells. D: VGAT-immunoreactive processes and tips in the OPL. E: Calbindin-immunoreactive horizontal cell bodies and processes. F: Merged image shows the co-localization of VGAT and calbindin immunoreactivities in the OPL, indicating that VGAT immunoreactivity is localized to horizontal cell bodies and processes. G–I: Enlarged images show distribution of VGAT and calbindin immunoreactivities in a horizontal cell. G: Weak VGAT immunoreactivity is localized at the cell body, whereas strong VGAT immunoreactivity is distributed to processes and tips. H: Calbindin immunoreactivity is in horizontal cell body, processes, and tips. I: Merged image indicating that VGAT immunoreactivity is in horizontal cell body and processes, with more intense immunoreactivity in the tips. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C), F (applies to D–F), and I (applies to G–I).
VGAT immunoreactivity was in putative horizontal cells in acute retinal dissociation preparations (Fig. 10A,E). Double immunostaining with VGAT (Fig. 10A,E) and calbindin (Fig. 10B,F) antibodies revealed co-localization of the two signals (Fig. 10C,G); putative A-type horizontal cells (Fig. 10A–D) and B-type horizontal cells (Fig. 10E–H) were seen in these preparations. VGAT immunoreactivity was distributed to all cellular compartments of dissociated horizontal cells, similar to the distribution of GAD65 in dissociated horizontal cells.
Figure 10.
VGAT and calbindin double staining of dissociated horizontal cells. A–D: Putative axonless A-type horizontal cell double labeled with VGAT and calbindin antibodies. A: VGAT immunoreactivity is present throughout the horizontal cell. B: Calbindin immunoreactivity is in the horizontal cell body, processes, and tips. C: Merged image shows the co-localization of VGAT and calbindin immunoreactivities. D: DIC image of the field of view. E–H: Putative axon-bearing B-type horizontal cell double labeled with VGAT and calbindin antibodies. E: VGAT immunoreactivity is localized at horizontal cell body and processes and tips. F: Calbindin immunoreactivity is in the horizontal cell body, processes, and tips. G: Merged image shows the co-localization of VGAT and calbindin immunoreactivities. H: DIC image of the field of view. Arrowheads point to the likely axonal process. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 10 μm in A–H.
GAT-1 and GAT-3 immunoreactivity
A proposed mechanism of GABA release from horizontal cells in nonmammalian retinas is based on the action of a plasmalemmal GAT that mediates GABA secretion (for review, see Schwartz, 2002). We therefore tested for the expression of the two GABA plasma membrane transporters GAT-1 and GAT-3 found in the mammalian retina (Brecha and Weigmann, 1994; Honda et al., 1995; Johnson et al., 1996; Guo et al., 2009).
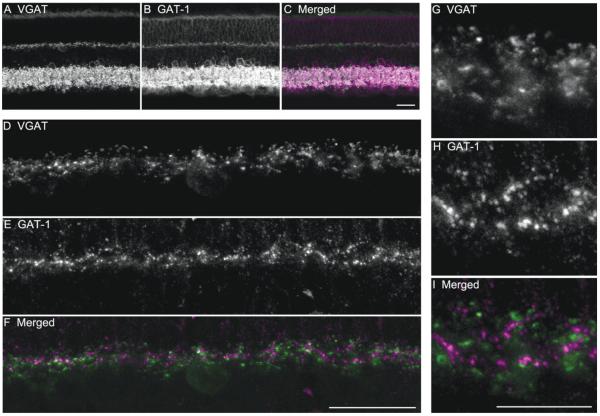
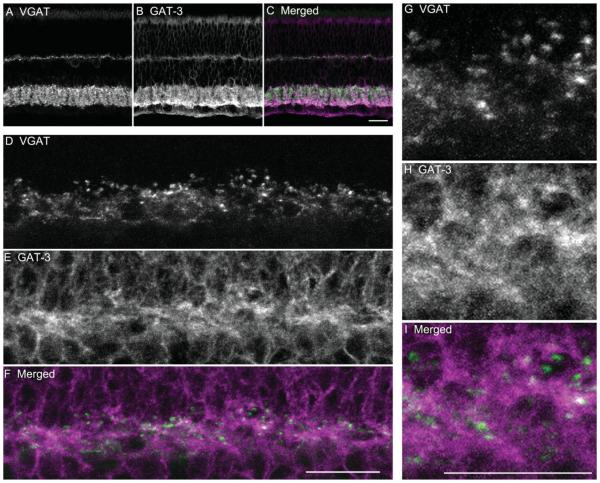
The pattern of GAT-1 (Fig. 11B) and GAT-3 (Fig. 12B) immunostaining in guinea pig retina was similar to the immunostaining pattern in mouse and rat retina (Honda et al., 1995; Johnson et al., 1996; Guo et al., 2009). GAT-1 immunoreactivity (Fig. 11B) was robust in the inner retina, with numerous, well-stained amacrine cell bodies, and their processes in all laminae of the IPL. GAT-1 immunoreactivity (Fig. 11B) was very weak in Müller cell bodies and processes, which were distributed across the retina. GAT-3 labeling (Fig.12B) showed a diffuse staining pattern of Müller cell bodies in the INL and their processes extending from the outer limiting membrane to the inner limiting membrane. In addition, GAT-3 immunoreactivity (Fig. 12B) was localized to some amacrine cell bodies and processes in the IPL.
Figure 11.
VGAT and GAT-1 immunostaining showed that GAT-1 immunoreactivity is not localized to horizontal cell processes in the outer retina. Images are of a vertical section through an adult guinea pig retina double labeled with antibodies to VGAT and GAT-1. A: VGAT immunostaining is distributed to horizontal cells and amacrine cells with strong signals mainly distributed to the processes and terminals. B: GAT-1 immunostaining occurs primarily in the plexiform layers as well as some amacrine cell bodies. C: Merged image shows the localization of VGAT and GAT-1 immunostaining in the retina. D–F: Enlarged images of the OPL show the lack of co-localization of VGAT and GAT-1 immunoreactivities. D: VGAT immunoreactivity is localized in horizontal cell processes and terminals in the OPL. E: GAT-1-immunostained processes in the OPL. F: Merged image shows that the GAT-1 and VGAT immunoreactivities do not co-localize in the OPL. G–I: High-magnification images show the distribution of VGAT (G) and GAT-1 (H) immunoreactivities in the OPL. I: Merged image shows that the VGAT and GAT-1 immunoreactivities do not co-localize. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C) and F (applies to D–F); 10 lm in I (applies to G–I).
Figure 12.
VGAT and GAT-3 immunolabeling showed that GAT-3 immunoreactivity was not distributed to horizontal cells or their processes in the outer retina. Images are of a vertical section through an adult guinea pig retina double labeled with antibodies to VGAT and GAT-3. A: VGAT immunostaining is present primarily in the plexiform layers with strong signals mainly distributed to the processes and terminals. B: GAT-3 immunostaining is localized to Müller cells and their processes, and at some amacrine cells and their processes in the IPL. C: Merged image shows the localization of VGAT and GAT-3 immunoreactivities in the retina. D–F: Enlarged images of the OPL show the lack of VGAT and GAT-3 co-localization. D: VGAT immunoreactivity is localized to horizontal cell processes and terminals in the OPL. E: GAT-3 labeling shows a typical honeycomb-like staining pattern of Müller cell processes in the OPL. F: Merged image shows that VGAT and GAT-3 immunoreactivities do not co-localize in the OPL. G–I: High-magnification images show the distribution of VGAT and GAT-3 in the OPL. G: VGAT immunoreactivity occurs in the horizontal cell processes and endings. H: GAT-3 immunoreactivity in the Müller cells processes. I: Merged image indicates that GAT-3 immunoreactivity is not distributed to horizontal cells. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C), F (applies to D–F), and I (applies to G–I).
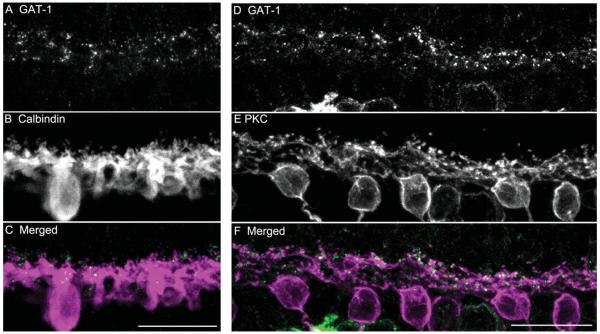
In the OPL, GAT-1 immunoreactivity (Fig. 11E) had a punctate appearance, and GAT-3 immunoreactivity (Fig. 12E) had a honeycomb-like appearance. Neither GAT-1 (Fig. 11F,I) nor GAT-3 (Fig. 12F,I) immunoreactive structures were co-localized with the VGAT-immunoreactive horizontal cell processes and terminals. In addition, double-labeling experiments using antibodies to GAT-1 and calbindin (Fig. 13A–C) or PKC (Fig. 13D–F) showed that GAT-1 immunoreactivity in the OPL was not distributed to horizontal cell processes and terminals labeled by calbindin (Fig. 12C), nor was it distributed to rod bipolar cell dendrites labeled by PKC (Fig. 13F). Finally, GAT-1 or GAT-3 immunoreactivity was not localized to isolated horizontal cells in dissociated retinal preparations (data not shown).
Figure 13.
Double labeling of GAT-1 with calbindin (A–C) or PKC (D–E) on vertical sections through an adult guinea pig retina. Images were taken at the OPL. A: GAT-1 immunoreactivity in the OPL. B: Calbindin immunolabeling of horizontal cell bodies, processes, and terminals in the OPL. C: Merged image shows that the punctate GAT-1 immunoreactivity is not co-localized with the calbindin immunoreactivity in the OPL, indicating that GAT-1 immunoreactivity is not distributed to the horizontal cell processes and terminals. D: GAT-1 immunoreactivity in the OPL. E: PKC immunoreactivity in the rod bipolar cell bodies and dendrites. F: Merged image shows that GAT-1 immunoreactivity is not co-localized with PKC immunoreactivity in the OPL, indicating that GAT-1-immunoreactivity is not distributed to rod bipolar cell dendritic terminals. Confocal images were scanned at 1-μm intervals, and six optical sections were obtained and compressed for viewing. Scale bar = 20 μm in C (applies to A–C) and F (applies to D–F).
DISCUSSION
These studies form a comprehensive examination of the presence of GABA, its synthetic enzyme GAD, and its transporters, GAT and VGAT, in the outer retina of the guinea pig, which revealed that both A- and B-type horizontal cells contain GABA, and express GAD65 and VGAT. These findings are in contrast to previous reports that GABA immunoreactivity was absent in the outer retina or present at low levels in a small number of horizontal cells in the adult guinea pig retina (Agardh et al., 1986; Osborne et al., 1986; Loeliger and Rees, 2005). Together, these findings fulfill three of the classical requirements for a neurotransmitter candidate in a neuron: 1) it is located at the presynaptic terminal; 2) it is synthesized by the neuron; and 3) mechanisms exist for removal or inactivation of the transmitter (Cooper et al., 2003). Findings from this study support the proposition that GABA is a horizontal cell transmitter.
GABA is in both A-type and B-type horizontal cells
GABA immunoreactivity is expressed in both A- and B-type horizontal cells in the guinea pig retina based on the co-localization of calbindin and GABA immunoreactivities in horizontal cell bodies in our preparations. This finding is in contrast to previous studies reporting that GABA immunoreactivity was absent in guinea pig horizontal cells (Agardh et al., 1986; Osborne et al., 1986). In addition, a more recent study by Loeliger and Rees (2005) indicated that GABA immunoreactivity was strong in horizontal cells during the postnatal development period, but by day 60, GABA immunostaining was faint and detected in only a few horizontal cells, using the same rabbit anti-GABA polyclonal antibody that was used for this study. Loeliger and Rees (2005) speculated that only one type of horizontal cell contained GABA in adult guinea pig retina. However, our study suggests that both types of horizontal cells contain GABA (Fig. 2), which is consistent with the presence of GAD65 immunoreactivity in both types of horizontal cells (Figs. 3–5). The discrepancy between our findings and those of others may be due to tissue preparation and fixation conditions.
For example, we found that the GABA immunostaining was best achieved by using retinas that were fixed for 15 minutes, which contrasts with previous studies that used fixation times greater than 2 hours (Agardh et al., 1986; Osborne et al., 1986; Loeliger and Rees, 2005). We determined that the longer fixation times have a deleterious effect on GABA immunostaining. In the present study, we observed GABA-containing horizontal cells distributed to all retinal regions of the guinea pig retina. This finding is similar to the distribution of GABA-containing horizontal cells in the cat retina (Chun and Wässle, 1989; Pourcho and Owczarzak, 1989; Wässle and Chun, 1989).
GAD65 is expressed in horizontal cells
GABA is synthesized from glutamic acid by the rate-limiting enzyme GAD, which has two well-characterized isoforms, GAD65 and GAD67. Only the GAD65 isoform was detected in guinea pig horizontal cells using GAD isoform-specific antibodies (Figs. 3–5). The suggestion that GAD65 is expressed in these cells was further strengthened by the detection of GAD65 mRNA in laser-captured horizontal cells using degenerate RT-PCR (Fig. 6).
All calbindin-immunoreactive horizontal cells appeared to contain GAD65 immunoreactivity in a series of serial vertical sections (~100 sections). However, double labeling of GAD65 and calbindin of horizontally sectioned retinas showed that about 80% of the horizontal cells contained GAD65. The difference in the co-localization between the vertical and horizontal sectioned retinas is likely due to technical reasons including tissue preparation, poor antibody penetration, and access to the antigen. Consistent with these findings, GABA and GAD immunostaining in horizontal cells has been reported in cat, rabbit, opossum, and primate retina (Mosinger et al., 1986; Osborne et al., 1986; Agardh et al., 1987; Mosinger and Yazulla, 1987; Chun and Wässle, 1989; Pourcho and Owczarzak, 1989; Wässle and Chun, 1989; Grünert and Wässle, 1990; Vardi et al., 1994; Johnson and Vardi, 1998; Marc et al., 1998; Calaza et al., 2006). Rabbit horizontal cells express both isoforms (GAD65 and GAD67) (Johnson and Vardi, 1998); whereas cat A- and B-type horizontal cells, identified with antibodies to parvalbumin (Röhrenbeck et al., 1987), express GAD67 mRNA (Sarthy and Fu, 1989a), consistent with GAD67 immunoreactivity in cat horizontal cells (Vardi et al., 1994). In contrast, monkey H1 and H2 horizontal cells express GAD65, but not GAD67 immunoreactivity (Vardi et al., 1994), also consistent with the lack of GAD67 mRNA in horizontal cells of the monkey (macaque) or human retina (Sarthy and Fu, 1989b). Together, these findings support the idea that the expression of GAD isoforms in horizontal cells is species dependent.
The subcellular localization of GAD also varies depending upon the species. In rabbit horizontal cells, GAD65 immunoreactivity was localized at the A-type horizontal cell somata and primary dendrites in the visual streak, and GAD67 was localized to the terminals of A- and B-type horizontal cells (Johnson and Vardi, 1998). In guinea pig horizontal cells, GAD65 was distributed predominantly to the dendritic terminals of A-type cells, and to the dendritic and axonal terminals of B-type cells, as well as in the region of the cell body closest to the OPL (Fig. 3D,G). The distribution of GAD65 in horizontal cell endings, where VGAT immunostaining is strongest, suggests that GABA is synthesized and packaged into the vesicles locally at the endings.
VGAT is expressed in horizontal cells
Specific VGAT immunoreactivity was detected primarily in horizontal cell processes and terminals in the OPL (Fig. 9D), supporting the idea that this vesicular transporter mediates GABA uptake into vesicles in guinea pig horizontal cells. The distribution of VGAT immunoreactivity to horizontal cell processes and terminals correlates well with the preferential localization of GAD65 to these subcellular compartments, further supporting the hypothesis that GABA is synthesized and packaged locally near or within the presynaptic terminals. An earlier study has shown that GABA synthesized by synaptic vesicle-associated GAD is preferentially transported into synaptic vesicles by VGAT (Jin et al., 2003), and a similar mechanism is likely for guinea pig horizontal cells based on the localization of GAD and VGAT to horizontal cell endings. Finally, the co-localization of GAD and VGAT to the tips of horizontal cells implies that GABA is released by a vesicular mechanism from these endings.
Plasmalemmal GABA transporters are expressed by glia but not by horizontal cells in the outer retina
GAT-1 and GAT-3 immunoreactivities were absent from guinea pig horizontal cells (Figs. 11–13), similar to macaque horizontal cells (Haverkamp et al., 2000). These findings are consistent with the failure of guinea pig horizontal cells to accumulate GABA and GABA analogs (Bruun and Ehinger, 1974; Marshall and Voaden, 1975; Bauer and Ehinger, 1978; Cunningham et al., 1981; Agardh and Ehinger, 1982, 1983), which are transported by these plasmalemmal transporters (Guastella et al., 1990; Borden et al., 1992; Clark et al., 1992). Instead, GAT-1 and GAT-3 are expressed by Müller cells of the guinea pig retina, based on their overall immunostaining patterns (Figs. 11, 12) and their Na+- and Cl− -dependent GABA uptake properties, which are similar to the properties of GAT-1 and GAT-3 in cell expression systems (Biedermann et al., 2002). These findings are thus in agreement with previous studies indicating the expression of GAT-1 and GAT-3 in Müller cells in the mammalian retina (Honda et al., 1995; Johnson et al., 1996; Biedermann et al., 2002; Guo et al., 2009). The action of GATs expressed on Müller cells is likely to involve the uptake of GABA released into the extracellular space (Neal and Iversen, 1972; Bruun and Ehinger, 1974; Blanks and Roffler-Tarlov, 1982), thus favoring a role for Müller cells in the rapid removal and metabolism of GABA in the mammalian retina (Biedermann et al., 2002).
Physiological significance
The discovery that most, if not all, horizontal cells express GABA and GAD65, as well as VGAT in guinea pig retina, suggests that mammalian horizontal cells in this species likely utilize GABA as a neurotransmitter. Moreover, the presence of VGAT strongly supports a vesicular release mechanism (for reviews, see Liu and Edwards, 1997; Gasnier, 2004). In the CNS, the expression of VGAT is localized to synaptic vesicles (Chaudhry et al., 1998; Dumoulin et al., 1999b; Takamori et al., 2000). VGAT expression in horizontal cell endings also suggests that GABA is packaged into synaptic vesicles in these cells. This finding is consistent with ultrastructural observations showing small, clear-core vesicles in horizontal cell processes and endings in the photoreceptor triad (Dowling and Boycott, 1966; Dowling et al., 1966; Gray and Pease, 1971; Fisher and Boycott, 1974; Brandon and Lam, 1983; Linberg and Fisher, 1988; Spiwoks-Becker et al., 2001), as well as the findings demonstrating that critical components (SNAP-25, complexins, syntaxin-1 and -4) of the soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) complex are present in mammalian horizontal cells (Hirano et al., 2005; Sherry et al., 2006; Hirano et al., 2007). In addition, the absence of plasmalemmal GABA transporters (GAT-1 and GAT-3) in guinea pig horizontal cells (Figs. 11, 12) argues against a plasma membrane transporter-mediated release mechanism for GABA.
GABA may act at multiple sites in the outer retina. Ionotropic GABA receptors have been localized to bipolar cell dendrites and horizontal cells in various species (Vardi et al., 1992; Greferath et al., 1993, 1994; Vardi and Sterling, 1994; Enz et al., 1996; Koulen et al., 1997; Wässle et al., 1998; Haverkamp et al., 2000). Ionotropic GABA receptors flux Cl− (Macdonald and Olsen, 1994) and can mediate hyperpolarizations as well as depolarizations (Ben-Ari et al., 1994) depending on the reversal potential for Cl− (Leinekugel et al., 1999). This mechanism may contribute in part to the formation of surround inhibition at bipolar cell dendrites of ON and OFF bipolar cells (Miller and Dacheux, 1983; Billups and Attwell, 2002; Varela et al., 2005; Duebel et al., 2006). Indeed, there is evidence that differential Cl− gradients exist in the two functional types of bipolar cells (Satoh et al., 2001; Duebel et al., 2006). In salamander OFF bipolar cells, GABA selectively blocked the surround response while leaving the center response unaltered (Wu, 1986). Finally, GABA is reported to modulate horizontal cell light responses via an autocrine action on horizontal cells (Blanco et al., 1996; Feigenspan and Weiler, 2004; Varela et al., 2005).
There are also a few studies reporting ionotropic GABA receptor immunostaining of mammalian cone photoreceptor terminals (Greferath et al., 1995; Haverkamp and Wässle, 2000; Pattnaik et al., 2000) suggesting GABA influences surround inhibition of photoreceptors. In fish retina, GABA hyperpolarized cone photoreceptors (Wu and Dowling, 1980). GABA evoked chloride currents or chloride-dependent membrane voltage changes in turtle, frog, mouse and pig cone photoreceptors (Kaneko and Tachibana, 1986; Picaud et al., 1998; Pattnaik et al., 2000; Liu et al., 2005, 2006; Tatsukawa et al., 2005; Liu and Yang, 2006). GABA elicited a conductance increase with a reversal potential consistent with a chloride conductance in salamander cones (Wu, 1986). At bullfrog cone terminals, GABA appears to affect calcium currents via GABAB receptor activation (Liu et al., 2005). However, other studies suggest that surround inhibition at macaque cone photoreceptors is not mediated by GABA (Verweij et al., 2003; McMahon et al., 2004).
Although it is generally accepted that horizontal cells provide inhibitory feedback onto photoreceptor terminals, the synaptic and cellular mechanisms underlying this process remain poorly understood. In addition to the evidence for a GABAergic mechanism presented here, there are several proposed mechanisms of synaptic transmission from horizontal cells to photoreceptors. One alternative mechanism for horizontal cell to photoreceptor feedback in both mammalian and nonmammalian retinas is a proton-dependent mechanism whereby a decrease in extracellular pH in the photoreceptor triad shifts the voltage activation of photoreceptor Ca2+ channels, which in turn regulates photoreceptor transmitter release (Barnes et al., 1993; DeVries, 2001; Barnes, 2003; Hirasawa and Kaneko, 2003; Vessey et al., 2005; Jonz and Barnes, 2007; Davenport et al., 2008; Thoreson et al., 2008; Babai and Thoreson, 2009). Finally, an ephaptic model of feedback onto cone terminals was originally conceived by Byzov and colleagues (Byzov and Shura-Bura, 1986), and later elaborated to involve hemichannels as current sinks near the presynaptic calcium channels (Kamermans et al., 2001).
The presence of high levels of GABA, GAD65, and VGAT in adult guinea pig horizontal cells suggests that the guinea pig retina may serve as a useful model system for future studies of GABA signaling mechanisms in mammalian horizontal cells and the outer retina.
ACKNOWLEDGMENTS
We thank Drs. Catia Sternini and Helen Lee for their comments on this manuscript. We also thank Dr. David Pow, University of Queensland, Brisbane, Australia, for providing us with the GABA polyclonal antibody. Finally, we thank Dr. Wenbin Tan for his helpful suggestions regarding PCR and sequencing.
Grant sponsor: National Institutes of Health; Grant number: EY 15573; Grant sponsor: Veterans Administration (Senior Career Research Scientist: N.C.B.).
LITERATURE CITED
- Agardh E, Ehinger B. (3H)-muscimol, (3H)-nipecotic acid and (3H)-isoguvacine as autoradiographic markers for GABA neurotransmission. J Neural Transm. 1982;54:1–18. doi: 10.1007/BF01249274. [DOI] [PubMed] [Google Scholar]
- Agardh E, Ehinger B. Retinal GABA neuron labelling with [3H]isoguvacine in different species. Exp Eye Res. 1983;36:215–229. doi: 10.1016/0014-4835(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Agardh E, Bruun A, Ehinger B, Storm-Mathisen J. GABA immunoreactivity in the retina. Invest Ophthalmol Vis Sci. 1986;27:674–678. [PubMed] [Google Scholar]
- Agardh E, Ehinger B, Wu JY. GABA and GAD-like immunoreactivity in the primate retina. Histochemistry. 1987;86:485–490. doi: 10.1007/BF00500621. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Babai N, Thoreson WB. Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J Physiol. 2009;587:2353–2364. doi: 10.1113/jphysiol.2009.169656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S. Center-surround antagonism mediated by proton signaling at the cone photoreceptor synapse. J Gen Physiol. 2003;122:653–656. doi: 10.1085/jgp.200308947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci U S A. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Ehinger B. Retinal uptake and release of [3H]DABA. Exp Eye Res. 1978;26:275–289. doi: 10.1016/0014-4835(78)90075-1. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. Gamma-aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippo-campal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, Saudou F, Elalouf JM, Hirsch E, Hantraye P, Deglon N, Brouillet E. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell. 2006;17:1652–1663. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann B, Bringmann A, Reichenbach A. High-affinity GABA uptake in retinal glial (Müller) cells of the guinea pig: electrophysiological characterization, immunohistochemical localization, and modeling of efficiency. Glia. 2002;39:217–228. doi: 10.1002/glia.10097. [DOI] [PubMed] [Google Scholar]
- Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J Physiol. 2002;545:183–198. doi: 10.1113/jphysiol.2002.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco R, Vaquero CF, de la Villa P. The effects of GABA and glycine on horizontal cells of the rabbit retina. Vision Res. 1996;36:3987–3995. doi: 10.1016/s0042-6989(96)00145-9. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Roffler-Tarlov S. Differential localization of radioactive gamma-aminobutyric acid and muscimol in isolated and in vivo mouse retina. Exp Eye Res. 1982;35:573–584. doi: 10.1016/s0014-4835(82)80071-7. [DOI] [PubMed] [Google Scholar]
- Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J Biol Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- Bragina L, Melone M, Fattorini G, Conti F. Clozapine upregulates the expression of the vesicular GABA transporter (VGAT) in rat frontal cortex. Mol Psychiatry. 2007;12:612–613. doi: 10.1038/sj.mp.4001987. [DOI] [PubMed] [Google Scholar]
- Brandon C. Retinal GABA neurons: localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase. Brain Res. 1985;344:286–295. doi: 10.1016/0006-8993(85)90806-6. [DOI] [PubMed] [Google Scholar]
- Brandon C, Lam DM. The ultrastructure of rat rod synaptic terminals: effects of dark-adaptation. J Comp Neurol. 1983;217:167–175. doi: 10.1002/cne.902170205. [DOI] [PubMed] [Google Scholar]
- Brecha NC, Weigmann C. Expression of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter in the rat retina. J Comp Neurol. 1994;345:602–611. doi: 10.1002/cne.903450410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun A, Ehinger B. Uptake of certain possible neurotransmitters into retinal neurons of some mammals. Exp Eye Res. 1974;19:435–447. doi: 10.1016/0014-4835(74)90052-9. [DOI] [PubMed] [Google Scholar]
- Byzov AL, Shura-Bura TM. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 1986;26:33–44. doi: 10.1016/0042-6989(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Calaza KC, Hokoç JN, Gardino PF. GABAergic circuitry in the opossum retina: a GABA release induced by L-aspartate. Exp Brain Res. 2006;172:322–330. doi: 10.1007/s00221-005-0338-x. [DOI] [PubMed] [Google Scholar]
- Casini G, Rickman DW, Brecha NC. Expression of the gamma-aminobutyric acid (GABA) plasma membrane transporter-1 in monkey and human retina. Invest Ophthalmol Vis Sci. 2006;47:1682–1690. doi: 10.1167/iovs.05-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani E, Gangitano C, Bosco L, Casini G. Expression of the neurokinin 1 receptor in the mouse retina. Neuroscience. 2004;128:519–530. doi: 10.1016/j.neuroscience.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Chun MH, Wässle H. GABA-like immunoreactivity in the cat retina: electron microscopy. J Comp Neurol. 1989;279:55–67. doi: 10.1002/cne.902790106. [DOI] [PubMed] [Google Scholar]
- Clark JA, Deutch AY, Gallipoli PZ, Amara SG. Functional expression and CNS distribution of a beta-alanine-sensitive neuronal GABA transporter. Neuron. 1992;9:337–348. doi: 10.1016/0896-6273(92)90172-a. [DOI] [PubMed] [Google Scholar]
- Conti F, Melone M, De Biasi S, Minelli A, Brecha NC, Ducati A. Neuronal and glial localization of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter, in human cerebral cortex: with a note on its distribution in monkey cortex. J Comp Neurol. 1998;396:51–63. doi: 10.1002/(sici)1096-9861(19980622)396:1<51::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. Oxford University Press; Oxford: 2003. [Google Scholar]
- Cueva JG, Haverkamp S, Reimer RJ, Edwards R, Wässle H, Brecha NC. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol. 2002;445:227–237. doi: 10.1002/cne.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J, Marshall J, Neal MJ. The radioautographical localization in the vertebrate retina of [3H]-(+ or −)-cisaminocyclohexane carboxylic acid (ACHC); a selective inhibitor of neuronal GABA transport. Exp Eye Res. 1981;32:445–450. doi: 10.1016/s0014-4835(81)80023-1. [DOI] [PubMed] [Google Scholar]
- Dalby NO. Inhibition of gamma-aminobutyric acid uptake: anatomy, physiology and effects against epileptic seizures. Eur J Pharmacol. 2003;479:127–137. doi: 10.1016/j.ejphar.2003.08.063. [DOI] [PubMed] [Google Scholar]
- Damiani D, Alexander JJ, O'Rourke JR, McManus M, Jadhav AP, Cepko CL, Hauswirth WW, Harfe BD, Strettoi E. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–4887. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci. 2008;28:456–464. doi: 10.1523/JNEUROSCI.2735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Wang L, Dong W, He S. Lateral components in the cone terminals of the rabbit retina: horizontal cell origin and glutamate receptor expression. J Comp Neurol. 2006;496:698–705. doi: 10.1002/cne.20959. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Ding JD, Weinberg RJ. Distribution of soluble guanylyl cyclase in rat retina. J Comp Neurol. 2007;500:734–745. doi: 10.1002/cne.21206. [DOI] [PubMed] [Google Scholar]
- Dkhissi O, Julien JF, Wasowicz M, Dalil-Thiney N, Nguyen-Legros J, Versaux-Botteri C. Differential expression of GAD(65) and GAD(67) during the development of the rat retina. Brain Res. 2001;919:242–249. doi: 10.1016/s0006-8993(01)03022-0. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Brown JE, Major D. Synapses of horizontal cells in rabbit and cat retinas. Science. 1966;153:1639–1641. doi: 10.1126/science.153.3744.1639. [DOI] [PubMed] [Google Scholar]
- Duebel J, Haverkamp S, Schleich W, Feng G, Augustine GJ, Kuner T, Euler T. Two-photon imaging reveals soma-todendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron. 2006;49:81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Levi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999a;112:811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Lévi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999b;112:811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Brain Res Mol Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstätter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest Ophthalmol Vis Sci. 2009;50:2417–2426. doi: 10.1167/iovs.08-3028. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Weiler R. Electrophysiological properties of mouse horizontal cell GABAA receptors. J Neurophysiol. 2004;92:2789–2801. doi: 10.1152/jn.00284.2004. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Boycott BB. Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proc R Soc Lond B Biol Sci. 1974;186:317–331. doi: 10.1098/rspb.1974.0052. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Kalloniatis M. Localisation of amino acid neurotransmitters during postnatal development of the rat retina. J Comp Neurol. 1997;380:449–471. doi: 10.1002/(sici)1096-9861(19970421)380:4<449::aid-cne3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fong AY, Stornetta RL, Foley CM, Potts JT. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol. 2005;493:274–290. doi: 10.1002/cne.20758. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Bonfield S, Gilmour GS, Kuny S, Mema SC, Martin BT, Smale L, Crowder N, Stell WK, Sauve Y. Retinal anatomy and visual performance in a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus) J Comp Neurol. 2008;510:525–538. doi: 10.1002/cne.21798. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Baetens D, Roth J, Norman AW, Orci L. Immunohistochemical mapping of calcium-binding protein immunoreactivity in the rat central nervous system. Brain Res. 1984;296:75–86. doi: 10.1016/0006-8993(84)90512-2. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Haverkamp S, Wässle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647. doi: 10.1523/JNEUROSCI.21-21-08636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DI, Chang YC, Schwob JE. Monoclonal antibodies to glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1986;83:8808–8812. doi: 10.1073/pnas.83.22.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG, Pease HL. On understanding the organisation of the retinal receptor synapses. Brain Res. 1971;35:1–15. doi: 10.1016/0006-8993(71)90591-9. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Möhler H, Wässle H. Cholinergic amacrine cells of the rat retina express the delta-subunit of the GABAA-receptor. Neurosci Lett. 1993;163:71–73. doi: 10.1016/0304-3940(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Müller F, Wässle H. Localization of GABAA receptors in the rabbit retina. Cell Tissue Res. 1994;276:295–307. doi: 10.1007/BF00306115. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Fritschy JM, Stephenson A, Möhler H, Wässle H. GABAA receptor subunits have differential distributions in the rat retina: in situ hybridization and immunohistochemistry. J Comp Neurol. 1995;353:553–571. doi: 10.1002/cne.903530407. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. GABA-like immunoreactivity in the macaque monkey retina: a light and electron microscopic study. J Comp Neurol. 1990;297:509–524. doi: 10.1002/cne.902970405. [DOI] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Guo C, Stella SL, Jr, Hirano AA, Brecha NC. Plasmalemmal and vesicular gamma-aminobutyric acid transporter expression in the developing mouse retina. J Comp Neurol. 2009;512:6–26. doi: 10.1002/cne.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano K, Kiyama H, Emson PC, Manabe R, Nakauchi M, Tohyama M. Localization of two calcium binding proteins, calbindin (28 kD) and parvalbumin (12 kD), in the vertebrate retina. J Comp Neurol. 1990;302:417–424. doi: 10.1002/cne.903020217. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Heusner CL, Beutler LR, Houser CR, Palmiter RD. Deletion of GAD67 in dopamine receptor-1 expressing cells causes specific motor deficits. Genesis. 2008;46:357–367. doi: 10.1002/dvg.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Brecha NC. Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. J Comp Neurol. 2005;488:70–81. doi: 10.1002/cne.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Vila A, Brecha NC. Robust syntaxin-4 immunoreactivity in mammalian horizontal cell processes. Vis Neurosci. 2007;24:489–502. doi: 10.1017/S0952523807070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Yamamoto M, Saito N. Immunocytochemical localization of three subtypes of GABA transporter in rat retina. Brain Res Mol Brain Res. 1995;33:319–325. doi: 10.1016/0169-328x(95)00150-q. [DOI] [PubMed] [Google Scholar]
- Hu M, Bruun A, Ehinger B. Expression of GABA transporter subtypes (GAT1, GAT3) in the adult rabbit retina. Acta Ophthalmol Scand. 1999;77:255–260. doi: 10.1034/j.1600-0420.1999.770302.x. [DOI] [PubMed] [Google Scholar]
- Ikegaki N, Saito N, Hashima M, Tanaka C. Production of specific antibodies against GABA transporter subtypes (GAT1, GAT2, GAT3) and their application to immunocytochemistry. Brain Res Mol Brain Res. 1994;26:47–54. doi: 10.1016/0169-328x(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Jellali A, Stussi-Garaud C, Gasnier B, Rendon A, Sahel JA, Dreyfus H, Picaud S. Cellular localization of the vesicular inhibitory amino acid transporter in the mouse and human retina. J Comp Neurol. 2002;449:76–87. doi: 10.1002/cne.10272. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between gamma-aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Chen TK, Rickman DW, Evans C, Brecha NC. Multiple gamma-aminobutyric acid plasma membrane transporters (GAT-1, GAT-2, GAT-3) in the rat retina. J Comp Neurol. 1996;375:212–224. doi: 10.1002/(SICI)1096-9861(19961111)375:2<212::AID-CNE3>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Vardi N. Regional differences in GABA and GAD immunoreactivity in rabbit horizontal cells. Vis Neurosci. 1998;15:743–753. doi: 10.1017/s0952523898154135. [DOI] [PubMed] [Google Scholar]
- Jonz MG, Barnes S. Proton modulation of ion channels in isolated horizontal cells of the goldfish retina. J Physiol. 2007;581:529–541. doi: 10.1113/jphysiol.2006.125666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Kraaij D, Spekreijse H. The dynamic characteristics of the feedback signal from horizontal cells to cones in the goldfish retina. J Physiol. 2001;534:489–500. doi: 10.1111/j.1469-7793.2001.t01-1-00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol. 2006;213:89–100. doi: 10.1007/s00232-006-0877-5. [DOI] [PubMed] [Google Scholar]
- Kao YH, Sterling P. Displaced GAD65 amacrine cells of the guinea pig retina are morphologically diverse. Vis Neurosci. 2006;23:931–939. doi: 10.1017/S0952523806230293. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Jami MM, Krishna L, Jackson R, Patchefsky AS, Cooper HS. Novel immunohistochemical localization of 28,000 molecular-weight (Mr) calcium binding protein (calbindin-D28k) in enterochromaffin cells of the human appendix and neuroendocrine tumors (carcinoids and small-cell carcinomas) of the midgut and foregut. Arch Pathol Lab Med. 1994;118:633–639. [PubMed] [Google Scholar]
- Koulen P, Brandstätter JH, Kröger S, Enz R, Bormann J, Wässle H. Immunocytochemical localization of the GABA(C) receptor rho subunits in the cat, goldfish, and chicken retina. J Comp Neurol. 1997;380:520–532. doi: 10.1002/(sici)1096-9861(19970421)380:4<520::aid-cne8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kyhn MV, Warfvinge K, Scherfig E, Kiilgaard JF, Prause JU, Klassen H, Young M, la Cour M. Acute retinal ischemia caused by controlled low ocular perfusion pressure in a porcine model. Electrophysiological and histological char-acterisation. Exp Eye Res. 2009;88:1100–1106. doi: 10.1016/j.exer.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Mann LB, Rickman DW, Lim EJ, Chun MH, Grzywacz NM. AII amacrine cells in the distal inner nuclear layer of the mouse retina. J Comp Neurol. 2006;494:651–662. doi: 10.1002/cne.20838. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben-Ari Y, Khazipov R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol. 1999;79:189–201. [PubMed] [Google Scholar]
- Lin CT, Li HZ, Wu JY. Immunocytochemical localization of L-glutamate decarboxylase, gamma-aminobutyric acid transaminase, cysteine sulfinic acid decarboxylase, aspartate aminotransferase and somatostatin in rat retina. Brain Res. 1983;270:273–283. doi: 10.1016/0006-8993(83)90601-7. [DOI] [PubMed] [Google Scholar]
- Linberg KA, Fisher SK. Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. J Comp Neurol. 1988;268:281–297. doi: 10.1002/cne.902680211. [DOI] [PubMed] [Google Scholar]
- Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu Rev Neurosci. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang XL. OFF response of bullfrog cones is shaped by terminal ionotropic GABA receptors. Brain Res Bull. 2006;71:219–223. doi: 10.1016/j.brainresbull.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhao JW, Du JL, Yang XL. Functional GABA(B) receptors are expressed at the cone photoreceptor terminals in bullfrog retina. Neuroscience. 2005;132:103–113. doi: 10.1016/j.neuroscience.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Liu J, Li GL, Yang XL. An ionotropic GABA receptor with novel pharmacology at bullfrog cone photoreceptor terminals. Neurosignals. 2006;15:13–25. doi: 10.1159/000094384. [DOI] [PubMed] [Google Scholar]
- Loeliger M, Rees S. Immunocytochemical development of the guinea pig retina. Exp Eye Res. 2005;80:9–21. doi: 10.1016/j.exer.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Corcuera B, Liu QR, Mandiyan S, Nelson H, Nelson N. Expression of a mouse brain cDNA encoding novel gamma-aminobutyric acid transporter. J Biol Chem. 1992;267:17491–17493. [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Marc RE, Murry RF, Fisher SK, Linberg KA, Lewis GP, Kalloniatis M. Amino acid signatures in the normal cat retina. Invest Ophthalmol Vis Sci. 1998;39:1685–1693. [PubMed] [Google Scholar]
- Marshall J, Voaden M. Autoradiographic identification of the cells accumulating 3H gamma-aminobutyric acid in mammalian retinae: a species comparison. Vision Res. 1975;15:459–461. doi: 10.1016/0042-6989(75)90102-9. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway. J Neurosci. 2004;24:3736–3745. doi: 10.1523/JNEUROSCI.5252-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Dacheux RF. Intracellular chloride in retinal neurons: measurement and meaning. Vision Res. 1983;23:399–411. doi: 10.1016/0042-6989(83)90087-1. [DOI] [PubMed] [Google Scholar]
- Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci. 1996;16:6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosinger J, Yazulla S. Double-label analysis of GAD- and GABA-like immunoreactivity in the rabbit retina. Vision Res. 1987;27:23–30. doi: 10.1016/0042-6989(87)90139-8. [DOI] [PubMed] [Google Scholar]
- Mosinger JL, Yazulla S, Studholme KM. GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res. 1986;42:631–644. doi: 10.1016/0014-4835(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Neal MJ, Iversen LL. Autoradiographic localization of 3 H-GABA in rat retina. Nat New Biol. 1972;235:217–218. doi: 10.1038/newbio235217a0. [DOI] [PubMed] [Google Scholar]
- O'Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Patel S, Beaton DW, Neuhoff V. GABA neurones in retinas of different species and their postnatal development in situ and in culture in the rabbit retina. Cell Tissue Res. 1986;243:117–123. doi: 10.1007/BF00221859. [DOI] [PubMed] [Google Scholar]
- Pattnaik B, Jellali A, Sahel J, Dreyfus H, Picaud S. GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. J Neurosci. 2000;20:6789–6796. doi: 10.1523/JNEUROSCI.20-18-06789.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, González-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Picaud S, Pattnaik B, Hicks D, Forster V, Fontaine V, Sahel J, Dreyfus H. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J Physiol. 1998;513:33–42. doi: 10.1111/j.1469-7793.1998.033by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho RG, Owczarzak MT. Distribution of GABA immunoreactivity in the cat retina: a light- and electron-microscopic study. Vis Neurosci. 1989;2:425–435. doi: 10.1017/s0952523800012323. [DOI] [PubMed] [Google Scholar]
- Pow DV, Crook DK. Extremely high titre polyclonal antisera against small neurotransmitter molecules: rapid production, characterisation and use in light- and electron-microscopic immunocytochemistry. J Neurosci Methods. 1993;48:51–63. doi: 10.1016/s0165-0270(05)80007-x. [DOI] [PubMed] [Google Scholar]
- Pow DV, Wright LL, Vaney DI. The immunocytochemical detection of amino-acid neurotransmitters in paraformaldehyde-fixed tissues. J Neurosci Methods. 1995;56:115–123. doi: 10.1016/0165-0270(94)00113-u. [DOI] [PubMed] [Google Scholar]
- Raven MA, Reese BE. Horizontal cell density and mosaic regularity in pigmented and albino mouse retina. J Comp Neurol. 2002;454:168–176. doi: 10.1002/cne.10444. [DOI] [PubMed] [Google Scholar]
- Renteria RC, Strehler EE, Copenhagen DR, Krizaj D. Ontogeny of plasma membrane Ca2+ ATPase isoforms in the neural retina of the postnatal rat. Vis Neurosci. 2005;22:263–274. doi: 10.1017/S0952523805223027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Röhrenbeck J, Wässle H, Heizmann CW. Immunocyto-chemical labelling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci Lett. 1987;77:255–260. doi: 10.1016/0304-3940(87)90508-8. [DOI] [PubMed] [Google Scholar]
- Rossi C, Strettoi E, Galli-Resta L. The spatial order of horizontal cells is not affected by massive alterations in the organization of other retinal cells. J Neurosci. 2003;23:9924–9928. doi: 10.1523/JNEUROSCI.23-30-09924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagné C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Fu M. Localization of L-glutamic acid decarboxylase mRNA in cat retinal horizontal cells by in situ hybridization. J Comp Neurol. 1989a;288:593–600. doi: 10.1002/cne.902880406. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Fu M. Localization of L-glutamic acid decarboxylase mRNA in monkey and human retina by in situ hybridization. J Comp Neurol. 1989b;288:691–697. doi: 10.1002/cne.902880413. [DOI] [PubMed] [Google Scholar]
- Satoh H, Kaneda M, Kaneko A. Intracellular chloride concentration is higher in rod bipolar cells than in cone bipolar cells of the mouse retina. Neurosci Lett. 2001;310:161–164. doi: 10.1016/s0304-3940(01)02120-6. [DOI] [PubMed] [Google Scholar]
- Schnitzer J, Rusoff AC. Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J Neurosci. 1984;4:2948–2955. doi: 10.1523/JNEUROSCI.04-12-02948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Transport-mediated synapses in the retina. Physiol Rev. 2002;82:875–891. doi: 10.1152/physrev.00010.2002. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Vollrath L, Seeliger MW, Jaissle G, Eshkind LG, Leube RE. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Penfold P, Pow DV. Neuronal migration and glial remodeling in degenerating retinas of aged rats and in nonneovascular AMD. Invest Ophthalmol Vis Sci. 2003;44:856–865. doi: 10.1167/iovs.02-0416. [DOI] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA-mediated component in the feedback response of turtle retinal cones. Vis Neurosci. 2005;22:317–324. doi: 10.1017/S0952523805223076. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Babai N, Bartoletti TM. Feedback from horizontal cells to rod photoreceptors in vertebrate retina. J Neurosci. 2008;28:5691–5695. doi: 10.1523/JNEUROSCI.0403-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Auerbach P. Specific cell types in cat retina express different forms of glutamic acid decarboxylase. J Comp Neurol. 1995;351:374–384. doi: 10.1002/cne.903510305. [DOI] [PubMed] [Google Scholar]
- Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res. 1994;34:1235–1246. doi: 10.1016/0042-6989(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Vardi N, Masarachia P, Sterling P. Immunoreactivity to GABAA receptor in the outer plexiform layer of the cat retina. J Comp Neurol. 1992;320:394–397. doi: 10.1002/cne.903200310. [DOI] [PubMed] [Google Scholar]
- Vardi N, Kaufman DL, Sterling P. Horizontal cells in cat and monkey retina express different isoforms of glutamic acid decarboxylase. Vis Neurosci. 1994;11:135–142. doi: 10.1017/s0952523800011172. [DOI] [PubMed] [Google Scholar]
- Varela C, Blanco R, De la Villa P. Depolarizing effect of GABA in rod bipolar cells of the mouse retina. Vision Res. 2005;45:2659–2667. doi: 10.1016/j.visres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Vaughn JE, Famiglietti EV, Jr, Barber RP, Saito K, Roberts E, Ribak CE. GABAergic amacrine cells in rat retina: immunocytochemical identification and synaptic connectivity. J Comp Neurol. 1981;197:113–127. doi: 10.1002/cne.901970109. [DOI] [PubMed] [Google Scholar]
- Veenstra TD, Johnson KL, Tomlinson AJ, Naylor S, Kumar R. Determination of calcium-binding sites in rat brain calbindin D28K by electrospray ionization mass spectrometry. Biochemistry. 1997;36:3535–3542. doi: 10.1021/bi9628329. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Pochet R, Nguyen-Legros J. Immunohistochemical localization of GABA-containing neurons during postnatal development of the rat retina. Invest Ophthalmol Vis Sci. 1989;30:652–659. [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Chun MH. GABA-like immunoreactivity in the cat retina: light microscopy. J Comp Neurol. 1989;279:43–54. doi: 10.1002/cne.902790105. [DOI] [PubMed] [Google Scholar]
- Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Wu SM. Effects of gamma-aminobutyric acid on cones and bipolar cells of the tiger salamander retina. Brain Res. 1986;365:70–77. doi: 10.1016/0006-8993(86)90723-7. [DOI] [PubMed] [Google Scholar]
- Wu SM, Dowling JE. Effects of GABA and glycine on the distal cells of the cyprinid retina. Brain Res. 1980;199:401–414. doi: 10.1016/0006-8993(80)90697-6. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Yamato E, Tashiro F, Sato T, Noso S, Ikegami H, Tamura S, Yanagawa Y, Miyazaki JI. Development of autoimmune diabetes in glutamic acid decarboxylase 65 (GAD65) knockout NOD mice. Diabetologia. 2004;47:221–224. doi: 10.1007/s00125-003-1296-0. [DOI] [PubMed] [Google Scholar]
- Yamasaki EN, Barbosa VD, De Mello FG, Hokoç JN. GABAergic system in the developing mammalian retina: dual sources of GABA at early stages of postnatal development. Int J Dev Neurosci. 1999;17:201–213. doi: 10.1016/s0736-5748(99)00002-7. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Kleinschmidt J. Dopamine blocks carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1982;233:211–215. doi: 10.1016/0006-8993(82)90944-1. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983;263:63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]
- Young WS., 3rd Expression of three (and a putative four) protein kinase C genes in brains of rat and rabbit. J Chem Neuroanat. 1988;1:177–194. [PubMed] [Google Scholar]
- Zhang LL, Fina ME, Vardi N. Regulation of KCC2 and NKCC during development: membrane insertion and differences between cell types. J Comp Neurol. 2006;499:132–143. doi: 10.1002/cne.21100. [DOI] [PubMed] [Google Scholar]