Abstract
Brain-based behavioral interventions targeting specific neurocognitive mechanisms show initial promise in the treatment of emotional disorders, but personalization of such approaches will be facilitated if brain targets are empirically established. As a preliminary step, we conducted a proof-of-concept study to test whether particular emotion regulatory neural circuitry can be differentially targeted by specific neurocognitive tasks, and whether these tasks effectively inhibit amygdala activity. Eleven healthy individuals underwent an idiographic sadness and guilt induction. Brain response was measured via fMRI during 4 subsequent emotion regulation conditions: fixation, cognitive reappraisal (selected to target the ventrolateral prefrontal cortex), working memory practice (selected to target the dorsolateral prefrontal cortex), and visual distraction (Tetris; selected to target occipital cortex). In whole-brain comparisons to fixation, hypotheses were upheld. Reappraisal uniquely activated left ventrolateral prefrontal cortex, working memory practice uniquely activated left dorsolateral prefrontal cortex, and Tetris uniquely activated bilateral occipitoparietal cortex, activations that were largely robust at the single-subject level. All tasks inhibited amygdala activity relative to fixation. Data support examining whether repeated exposure to these tasks in psychiatric patients affects neural abnormalities implicated in emotional disorders. Ideally, psychiatric treatment will be accelerated by matching specific treatments to patients with specific neural profiles.
Keywords: emotion regulation, personalized medicine, fMRI, prefrontal cortex, amygdala
Introduction
Conventional treatments for emotional disorders (e.g., anxiety, depression) are moderately effective (Ballenger, 2004; Dimidjian et al., 2006; Kirsch, Moore, Scoboria, & Nicholls, 2002; Rush et al., 2006), take months to work, can be associated with significant side effects and/or aversive emotional experiences, and are expensive to the healthcare system requiring either expert psychotherapists or pharmacologists’ time. In response, calls for a new generation of treatments that address specific brain mechanisms quickly, efficiently, and ideally in a rehabilitative computer-based automated format have begun to emerge (Siegle, Ghinassi, & Thase, 2007). Here, we examine the extent to which a series of such rehabilitative techniques are, indeed, associated with activation of specific identified brain mechanisms.
The promise of this work is that at some point, patients who are identified as having specific brain abnormalities could thus be directed to specific automated rehabilitative treatments, either as adjunctive or as stand-alone interventions. Without a well-developed mechanistic understanding of treatments, the individual difference variables by which a given patient might be matched to a given treatment targeting his/her individual deficits are left obscure, creating difficulty in selecting appropriate predictor variables and hindering progress in treatment outcome predictor research (Kraemer, Wilson, Fairburn, & Agras, 2002). Research aimed at uncovering neural mechanisms of neurocognitive intervention in healthy samples can provide an important initial step in this process, allowing for experimental control over a unitary symptom construct of interest (depressotypic mood), separable from other symptom clusters (e.g., vegetative symptoms), in order to explore the acute neural effects of intervention components prior to attempting treatment-matching in clinical patients.
Initial efforts attest to the potential of neuroscience-guided treatment strategies that target specific neurocognitive mechanisms directly. Such novel behavioral methods (“neurocognitive” methods), guided by cognitive science findings, offer the opportunity to target brain mechanisms non-invasively. For example, neurofeedback, a form of biofeedback in which patients learn to correct relevant alterations in brain function through operant conditioning, has shown promise in the treatment of attention-deficit disorder (Butnik, 2005), anxiety (Hammond, 2005), depression (Baehr, Rosenfeld, & Baehr, 1997), and chronic pain (deCharms et al., 2005). Attention bias modification, a novel cognitive science-based intervention that targets alterations in attention to threat through repeated practice in orienting away from threat, has shown promise in the treatment of anxiety, potentially reducing symptoms through modulation of top-down prefrontal attention control mechanisms (Hakamata et al., 2010; Hallion & Ruscio, 2011). Similarly, a neurocognitive intervention designed to target depression-related prefrontal deficits through repeated practice in selective attention and working memory tasks has been associated with reduced severity of clinical depression in comparison to treatment-as-usual (between-groups d=1.3, a large effect), as well as alterations in targeted brain mechanisms (Siegle, Ghinassi et al., 2007). A large body of evidence also supports the efficacy of neurocognitive training interventions for treatment of schizophrenia (Twamley, Jeste, & Bellack, 2003), brain injury (Gillen, 2010), and attention deficit disorder (Tucha et al., 2011). It must be acknowledged that data on many of these approaches are quite preliminary, based on small sample sizes or case studies, with little long-term follow-up, yielding variable effect sizes across studies, and leaving unanswered questions regarding their utility as adjunctive vs. stand-alone treatments. Nevertheless, these data cumulatively support the potential to induce symptom reduction through computer-assisted manipulation of neurocognitive mechanisms, and hold promise for advancing personalized medicine by allowing for selection of specific mechanistic targets for specific patients (Forgeard et al., 2011).
One important set of targets for neurocognitive therapies are the brain pathways involved in emotion regulation (ER). Recent transdiagnostic conceptualizations of emotional disorders emphasize emotion dysregulation as a key variable promoting vulnerability to symptoms of anxiety and depression (Aldao, Nolen-Hoeksema, & Schweizer, 2010; Gross & Munoz, 1995; Hofmann, Sawyer, Fang, & Asnaani, 2012; Kring & Sloan, 2010). Broadly speaking, these deficits are thought to involve disruptions in prefrontal-limbic circuitry, resulting in an imbalance between bottom-up salience systems responsive to emotional stimuli (e.g., amygdala) and top-down regulatory regions capable of modulating emotional responses (e.g., prefrontal cortex, PFC; (Bishop, 2007; Davidson, 2003; Hofmann, Ellard, & Siegle, 2012; Pessoa, Kastner, & Ungerleider, 2002).
However, emotion dysregulation can theoretically derive from alterations in any one of a large number of structures involved in ER pathways. For instance, depression has been linked to functional deficits in the dorsolateral prefrontal cortex (DLPFC; (Siegle, Thompson, Carter, Steinhauer, & Thase, 2007), a region implicated in working memory and top-down control of attention (Nee, Wager, & Jonides, 2007; Wager & Smith, 2003). DLPFC deficits have been hypothesized to contribute specifically to sustained emotional and amygdalar processing observed in depressed patients following brief exposure to negative stimuli (Siegle, Steinhauer, Carter, Ramel, & Thase, 2003; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002).
Dysfunction in a distinct ventrolateral prefrontal cortex (VLPFC)-amygdala pathway may underlie difficulties in the ability to flexibly and effortfully down-regulate negative affect in both depressed (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007) and anxious individuals (Campbell-Sills et al., 2011) during a form of explicit ER known as cognitive reappraisal (henceforth, reappraisal; changing one's interpretation of a situation in order to change one's emotional response). The VLPFC, a region involved in cognitive control and selection of goal-relevant information (Blumenfeld & Ranganath, 2007; Nee et al., 2007), has been consistently implicated in studies of healthy volunteers engaged in reappraisal (Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner et al., 2004; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008), and is correlated with self-reported reappraisal success in both unselected volunteers (Wager et al., 2008) and individuals with high trait anxiety (Campbell-Sills et al., 2011). This brain-behavior relationship has been shown to be mediated by decreases in the amygdala (Wager et al., 2008), suggesting VLPFC-amygdala pathway dysfunction is another viable target for neurocognitive intervention in emotional disorders.
Finally, visuospatial processing in the occipital cortex may be pertinent to ER. For instance, in posttraumatic stress disorder, emotion dysregulation involves uncontrollable sensory-perceptual flashbacks involving potent visuospatial components (Ehlers, Hackmann, & Michael, 2004). Manipulating the availability of visuospatial processing resources by presenting a visuospatial cognitive task (“Tetris”) during key memory consolidation windows has been shown to modulate the frequency of flashbacks in healthy volunteers (Holmes, James, Coode-Bate, & Deeprose, 2009; Holmes, James, Kilford, & Deeprose, 2010).
Developing strategies that target each of these pathways to emotion dysregulation directly may increase the potency of psychiatric treatments by allowing for identification of individual differences in the pathway disruptions that lead to emotion dysregulation and then targeting these mechanisms directly. A preliminary step in this process is to identify behavioral strategies that induce regulation of negative emotion via specific brain pathways. Such empirical validation of hypothesized treatment mechanisms is part of standard best practices for rehabilitation generally, and cognitive rehabilitation specifically (e.g., (Johnstone & Stonnington, 2001), allowing for delineation of the full pathway from intervention to brain targets to symptom reduction. Furthermore, a focus on neural mechanisms during initial stages of treatment development and refinement, rather than after a treatment is well-established and has entered widespread use, allows for iterative, mechanistic treatment refinement and ultimately, the potential to disseminate high-impact interventions to patients as efficiently as possible.
In a sample of 11 healthy volunteers, we tested the hypothesis that specific regulatory brain regions could be differentially activated by specific ER strategies. We studied neural features of three automated emotion regulation tasks that lie on a continuum from explicit ER, or intentional down-regulation of negative emotion, to distraction—a relatively implicit form of ER. First, reappraisal, an integral skill in first-line cognitive behavioral treatments, was selected as an unambiguously explicit ER strategy involving intentional top-down regulation of negative affect. Second, a working memory practice task [the Paced Auditory Serial Addition Task; PASAT (Gronwall, 1977)] was selected as a PFC activation strategy specifically designed to strengthen the ability to flexibly modulate sustained forms of attention to negative information (e.g., rumination) via a DLPFC-amygdalar pathway. Thus, the task is conceived as a method to improve explicit ER capability through neurocognitive training, though completing the task does not necessarily involve explicit ER practice per se. Finally, a visual puzzle-solving task, “Tetris,” was selected as a powerful visual distraction strategy that may have therapeutic benefits in terms of 1) initial formation of pathological memories, as in previous research (Holmes et al., 2009; Holmes et al., 2010), and 2) as a method of engaging visual processing regions following emotional triggers in order to reduce negative emotion implicitly, particularly given the task's tenacious effects on visual memory that can last up to several days after playing (Stickgold, Malia, Maguire, Roddenberry, & O'Connor, 2000). Each strategy represents a principal component of emerging and/or established interventions for psychiatric disorders and was selected to target a distinct brain pathway to emotion dysfunction.
To evoke a depressotypic state, participants underwent 7 minutes of sad mood induction during which they were asked to recall a sad autobiographical memory involving significant guilt while listening to sad music individually selected from a choice of four pieces of music. Participants then completed blocks of reappraisal, selected to target the VLPFC (Ochsner et al., 2004); PASAT, selected to target the DLPFC (Siegle, Ghinassi et al., 2007); and Tetris, selected to target the occipital cortex (Haier et al., 1992), in a within-subjects design. Each of these ER strategies has previously shown promise in effecting lasting decreases in negative emotional symptoms (DeRubeis et al., 2005; Holmes et al., 2009; Holmes et al., 2010; Resick et al., 2008; Siegle, Ghinassi et al., 2007), but no previous study has examined whether dissociable or overlapping brain pathways are implicated in these clinical effects. We hypothesized that each ER strategy would elicit activation in its targeted regulatory area, accompanied by decreases in self-reported negative affect and amygdala activation.
Method
Participants
Participants were 11 healthy volunteers (age: M=22.2; SD=2.2; range=20-26; 8 female) recruited by contacting a list of community members interested in participating in brain imaging studies. Study procedures were approved by the Institutional Review Board of the University of Pittsburgh. Written informed consent was obtained from all participants. Participants were required to be right-handed, native English speakers, able to meet MRI safety requirements, and to have no greater than mild self-reported depressive symptoms as indicated by the Quick Inventory for Depressive Symptoms Self-Report (QIDS-SR; (Rush et al., 2003) score ≤10 (M=4.4; SD=1.6; range=2-7). One additional participant completed all study procedures but was excluded due to excessive head movement (total movement >8mm in x-plane).
Autobiographical memories
For use as a negative mood induction, participants were asked to provide a one-paragraph written narrative of a personally relevant depressotypic autobiographical memory in response to the following prompt:
“We ask that in the space below, you describe one of the worst times in your life, when you felt very sad or depressed. We are specifically looking for a situation in which you felt really bad about yourself, for example, because you could have been at least partially at fault, or in which you felt guilty, inadequate, worthless, hopeless, or like a bad person. On a scale of 1 (neutral) to 9 (extremely sad), we ask that you try to pick an experience that you would rate as at least a 7.”
Instructions further stated that participants should select a vivid, emotionally salient memory, capable of evoking strong negative mood upon recall: “We will ask you to read this during the experiment and to try to feel as you felt at that time, so please try to pick an experience that is vivid enough that you will be able to re-create your mood. Please describe it in a way that will allow you to recreate this mood during the study. Describe the event clearly and in detail. Please describe your thoughts and feelings at the time of the event.” Participants were then asked to provide 12 short phrases (1-4 words) to serve as visual reminders to help them vividly visualize their sad experience. Finally, participants listened to 30-second clips from 4 pieces of sad music (Gemar, Kapur, Segal, Brown, & Houle, 1996) and made a single qualitative selection of the piece that would most likely help them experience a sad mood. To facilitate the participants’ return to baseline mood at the end of the experiment, they were also asked to provide a one-paragraph narrative of a happy autobiographical memory and to select one of eight happy music options.
Reappraisal task stimulus elicitation
Participants underwent a brief training in cognitive reappraisal with assistance from an experimenter trained in Cognitive Therapy (BSP). The experimenter used the downward-arrow method (Beck, 1995), in which respondents were repeatedly and progressively asked to consider what their sad autobiographical memory means about themselves, others and/or the world around them, until an absolute or conclusive statement is reached (e.g., “I am worthless”). This procedure was used to elicit 8 dysfunctional negative thoughts related to the participant's sad story, 6 for use as prompts on scan day and 2 for practice. Participants were assisted in reframing 2 practice thoughts by identifying an alternative, more benign interpretation of the event that was also believable. Following a standard Cognitive Therapy technique (e.g., (Greenberger & Pandesky, 1995), if the participant did not endorse > 50% belief in the alternative explanation, they were guided to identify a more plausible explanation.
Emotion Regulation (ER) Task
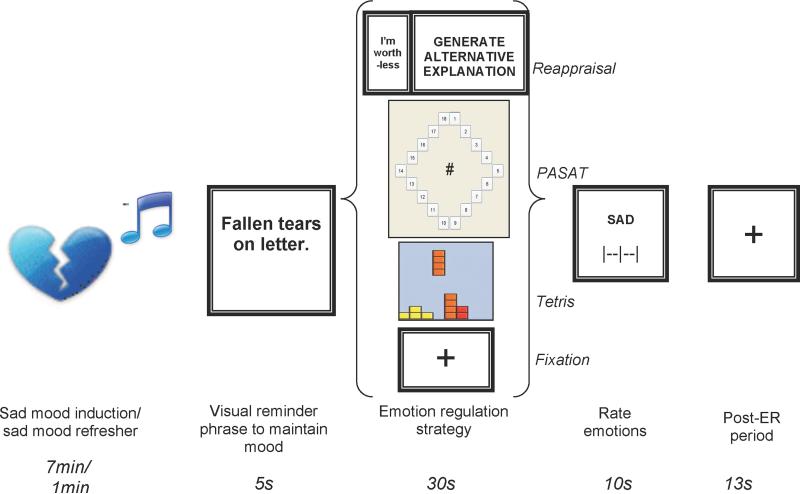
In the scanner, the ER task consisted of 24 trials (presented across 3 runs), each consisting of a visual reminder phrase to maintain negative mood (5.01s), a single ER condition (Reappraisal, Paced Auditory Serial Addition Test (PASAT), Visual Processing (Tetris), or fixation (a baseline comparison condition in which participants were instructed to keep their eyes on a cross) (30.34s), reappraisal success ratings (Reappraisal trials only), visual analog scale ratings of sad, happy, and anxious mood using a sliding scale with three anchors (“neutral”, “moderately”, and “extremely”; 3.34s each), and a post-ER period consisting of a fixation cross (13.36s; Figure 1). Six repetitions of each of 4 ER conditions—Fixation, Reappraisal, PASAT, and Tetris—were completed in an individually randomized order, with the 6 trials of each type randomly intermixed with trials of all other types (rather than presented in sequence). The post-ER period at the close of each trial (13.36s) was intended to allow return to a baseline state of neural and cognitive activity following engagement in a specific ER strategy, prior to presentation of the visual negative mood reminder phrase at the onset of the next trial. Prior to onset of runs 2 and 3 (i.e., prior to the 9th and 17th trials), a 1min sad mood refresher consisting of sad music along with the text “vividly imagine sad story” was presented.
Figure 1.
Trial design. Emotion regulation conditions, depicted as a representative screen shot, from top to bottom are: Cognitive Reappraisal, Paced Auditory Serial Addition Task, Tetris, and Fixation. Sad mood induction occurred at the start of the experiment only; sad mood refresher occurred prior to onset of the 9th and 17th trials.
ER Task: Reappraisal Trials
Each Reappraisal trial consisted of one of the participant's negative thoughts displayed for 3.34 seconds followed by the prompt “Generate Alternative Explanation” displayed for 23.38 seconds.
ER Task: Paced Auditory Serial Addition Trials (PASAT)
Participants completed an adaptive version of the PASAT (Gronwall, 1977) as previously described (Siegle, Ghinassi et al., 2007). The task involves continuously adding serially presented digits in working memory by adding each new digit appearing on the screen to the digit that preceded it, and inhibiting the impulse to sum the current digit with the last answer given. For consistency with previous clinical research in depressed samples (Siegle, Ghinassi et al., 2007), digits were presented verbally through headphones and responses were made using a mouse to select from a diamond-shaped panel of possible sums. Performance was held at a constant level by selecting a maximally difficult presentation speed. We selected a speed for each individual using a 5-minute practice session prior to the scan, in which the inter-stimulus interval (ISI) increased automatically by 100ms whenever 75% accuracy on 4 consecutive responses was achieved. Scan ISI was defined by the speed attained at the end of the 5-minute interval (baseline ISI: M=2.8s; SD=.2; range=2.4-3.1s).
ER Task: Tetris
Tetris (akin to www.tetris.com) is a visuospatial computer game requiring mental rotation of 7 geometrically shaped blocks that fall from the top of the screen. The goal is to manipulate the orientation and position of the blocks as they fall so as to produce solid rows of blocks, or “lines”, at the bottom of the screen. Participants moved blocks left and right by moving the mouse left and right. Each click of the right mouse button rotated blocks 90° clockwise. The left mouse button could be used to accelerate the descent of a block.
Procedure
Participants completed two study visits scheduled within 7 days of one another (mean=2.5 days). Visit 1 (the pre-scan session) included informed consent procedures, autobiographical memory and visual reminder phrase elicitation, reappraisal task stimulus elicitation and training, music selection, and practice in the three ER tasks. Visit 2 (scan day) included additional practice in the 3 ER tasks outside the scanner followed by completion of the scan procedure. The scan procedure included a 7-minute sad mood induction performed during structural scan acquisition, during which participants viewed the full text of their sad narrative, listened to individually selected sad music, and made continuous ratings of sad mood on a visual sliding scale using a mouse-operated slider bar. Continuous ratings were taken throughout the 7-minute period to facilitate attention to, and engagement with, the emotional content of the induction. Mean post-induction sadness ratings were used as a manipulation check to assess the cumulative sad mood achieved, and fell between the anchors for “somewhat ” and “very” sad. In quantitative terms, converting the final mouse coordinate to a percentage of the maximum possible rating (100%=“very sad”; 0%=“neutral”), a mean of 67.1% was obtained (SD=30.8). Participants next completed the ER task. At the conclusion of the ER task, a 7-min happy mood induction was presented consisting of the text from the participant's happy narrative and their happy music selection, to facilitate return to baseline mood.
fMRI acquisition
Images were acquired using a 3Tesla head-only Siemens Trio scanner (Siemens Medical Systems, Ehrlangen, Germany) equipped with a fast gradient system for echoplanar imaging. Head motion was restricted using a standard radiofrequency head coil with foam padding. A 7-minute 3D T1-weighted Magnetization Prepared Rapid Gradient Echo Imaging (MPRAGE) sequence was used to acquire a high-resolution anatomical scan for spatial normalization. Functional images were acquired using a T2*-weighted echoplanar imaging sequence (TR=1.67s, TE=29ms, FOV=205 × 205, flip=75°, 3.2mm isotropic voxels, 32 axial slices). Data were collected in 3 runs (8 trials/run). One participant completed only two runs due to time constraints.
fMRI analysis
Functional volumes were corrected for slice-timing and spatially realigned to correct for motion using Analysis of Functional NeuroImaging (AFNI; Cox 1996). Trials exhibiting movement of more than 1.5mms or degrees were excluded from analysis (5.9% of trials). Linear trends over runs were removed and outliers were Windsorized (e.g., values over 1.5 inter-quartile ranges from the 25th or 75th percentiles were rescaled to the Tukey Hinges using niscorrect from the NeuroImaging Software (NIS) suite (Fissell et al., 2003)). Data were temporally smoothed using a seven-point Gaussian filter (nisfilter) and converted to %-change from the median of all scans (custom code available upon request). Images were co-registered to the MNI reference brain using a 30 parameter non-linear automated warping algorithm (Woods, Mazziotta, & Cherry, 1993).
Single-subject averages were calculated for each of the 4 conditions during the ~30s ER task period. These averages were baseline-corrected to the first scan in the ER task period and subjected to paired t-tests comparing the fixation condition to each of the 3 active ER conditions, allowing for whole-brain tests assessing the primary research question: what brain circuits were targeted or “exercised” by each task in relation to baseline? Type I error for voxelwise tests was controlled using contiguity thresholds derived based on the autocorrelation of the statistical maps (AFNI's AlphaSim with smoothing via 3dFWHM). Using a voxel-wise threshold of p < .005, cluster volume thresholds ranging from 82 to 139 contiguous voxels were determined necessary to hold the probability of map-wise false positive detection at p < .05, depending on the smoothness of the data included in a particular pairwise comparison. Because of an a priori interest in task-related activity in the amygdala, mean time series data for each subject for each condition was extracted and subjected to mixed models GLM analysis. Amygdalae were identified using AFNI's Talairach masks rendered on the MNI reference brain.
Post hoc analyses of targeted regions were performed by extracting the mean signal from all voxels in the clusters identified in whole-brain analyses above (voxels significant at p<.005 uncorrected, p<.05 map-wise corrected) to create functional regions of interest (ROIs) for each targeted region. Data extraction was performed using the nistime command from the NIS suite (Fissell et al., 2003). This approach provides a related set of primary and secondary (post hoc) analyses, as is standard in modern statistical analysis, with post hoc functional ROI analyses designed to provide a more complete understanding of the data in identified regions, particularly with regard to clinical implications, and to aid in hypothesis generation for future work. However, it should be noted that the presented post hoc analyses are non-independent with respect to the selection method (i.e., voxels significant in a primary fixation vs. active ER contrast). Because such non-independent analyses may bias post hoc results towards hypothesis confirmation (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009), these analyses are presented for descriptive purposes only; primary inferences are based on the results from independent whole-brain analyses.
Results
Subjective ratings
Post-task mood ratings and cognitive reappraisal success ratings are presented in Table 1. Due to unanticipated difficulties with slider bar motility during the mood ratings (i.e., some subjects reported the slider was “jerky” and hard to control), a significant number of ratings (16%) were missing and the accuracy of acquired data may have been compromised. For trials with acquired data, mixed model GLM analysis with subject as a random variable and trial and condition as repeated factors revealed a main effect of condition on sadness ratings (F3,149.4=5.3, p=.002). Planned pairwise comparisons comparing each active condition to fixation revealed decreased sadness following the PASAT only (t150=3.3, p=.001; all other p's>.80). Identical mixed models analysis of happy and anxious ratings revealed no main effect of condition (p's>.15). For completeness, planned pairwise comparisons between fixation and active tasks were performed for happy and anxious mood ratings as above, and revealed only one significant result: anxious mood was rated slightly higher post-PASAT than post-fixation (t174.4=2.3, p=.025).
Table 1.
Subjective mood ratings following trials of each emotion regulation task
| Task | Sad | Happy | Anxious |
|---|---|---|---|
| After sad mood induction | 67.1% (30.8)† | -- | -- |
| Fixation | 49.5% (13.1)** | 27.1% (16.0) | 34.8% (20.6)* |
| Reappraisal | 46.8% (13.4) | 27.0% (15.1) | 35.3% (20.9) |
| PASAT | 45.0% (12.7)** | 27.2% (18.4) | 38.7% (19.1)* |
| Tetris | 49.4% (14.9) | 28.7% (16.9) | 37.2% (22.9) |
Note: Values presented as mean (SD). Ratings converted to % of possible maximum from visual analog slider bar ratings. PASAT= Paced Auditory Serial Addition Test. Mixed models analysis revealed main effect of trial type (p<.05) for sad ratings only.
PASAT<Fixation, p=.001; significant omnibus effect of trial type (p = .002)
PASAT>Fixation, p=.03; omnibus effect of trial type was not significant (p=.15)
Ratings immediately following sad mood induction greater than ratings following trials of all other types (post-mood induction rating vs. mean of all end-of-trial ratings): p=.04
Task-related activations: comparisons to fixation
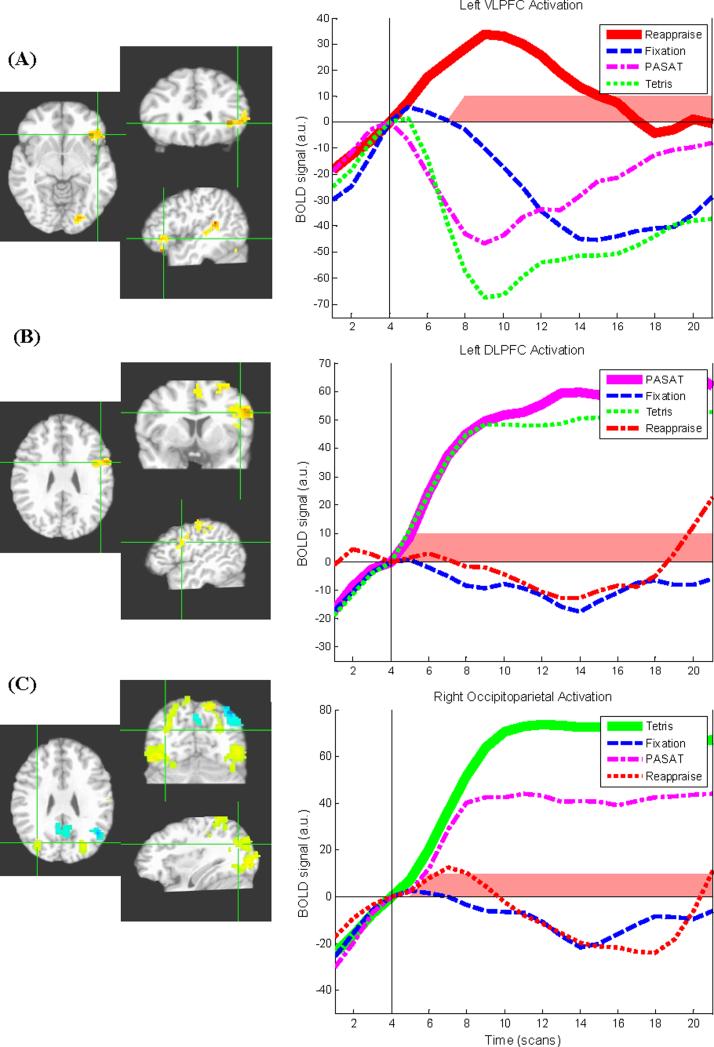
Brain regions showing differential activity as a function of task during the task period are shown in Table 2. Consistent with hypotheses, each of the 3 active tasks produced increases in the regulatory regions targeted by each task (Figure 2). Specifically, VLPFC increases in comparison to fixation were found for the reappraisal task alone, DLPFC increases in comparison to fixation were found for the PASAT alone, and extensive bilateral occipitoparietal activations in comparison to fixation were found for Tetris alone. However, a portion of the left occipital cortex was also active during reappraisal in comparison to fixation.
Table 2.
Within-subject comparisons of BOLD signal during emotion regulation tasks: comparisons to fixation
| Region | Location of centroid voxel | Brodmann's areas | x | y | z | Cluster extent (mm3) | Peak d |
|---|---|---|---|---|---|---|---|
| Reappraisal | |||||||
| Reappraisal>Fixation | |||||||
| Ventrolateral PFC* | L Inferior Frontal Gyrus | 47, 45 | -44 | 26 | 3 | 2211 | 4.16 |
| Cuneus/Lingual Gyrus/Middle Occipital Gyrus | L Cuneus | 17, 18 | -21 | -83 | 6 | 2766 | 3.39 |
| Superior Temporal Gyrus/Insula | L Superior Temporal Gyrus | 41, 13 | -44 | -36 | 15 | 2073 | 4.80 |
| Fixation>Reappraisal | |||||||
| L amygdala (anatomical mask) | -- | -- | -23 | -5 | -15 | 2073 | -- |
| Paced Auditory Serial Addition Task (PASAT) | |||||||
| PASAT>Fixation | |||||||
| Dorsolateral PFC* | L Inferior Frontal Gyrus | 6, 9, 44 | -47 | 4 | 32 | 2980 | 3.98 |
| Precentral/Postcentral Gyrus | L Middle Frontal Gyrus | 6, 1 | -32 | -24 | 57 | 14838 | 4.24 |
| Culmen/Lingual Gyrus | R Culmen | 18, 19 | 13 | -54 | -4 | 5818 | 3.86 |
| Dorsomedial PFC/Supplementary Motor Area | R Superior Frontal Gyrus | 6, 8 | 0 | 11 | 49 | 3162 | 3.16 |
| Thalamus | L Thalamus | -- | -12 | -18 | 16 | 2622 | 4.22 |
| Fixation>PASAT | |||||||
| Medial PFC/Anterior Cingulate | R Anterior Cingulate | 32, 24 | 2 | 33 | 3 | 7101 | 5.17 |
| L amygdala (anatomical mask) | -- | -- | -23 | -5 | -15 | 2073 | -- |
| R amygdala (anatomical mask) | -- | -- | 24 | -5 | -15 | 2244 | -- |
| Tetris | |||||||
| Tetris>Fixation | |||||||
| Occipitoparietal* | R Middle Occipital Gyrus | 19, 7 | 33 | -64 | 31 | 30477 | 5.27 |
| Occipitoparietal | L Inferior Parietal Lobule | 6, 7, 1 | -30 | -41 | 43 | 36508 | 5.04 |
| Fixation>Tetris | |||||||
| Precuneus/Posterior Cingulate | L Precuneus | 7, 31 | -3 | -50 | 37 | 11787 | 5.01 |
| Inferior Parietal Lobule | L Inferior Parietal Lobule | 7, 40 | -41 | -61 | 43 | 7251 | 6.86 |
| L amygdala (anatomical mask) | -- | -- | -23 | -5 | -15 | 2073 | -- |
| R amygdala (anatomical mask) | -- | -- | 24 | -5 | -15 | 2244 | -- |
Note: Coordinates for each cluster's center-of-mass are presented in Talairach space. Anatomical mask for the amygdala (left and right) created as previously described (Siegle et al., 2002; Siegle, Thompson et al., 2007); mean signal from anatomical region extracted and analyzed in mixed models GLM. All other findings are from unrestricted whole brain analysis with map-wise error rate p < .05. BOLD = blood-oxygen-level dependent; PFC = prefrontal cortex; PASAT=Paced Auditory Serial Addition Test.
Targeted region used for functional region of interest (ROI) follow-up analyses.
Figure 2.
Regulatory regions targeted by specific neurocognitive tasks exhibiting greater activation during task than fixation. Crosshairs indicate center-of-mass of targeted regulatory regions. Images shown in radiological convention (left = right). Yellow/red shading indicates activation; blue shading indicates deactivation (in comparison to fixation). Line graphs show baseline-corrected BOLD signal over time during each of the four task conditions. Vertical black line indicates the start of the active task period. Time points showing significant differences between condition of interest and fixation are indicated with red shading. a.u. = arbitrary units. NOTE: data used in graphs and significance tests are non-independent from the primary t-test analysis and are presented for illustrative rather than inferential purposes. (A) Left ventrolateral prefrontal cortex region exhibiting greater activation during cognitive reappraisal than fixation. (B) Left dorsolateral prefrontal cortex region exhibiting greater activation during Paced Auditory Serial Addition Task (PASAT) than fixation. (C) Right occipitoparietal region exhibiting greater activation during Tetris than fixation.
Did neurocognitive tasks produce decreases in amygdalar activity?
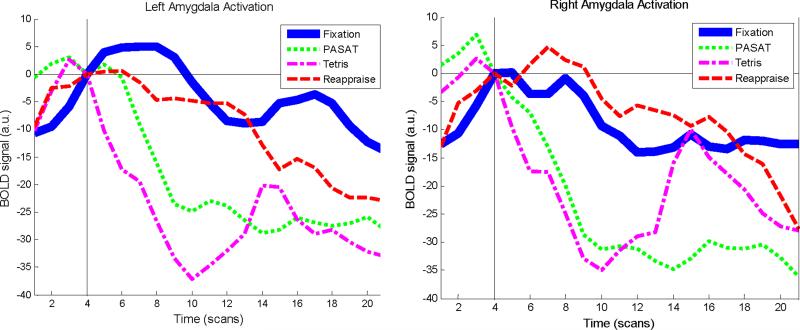
Consistent with hypotheses, time-series data extracted from the anatomically defined left and right amygdalae during the task period showed a decrease in amygdalar activity in all active conditions compared to fixation. The effect was qualified by a condition × hemisphere (left vs. right) interaction [Table 2, Figure 3; mixed model GLM analysis with subject as a random variable and condition, time, and hemisphere as fixed factors: main effect of condition (F3,1451=23.3, p<.001) and condition-by-hemisphere interaction (F3,1414=4.1, p=.006)]. Pairwise comparisons of estimated marginal means were designed to test the primary hypothesis that amygdalar activity would be decreased in each active condition in comparison to fixation. These analyses revealed decreased left amygdala activity in all 3 active conditions in comparison to fixation (p's<.007, d's=.41-.82) and decreased right amygdala activity during the PASAT and Tetris (p's<.001, d's=.50-.53) but not reappraisal (p=.84, d=.04).
Figure 3.
Line graphs showing baseline-corrected BOLD signal over time during each of the four task conditions in anatomically defined left and right amygdala. Vertical black line indicates the start of the active task period. Mixed models analysis revealed a significant main effect of condition and a significant condition × hemisphere interaction.
Non-independent post-hoc analysis: are activations in targeted regions specific to their respective neurocognitive task?
Non-independent, post hoc analyses of each of the targeted regulatory regions (VLPFC, DLPFC, occipitoparietal cortex) were designed to provide further descriptive information regarding whether each target region was specifically and uniquely activated by the task selected to target it.
VLPFC
Follow-up, non-independent pairwise comparisons assessing the specificity of VLPFC activation to reappraisal were conducted by extracting mean values from this VLPFC functional ROI (averaged across the ER period) for each of the four task conditions. In contrast to reappraisal, activation in the VLPFC ROI did not differ for PASAT and fixation (t10=.16, p=.87, d=0.05), while VLPFC values were significantly smaller for Tetris compared to fixation (t10=-2.4, p=.04, d=-0.77), suggesting activation of VLPFC above baseline (fixation) was specific to reappraisal. While deactivation of this region during Tetris could indicate a relevant change in brain state, this pattern was not hypothesized nor observed in primary independent analyses (Table 2) and thus should be interpreted with caution.
DLPFC
As above, mean values from the DLPFC were averaged across the ER period for use in non-independent descriptive analysis. While activation in the DLPFC ROI did not differ for reappraisal and fixation (t10=-.89, p=.40, d=-0.31), DLPFC values were significantly greater for Tetris than fixation (t10=4.0, p=.003, d=1.80), suggesting this region's activation may not be fully specific to the PASAT.
Occipital cortex
As above, mean functional ROI values were extracted from the right occipitoparietal cluster uniquely activated during Tetris and averaged across the ER period. In non-independent post hoc analysis, while activation in the right occipitoparietal ROI did not differ for reappraisal and fixation (t10=.33, p=.75, d=0.13), values were greater for PASAT than fixation (t10=3.2, p=.009, d=1.21), suggesting this region's activation may not be fully specific to Tetris.
Between-task comparisons
Is the magnitude of activity in targeted regions largest during its respective neurocognitive task (in comparison to other active tasks)?
In the task vs. fixation analyses described above, target regions (VLPFC, DLPFC, occipitoparietal cortex) showed evidence of specificity of activation to the respective ER tasks selected to target them. We were also interested in whether target regions would show greater magnitude of activations during their respective tasks in direct comparisons to the other active tasks. We first tested the omnibus hypothesis that activations in targeted regions differed as a function of which active task was being performed. A whole-brain ANOVA comparing the three active tasks (reappraisal, PASAT, Tetris) was performed on single-subject means from the active ER period and a mask extracted for all voxels significant at p<.005. This mask was applied to the primary task vs. fixation maps reported in Table 2 to determine which activated clusters also differed significantly as a function of task. Subclusters within all three a priori targeted regions survived this additional constraint [left VLPFC (22 voxels), left DLPFC (57 voxels), right occipitoparietal (286 voxels)], suggesting that the regions were differentially activated as a function of the specific neurocognitive strategy used.
In addition to this whole-brain strategy, we also used non-independent, post hoc analysis of the mean signal extracted from functional ROIs in each of the three targeted regions to obtain further descriptive information as to whether activity in these functional ROIs differed in direct comparisons across tasks. Consistent with hypotheses, the three functional ROIs showed differential activation across the three tasks in an omnibus test [repeated measures ANOVA with task (reappraisal vs. PASAT vs. Tetris) and region (left VLPFC vs. left DLPFC vs. right occipitoparietal cortex) as fixed factors and mean activation contrast values (active task – fixation) as the dependent variable: task × region interaction (F4,40=34.5, p<.001)]. Planned pairwise contrasts were consistent with the claim that activations were strongest in each targeted region during the task intended to target that region, with one exception: while left VLPFC activation was greater during reappraisal than during PASAT (t7=4.4, p=.003, d=1.04) and Tetris (t7=6.8, p<.001, d=2.46), and right occipitoparietal activation was greater during Tetris than during PASAT (t7=2.8, p=.03, d=0.55) and reappraisal (t7=7.9, p<.001, d=1.45), left DLPFC activation was greater during PASAT than during reappraisal (t7=6.2, p<.001, d=1.64), but not greater during PASAT than during Tetris (t7=.76, p=.47, d=0.16).
Exploratory single-subject analysis
Were targeted activations robust at the single-subject level?
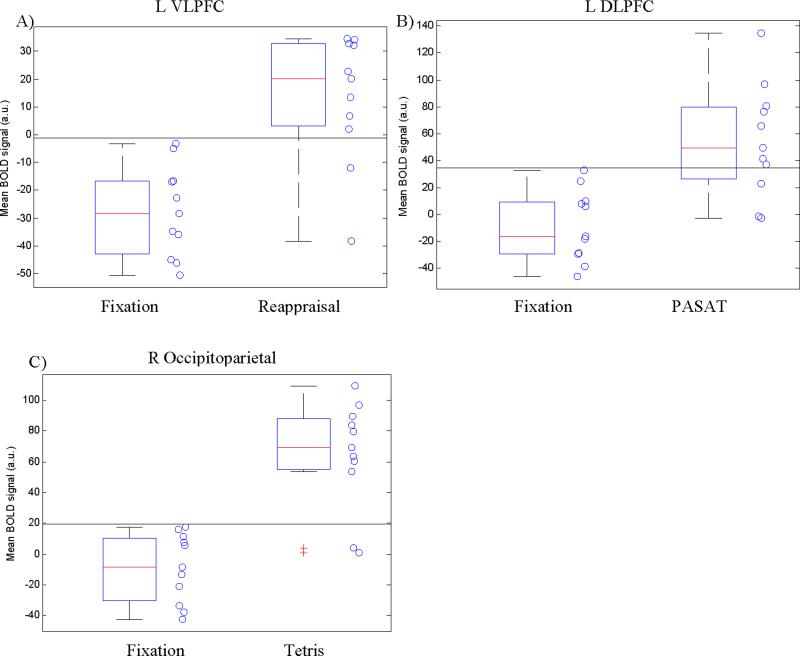
Because we are ultimately interested in clinical, single-subject applications of neurocognitive interventions, we explored whether observed group-level findings in targeted regions for primary task vs. fixation t-tests were robust at the individual subject level. Mean BOLD signal from each subject in each targeted ROI was extracted and BOLD signal distributions were inspected to determine the number of individual subjects showing greater activation during the relevant task compared to fixation baseline values. For each ROI, we determined the BOLD threshold that allowed for maximum discrimination between fixation and active tasks using individual subjects’ values and tested the significance of correctly classified BOLD values using Fisher's exact two-tailed tests.
Reappraisal task
Ten out of 11 subjects showed BOLD signal increase in the L VLPFC during reappraisal compared to fixation (mean signal change=41.3; SD=20.1, range=-3.5-66.5). A BOLD threshold of -3.3 (horizontal line in Figure 4a) maximized discrimination between fixation and reappraisal, resulting in 11/11 correct classifications for fixation and 9/11 (81.8%) correct classifications for reappraisal (90.9% overall correct classification; Fisher's exact p=.0002).
Figure 4.
Boxplot and scatterplot of individual subjects’ mean BOLD signal values for (A) fixation vs. reappraisal; (B) fixation vs. PASAT; and (C) fixation vs. Tetris. Horizontal line represents value that maximized discrimination between fixation and active tasks.
PASAT task
Ten out of 11 individual subjects showed BOLD signal increase in the L DLPFC during PASAT compared to fixation (mean signal change=63.4; SD=37.3, range=-9.0-128.5). A threshold of 32.8 (horizontal line in Figure 4b) maximized discrimination between fixation and PASAT, resulting in 11/11 correct classifications for fixation and 8/11 (72.7%) correct classifications for the PASAT (86.4% overall correct classification; Fisher's exact p=.001).
Tetris task
Ten out of 11 subjects showed BOLD signal increase in the right occipitoparietal cortex during Tetris compared to fixation (mean signal change=73.5; SD=36.9, range=-11.9-111.4). A BOLD threshold of 17.8 (horizontal line in Figure 4c) maximized discrimination between fixation and Tetris, resulting in 11/11 correct classifications for fixation and 9/11 (81.8%) correct classifications for Tetris (90.9% overall correct classification; Fisher's exact p=.0002).
Individual differences: do the same participants show large responses in target areas across the three tasks?
DLPFC activation during the PASAT was strongly and significantly correlated with occipitoparietal activation during Tetris across participants (r=.64, p=.03). However, correlations between VLPFC activation during reappraisal and target region activations during the other two tasks were small and non-significant (Reappraisal-VLPFC and Tetris-Occipitoparietal: r=.17, p=.63; Reappraisal-VLPFC and PASAT-DLPFC: r=-.02, p=.95). These results suggest greater congruence between individual differences in Tetris and PASAT target activation than between reappraisal target activation and either of the other two tasks.
Discussion
The current study provides proof-of-concept for the notion that distinct neurocognitive intervention components can be selected to activate distinct regulatory regions chosen a priori, with a common consequence of decreased amygdala activation, in the context of a sustained depressotypic mood state. Amygdalar decreases were observed during all 3 active tasks, while self-report ratings of mood regulation were more equivocal, suggesting the tasks in the current context might best be construed as neurocognitive “amygdala modulators” rather than emotion regulation (ER) tasks per se. Nevertheless, each of the tasks included in the present study have previously been shown to reduce negative affect more enduringly in clinical and/or analogue samples (DeRubeis et al., 2005; Holmes et al., 2009; Holmes et al., 2010; Resick et al., 2008; Siegle, Ghinassi et al., 2007), suggesting these tasks are promising candidates for neuroscience-guided intervention development. Primary analyses supported specificity of targeted activations to each of the three tasks, confirming hypotheses regarding intervention mechanisms; however, non-independent post hoc exploration of the data suggested a more nuanced pattern across tasks that may help refine hypotheses for future research in independent samples. Specifically, two of the selected tasks (PASAT and Tetris; both involving relatively implicit methods of amygdala modulation) produced similar magnitude of activation in targeted regions (DLPFC and occipital cortex) according to post hoc exploration, while the task with the most explicit ER goals (reappraisal) showed evidence of greater specificity in its target region (VLPFC). Targeted activations during each task compared to fixation were robust in the vast majority of individual subjects. These findings lay initial groundwork for the development of an array of behavioral options that can be selected to target particular neurocognitive profiles of emotion dysregulation in individual patients.
Findings in targeted regions
Reappraisal, a key skill taught in CBT for depression and anxiety, produced activation in the left VLPFC. VLPFC activation is explicitly correlated with reappraisal success and limbic modulation in healthy volunteers (Wager et al., 2008) and has been implicated in impaired top-down ER in depressed (Johnstone et al., 2007) and anxious (Campbell-Sills et al., 2011) patients, suggesting that individual differences in the ability to recruit this region during reappraisal may have high clinical relevance warranting targeted intervention. The decreased amygdala activity observed during reappraisal was left-lateralized, consistent with left-lateralized findings in the majority of neuroimaging studies of emotion (Baas, Aleman, & Kahn, 2004; Wager, Phan, Liberzon, & Taylor, 2003), particularly those involving verbal processing (Markowitsch, 1998), as is likely to occur during reappraisal. The current study extends previous findings in healthy volunteers through the use of highly personally relevant, negative autobiographical information and the induction of a sustained depressotypic mood state. These features of the task design provide a tighter analogue for the skill taught clinically during CBT in comparison to previous studies which have 1) primarily used normative negative scenes (Johnstone et al., 2007; Ochsner et al., 2002; Ochsner et al., 2004), c.f. (Goldin, Manber-Ball, Werner, Heimberg, & Gross, 2009), 2) instructed participants to reappraise using non-clinically relevant methods (e.g., imagining that the negative scenes are actually staged photographs), and 3) allotted substantially less time for each reappraisal trial (e.g., 10s) than what is typical in a clinical setting.
In contrast to the current study, previous neuroimaging studies of reappraisal have elicited activation in a broader network of prefrontal regions including the medial PFC, anterior cingulate cortex (ACC) and DLPFC (e.g., (Ochsner et al., 2004). This discrepancy may be related to procedural variables such as the longer duration of reappraisal in the current study and could suggest that the VLPFC is most relevant to sustaining reappraisal-related negative affect reductions over a longer period of time and/or in response to self-relevant material.
In contrast to reappraisal, which is an explicit strategy requiring attention to be directed towards the source of negative emotion, Tetris and PASAT could operate through distraction, producing incidental reductions in amygdalar activity due to reallocation of mental resources and reciprocal inhibition of cognitive and affective processing pathways (Drevets & Raichle, 1998). Our findings are consistent with previous studies finding amygdalar decreases and PFC and parietal cortex increases during distraction from emotional stimuli (Kanske, Heissler, Schönfelder, Bongers, & Wessa, 2011; McRae et al., 2010), but extend this literature through use of personally relevant material and a prolonged (30s per trial), post-mood induction task period, during which strategies were applied without additional external emotional provocation from stimuli remaining on-screen. This mood induction was designed to provide a tighter analogue for clinical manifestations of negative affect and cognition, intrusive imagery, and related limbic hyperactivation, which are protracted even in the absence of ongoing external provocation (e.g., (Ehlers et al., 2004; Siegle, Granholm, Ingram, & Matt, 2001; Siegle et al., 2003; Siegle et al., 2002; Siegle, Thompson et al., 2007).
Although short-term distraction effects alone are useful in the context of chronic emotion dysregulation (Nolen-Hoeksema & Morrow, 1993) and commonly employed in first-line psychotherapies to improve both acute and chronic ER ability (Linehan, 1993; Martell, Dimidjian, & Herman-Dunn, 2010), there is also initial evidence that both the PASAT and Tetris are capable of producing enduring changes in the way the brain processes negative emotional material. Six sessions of repeated PASAT practice have been associated with symptom reductions as well as decreased DLPFC-amygdala circuit disruptions in a clinically depressed sample (Siegle, Ghinassi et al., 2007), ostensibly producing symptom relief not through distraction alone, but through strengthening of a top-down DLPFC-amygdalar pathway that is deficient in depression (Siegle, Thompson et al., 2007). Playing Tetris after viewing a traumatic film involving scenes of injury and death reduced intrusive “flashback” memories and PTSD symptomatology in a healthy volunteer sample for up to 1-week post-viewing, ostensibly due to memory consolidation interference from competing visuospatial material (Holmes et al., 2009; Holmes et al., 2010). Furthermore, given Tetris's ability to induce potent, highly stereotyped visual images of the game itself for several days subsequent to playing (e.g., during hypnagogic states; (Stickgold et al., 2000), it could hold promise as a method of introducing enduring interference for potent emotional images that are recurrent and tenacious.
Specificity and magnitude of targeted activations
Primary whole-brain analyses, supplemented by non-independent post hoc comparisons across tasks, largely indicated that each targeted region was most strongly activated during the task designed to target it. The VLPFC, in particular, demonstrated full specificity of activation (greater than fixation) to reappraisal in all analyses. Tetris, reappraisal, and (in post hoc analyses only) PASAT were associated with some level of occipital activity, though activations were both stronger and more extensive during Tetris than the other two tasks. DLPFC activation was unique to PASAT in primary analyses, although non-independent post hoc exploration ran contrary to hypotheses in suggesting potential overlap in the strength of DLPFC activation between PASAT and Tetris, two higher-order cognitive tasks requiring working memory manipulations (Nee et al., 2007; Wager & Smith, 2003). Collectively, post hoc descriptive comparisons suggest that with regard to specificity and magnitude of activation in targeted regions, the largest neural distinctions were those separating the most explicit strategy (reappraisal) from the two strategies selected to lie closer to the implicit end of an explicit-implicit continuum (PASAT and Tetris).
Notably, individual differences in the magnitude of targeted activation also suggested an explicit vs. implicit distinction: the degree of success with which individuals recruited targeted regions during Tetris and PASAT were highly and significantly correlated, while correlations between reappraisal-related targeted activation and targeted activation during the other two conditions were small and non-significant. A critical empirical question is therefore whether clinical patients will show a similar implicit vs. explicit distinction, and if so, whether individual patients might benefit most from practice in a neurocognitive strategy type that plays to their neural “strengths,” or alternatively, a strategy targeting a neurocognitive deficit or “weakness.”
Additional task-related findings
PASAT and Tetris were more effective than reappraisal in decreasing activity in medial PFC/ACC (PASAT) and inferior parietal and posterior cingulate cortex (Tetris; Table 2), potentially indicating more sustained inhibition of “default-mode” (Raichle et al., 2001) and/or self-referential processing (Northoff et al., 2006) during these two cognitively-demanding tasks. PASAT also elicited activation in a broad executive network of fronto-thalamic and cerebellar regions, while reappraisal activated a region encompassing portions of the left superior temporal gyrus and insula, consistent with previous suggestions of superior temporal cortex involvement in reappraisal (Ochsner et al., 2004) and/or with increased insular interoceptive processing (Craig, 2009) during reappraisal (e.g., monitoring for signs of decreased physiological arousal in order to assess reappraisal success).
Subjective ratings
Reliable task-related change in subjective mood ratings was observed only for the PASAT in the current study, suggesting that amygdalar function provided a more sensitive measure of task-related effects on emotional processing. Sensitivity of mood ratings was likely reduced by limited power due to the small sample size, missing data and difficulties controlling the computer mouse in a short period of time, and individual differences in ER success across distinct tasks. In addition, in order to provide an uninterrupted period of task engagement, mood ratings were acquired only at the end of the 30s task period, which likely provided ample time for gradual attenuation of negative mood in the comparison (fixation) condition. Consistent with this interpretation, sadness ratings were decreased for all conditions, including fixation, in comparison to ratings made immediately following mood induction (Table 1). By contrast, the amygdala fMRI data, which did demonstrate hypothesized decreases in all 3 active tasks, was measured continuously every 1.67s throughout the course of each trial, and therefore had greater sensitivity to detect emotional effects varying across time.
In addition to decreased sadness during the PASAT, there was some suggestive evidence for a small increase in anxious mood observed following the PASAT task. Though not specifically hypothesized, this pattern in anxiety ratings is not entirely unexpected given the difficulty level of the task and its previously demonstrated ability to induce anxiety and frustration (Gratz, Rosenthal, Tull, Lejuez, & Gunderson, 2010; Holdwick & Wingenfeld, 1999; Tombaugh, 2006). The current, adaptive version of the PASAT has previously been conceptualized as providing an appropriate level of frustration for practice in top-down prefrontal control to occur in the context of elevated negative affect (Siegle, Ghinassi et al., 2007), an important feature of the real-world scenarios in which emotion dysregulation occurs.
Limitations
Although the sample size of the current study was small, detection of predicted effects suggests the study was adequately powered to test hypotheses, and the demonstration of activations that were largely robust even in individual subjects supports the potential utility of these tasks for single-subject, clinical applications. Nevertheless, both the sample size and the number of trials per condition were low by current standards, which may have constrained power to detect additional effects of interest; findings should thus be considered preliminary. The lack of significant differences in subjective mood ratings for two of the three ER tasks compared to fixation (reappraisal, Tetris) limit conclusions regarding the viability of these tasks as ER strategies and may constrain the interpretation of observed brain effects. As the selected tasks have indeed shown emotion regulation properties in previous studies, power to detect such effects was likely insufficient in the present sample due to sample size, task design, and technical difficulties with mood rating collection. However, amygdalar findings, which were fully consist with hypothesized mood regulation during all three neurocognitive tasks, may provide a more sensitive index of task-related effects on emotional processing that are both more proximal to the putative mechanisms of these neurocognitive strategies and free of behavioral demand effects.
The four task conditions compared in the current study involved highly variable stimulus properties (e.g., visually complex, changing arrays of shapes vs. stationary words vs. auditory digits); thus, the study was not designed to provide a tightly controlled assessment of neural mechanisms involved in the specific components of each task, but rather to explore the neural bases of automated interventions in a format closely resembling their prior and potential applications in clinical settings. The use of an analogue manipulation in healthy volunteers may reduce generalizability to patient groups, but improves internal validity in terms of isolating a unitary symptom construct (depressotypic mood) and assessing robustness of single-subject activations in participants who represent a healthy goal-state rather than an impaired state. However, the clinical screening procedure was limited to a single self-report measure of depression; therefore, we cannot rule out the presence of psychopathology in our sample. Future studies should aim to extend these findings to clinical populations and to examine the neural substrates of enduring changes in neurocognitive pathways occurring through repeated practice.
Conclusions
In summary, this investigation tested whether brief practice in three neurocognitive interventions, delivered in an automated, computer-based format—reappraisal, working memory practice (PASAT), and visual distraction (Tetris)—would produce amygdalar reductions through distinct pathways involving the VLPFC, DLPFC, and occipital cortex, respectively. Data suggested each task 1) robustly targeted its respective structure and 2) decreased amygdalar activation, confirming hypothesized neural mechanisms. Primary and post hoc results were most consistent for the left VLPFC, which was specifically and maximally activated by reappraisal, the most explicit regulation strategy employed. The current findings support the preliminary premise that neurocognitive intervention strategies can be selected to maximize practice or “brain-training” for targeted regions of interest. These findings in a healthy sample, where mood is isolated from other symptom clusters (e.g., vegetative symptoms) and subjected to experimental control, lay initial groundwork for development of a patient-treatment matching algorithm, e.g., personalized explicit/VLPFC vs. implicit/DLPFC cognitive training. Upon further replication in clinical samples, these findings may provide a preliminary step towards the development of a personalized medicine approach to psychiatric care–one that eschews heterogeneous diagnostic categories and heterogeneous treatments in favor of individualized neurocognitive profiles and mechanistic, neurocognitive strategies.
Acknowledgments
This research was supported by Defense Advanced Research Projects Agency (DARPA) N00014-05-1-0881 and NIH MH082998 & MH018269-25.
Footnotes
Conflict-of-Interest Statement: Greg Siegle is an unpaid consultant for TrialIQ. No other authors have conflicts of interest to declare.
References
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research. Brain Research Reviews. 2004;45(2):96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baehr E, Rosenfeld JP, Baehr R. The Clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression. Journal of Neurotherapy. 1997;2:10–23. [Google Scholar]
- Ballenger JC. Remission rates in patients with anxiety disorders treated with paroxetine. Journal of Clinical Psychiatry. 2004;65(12):1696–1707. doi: 10.4088/jcp.v65n1216. [DOI] [PubMed] [Google Scholar]
- Beck JG. Cognitive Therapy: Basics and Beyond. The Guilford Press; New York, NY: 1995. [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Butnik SM. Neurofeedback in adolescents and adults with attention deficit hyperactivity disorder. Journal of Clinical Psychology. 2005;61:621–625. doi: 10.1002/jclp.20124. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Simmons AN, Lovero KL, Rochlin A. a., Paulus MP, Stein MB. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. NeuroImage. 2011;54:689–696. doi: 10.1016/j.neuroimage.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, et al. Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Drevets W, Raichle M. Suppression of regional cerebral blood during emotional versus higher cognitive implications for Interactions between emotion and cognition. Cognition & Emotion. 1998;12:353–385. [Google Scholar]
- Ehlers A, Hackmann A, Michael T. Intrusive re-experiencing in post-traumatic stress disorder: Phenomenology, theory, and therapy. Memory. 2004;12:403–415. doi: 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- Fissell K, Tseytlin E, Cunningham D, Carter CS, Schneider W, Cohen JD. Fiswidgets: A graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1(1):111–125. doi: 10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- Forgeard MJC, Haigh EAP, Beck AT, Davidson RJ, Henn FA, Maier SF, et al. Beyond depression: Towards a process-based approach to research, diagnosis, and treatment. Clinical Psychology: Science & Practice. 2011;18(4):275–299. doi: 10.1111/j.1468-2850.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemar MC, Kapur S, Segal ZV, Brown GM, Houle S. Effects of self generated sad mood on regional cerebral activity: A PET study in normal subjects. Depression. 1996;4(2):81–88. doi: 10.1002/(SICI)1522-7162(1996)4:2<81::AID-DEPR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gillen G. Rehabilitation research focused on neurorehabilitation. American Journal of Occupational Therapy. 2010;64:341–356. doi: 10.5014/ajot.64.2.341. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry. 2009;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Rosenthal MZ, Tull MT, Lejuez CW, Gunderson JG. An experimental investigation of emotional reactivity and delayed emotional recovery in borderline personality disorder: the role of shame. Comprehensive Psychiatry. 2010;51:275–285. doi: 10.1016/j.comppsych.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Greenberger D, Pandesky CA. Mind Over Mood: Change How You Feel by Changing the Way You Think. The Guilford Press; New York: 1995. [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual & Motor Skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–164. [Google Scholar]
- Haier RJ, Siegel BV, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: A positron emission tomographic study. Brain Research. 1992;570:134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox N. a., Leibenluft E, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011 doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hammond DC. Neurofeedback with anxiety and affective disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:105–123. vii. doi: 10.1016/j.chc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Ellard KK, Siegle GJ. Neurobiological correlates of cognitions in fear and anxiety : A cognitive–neurobiological information-processing model. Cognition & Emotion. 2012;26(2):37–41. doi: 10.1080/02699931.2011.579414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, Asnaani A. Emotion dysregulation model of mood and anxiety disorders. Depression and Anxiety. 2012;29:409–416. doi: 10.1002/da.21888. [DOI] [PubMed] [Google Scholar]
- Holdwick DJ, Wingenfeld S. a. The subjective experience of PASAT testing. Does the PASAT induce negative mood? Archives of Clinical Neuropsychology. 1999;14:273–284. doi: 10.1093/arclin/14.3.273. [DOI] [PubMed] [Google Scholar]
- Holmes E. a., James EL, Coode-Bate T, Deeprose C. Can playing the computer game “Tetris” reduce the build-up of flashbacks for trauma? A proposal from cognitive science. PloS one. 2009;4:e4153. doi: 10.1371/journal.pone.0004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. a., James EL, Kilford EJ, Deeprose C. Key steps in developing a cognitive vaccine against traumatic flashbacks: visuospatial Tetris versus verbal Pub Quiz. PloS one. 2010;5:e13706. doi: 10.1371/journal.pone.0013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Stonnington HH. Rehabilitation of Neuropsychological Disorders: A Practical Guide for Rehabilitation Professionals. Taylor & Francis; Philadelphia, PA: 2001. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor's new drugs: An analysis of antidepressant medication data submitted to the U.S. Food and Drug Administration. Prevention & Treatment. 2002;5:21–11. article23. [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience–the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring A, Sloan D. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. Guilford Press; New York: 2010. [Google Scholar]
- Linehan M. Skills Training Manual for Treating Borderline Personality Disorder. The Guilford Press; New York: 1993. [Google Scholar]
- Markowitsch HJ. Differential contribution of right and left amygdala to affective information processing. Behavioral Neurology. 1998;11(4):233–244. doi: 10.1155/1999/180434. [DOI] [PubMed] [Google Scholar]
- Martell CR, Dimidjian S, Herman-Dunn R. Behavioral Activation for Depression: A Clinician's Guide. The Guilford Press; New York: 2010. [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affectective, and Behavioral Neuroscience. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition and Emotion. 1993;7:561–570. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Research: Cognitive Brain Research. 2002;15(1):31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod a. M., Snyder a. Z., Powers WJ, Gusnard D. a., Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resick P. a., Galovski TE, O'Brien Uhlmansiek M, Scher CD, Clum G. a., Young-Xu Y. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. Journal of Consulting and Clinical Psychology. 2008;76:243–258. doi: 10.1037/0022-006X.76.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): A Psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Rush J, Trivedi MH, Wisniewski SR, Nierenberg A, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research. 2007;31(2):235–262. [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Malia A, Maguire D, Roddenberry D, O'Connor M. Replaying the game: Hypnagogic images in normals and amnesics. Science. 2000;290(5490):350–353. doi: 10.1126/science.290.5490.350. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Tucha O, Tucha L, Kaumann G, König S, Lange KM, Stasik D, et al. Training of attention functions in children with attention deficit hyperactivity disorder. Attention Deficit and Hyperactivity Disorders. 2011;3:271–283. doi: 10.1007/s12402-011-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophrenia Bulletin. 2003;29:359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive Affective and Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]