Abstract
Aim:
To investigate the effects of diets rich in n-6 polyunsaturated fats (PUFA) fed during pre- and post-weaning time periods on the lipid metabolism and vascular reactivity in adult C57Bl/6 mice, in order to assess the impact of maternal nutrition and its interaction with the offspring diet on the metabolism of adult offspring.
Methods:
Female C57Bl/6 mice were fed a high-fat diet enriched with n-6 PUFA (P) or control diet (C) for 2-weeks before, during mating, gestation and lactation, while their pups received either P or C for 8-weeks post-weaning.
Results:
A significant interaction between the maternal and post-weaning diets was observed for the offspring body weight, food-, caloric-intake, plasma lipids, hepatic mRNA expression of lecithin cholesterol acyltransferase, aortic contractile and relaxation responses (P < 0.05).
Conclusion:
The overall metabolic and physiological outcome in the offspring is dependent upon the interaction between the pre- and post-weaning dietary environments.
Keywords: dietary n-6 PUFA, developmental programming, predictive adaptive responses, lipid metabolism, vascular reactivity
Introduction
Recent evidence suggests that in utero nutrition can play a significant role in determining the cardiovascular health of an individual in later life. A typical North American diet is rich in dietary fats, a fact that has been linked to the increased prevalence of cardiovascular disease (CVD).1 As a result, a number of studies have focused on the effects of maternal consumption of high-fat diets during gestation and lactation on the development of insulin resistance,2 obesity3 and hypertension4 in the offspring. It is well known that besides the quantity of fat, the quality of dietary fats also plays an important role in determining the outcome of CVD. While an increased consumption of saturated fatty acids (SFA) has largely been associated with higher incidence of CVD,5,6 a diet rich in polyunsaturated fatty acids (PUFA) is known to lower the risk of developing CVD.7,8 Therefore, replacement of dietary SFA with PUFA is recommended as a preventive measure for CVD by the general population.9,10 However, in the past decades a steady increase in the dietary n-6 PUFA consumption has been associated with increased incidence of obesity and metabolic syndrome.11 Moreover, an exposure to n-6 PUFA via breast milk and infant formulas during early life has also been associated with the increased incidence of childhood obesity in humans.12 The n-6 PUFA constitutes ∼85% of the total dietary PUFA intake in modern-day Western diet.10 This prevalence would also point towards an increased intake of n-6 PUFA during pregnancy, thus emphasizing the need to understand the effects of a maternal diet rich in n-6 PUFA fed during gestation and lactation on the metabolic and cardiovascular health of the adult offspring.
Mechanistically, dietary fat intake can alter the cellular and systemic lipid metabolism, thus it is proposed that a maternal diet rich in n-6 PUFA would ‘program’ the offspring lipid metabolism in a way that would affect their cardiovascular health in later life. We have previously reported lower hepatic mRNA expression of LDL-receptor, higher plasma LDL-cholesterol and reduced aortic contractile reactivity in the female offspring of C57Bl/6 mice fed a diet rich in SFA during gestation and lactation.13 The current study was designed on the basis of these previous findings to investigate whether a maternal diet rich in n-6 PUFA fed during gestation and lactation would affect the lipid metabolism and vascular reactivity of the adult offspring.
In addition, there is emerging evidence suggesting that the interaction between maternal and postnatal nutrition plays an important role in modulating the metabolic and cardiovascular health of the offspring in adult life. According to the predictive adaptive response (PAR) hypothesis, it is proposed that the developing fetus in utero senses its nutritional environment and undergoes certain metabolic adaptations while predicting a similar postnatal nutritional environment. If the post-natal nutrition is similar to the expected (prenatal) nutrition, the fetal metabolic adaptations would be able to deal with this nutritional environment and the offspring would be protected from the ill-effects of the postnatal nutritional environment, if any. However, when the postnatal nutrition is considerably different from the prenatal nutrition, the fetal metabolic adaptations are incapable of dealing with the ‘unpredicted’ postnatal environment and the disease becomes manifest.14 In the current study, we also investigated the effects of interaction between the maternal and post-weaning diet on the lipid metabolism and vascular reactivity of the adult offspring with respect to the PAR hypothesis.
Materials and Methods
Animals
All the experimental procedures were in accordance with the principles and guidelines of the Canadian Council on Animal Care, and were approved by the Institutional Animal Care Committee of Memorial University. Animals were housed in a single room with a 12 hr light/12 hr dark period cycle. The temperature and humidity were maintained at 21 °C and 35% ± 5%, respectively.
Female C57Bl/6 mice (8-week-old) were fed ad-libitum, for 2-weeks prior to mating and throughout gestation and lactation, either a control diet or a diet rich in n-6 PUFA. At weaning, the offspring from each group of mothers were divided into two groups, where one half continued on the control diet whereas the other half were fed high-fat diet rich in n-6 PUFA for next 8-weeks. The resulting offspring groups were identified by their pre-/post-weaning diet combination: PUFA/PUFA (P/P), PUFA/control (P/C), control/control (C/C) and control/PUFA (C/P). The current study was conducted in parallel to our previous study, where offspring lipid metabolism and vascular reactivity was studied in response to pre- and post-weaning diets rich in SFA vs. control.13 Thus, the data originating from the control group of female animals ie, C/C is shared with our previous study. Body weights and food consumption of the offspring were recorded weekly. At the end of study period, mice were fasted for 12 hr overnight and then sacrificed by anaesthetizing the animals with halothane vapor in a closed chamber.
Diets
The experimental high-fat diet rich in n-6 PUFA was prepared using a base semi-synthetic diet [casein, 200; DL-methionine, 3; sucrose, 305; corn starch, 190; safflower oil, 200; alphacel non-nutritive bulk, 50; vitamin mix, 11 and mineral mix, 40 g/kg of the diet supplied by MP Biomedicals, OH, USA]. The diet was obtained in powdered form with fat source omitted, designed specifically to permit the control of fat level at 20% w/w. Safflower oil obtained from a local supermarket was used as a source of n-6 PUFA. The control chow diet contained proteins, 23.2%; fiber, 3.8%; starch, 39.5%; glucose, 0.29%; fructose, 0.34%; sucrose, 3.38%; ash, 6.6% and 5% fat (Agribrands Purina Inc, ON, Canada). The fatty acid composition of the experimental diets was determined using the methods of gas-liquid chromatography described previously15 (Table 1).
Table 1.
Fatty acid composition of the experimental diets.
| Fatty acids | High-fat n-6 PUFA | Control |
|---|---|---|
| C14:0 (myristic acid) | ND | 1 |
| C16:0 (palmitic acid) | 8 | 18 |
| C18:0 (stearic acid) | 3 | 5 |
| ∑ SFA | 11 | 24 |
| C16:1 (palmitoleic acid) | ND | 1 |
| C18:1 (oleic acid) | 15 | 22 |
| C20:1 (eicosenoic acid) | ND | ND |
| ∑ MUFA | 15 | 23 |
| C18:2 (linoleic acid) | 70 | 38 |
| C18:3 (linolenic acid) | 4 | 4 |
| C20:5 (eicosapentaenoic acid) | ND | 1 |
| C22:6 (docosahexaenoic acid) | ND | 2 |
| ∑ PUFA | 74 | 45 |
Note: Given as % area covered by each fatty acid peak.
Abbreviations: ∑ SFA, sum of saturated fatty acids; ∑ MUFA, sum of monounsaturated fatty acids; ∑ PUFA, sum of polyunsaturated fatty acids; ND, not detected.
Plasma lipid profile
Fasting blood was collected using cardiac puncture into tubes containing 4.5 mM EDTA, pH 7.5. Plasma was collected after centrifugation of whole blood at 3000 g for 15 min. Plasma triglycerides (TG) and total- cholesterol concentrations were determined using TG assay kit # 2150-101 and cholesterol assay kit # 1010-430 (Stanbio Laboratories, TX, USA). Plasma samples were treated with the reagent # 200-26A (Diagnostics Chemicals Ltd., PEI, Canada) and the supernatant was used for assaying high-density lipoprotein (HDL) concentration using the cholesterol assay kit # 1010-430 (Stanbio Laboratories, TX, USA). The non-HDL cholesterol concentration was determined by subtracting the HDL-cholesterol concentration from the total-cholesterol concentration. The plasma low-density lipoprotein (LDL)- cholesterol concentration was calculated from plasma total-cholesterol, HDL- cholesterol, and TG concentrations according to the method of Friedewald et al.16 Plasma non esterified fatty acids (NEFA) concentration was determined using commercially available kit # 999-34691 (Wako Chemicals Inc., USA). Blood glucose concentrations were measured using a commercially available glucometer (Lifescan Inc, CA, USA) in the fasted animals from the tail blood at the time of sacrificing the animals.
Quantitative-PCR analysis
Liver tissues from sacrificed animals were snap frozen in liquid nitrogen and stored at −70 °C until further analysis. Total RNA was isolated from the liver samples as previously described.17 Reverse transcription of total RNA into cDNA was performed using one-step reverse transcription kit from Roche Diagnostics (PQ, Canada). The mRNA expression levels were determined on a Lightcycler 2.0 Detection System (Roche Diagnostics, PQ, Canada). The primer sequence in the order of sense (S) and anti sense (AS) used for the amplification of various genes were as following: Scavenger receptor-B1 (SR-B1) (S): 5′- TTTGGAGTGGTAGTAAAAAGGGC-3′, SR-B1 (AS): 5′- TGACATCAGGGACTCAGAGTAG-3′; Lecithin cholesterol acyltransferase (LCAT) (S): 5′- GTAACCACACACGGCCTGTC-3′, LCAT (AS): 5′- TCTTACGGTAGCACATCCAGTT-3′; Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (S): 5′- TGAAGCAGGCATCTGAGGG-3′, GAPDH (AS): 5′- CGAAGGTGGAAGAGTGGGAG-3′, where GAPDH was used as housekeeping gene. Briefly, standard curves were generated using the serial dilution of a control sample for various target and GAPDH genes and the PCR efficiency for each reaction was calculated. No differences were found in the expression of GAPDH among various groups. The expression of a gene for each sample was then calculated in relation to the expression of GAPDH, thus, normalizing and correcting the data for the differences in PCR efficiencies for each set of primers.
Vascular function analysis
Thoracic descending aortas were isolated and cleaned of adherent fat, and then 2 mm length segments were used for determining the drug concentration-isometric contractile response curves for KCl (30–120 mM), phenylephrine (PE; 1 nM to 10 μM) and thromboxane A2 mimetic (U46619; 1 nM to 1 μM) as previously described.13 Then endothelium-dependent and -independent relaxation responses were measured using acetylcholine (ACh; 1 nM to 10 μM) and sodium nitroprusside (SNP; 1 nM to 10 μM), respectively, in vessels contracted submaximally with U46619 (50%–75% of Emax). All chemicals were purchased from Sigma Aldrich (ON, Canada).
Statistical analysis
Data were expressed as means ± SEM, n = 6–10, where P/P, n = 8; P/C, n = 6; C/C, n = 10 and C/P, n = 6. Main effects of pre-weaning diet, post-weaning diet and their interaction (pre-weaning X post- weaning diet) were assessed using two-way ANOVA. Effects of the significant interaction were further analyzed using Tukey’s HSD post hoc tests (SYSTAT for Windows, version 12.02; SYSTAT Software Inc., Richmond, California). Differences having a P < 0.05 were considered significant.
Constrictor responses were reported as the force generated in response to each concentration of the agonist and relaxant responses as the percentage reversal of U46619-induced contraction. Cumulative concentration–response curves to agonists were analyzed by fitting to a four-parameter logistic equation using non-linear regression to obtain the -log effective concentration equal to 50% of the maximal response (pEC50) and maximum response (Emax) (Prism 3.0, GraphPAD Software Inc). pEC50 and Emax values were then compared using two-way ANOVA with Tukey’s HSD post hoc analysis. Since the KCl-response curves were not sigmoidal, pEC50 values were not calculated and only maximal responses were compared among various groups.
Results
Offspring body weight, food and caloric intake and serum variables
A significant interaction between the pre- and post-weaning diets affected the offspring body weight at 11-weeks of age (P = 0.002) (Table 2). Multiple comparisons further revealed that a continuous exposure to n-6 PUFA-rich diets during pre- and post-weaning time periods was associated with higher body weight in the P/P offspring compared to the P/C offspring (P/P vs. P/C, P < 0.001) (Table 2). A significant interaction between the pre- and post-weaning diets also affected the food and caloric intake in the female offspring (P = 0.028 and P = 0.037, respectively) (Table 2). Multiple comparisons further revealed that n-6 PUFA-rich diets fed post-weaning was associated with lower food intake in P/P and C/P offspring compared to the P/C (P < 0.001) and C/C offspring (P < 0.001) (Table 2). In addition, both P/P and C/P offspring exhibited reduced caloric intake compared to the C/C offspring (P < 0.005) (Table 2). No differences were found among the offspring for either fasting plasma glucose or NEFA concentrations (Table 2).
Table 2.
Body weight, food-, caloric-intake and plasma variables of various offspring.
| P/P | P/C | C/C | C/P | Pre | Post | Pre × Post | |
|---|---|---|---|---|---|---|---|
| Body weight (g) | 21.3 ± 0.8a | 16.7 ± 0.3b | 18.6 ± 0.8ab | 18.3 ± 0.4ab | NS | P = 0.014 | P = 0.002 |
| Food intake (g/day) | 2.5 ± 0.2b | 3.6 ± 0.1a | 4.1 ± 0.2a | 2.3 ± 0.0b | NS | P < 0.001 | P = 0.028 |
| Caloric intake (Kcal/day) | 12.8 ± 1.0b | 14.7 ± 0.3ab | 17.0 ± 1.0a | 11.7 ± 0.2b | NS | P < 0.001 | P = 0.037 |
| Glucose (mM) | 7.4 ± 0.7 | 8.5 ± 0.6 | 8.3 ± 0.4 | 8.5 ± 0.4 | NS | NS | NS |
| NEFA (mM) | 1.4 ± 0.3 | 1.2 ± 0.0 | 1.0 ± 0.1 | 1.4 ± 0.1 | NS | NS | NS |
| TG (mM) | 0.4 ± 0.0 | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.6 ± 0.1 | NS | NS | P = 0.014 |
| Total-cholesterol (mM) | 1 ± 0.2b | 1.2 ± 0.2ab | 0.9 ± 0.1b | 1.7 ± 0.1a | NS | P = 0.048 | P = 0.006 |
| LDL-cholesterol (mM) | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.1 | NS | P = 0.015 | NS |
| HDL-cholesterol (mM) | 0.3 ± 0.1b | 0.8 ± 0.1a | 0.4 ± 0.0b | 0.6 ± 0.1ab | NS | P = 0.005 | P < 0.001 |
| non-HDL-cholesterol (mM) | 0.8 ± 0.1ab | 0.5 ± 0.1b | 0.4 ± 0.1b | 0.9 ± 0.1a | NS | P = 0.001 | NS |
Notes: Data represents means ± SEM (n = 6–8, specified in section 2.6). Two way ANOVA followed by Tukey’s HSD post Hoc analysis was performed to assess the effects of pre-weaning diet, post-weaning diet and of their interaction (Pre × Post) on various parameters. Different superscripts represent significant differences of P < 0.05.
Abbreviations: P/P = offspring fed n-6 PUFA-rich diet both during pre- and post-weaning time periods; P/C, offspring fed n-6 PUFA-rich diet during pre- and post-weaning time periods; C/C, offspring fed control diet both during pre- and post-weaning time periods; C/P, offspring fed n-6 PUFA-rich diet during post-weaning alone; NEFA, non-esterified fatty acids; TG, triglycerides; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Pre, pre-weaning; Post, post-weaning; NS, non-significant.
Offspring plasma lipid levels
A significant interaction between the pre- and post-weaning diets was observed for the offspring plasma TG (P = 0.014), total-cholesterol (P = 0.006) and HDL-cholesterol concentrations (P < 0.001) (Table 2). Multiple comparisons further revealed that C/P offspring had higher plasma total-cholesterol concentration compared to the P/P and C/C offspring (P/P vs. C/P, P = 0.032; C/C vs. C/P, P = 0.006) (Table 2). In contrast, P/C offspring exhibited higher plasma HDL cholesterol concentration compared to both P/P and C/C offspring (P/P vs. P/C, P < 0.001; P/C vs. C/C, P < 0.001) (Table 2). An n-6 PUFA-rich diet fed post-weaning was associated with higher plasma LDL- (P = 0.015) and non-HDL cholesterol concentrations (P = 0.001) in the offspring compared to the control diet (Table 2). Multiple comparisons further revealed that C/P had higher non-HDL cholesterol concentration compared to the P/C and C/C offspring (C/P vs. P/C, P = 0.039; C/P vs. C/C, P = 0.006) (Table 2).
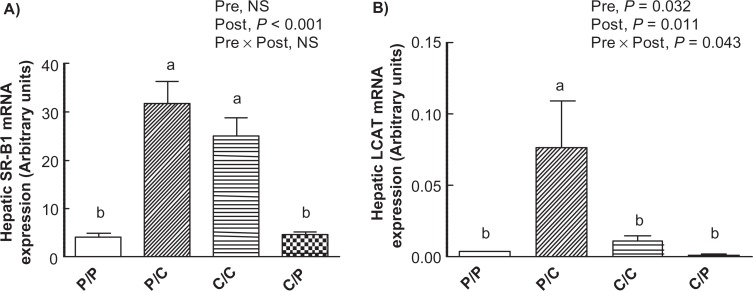
Offspring hepatic mRNA expression of LCAT and Sr-B1
An n-6 PUFA-rich diet fed post-weaning was associated with lower hepatic mRNA expression of SR-B1 in the P/P and C/P offspring compared to the P/C and C/C offspring (P < 0.001) (Fig. 1). In contrast, a significant interaction between the pre- and post-weaning diets affected offspring hepatic mRNA expression of LCAT (P = 0.043) (Fig. 1). Multiple comparisons further revealed that a diet rich in n-6 PUFA fed pre-weaning was associated with the highest mRNA expression of hepatic LCAT in the P/C offspring compared to all other offspring (P/C vs. P/P, P = 0.015; P/C vs. C/C, P = 0.024; P/C vs. C/P, P = 0.009) (Fig. 1).
Figure 1.
Hepatic mRNA expression of (A) SR-B1 (B) and LCAT in various offspring.
Data represents means ± SEM (n = 6–8, specified in section 2.6). Two way ANOVA followed by Tukey’s HSD post Hoc analysis was performed to assess the effects of pre-weaning diet, post-weaning diet and of their interaction (Pre X Post) on LCAT and SR-B1 mRNA expression. Different superscripts represent significant differences of P < 0.05.
Abbreviations: P/P, offspring fed n-6 PUFA-rich diet both during pre- and post-weaning time periods. P/C,offspring fed n-6 PUFA-rich diet during pre-weaning alone; C/C, offspring fed control diet both during pre- and post-weaning time periods; C/P, offspring fed n-6 PUFA-rich diet during post-weaning alone. SR-B1, scavenger receptor-B1; LCAT, Lecithin cholesterol acyltransferase; Pre, pre-weaning; Post, post-weaning; NS, non-significant.
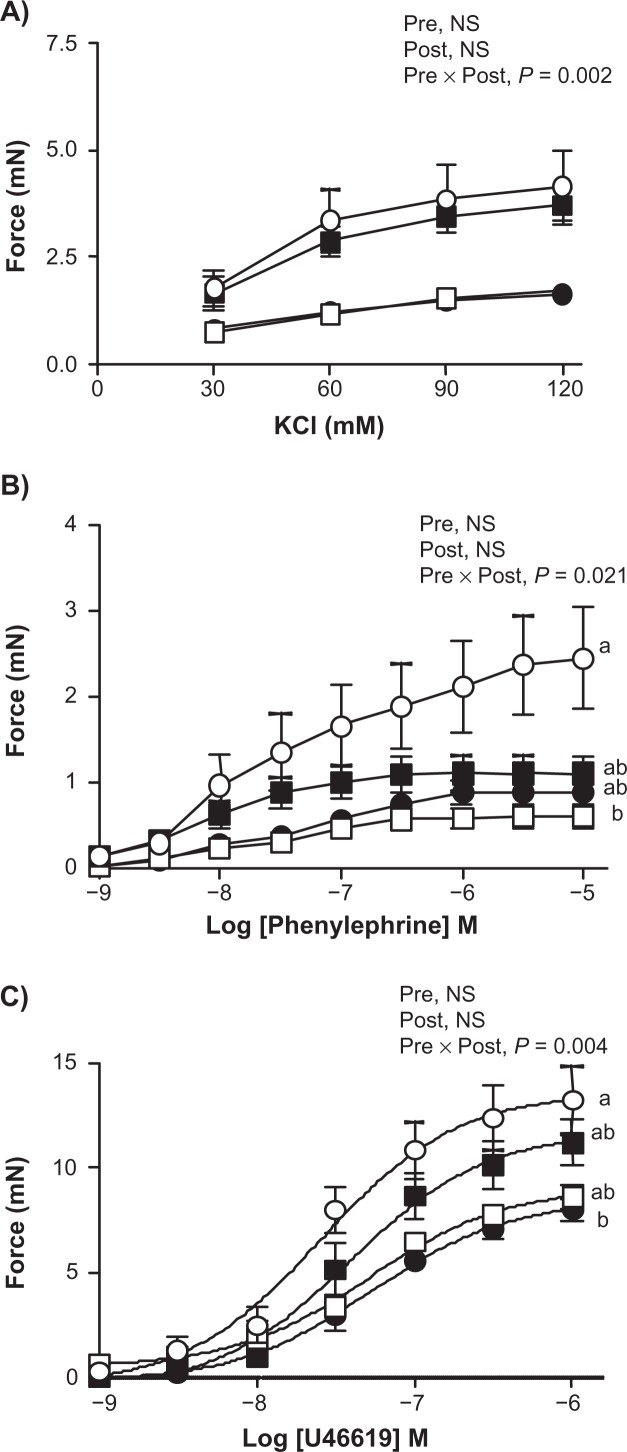
Offspring aortic contractile and relaxation responses
A significant interaction between the pre- and post-weaning diets affected the maximal contractile responses of the offspring aortas to KCl (P = 0.002), PE (P = 0.021) and U46619 (P = 0.004) (Fig. 2). Multiple comparisons further revealed that P/C offspring exhibited lower aortic maximal contractile responses to PE when compared to the C/C offspring (P = 0.020), whereas C/P offspring exhibited lower maximal aortic contractile responses to U46619 compared to the C/C offspring (P = 0.035). In contrast, a pre-weaning diet rich in n-6 PUFA was associated with higher sensitivity to PE in the P/P offspring aortas compared to the C/P offspring aortas (P = 0.009), whereas no differences were observed for the sensitivity to U44619 among the aortas of various offspring (Table 3).
Figure 2.
Dose-dependent contractions of the aorta from various offspring by (A) KCl, (B) phenylephrine and (C) U46619.
Data represents means ± SEM (n = 6–8, specified in section 2.6). Two way ANOVA followed by Tukey’s HSD post Hoc analysis was performed to assess the effects of pre-weaning diet, post-weaning diet and of their interaction (Pre X Post) on the aortic contractile responses. Different superscripts represent significant differences of P < 0.05.
Abbreviations: P/P (▪), offspring fed n-6 PUFA-rich diet both during pre- and post-weaning time periods; P/C (□), offspring fed n-6 PUFA-rich diet during pre-weaning alone, C/C (○), offspring fed control diet both during pre- and post-weaning time periods; C/P (•), offspring fed n-6 PUFA-rich diet during post-weaning alone. Pre, pre-weaning; Post, post-weaning; NS, non-significant.
Table 3.
Half-maximal dose concentration (pEC50) of the offspring aortic rings towards various drugs.
| P/P | P/C | C/C | C/P | Pre | Post | Pre × Post | |
|---|---|---|---|---|---|---|---|
| Phenylephrine | 8.2 ± 0.1a | 7.8 ± 0.2ab | 7.6 ± 0.2ab | 7.3 ± 0.1b | P = 0.009 | NS | NS |
| U46619 | 7.4 ± 0.1 | 7.4 ± 0.2 | 7.7 ± 0.1 | 7.3 ± 0.1 | NS | NS | NS |
| Acetylcholine | 7.4 ± 0.2 | 7.3 ± 0.1 | 7.1 ± 0.1 | 6.9 ± 0.1 | P = 0.012 | NS | NS |
| Sodium nitroprusside | 8.1 ± 0.1 | 8.1 ± 0.1 | 7.7 ± 0.1 | 7.9 ± 0.1 | P = 0.014 | NS | NS |
Notes: Data represents means ± SEM (n = 6–8, specified in section 2.6). Two way ANOVA followed by Tukey’s HSD post Hoc analysis was performed to assess the effects of pre-weaning diet, post-weaning diet and of their interaction (Pre × Post) on various parameters. Different superscripts represent significant differences of P < 0.05.
Abbreviations: P/P = offspring fed n-6 PUFA-rich diet both during pre- and post-weaning time periods; P/C, offspring fed n-6 PUFA-rich diet during pre-weaning alone; C/C, offspring fed control diet both during pre- and post-weaning time periods; C/P, offspring fed n-6 PUFA-rich diet during post-weaning alone; Pre, pre-weaning; Post, post-weaning; NS, non-significant.
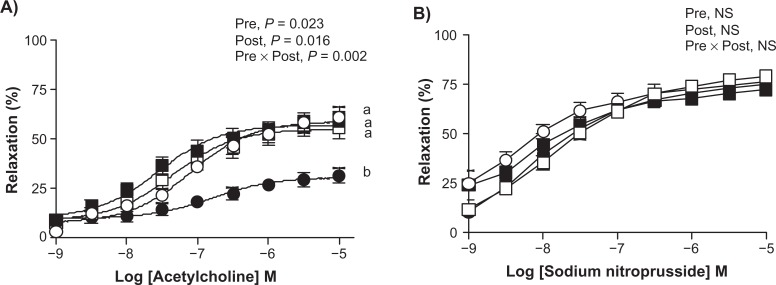
Similar to the offspring aortic contractile responses, a significant interaction between the pre- and post-weaning diets was observed for their relaxation responses to ACh (P = 0.002) (Fig. 3). Multiple comparisons further revealed that C/P offspring aortas exhibited the lowest relaxation responses to ACh compared to all other offspring (P/P vs. C/P, P = 0.002; C/C vs. C/P, P = 0.001; P/C vs. C/P, P = 0.017) (Fig. 3). No differences were observed for the maximal relaxation responses to SNP in the aortas from various offspring (Fig. 3). However, a diet rich in n-6 PUFA fed pre-weaning was associated with higher sensitivity towards ACh (P = 0.012) and SNP (P = 0.014) in the offspring aortas compared to the control diet (Table 3).
Figure 3.
Dose-dependent relaxation responses of the aorta from various offspring by (A) acetylcholine and (B) sodium nitroprusside.
Data represents means ± SEM (n = 6–8, specified in section 2.6). Two way ANOVA followed by Tukey’s HSD post Hoc analysis was performed to assess the effects of pre-weaning diet, post-weaning diet and of their interaction (Pre X Post) on the aortic relaxation responses. Different superscripts represent significant differences of P < 0.05.
Abbreviations: P/P (▪), offspring fed n-6 PUFA-rich diet both during pre- and post-weaning time periods; P/C (□), offspring fed n-6 PUFA-rich diet during pre-weaning alone, C/C (○), offspring fed control diet both during pre- and post-weaning time periods; C/P (•), offspring fed n-6 PUFA-rich diet during post-weaning alone. Pre, pre-weaning; Post, post-weaning; NS, non-significant.
Discussion
Increased consumption of n-6 PUFA has been argued to be associated with increased prevalence of obesity and metabolic syndrome in Western population.11,12 The objective of the current study was to investigate the effects of pre- and post-weaning high-fat diets rich in n-6 PUFA on the lipid metabolism and vascular reactivity of the adult offspring. Results indicate that most of the parameters under study were affected by an interaction between the pre- and post- weaning diets enriched with n-6 PUFA, underscoring the importance of both pre- and post-weaning nutritional environment in maintaining the metabolic and cardiovascular health of the offspring in later life.
An effect of the interaction between pre- and post-weaning diets rich in n-6 PUFA was observed for offspring body weight, food and caloric intake as well as on the plasma lipid levels (Table 2). A continuous exposure to diets rich in n-6 PUFA during pre- and post-weaning diets was however not associated with changes in the plasma lipid levels between P/P and C/C offspring, which can be explained based on the PAR hypothesis. The PAR hypothesis suggests that if the post-natal diet is similar to the prenatal diet, it confers a protective effect in terms of disease outcome in the offspring. However, if the postnatal diet is considerably different from the prenatal diet, the disease becomes manifest.14 Thus, it can be suggested that an exposure to the n-6 PUFA-rich pre-weaning environment was associated with metabolic adaptations in the offspring that resulted in normal plasma lipid levels when the offspring continued on the n-6 PUFA-rich diet post-weaning. This proposal is further corroborated by our observations that an exposure to n-6 PUFA-rich diet during post-weaning alone was associated with higher total-cholesterol in the C/P offspring, whereas an exposure to the n-6 PUFA-rich diet pre-weaning was associated with higher HDL-cholesterol in the P/C offspring compared to the C/C offspring.
A diet rich in linoleic acid (LA), an n-6 PUFA, has previously been shown to increase HDL-cholesterol concentration, which was associated with reduced aortic atherosclerotic lesion area in apo-E deficient mice.18 The n-6 PUFA diet used in the current study was particularly rich in LA (Table 1), thus it is likely that LA is the causal factor in the maternal diet that would have contributed to higher HDL-cholesterol concentration in the adult C57Bl/6 mice offspring (Table 2). HDL-cholesterol is involved in the reverse cholesterol transport pathway, thus presence of higher HDL-cholesterol concentration can be expected to have anti-atherogenic effects in the offspring. We have previously reported that a maternal diet rich in SFA was associated with higher total- and LDL-cholesterol in the offspring fed control diet post-weaning compared to the C/C offspring.13 In the current study, we observed that a maternal diet rich in n-6 PUFA did not increase LDL-cholesterol, instead it was associated with significantly higher HDL-cholesterol in the offspring, which suggests that the type of maternal dietary fat intake can specifically program offspring lipid metabolism during adult life.
LCAT and SR-B1 play an important role in the reverse cholesterol transport pathway and have been shown to be up-regulated by diets rich in PUFA.19 Thus, in an attempt to pinpoint the plausible underlying mechanisms of an increase in HDL-cholesterol by maternal intake of n-6 PUFA-rich diets, the hepatic gene expression of LCAT and SR-B1 was assessed. The hepatic mRNA expression of LCAT was significantly higher in the offspring obtained from mothers fed an n-6 PUFA-rich diet and those who continued on the control diet post-weaning (ie, P/C) compared to all other offspring (Fig. 1). The hepatic mRNA expression of LCAT has been shown to increase plasma HDL cholesterol in rats.20 Since LCAT is responsible for converting free cholesterol in HDL particle to cholesterol esters thereby assisting in further removal of cholesterol from the peripheral cells,21 an increased expression of LCAT may have contributed to an increase in plasma HDL-cholesterol concentrations observed in case of the P/C offspring.
SR-B1 plays an important role in the selective uptake of HDL-cholesterol by the liver.22 The n-6 PUFA-rich diet fed post-weaning was associated with reduced expression of hepatic SR-B1 and a concomitant increase in non-HDL cholesterol concentrations in the P/P and C/P offspring compared to the P/C and C/C offspring (Fig. 1). Besides clearing the HDL particles, hepatic SR-B1 has been reported to act as a remnant receptor and bind to LDL, VLDL and apo-B containing lipoprotein-particles resulting in their clearance from the plasma.23 It is likely that down-regulation of hepatic SR-B1 expression by n-6 PUFA-rich diets resulted in an increase in the plasma non-HDL cholesterol concentration of the offspring exposed to these diets post-weaning.
Changes in plasma cholesterol concentrations have been associated with changes in endothelial cell functions in various studies. Whilst higher LDLcholesterol concentrations have been associated with endothelial dysfunction in vitro24 and in vivo studies,25 an increase in plasma HDL-cholesterol concentration has been associated with improved endothelial function in humans26 and in mice.27 Since P/C offspring exhibited higher HDL-cholesterol concentrations, it was expected that maternal diet rich in n-6 PUFA would have beneficial effects on the aortic smooth muscle and endothelium function in these offspring. However, P/C offspring exhibited reduced aortic contractile responses towards PE when compared to the C/C offspring. In contrast, C/P offspring exhibited reduced aortic contractile responses towards thromboxane mimetic U46619 and reduced relaxation responses to ACh when compared to the C/C offspring. Previous studies investigating the effects of maternal high-fat diets on the offspring vascular function have reported reduced relaxation responses and enhanced constrictor responses in the offspring vessels,28,29 albeit in different vascular beds such as mesenteric and femoral arteries. The discrepancies in our observations may be attributed to the difference in species and vascular beds studied. In a recent study, Byers et al reported smaller contractions by PE and U46619 as well as attenuated relaxations to ACh in the carotid artery of the offspring obtained from the CD-1 mice made obese by feeding high-fat diets and pre-eclamptic by injecting adenoviruses containing soluble fms-like tyrosine kinase-1.30 This study also highlighted that the contractile responses to PE were significantly lower in the carotid arteries of the female offspring obtained from the mothers fed a high-fat diet compared to male offspring, pointing towards complex gender- associated differences. We13 and others31 have also reported gender-associated differences in the vascular function of the offspring exposed to lard-rich diets during pre- and post-weaning time periods previously. Since we only studied the female offspring in the current study, it is acknowledged that complex and yet to be understood gender-mediated mechanisms may also have played a role behind our observations.
The C/P offspring showed higher circulating total-cholesterol concentrations (Table 2) and lower aortic contractions and relaxations by U46619 and ACh, respectively, compared to the C/C offspring (Figs. 2 and 3). Previously, Jiang et al reported reduced aortic contractile responses to U46619 and reduced aortic relaxation responses to ACh in apo E−/− mice fed high-fat diets.32 These mice exhibited higher circulating plasma total- cholesterol concentrations along with aortic lesions compared to the wild-type C57Bl6 mice.32 In contrast, Ellis et al reported reduced aortic contractility to α1-adrenoreceptor agonist in C57Bl/6 mice fed a high-fat Western-style diet for a period of 8-weeks. Interestingly, these mice exhibited less relaxations of aortas by ACh only when incubated with high glucose (ie, 30 mM) for a 20-hour period. These authors concluded that dyslipidemia on a short term basis was associated with altered aortic smooth muscle function, but the added presence of hyperglycemia was required to produce the endothelial cell dysfunction in the C57Bl/6 mice vasculature.33 These studies suggest that the effects of diet on vascular function are complex where more than one factor may affect the vascular tone.
A diet rich in LA has been found to inhibit the basal endothelial NOS activity in an immortalized human endothelial cell line.34 Moreover, LA-rich diets have also been shown to increase urinary 8-isoprostaglandin F2α”. that was further associated with reduced urinary NO metabolites in healthy young individuals.35 Thus, it is suggested that LA-mediated increase in oxidative stress could yet be another causative mechanism behind the endothelial dysfunction observed in case of the C/P offspring besides their hyperlipidemic state. In comparison to P/C and C/P offspring, the P/P offspring exhibited no differences in their aortic contractile and relaxation responses towards various drugs used, which is indicative of the protective effects of PAR hypothesis. Our results are in line with previous studies where a continuous exposure to high-fat and atherogenic diets during pre- and post-weaning time periods was associated with protective effects on the endothelial function of mesenteric arteries in the adult rats36 and aortic fat deposition in a pig model of developmental programming.37 The protective effects observed in each of the above studies point towards the plasticity and versatility of the PAR hypothesis, which seem to exist beyond species and nature of the dietary insults.
In conclusion, the current study provides evidence to support the PAR hypothesis and highlights the importance of the interaction between pre- and post-weaning dietary environment on the overall metabolic and cardiovascular health of the offspring. Our findings also suggest a complex association between diet and vascular function. Future studies are warranted to understand the mechanisms by which pre- and post-weaning diets interact to regulate metabolic pathways in the offspring.
Acknowledgments
Authors would like to thank the Natural Science and Engineering Research Council of Canada (NSERC), Canada Foundation for Innovation New Opportunities, Industrial Research and Innovation Fund and the Canadian Institutes of Health Research (ROP-72465; RSH-78370) for supporting our work.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Cordain L, Eaton S, Sebastian A, Mann N, Lindeberg S, Watkins B, et al. Origins and evolution of the western diet: Health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 2.Cerf M. High fat programming of beta-cell failure. Adv Exp Med Biol. 2010;654:77–89. doi: 10.1007/978-90-481-3271-3_5. [DOI] [PubMed] [Google Scholar]
- 3.Metges C. Early nutrition and later obesity: Animal models provide insights into mechanisms. Adv Exp Med Biol. 2009;646:105–12. doi: 10.1007/978-1-4020-9173-5_11. [DOI] [PubMed] [Google Scholar]
- 4.Stocker CJ, Arch JRS, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc. 2005;64:143–51. doi: 10.1079/pns2005417. [DOI] [PubMed] [Google Scholar]
- 5.Artaud-Wild S, Connor S, Sexton G, Connor W. Differences in coronary mortality can be explained by differences in cholesterol and saturated fat intakes in 40 countries but not in France and Finland. Cir Res. 1993;88:2771–9. doi: 10.1161/01.cir.88.6.2771. [DOI] [PubMed] [Google Scholar]
- 6.Denke M. Dietary fats, fatty acids, and their effects on lipoproteins. Curr Atheroscler Rep. 2006;8:466–71. doi: 10.1007/s11883-006-0021-0. [DOI] [PubMed] [Google Scholar]
- 7.Dolecek T. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200:177–82. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 8.Russo G. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–46. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Sanders T. Polyunsaturated fatty acids in the food chain in europe. Arterioscler Thromb Vasc Biol. 2000;71:176S–8. doi: 10.1093/ajcn/71.1.176s. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos A, Leaf A, Salem N. Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. 1999;43:127–30. doi: 10.1159/000012777. [DOI] [PubMed] [Google Scholar]
- 11.Ailhaud G, Guesnet P, Cunnane S. An emerging risk factor for obesity: Does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? Br J Nutr. 2008;100:461–70. doi: 10.1017/S0007114508911569. [DOI] [PubMed] [Google Scholar]
- 12.Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri J-M, Guesnet P. Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res. 2006;45:203–36. doi: 10.1016/j.plipres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Chechi K, McGuire JJ, Cheema SK. Developmental programming of lipid metabolism and aortic vascular function in C57Bl/6 mice: A novel study suggesting an involvement of ldl-receptor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1029–40. doi: 10.1152/ajpregu.90932.2008. [DOI] [PubMed] [Google Scholar]
- 14.Gluckman P, Hanson M. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–7. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Chechi K, Herzberg GR, Cheema SK. Maternal dietary fat intake during gestation and lactation alters tissue fatty acid composition in the adult offspring of C57Bl/6 mice. Prostaglandins Leukot Essent Fatty Acids. 2010;83:97–104. doi: 10.1016/j.plefa.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of rna isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Shibata K, Nomura R, Kawamoto D, Nagamine R, Imaizumi K. Linoleic acid-rich fats reduce atherosclerosis development beyond its oxidative and inflammatory stress-increasing effect in apolipoprotein e- deficient mice in comparison with saturated fatty acid-rich fats. Br J Nutr. 2005;94:896–901. doi: 10.1079/bjn20051409. [DOI] [PubMed] [Google Scholar]
- 19.Spady DK, Kearney DM, Hobbs HH. Polyunsaturated fatty acids upregulate hepatic scavenger receptor b1 (SR-B1) expression and hdl cholesteryl ester uptake in the hamster. J Lipid Res. 1999;40:1384–94. [PubMed] [Google Scholar]
- 20.Aizawa K, Inakuma T. Dietary capsanthin, the main carotenoid in paprika (capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br J Nutr. 2009;102:1760–6. doi: 10.1017/S0007114509991309. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Burton C, Song X, Mcnamara L, Langella A, Cianetti S, et al. An ApoA-1 mimetic peptide increases LCAT activity in mice through increasing HDL concentration. Int J Biol Sci. 2009;5:489–99. doi: 10.7150/ijbs.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita M, Fujita M, Usui S, Maeda Y, Kudo M, Hirota D, et al. Scavenger receptor type bi potentiates reverse cholesterol transport system by removing cholesterol ester from HDL. Atherosclerosis. 2004;173:197–202. doi: 10.1016/j.atherosclerosis.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Fu T, Kozarsky KF, Borensztajn J. Overexpression of sr-bi by adenoviral vector reverses the fibrateinduced hypercholesterolemia of apolipoprotein e-deficient mice. J Biol Chem. 2003;278:52559–63. doi: 10.1074/jbc.M310892200. [DOI] [PubMed] [Google Scholar]
- 24.Liao J. Inhibition of gi proteins by low density lipoprotein attenuates bradykinin-stimulated release of endothelial-derived nitric oxide. J Biol Chem. 1994;269:12987–92. [PubMed] [Google Scholar]
- 25.Cohen R, Zitnay K, Haudenschild C, Cunningham L. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988;63:903–10. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- 26.Hovingh GK, Brownlie A, Bisoendial RJ, Dube MP, Levels JHM, Petersen W, et al. A novel Apaa-1 mutation (l178p) leads to endothelial dysfunction, increased arterial wall thickness, and premature coronary artery disease. J Am Coll Cardiol. 2004;44:1429–35. doi: 10.1016/j.jacc.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 27.Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, et al. Abcg1 and hdl protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–13. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor P, Khan I, Lakasing L, Dekou V, O’Brien-Coker I, Mallet A, et al. Uterine artery function in pregnant rats fed a diet supplemented with animal lard. Exp Physiol. 2003;88:389–98. doi: 10.1113/eph8802495. [DOI] [PubMed] [Google Scholar]
- 29.Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, et al. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–33. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- 30.Byers BD, Betancourt A, Lu F, Hankins GDV, Longo M, Saade GR, et al. The effect of prepregnancy obesity and sflt-1-induced preeclampsia-like syndrome on fetal programming of adult vascular function in a mouse model. Am J Obstet Gynecol. 2009;200:432.e1–e7. doi: 10.1016/j.ajog.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 31.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, et al. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–75. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- 32.Jiang F, Gibson AP, Dusting GJ. Endothelial dysfunction induced by oxidized low-density lipoproteins in isolated mouse aorta: A comparison with apolipoprotein-e deficient mice. Eur J Pharmacol. 2001;424:141–9. doi: 10.1016/s0014-2999(01)01140-2. [DOI] [PubMed] [Google Scholar]
- 33.Ellis A, Cheng Z-J, Li Y, Jiang YF, Yang J, Pannirselvam M, et al. Effects of a western diet versus high glucose on endothelium-dependent relaxation in murine micro- and macro-vasculature. Eur J Pharmacol. 2008;601:111–7. doi: 10.1016/j.ejphar.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 34.Couloubaly S, Deloménie C, Rousseau D, Paul JL, Grynberg A, Pourci ML. Fatty acid incorporation in endothelial cells and effects on endothelial nitric oxide synthase. Eur J Clin Inves. 2007;37:692–9. doi: 10.1111/j.1365-2362.2007.01843.x. [DOI] [PubMed] [Google Scholar]
- 35.Turpeinen AM, Basu S, Mutanen M. A high linoleic acid diet increases oxidative stress in vivo and affects nitric oxide metabolism in humans. Prostagland, Leukot Essen Fatty Acids. 1998;59:229–33. doi: 10.1016/s0952-3278(98)90067-9. [DOI] [PubMed] [Google Scholar]
- 36.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation. 2004;110:1097–102. doi: 10.1161/01.CIR.0000139843.05436.A0. [DOI] [PubMed] [Google Scholar]
- 37.Norman JF, LeVeen RF. Maternal atherogenic diet in swine is protective against early atherosclerosis development in offspring consuming an atherogenic diet post-natally. Atherosclerosis. 2001;157:41–7. doi: 10.1016/s0021-9150(00)00668-7. [DOI] [PubMed] [Google Scholar]