Abstract
Aim:
To assess serum HGF concentration in individuals with bipolar disorder and investigate the efficacy of zinc therapy on these levels.
Subjects and methods:
Serum from 35 individuals diagnosed with bipolar disorder and 19 age and gender similar controls were tested for HGF concentration using ELISAs, and copper and zinc plasma levels using inductively-coupled plasma-mass spectrometry.
Results:
HGF serum levels of individuals with bipolar disorder were significantly lower than age and gender similar controls (P = 0.0021). HGF serum concentration was significantly lower in Bipolar patients pre-therapy (P = 0.0009) and HGF levels normalized post-therapy. Zinc levels in these same individuals also normalized (P = 0.0046) and patient’s perceived severity of Bipolar symptoms significantly decreased after therapy (P = 0.0003). We also found a significant direct correlation between Zinc and HGF serum concentration in the bipolar patients (P = 0.04).
Discussion:
These results suggest an association between low HGF levels and bipolar disorder and also demonstrate that zinc therapy may be associated with the normalization of HGF levels and decrease in severity of disease.
Keywords: bipolar disorder, hepatocyte growth factor, HGF
Introduction
Bipolar Disorder is characterized by episodes of mania and depression that are frequently acute, debilitating, and may be associated with gross social dysfunction and an elevated suicide rate.1–4
Recent findings indicate that the pathologic process of this disorder is involved in complex interactions between multiple susceptibility genes and environmental factors.5,6
More than two-thirds of people with bipolar disorder have at least one close relative with the disorder or with unipolar major depression, indicating that the disease has a genetic component. The monozygotic concordance rate for the disorder is 40%–70% and dizygotic twins have a 10%–23% concordance rate.7,8
There is also a correlation between DGKH (diacylglycerol kinase eta) and bipolar disorder. DGKH is a key protein in the lithium-sensitive phosphatidyl inositol pathway,9 and more recently a link between environmental stress and schizophrenia and possibly bipolar disorder has been revealed.10
Recent advances in genetics, molecular and cellular biology, molecular neuroscience, and imaging technology have provided a variety of new and sophisticated approaches to evaluate the complex features of this disease. Important among these advances, high density DNA expression microarray technology has recently been widely adapted by scientists in the search for susceptibility genes for bipolar disorder,11–13 and has helped reveal gene candidates coding for proteins related to mitochondrial function and apoptosis, glutamate receptors, markers of GABAergic neurons, molecular chaperones, and oligodendrocyte proteins are differentially expressed in postmortem brain tissue, peripheral blood cells, and olfactory neuroepithelium between control and bipolar disorder subjects.14–16
There is also an emerging body of data suggesting that oxidative stress may be associated with the pathophysiology of Bipolar Disorder,17–19 including increased levels of glutamate found in the brains of patients with mood disorders.20
Hepatocyte growth factor (HGF), an 82 kDa, 674 amino acid residue heterodimeric glycoprotein, was originally isolated from rat platelets.21,22 This growth factor has also been called scatter factor, hepatopoietin A, and mammary growth factor.23 It is one of a small family of factors lacking significant homology with other known growth factors, but including an HGF-like factor known as macrophage stimulating protein (MSP).24–27 HGF has mitogenic, morphogenic, and motogenic effects on hepatocytes, as well as endothelial, mesenchymal and hematopoietic cell types,26,28,29 and demonstrates noticeable species cross-reactivity.30
HGF regulates cell growth, cell motility, and morphogenesis by activating a tyrosine kinase signaling cascade after binding to the proto-oncogenic c-Met receptor (translated by the MET gene). HGF is secreted by mesenchymal cells and, although it was first considered to exert biological effects only on specific target cells, it has since been demonstrated to mediate inflammatory responses to tissue injury, and regulate cell growth, cell motility, and morphogenesis in a wide variety of cells. Its ability to stimulate branching morphogenesis, cell migration, survival and proliferation gives it a central role in angiogenesis, tissue regeneration, as well as tumorogenesis.31–37
Signaling by HGF has also been found to have anti-inflammatory, antifibrotic, and pro-regenerative activity on various types of tissue. But it seems to be particularly active in the nervous system, where it has been found to have neurotrophic and angiogenetic activity on CNS neurons, promote both the survival of neurons and the regeneration of injured nerves, and function as a target-derived axonal chemoattractant, guiding axons to their target. As a result, it plays significant roles in the development of the CNS.38
Zinc is well known as one of the most important trace elements in the body. Dietary zinc deficiency is associated with a variety of physiological defects including anorexia, skin lesion, and growth retardation.39 Mechanistic studies demonstrated that zinc deficiency affects a large number of hepatic genes involved in multiple cellular functions. In particular, zinc deficiency has been shown to down-regulate hepatic gene expression of metallothionein (MT), insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein 1 (IGFBP1), cyclin D1, and HGF, which are involved in cell proliferation.40–42
Genes involved in GABAergic neurotransmission (43.44) and decreased levels of serum GABA45 have been associated with Bipolar Disorder. HGF has been shown to modulate GABAergic activity46 and enhance NMDA currents in the hippocampus.47
Because of the potential association between HGF, GABA and neurological development and differentiation, and its association with the etiology of neurological diseases, and because zinc deficiency may down regulate HGF synthesis, we tested patients with Bipolar Disease for serum concentration of HGF and then compared those levels with zinc and copper levels as well as perceived severity of disease, before and after zinc therapy.
Materials and Methods
Elisa to measure serum HGF (Elisa Kit, R&D Systems, Minneapolis, Minn.)
All reagents and specimens were equilibrated to room temperature before the assay was performed. A 1:51 dilution of the patient samples was prepared by mixing 10 μl of the patient’s sera with 0.5 ml of Serum Diluent. One hundred microliters of calibrators (20–200 Eu/ml antibodies), positive and negative control serums, serum diluent alone, and diluted patient samples were added to the appropriate microwells of a microculture plate (each well contained affinity purified polyclonal IgG to HGF). Wells were incubated for 60 minutes (±5 min) at room temperature, then washed 4x with wash buffer. One hundred microliters of pre-diluter anti-human IgG conjugated with HRP was added to all microwells, incubated for 30 minutes (±5 min) at room temperature, then wash 4x with wash buffer. One hundred microliters of enzyme substrate was added to each microwell. After approximately 30 minutes at room temperature, the reaction was stopped by adding 50 μl of 1M sulfuric acid, then the wells were read at 405 nm with an ELISA reader (BioRad Laboratories, Inc., Hercules, CA, USA).
Copper and zinc serum concentration
Assay to establish copper and zinc plasma concentration was performed by LabCorp, Inc. (Naperville, IL 60563) using inductively-coupled plasma-mass spectrometry.
Serums
All serums, experimental and control serums were treated in an identical fashion—frozen at −70C immediately after collection and cell/serum separation, then stored at −70C until thawed for use in ELISAs.
Subjects
Experimental and controls
Serum from individuals with diagnosed (psychiatric interview using DSM criteria) Bipolar Disorder (n = 35; 18 male; mean age 34 ± 12 years) and controls (n = 19; 10 male mean age 41 ± 14 years) was obtained from patients at the Health Research Institute/Pfeiffer Treatment Center. Most of these individuals were previously diagnosed with Bipolar Disorder, then all were assessed using the modified Mood Disorder Questionnaire, before presenting at the Pfeiffer Treatment Center, Warrenville, Il.*
All patients in this study received zinc and B-6 therapy.
Patients (n = 35) for this study were chosen randomly as they presented to The Pfeiffer Treatment Center. At the time of the blood draw, six were taking anti-depressants, four were taking anti-epileptic drugs, eight were taking anti-psychotics, eight were taking lithium and nine presented not taking any associated drugs. We did not find any significant difference in HGF serum concentration in any of these groups (ANOVA; P = 0.995; F = 0.157). All patients presented after having mixed manic and depression episodes.
Patient consent was obtained from all patients involved in this study.
Controls were normal healthy volunteers, all employees of the Pfeiffer Treatment Center.
Severity of disease
A modified questionnaire asking patients to rank the severity of the signs and symptoms of Bipolar Disease, found in the Mood Disorder Questionnaire,56 was used to determine the overall severity. Patients were asked to rate on a scale of 0–5 (5 being the highest), behaviors such as; irritability and anger, lack of ability to focus/concentrate, obsessive behavior, trouble sleeping, paranoia, migraines, intrusive thoughts, anxiety, depression and panic. We evaluated the overall severity of Bipolar Disease by establishing the mean of all of the scores for each patient. The overall bipolar behavior assessment includes assessment of all the behaviors (above).
Zinc and anti-oxidant therapy
Individuals in this study who presented to the Pfeiffer Treatment Center with Bipolar Disease were tested for zinc, copper and anti-oxidant levels. Based on deficiencies, they were then prescribed the appropriate dose of anti-oxidants. Pre-therapy patients represent those who were tested when they first presented and were not previously taking any zinc or anti-oxidants. Post-therapy patients received anti-oxidant therapy (One dose—Vitamin C (125 mg), E (50 IU), B6 (37.5 mg) as well as Magnesium (11 mg), and Manganese (3.75 mg) if warranted), and zinc supplementation (as zinc picolinate) (25 mg), for a minimum of 8 weeks. Pre and post therapy patients were different groups.
Statistics
Inferential statistics were derived from unpaired t-test and odds ratios with 95% confidence intervals. ANOVA was used to assess variance between groups.
Results
Serum from 35 individuals diagnosed with bipolar disorder and 19 age and gender similar controls were tested for HGF concentration using ELISAs, and copper and zinc plasma levels were established using inductively-coupled plasma-mass spectrometry (Labcorp, Naperville IL).
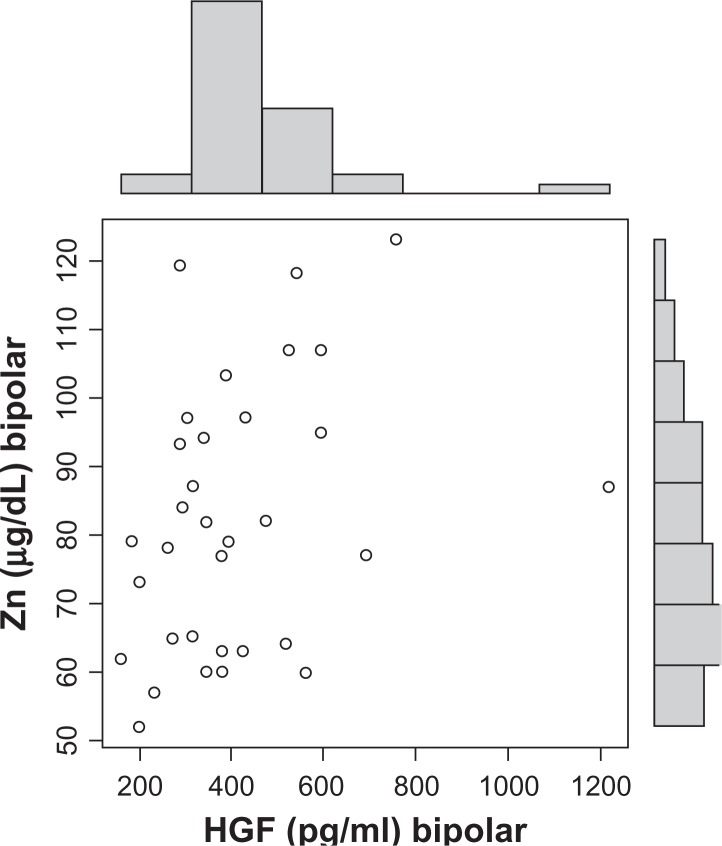
HGF serum hilda levels of individuals with bipolar disorder were significantly lower than age and gender similar controls (P = 0.0018) (Table 1). HGF serum concentration before zinc therapy was significantly lower in Bipolar patients when compared to levels post-therapy (P = 0.0009) and HGF levels normalized (increased to the level of controls) post-therapy (Table 2). We found a dose dependent correlation between zinc and HGF (P = 0.04) (Fig. 1), and zinc levels in these same individuals also rose (P = 0.0046) and normalized post-therapy (Table 3) (normal zinc is 90–150 μg/dL). Copper levels decreased post therapy, but not significantly (P = 0.59). Patient’s perceived severity of overall Bipolar symptoms decreased significantly post therapy (P = 0.0003) (Table 4).
Table 1.
HGF levels in bipolar disorder patients and controls.
| HGF bipolar | HGF controls | |
|---|---|---|
| Mean | 402.3 | 573.9 |
| SD | 204.5 | 142.8 |
| SEM | 34.6 | 32.8 |
| N | 35 | 19 |
Notes: The two-tailed P value equals 0.0021; t = 3.2457; df = 52; Standard error of difference = 52.859.
Table 2.
HGF levels pre and post therapy in bipolar disorder patients.
| HGF pre-therapy | HGF post-therapy | |
|---|---|---|
| Mean | 314.1 | 534.8 |
| SD | 108.4 | 244.6 |
| SEM | 23.7 | 65.4 |
| N | 21 | 14 |
Notes: The two-tailed P value equals 0.0009; t = 3.6511; df = 33; Standard error of difference = 60.449.
Figure 1.
Significant dose dependent correlation between zinc and HGF levels (P = 0.04).
Table 3.
Zn levels pre and post therapy in bipolar disorder patients.
| Zn pre therapy | Zn post therapy | |
|---|---|---|
| Mean | 74.0 | 92.4 |
| SD | 13.9 | 21.5 |
| SEM | 3.1 | 5.7 |
| N | 20 | 14 |
Notes: The two-tailed P value equals 0.0046; t = 3.0490; df = 32; Standard error of difference = 6.044.
Table 4.
Overall severity of bipolar symptoms pre and post therapy.
| Overall severity pre-therapy | Overall severity post-therapy | |
|---|---|---|
| Mean | 3.64 | 2.54 |
| SD | 0.74 | 0.61 |
| SEM | 0.17 | 0.18 |
| N | 19 | 11 |
Notes: The two-tailed P value equals 0.0003; t = 4.1391; df = 28; Standard error of difference = 0.265.
Discussion
HGF has been found to be associated with a variety of diseases of the CNS. For instance, immunohistochemistry using anti-HGF antibody has revealed more intense immunolabeling in Alzheimer’s disease (AD) than in control brains, and there appears to be a significant correlation between CSF HGF levels and white matter high-signal foci determined on brain magnetic resonance imaging (MRI) in AD patients.48 In Amyotrophic lateral sclerosis (ALS), overexpression of hepatocyte growth factor (HGF) in the nervous system attenuates motoneuron death and axonal degeneration and prolongs the life span of transgenic mice overexpressing mutated Cu2+/Zn2+ superoxide dismutase.49 Overexpression of HGF after gene transfer prevented neuronal death in a Parkinson’s Disease rat model,50 and decreased levels of HGF has been found in autistic children with GI disease.51
Previous studies have shown a positive correlation between oxidative stress markers and bipolar disorder and have suggested a role of mitochondrial dysfunction in the etiology of the disease.52
Mitochondria-related genes may be abnormally expressed in bipolar disorder. Increasing evidence has shown the presence of mitochondrial dysfunctions such as deletion, mutation and abnormal expression of mitochondria-related genes, increased anaerobic glycolysis, and impaired phospholipid metabolism. Because mitochondrial oxidative phosphorylation is the major resource for the generation of reactive oxygen species, mitochondrial dysfunction indicates that oxidative stress may occur in bipolar disorder and that oxidative stress induced damage may be prevented by mood-stabilizing treatment.53
Zinc supplementation has also been found to prevent liver cell injury through attenuation of oxidative stress,54 and there is evidence suggesting that alcohol-induced liver damage initiates hepatocyte proliferation, and zinc supplementation accelerates liver regeneration, through up-regulating cell proliferation-related proteins such as HGF.55
Our results demonstrate that HGF is significantly lower in Bipolar patients compared to controls and we suggest a potential role for HGF deficiency in the etiology of Bipolar Disease by modulating NMDA and GABAergic activity.
The present data, which demonstrates a dose dependent relationship between zinc and HGF, raise the intriguing possibility that HGF levels are raised by zinc therapy and that these higher levels may be associated with improved symptoms, also suggesting that HGF may be playing a role in the etiology of the disease.
Acknowledgments
The author thanks Scott Filer, Executive Director and the entire staff of The Health Research Institute/Pfeiffer Treatment Center, for their support of this research.
The author also thanks Laurie Myers and Kyle Andrews for their technical assistance.
Footnotes
The Pfeiffer Treatment Center is a comprehensive treatment and research center, specializing in the care of with neurological disorders, including Bipolar Disorders.
Disclosure
This manuscript has been read and approved by the author. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The author and peer reviewers of this paper report no conflicts of interest. The author confirms that they have permission to reproduce any copyrighted material.
References
- 1.Tondo L, Isacsson G, Baldessarini R. Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs. 2003;17:491–511. doi: 10.2165/00023210-200317070-00003. [DOI] [PubMed] [Google Scholar]
- 2.Manji HK, Lenox RH. The nature of bipolar disorder. J Clin Psychiatry. 2000;61(Suppl 13):42–57. [PubMed] [Google Scholar]
- 3.Angst F, Stassen HH, Clayton PJ, et al. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–81. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 4.Moller HJ, Curtis VA. The bipolar spectrum: diagnostic and pharmacologic considerations. Expert Rev Neurother. 2004;6(Suppl 2):S3–8. doi: 10.1586/14737175.4.6.S3. [DOI] [PubMed] [Google Scholar]
- 5.Carter CJ. Multiple genes and factors associated with bipolar disorder converge on growth factor and stress activated kinase pathways controlling translation initiation: implications for oligodendrocyte viability. Neurochem Int. 2007;50:461–90. doi: 10.1016/j.neuint.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Sanders AR, Duan J, Gejman PV. Complexities in psychiatric genetics. Int Rev Psychiatry. 2004;16:284–93. doi: 10.1080/09540260400014393. [DOI] [PubMed] [Google Scholar]
- 7.Kieseppä T, et al. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004 Oct;161(10):1814–21. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- 8.Cardno AG, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry. 1999 Feb;56(2):162–8. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 9.Baum AE, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Molecular Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll LS, Owen MJ. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Medicine. 2009;1:102. doi: 10.1186/gm102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Wang JF, Tseng M, et al. Down regulation in components of mitochondrial electron transport chain in post-mortem frontal cortex from subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–96. [PMC free article] [PubMed] [Google Scholar]
- 12.Konradi C, Eaton M, MacDonald ML, et al. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–8. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14:241–53. doi: 10.1093/hmg/ddi022. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen DV, Arpat AB, Wang N, et al. DNA microarray experiments: biological and technological aspects. Biometrics. 2002;58:701–17. doi: 10.1111/j.0006-341x.2002.00701.x. [DOI] [PubMed] [Google Scholar]
- 15.Draghici S, Khatri P, Eklund AC, et al. Reliability and reproducibility issues in DNA microarray measurements. Trends Genet. 2006;22:101–9. doi: 10.1016/j.tig.2005.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konradi C. Gene expression microarray studies in polygenic psychiatric disorders: applications and data analysis. Brain Res Rev. 2005;50:142–55. doi: 10.1016/j.brainresrev.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Andreazza A, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J of Affective Disorders. 2008;2:135–44. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Gama C. Markers of oxidative stress in bipolar disorder and in schizophrenia. European Psychiatry. 2009;24:S159. [Google Scholar]
- 19.Frey, et al. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):283–5. doi: 10.1016/j.pnpbp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–16. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, et al. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A. 1986;83:6489. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, et al. Partial purification and characterization in hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:450. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki M, et al. Identification of mouse mammary fibroblast-derived mammary growth factor as hepatocyte growth factor. Biochem Biophys Res Commun. 1994;199:772. doi: 10.1006/bbrc.1994.1296. [DOI] [PubMed] [Google Scholar]
- 24.Michalopoulos G, et al. Control of hepatocyte replication by two serum factors. Cancer Res. 1984;44:4414. [PubMed] [Google Scholar]
- 25.Thaler FJ, Michalopoulos G. Hepatopoietin A: partial characterization and trypsin activation of a hepatocyte growth factor. Cancer Res. 1985;45:2545. [PubMed] [Google Scholar]
- 26.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidner KM, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991;88:7001–5. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comoglio PM, Graziani A. In: Guidebook to Cytokines and their Receptors. Nicola NA, editor. Oxford University Press; 1994. p. 182. [Google Scholar]
- 29.Comoglio PM, Graziani A. In: Guidebook to Cytokines and their Receptors. Nicola NA, editor. Oxford University Press; 1994. p. 185. [Google Scholar]
- 30.Grant DS, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1937–41. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura T, et al. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A. 1986;83:6489. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura T, et al. Partial purification and characterization in hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1994;122:1450. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki M, et al. Identification of mouse mammary fibroblast-derived mammary growth factor as hepatocyte growth factor. Biochem Biophys Res Commun. 1994;199:772. doi: 10.1006/bbrc.1994.1296. [DOI] [PubMed] [Google Scholar]
- 34.Michalopoulos G, et al. Control of hepatocyte replication by two serum factors. Cancer Res. 1994;44:4414. [PubMed] [Google Scholar]
- 35.Warn R, et al. HGF/SF induces mesothelial cell migration and proliferation by autocrine and paracrine pathways. Exp Cell Res. 2001 Jul 15;267(2):258–266. doi: 10.1006/excr.2001.5240. [DOI] [PubMed] [Google Scholar]
- 36.Masakazu Y, et al. Hepatocyte Growth Factor (HGF) Produced By Peritoneal Fibroblasts May Affect Mesothelial Cell Morphology And Promote Peritoneal Dissemination. Int. J. Cancer. 1996;47:289–293. doi: 10.1002/(SICI)1097-0215(19960717)67:2<289::AID-IJC22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Weidner KM, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991;88:7001–5. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamanoue M, et al. Neurotrophic effect of hepatocyte growth factor on central nervous system neurons in vitro. J Neurosci Res. 1996;43(5):554–64. doi: 10.1002/(SICI)1097-4547(19960301)43:5<554::AID-JNR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.McClain CJ, Adams L, Shedlofsky S. Zinc and the gastrointestinal system. In: Prasad AS, editor. Essential and Toxic Trace Elements in Human Health and Disease. New York: Alan R; Liss Inc; 1988. pp. 55–73. [Google Scholar]
- 40.McNall AD, Etherton TD, Fosmire GJ. The impaired growth induced by zinc deficiency in rats is associated with decreased expression of the hepatic insulin-like growth factor I and growth hormone receptor genes. J Nutr. 1995;125:874–9. doi: 10.1093/jn/125.4.874. [DOI] [PubMed] [Google Scholar]
- 41.Pfaffl MW, Gerstmayer B, Bosio A, Windisch W. Effect of zinc deficiency on the mRNA expression pattern in liver and jejunum of adult rats: monitoring gene expression using cDNA microarrays combined with real-time RT-PCR. J Nutr Biochem. 2003;2002;14:691–702. doi: 10.1016/j.jnutbio.2003.08.007. 283:C623–30. [DOI] [PubMed] [Google Scholar]
- 42.Dieck HT, Döring F, Roth HP, Daniel H. Changes in rat hepatic gene expression in response to zinc deficiency as assessed by DNA arrays. J Nutr. 2003;133:1004–10. doi: 10.1093/jn/133.4.1004. [DOI] [PubMed] [Google Scholar]
- 43.Oruc L, et al. Association study between bipolar disorder and candidate genes involved in dopamine serotonin metabolism and GABAergic neuro transmission: a preliminary report. Psychiatric Genetics. 1996;6:213–7. doi: 10.1097/00041444-199624000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Kelsoe JR, et al. Possible locus for bipolar disorder near the dopamine transporter on chromosome 5. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 1998;67:533–40. doi: 10.1002/(SICI)1096-8628(19961122)67:6<533::AID-AJMG4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 45.Petty F, et al. Low plasma GABA is a trait-like marker for bipolar illness. Neuropsychopharmacology. 1993;9(2):125–32. doi: 10.1038/npp.1993.51. [DOI] [PubMed] [Google Scholar]
- 46.Bae MH, et al. Hepatocyte growth factor (HGF) modulates GABAergic inhibition and seizure susceptibility. Experimental Neurology. 2010;221:129–35. doi: 10.1016/j.expneurol.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akimoto M, et al. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128:155–62. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 48.Tsuboi Y, et al. Increased hepatocyte growth factor level in cerebrospinal fluid in Alzheimer’s disease. Acta neurologica scandinavica. 2003;107:81–6. doi: 10.1034/j.1600-0404.2003.02089.x. [DOI] [PubMed] [Google Scholar]
- 49.Sun W, et al. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. The Journal of Neuroscience. 2002;22(15):6537–48. doi: 10.1523/JNEUROSCI.22-15-06537.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koike H, et al. Prevention of onset of Parkinson’s disease by in vivo gene transfer of human hepatocyte growth factor in rodent model: a model of gene therapy for Parkinson’s disease. Gene Therapy. 2006;13:1639–44. doi: 10.1038/sj.gt.3302810. [DOI] [PubMed] [Google Scholar]
- 51.Russo AJ, et al. Decreased serum hepatocyte growth factor (HGF) in autistic children with severe gastrointestinal disease. Biomarker Insights. 2009;2:181–90. doi: 10.4137/bmi.s3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreazza A. Mitochondrial dysfunction and oxidative stress in bipolar disorder. European Psychiatry. 2009;24:S15. [Google Scholar]
- 53.Wang J-F. Defects of mitochondrial electron transport chain in bipolar disorder: implications for mood-stabilizing treatment. Can J Psychiatry. 2007;52(12):753–62. doi: 10.1177/070674370705201202. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681–90. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang X, et al. Zinc supplementation enhances hepatic regeneration by preserving hepatocyte nuclear factor-4 (alpha) in mice subjected to long-term ethanol administration. The American Journal of Pathology. 2008;172:916–25. doi: 10.2353/ajpath.2008.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirschfeld RMA. The mood disorder questionnaire: a simple, patient-rated screening instrument for bipolar disorder. Journal of Clinical Psychiatry. 2002;4:9–11. doi: 10.4088/pcc.v04n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]