Abstract
Aim:
To assess serum Cu/Zn SOD (Superoxide Dismutase) concentration in individuals with clinical depression.
Subjects and methods:
Serum from 36 individuals diagnosed with clinical depression and 18 healthy controls were tested for Cu/Zn SOD serum concentration using ELISAs.
Results:
Serum Cu/Zn SOD levels of depressed individuals (both with and without secondary anxiety) were significantly higher than age and gender similar controls. We also found that, post anti-oxidant therapy, Cu/Zn SOD levels normalized to the level of normal healthy controls.
Discussion:
These results suggest an association between Cu/Zn SOD serum levels and clinical depression.
Keywords: depression, Cu/Zn SOD, super oxide dismutase, oxidative stress
Introduction
Clinical depression is a considerable public health problem.1 Approximately 32 to 35 million adults in the United States have experienced depression at some point in their life and approximately 13 million adults have experienced depression within the past year.2 Clinical depression is also a considerable medical problem as those with major depressive disorder (MDD) are at increased risk for serious medical illness, including cardiovascular disease,3–5 diabetes,6–8 cancer,9–11 and stroke.12 This risk is often independent of traditional risk factors, suggesting that depression may function as a causal factor in the pathogenesis of multiple diseases. To understand the pathophysiological effects of depression, we examined one of the biologic mechanisms common to multiple diseases: oxidative damage to DNA.
Oxidative damage results from biochemical interactions between reactive oxygen species (ROS) and target biomolecules. ROS can damage nucleic acids, lipids, and proteins, and figures prominently in the etiology and progression of numerous cancers13–15 as well as coronary and carotid atherosclerosis.16–19
Oxidative stress, occurring as a consequence of imbalance between the formation of free oxygen radicals and inactivation of these species by antioxidant defense system, is capable of causing damage to various cellular and extracellular constituents. The deleterious effects of increased oxidative stress are termed oxidative damage. These effects generally appear after exposure to a relatively high concentration of reactive oxygen species (ROS) and/or a decrease in antioxidant (AO) defense system against ROS.20
In vivo, oxygen radicals are produced as byproducts of normal oxidative metabolism.21 Hence, activated cells with increased metabolism produce more oxygen radicals. It has long been known that control of the intracellular redox environment is vital for proper cellular function. To protect themselves from the constant oxidative challenge, cells have developed defense mechanisms that ensure a proper balance between pro- and antioxidant molecules.22
Cu/Zn superoxide dismutase (SOD-1) is a key enzyme in the dismutation of superoxide radicals resulting from cellular oxidative metabolism, converting them into hydrogen peroxide,23 and, as a result, serves a key antioxidant role. In fact, mice lacking SOD die several days after birth, amidst massive oxidative stress.24 Copper, and zinc participate in SOD enzymatic mechanisms that protect against free radicals and therefore serve an important adjunct role in oxidative balance.25 Our study was designed to determine Cu/Zn SOD serum levels in clinically depressed individuals and test the hypothesis that this oxidative stress marker is increased in depressed individuals. This oxidative imbalance may lead to depressive symptoms.
Materials and Methods
ELISA to measure serum Cu/Zn SOD (bender systems)
All reagents and specimens were equilibrated to room temperature before the assay was performed. A 1:51 dilution of the patient samples was prepared by mixing 10 μl of the patient’s sera with 0.5 ml of Serum Diluent. One hundred microliters of calibrators (0.08–2.5 ng/ml Cu/Zn SOD), serum diluent alone, and diluted patient samples were added to the appropriate microwells of a microculture plate (each well contained affinity purified polyclonal IgG to Cu/Zn SOD). Wells were incubated for 60 minutes (± 5 min) at room temperature, then washed 4× with wash buffer. One hundred microliters of pre-diluter anti-human Cu/Zn SOD IgG conjugated with HRP was added to all microwells, incubated for 30 minutes (± 5 min) at room temperature, then wash 4× with wash buffer. One hundred microliters of enzyme substrate was added to each microwell. After approximately 30 minutes at room temperature, the reaction was stopped by adding 50 μl of 1M sulfuric acid, then the wells were read at 450 nm with an ELISA reader (BioRad Laboratories, Inc., Hercules, CA, USA).
Subjects
Experimental and controls
Serum from individuals with diagnosed clinical depression (n = 26; 10 male; mean age 35.2 years) and controls (n = 19; 5 male; mean age 41.7 years) was obtained from patients presenting at the Health Research Institute/Pfeiffer Treatment Center Warrenville, Il*. Patients were chosen for this study by presenting to the Pfeiffer Treatment Center for depression and agreeing to be involved in research at the Center. Most of these individuals were diagnosed using The Hamilton Rating Scale for Depression before presenting at the Pfeiffer Treatment Center. Healthy controls included individuals with no documented mental illness, recruited from the community and agreeing to the research.
The sample size was determined by the number of depression patients and controls available at the time of the study.
Cases and controls were age and gender similar (see above). Smokers and individuals taking medications which might affect oxidative stress, such as steroids, statins and indole-derived drugs, were eliminated from the study.
Anti-oxidant Therapy
Individuals in this study who presented to the Pfeiffer Treatment Center with depression were tested for anti-oxidant levels. Based on deficiencies, they were then prescribed the appropriate dosage of anti-oxidant. Pre-therapy patients represent those who were tested when they first presented and were not previously taking any anti-oxidants. Post-Therapy patients received anti-oxidant therapy, including zinc supplimentation if necessary, for a minimum of 8 weeks.
Serum/Plasma
All experimental and control serums were drawn using venipuncture then treated in an identical fashion—frozen at −70 C immediately after collection and cell/serum separation, then stored at −70 C until thawed for use in ELISAs.
Statistics
Inferential statistics were derived from unpaired t-tests.
Results
Serum from 36 individuals diagnosed with clinical depression and 18 age and gender matched controls was tested for Cu/Zn SOD plasma concentration using an ELISA (described above). Each assay was repeated two or more times, with multiple wells for each serum in each assay. Results of a typical assay with assay controls and depressed and control individuals are shown in Figures 1 and 2, respectively.
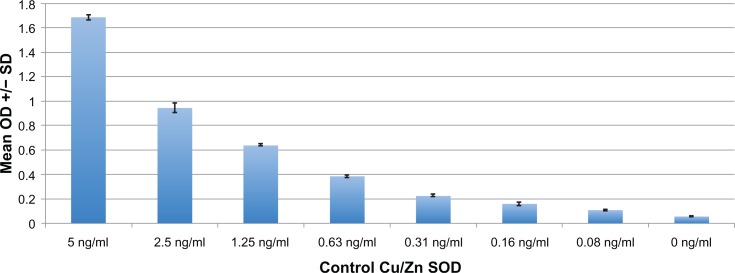
Figure 1.
Positive and negative controls of typical ELISA to measure Cu/Zn SOD plasma levels.
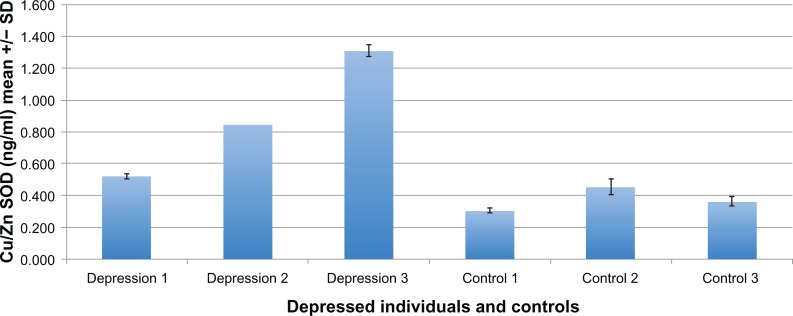
Figure 2.
In a typical ELISA, plasma from 3 individuals diagnosed with clinical depression and three normal (neurotypical) age/gender matched individuals was tested for the Cu/Zn SOD concentration. Cu/Zn SOD concentration is significantly higher in the depressed individuals.
Serum Cu/Zn SOD levels of depressed individuals were significantly higher than all non-depressed controls (P < 0.0025) (Table 1). Although Cu/Zn SOD levels were higher in depressed individuals with secondary anxiety, when compared to depressed individuals without anxiety, this difference was not significant.
Table 1.
Cu/Zn SOD plasma levels in depressed individuals are significantly higher than in normal controls.
| Depression | Normal controls | |
|---|---|---|
| Mean | 0.695 | 0.326 |
| SD | 0.479 | 0.149 |
| SEM | 0.080 | 0.035 |
| N | 36 | 18 |
Note: The two-tailed P value equals 0.0025. t = 3.1769; df = 52; standard error of difference = 0.116.
Medical records were available for 28 of the 36 depressed individuals in this study. Cu/Zn SOD levels in 18 of these same individuals after anti-oxidant therapy was significantly lower than 10 who presented with depression for the first time (before therapy) (P = 0.037) (Table 2) and Cu/Zn SOD levels normalized post therapy when compared to controls (P = 0.305) (Table 4).
Table 2.
Individuals with clinical depression had significantly lower serum Cu/Zn SOD concentration (ng/ml) after antioxidant therapy.
| Cu/Zn SOD before therapy | Cu/Zn SOD after therapy | |
|---|---|---|
| Mean | 0.751 | 0.437 |
| SD | 0.331 | 0.412 |
| SEM | 0.078 | 0.130 |
| N | 18 | 10 |
Note: The two-tailed P value equals 0.0368. t = 2.2018; df = 26; standard error of difference = 0.142.
Table 4.
Cu/Zn SOD levels normalized in depressed individuals after anti-oxidant therapy and zinc supplimentation.
| Cu/Zn SOD after therapy | Cu/Zn SOD controls | |
|---|---|---|
| Mean | 0.437 | 0.326 |
| SD | 0.412 | 0.149 |
| SEM | 0.130 | 0.035 |
| N | 10 | 18 |
Note: The two-tailed P value equals 0.3049. t = 1.0466; df = 26; standard error of difference = 0.107.
Perceived severity of depression (scale of 0–5 (5 being the most severe depression)) was asked of 21 of the 36 depressed individuals in this study. There was a significantly lower level of perceived depression post anti-oxidant therapy (P = 00031) (Table 3).
Table 3.
When individuals with depression were asked to assess their levels of depression (0–5; 5 being the most severe), depression severity was significantly lower after therapy.
| Depression severity no therapy | Depression severity after therapy | |
|---|---|---|
| Mean | 4.154 | 2.688 |
| SD | 0.718 | 1.28 |
| SEM | 0.199 | 0.453 |
| N | 13 | 8 |
Note: The two-tailed P value equals 0.0031. t = 3.3846; df = 19; standard error of difference = 0.433.
Discussion
Oxidative stress has been implicated in the pathogenesis of a diverse group of disease states, and, because the brain has comparatively greater vulnerability to oxidative damage, may be a common pathogenic mechanism underlying many major psychiatric disorders.
This study represents an attempt to assess levels of the oxidative damage expressed by Cu/Zn SOD levels in the plasma of clinically depressed, but otherwise healthy adults. As our results demonstrate, compared with matched control subjects, those with clinical depression show significantly higher serum levels of Cu/Zn SOD.
Other studies have shown positive correlations between levels of 8-OhdG,40 negative mood, as measured by Profile of Mood States,26 perceived workload, psychological distress and the perceived impossibility of alleviating distress,27 and severe depression as measured by the General Health Questionnaire.28
Clinical depression associated with increased oxidative damage may represent a common pathophysiological mechanism, whereby these patients become vulnerable to multiple comorbid medical illnesses.29 Depression is associated with an activation of innate immune responses,30 inflammation31 and heightened vulnerability to latent infection.32,33 Activated phagocytes are significant sources of ROS and produce superoxide, hydrogen peroxide, nitric oxide, and peroxynitrite as part of the cytotoxic host response against invading pathogens.34 Depression may contribute to oxidative damage through an increase in the production of reactive oxygen species (ROS). ROS can be antimicrobial; they damage lipid membranes and protein structures, thus destroying antigen-bearing cells. Oxidative damage is not limited to microbial targets, however, and extensive host tissue damage may result.35 ROS from activated phagocytes can damage DNA bases36 and induce strand breaks in neighboring cells,37 leading some to argue that the hydroxyl radicals and peroxynitrite formed during inflammation are the greatest contributors to the oxidation of DNA.38
Depression may not increase production or exposure to ROS, but rather decrease repair of damaged DNA. There is some evidence that repair of x-ray-damaged DNA is slower among highly distressed psychiatric inpatients, the majority of whom were hospitalized for depression.39
Recent research demonstrates that, in a chronic mild stress (CMS) rat model, which induces some symptoms of a major depressive episode in humans, superoxide production was increased in all brain structures analyzed.41 Despite the fact that Cu/Zn SOD is a very non specific marker of oxidative stress, the levels of which basically signify the overall redox balance in body, others have found it to be a useful clinical biomarker of oxidative stress,42,43 Our data suggests that Cu/Zn SOD levels in plasma may reflect this overproduction and may make Cu/Zn SOD a good biomarker for the monitoring of clinical depression.
The clinical relevance of increased oxidative damage, evidenced by clinically depressed individuals, is currently unknown, and future studies should appropriately address this deficit. Our data are consistent with the hypothesis that oxidative damage is a potential common pathophysiological mechanism underlying multiple comorbid conditions in depressed people. Future studies should include measurement of multiple oxidative damage markers to different macromolecules, associated dietary deficiencies associated with these markers, associated severity of depression, and should address whether depression remission remediates underlying oxidative damage.
Acknowledgments
I’d like to thank Scott Filer, Executive Director, and Allen Lewis, Medical Director of The Pfeiffer Treatment Center for their support and help in this research and manuscript preparation.
I’d also like to thanks Laurie Myers and Kyle Andrews for their technical assistance.
Footnotes
The Pfeiffer Treatment Center is a comprehensive research and treatment center, specializing in the care of with neurological disorders, including Depression.
References
- 1.Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53:849–57. doi: 10.1016/s0022-3999(02)00304-5. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–15. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 4.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–40. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 5.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–92. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 6.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54:317–29. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 7.Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. J Psychosom Res. 2002;53:903–6. doi: 10.1016/s0022-3999(02)00302-1. [DOI] [PubMed] [Google Scholar]
- 8.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097–102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- 9.Penninx B, Guralnik J, Pahor M, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–93. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- 10.Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry. 2003;54:283–94. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–82. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 12.Larson SL, Owens PL, Ford D, Eaton W. Depressive disorder, dysthymia, and risk of stroke: thirteen-year follow-up from the Baltimore epidemiologic catchment area study. Stroke. 2001;32:1979–83. doi: 10.1161/hs0901.094623. [DOI] [PubMed] [Google Scholar]
- 13.Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 14.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 15.Floyd RA. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11:1447–50. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- 16.Andreassi MG, Botto N. DNA damage as a new emerging risk factor in atherosclerosis. Trends Cardiovasc Med. 2003;13:270–5. doi: 10.1016/s1050-1738(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 17.Botto N, Masetti S, Petrozzi L, et al. Elevated levels of oxidative DNA damage in patients with coronary artery disease. Coron Artery Dis. 2002;13:269–74. doi: 10.1097/00019501-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Martinet W, Knaapen MWM, De Meyer GRY, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–32. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 19.Botto N, Rizza A, Colombo MG, et al. Evidence for DNA damage in patients with coronary artery disease. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2001;493:23–30. doi: 10.1016/s1383-5718(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 20.Sies H. Oxidative stress: Oxidants and Antioxidants. Academic Press; New York: 1991. [DOI] [PubMed] [Google Scholar]
- 21.Malmstrom BG. Enzymology of oxygen. Annu Rev Biochem. 1982;51:21. doi: 10.1146/annurev.bi.51.070182.000321. [DOI] [PubMed] [Google Scholar]
- 22.Forman HJ, Torres M. Redox signaling in macrophages. Mo Aspects Med. 2001;22:189. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 23.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 24.Bonham M, O’Connor JM, Hannigan BM, Strain JJ. The immune system as a physiological indicator of marginal copper status? Br J Nutr. 2002;87(5):393–403. doi: 10.1079/BJNBJN2002558. [DOI] [PubMed] [Google Scholar]
- 25.Ozcelik D, et al. Copper-mediated oxidative stress in rat liver. Biological Trace Element Research. 2007;96:209–15. doi: 10.1385/BTER:96:1-3:209. [DOI] [PubMed] [Google Scholar]
- 26.Irie M, Asami S, Nagata S, Ikeda M, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Jpn J Cancer Res. 2001;92:367–76. doi: 10.1111/j.1349-7006.2001.tb01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irie M, Asami S, Nagata S, Miyata M, Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. Int Arch Occup Environ Health. 2001;74:153–7. doi: 10.1007/s004200000209. [DOI] [PubMed] [Google Scholar]
- 28.Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun. 2003;311:1014–8. doi: 10.1016/j.bbrc.2003.10.105. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan KRR, Delong M, Kraemer H, et al. Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry. 2002;52:559–88. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- 30.Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993;113:472–86. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 31.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–85. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 32.Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. 2005;95:317–21. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–83. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 34.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 35.Simic MG. DNA markers of oxidative processes in vivo: relevance to carcinogenesis and anticarcinogenesis. Cancer Res. 1994;54:1918–23. [PubMed] [Google Scholar]
- 36.Jackson JH, Gajewski E, Schraufstatter IU, et al. Damage to the bases in DNA induced by stimulated human neutrophils. J Clin Invest. 1989;84:1644–9. doi: 10.1172/JCI114342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shacter E, Beecham EJ, Covey JM, Kohn KW, Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988;9:2297–304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- 38.Aust AE, Eveleigh JF. Mechanisms of DNA oxidation. Proc Soc Exp Biol Med. 1999;222:246–52. doi: 10.1046/j.1525-1373.1999.d01-141.x. [DOI] [PubMed] [Google Scholar]
- 39.Kiecolt-Glaser JK, Stephens RE, Lipetz PD, Speicher CE, Glaser R. Distress and DNA repair in human lymphocytes. J Behav Med. 1985;8:311–20. doi: 10.1007/BF00848366. [DOI] [PubMed] [Google Scholar]
- 40.Forlenza MJ, et al. Increased Serum Levels of 8-Hydroxy-2’-Deoxyguanosine in Clinical Depression. Psychosomatic Medicine. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 41.Lucca G, et al. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. Journal of Psychiatric Research. 2009;43:864–69. doi: 10.1016/j.jpsychires.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Pawlak K, Pawlak D, Mysliwiec M. Cu/Zn superoxide dismutase plasma levels as a new useful clinical biomarker of oxidative stress in patients with end-stage renal disease. Clin Biochem. 2005 Aug;38(8):700–5. doi: 10.1016/j.clinbiochem.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Olivares M, et al. Present situation of biomarkers for copper status American Journal of Clinical Nutrition. 2008;88(3):859S–62. doi: 10.1093/ajcn/88.3.859S. [DOI] [PubMed] [Google Scholar]