Abstract
Clozapine is a widely used atypical antipsychotic with a unique effectiveness in treatment-resistant schizophrenia. An important adverse effect is seizures, which have been observed at all stages of clozapine treatment. Valproate has traditionally been considered the drug of choice for the prophylaxis of clozapine seizures, however it may not be the most suitable choice for all patients. There is disagreement as to the best point to prescribe valproate or a suitable antiepileptic: as seizure prophylaxis at a certain clozapine dose or level, or only as remedial treatment. In this review, we examine the relevant literature with an aim to evaluate the following relationships: clozapine dose and electroencephalogram (EEG) abnormalities, plasma levels and EEG abnormalities, dose and occurrence of seizures and plasma levels and occurrence of seizures. Weighted linear regression models were fitted to investigate these relationships. There was a strong relationship between clozapine dose and plasma level and occurrence of clozapine-induced EEG abnormalities. However, a statistically significant relationship between dose and occurrence of seizures was not found. A relationship between clozapine plasma level and occurrence of seizures was not established because of the scarcity of useful data although our review found three case reports which suggested that there is a very substantial risk of seizures with clozapine plasma levels exceeding 1300 μg/l. Seizures are more common during the initiation phase of clozapine treatment, suggesting a slow titration to target plasma levels is desirable. An antiepileptic drug should be considered when the clozapine plasma level exceeds 500 μg/l, if the EEG shows clear epileptiform discharges, if seizures, myoclonic jerks or speech difficulties occur and when there is concurrent use of epileptogenic medication. The antiepileptics of choice for the treatment and prophylaxis of clozapine-induced seizures are valproate (particularly where there is mood disturbance) and lamotrigine (where there is resistance to clozapine).
Keywords: antiepileptic, clozapine, seizure, valproate
Background
Clozapine is an atypical antipsychotic agent displaying unique effectiveness in the treatment of refractory schizophrenia. Its superior efficacy over other antipsychotics [Kane, 1998] has been confirmed by numerous studies [Wahlbeck et al. 1999] and clozapine is widely used despite its broad range of adverse effects. An important adverse effect is seizures, which have been observed at all stages of treatment [Sajatovic and Meltzer, 1996]: at low doses during the titration phase and at high doses during the maintenance phase of clozapine [Pacia and Devinsky, 1994]. As many as 8% of patients taking clozapine have seizures [Wilson, 1992] and resulting fatalities have been reported [Taylor et al. 2009b; Atkinson et al. 2007].
Valproate has, in the past, been considered as the drug of choice for the prophylaxis of clozapine seizures [Devinsky and Pacia, 1994]; however, since the introduction of other antiepileptic drugs (AEDs), it might not be the best choice and it is not prescribed to every patient receiving clozapine [Atkinson et al. 2007]. There is disagreement as to when best to prescribe valproate during clozapine treatment. Suggestions have included prescribing valproate prophylactically before the occurrence of a seizure [Taner et al. 1998], remedially after the occurrence of one seizure [Haller and Binder, 1990] or remedially after two seizures [Wong and Delva, 2007; Liukkonen et al. 1992]. Some guidelines suggest using prophylactic valproate in individuals on clozapine who are prescribed clozapine at doses of 600 mg a day or more or whose clozapine plasma levels are above 500 μg/l [Taylor et al. 2009a].
In the absence of any definitive and widely accepted guidance on the prevention and treatment of clozapine-induced seizures we undertook a systematic review of the relevant literature.
Method
Searches of the databases PubMed and Embase were undertaken in June 2009 using the keywords ‘clozapine’, ‘seizure’, ‘anticonvulsant’, ‘antiepileptic’, ‘EEG’ and ‘valproate’ restricted to the English language and humans. All retrieved papers were examined for additional relevant references. Authors were contacted where necessary for additional information. We aimed to investigate and evaluate the following relationships: clozapine dose and electroencephalogram (EEG) abnormalities, plasma levels and EEG abnormalities, dose and occurrence of seizures and plasma levels and occurrence of seizures.
Data obtained were tabulated and weighted linear regression models were fitted to investigate the relationship between clozapine (mean dose and plasma level) and percentage of patients with abnormal EEG and also percentage of patients with seizures. The model was fitted using the Metareg command in Stata version 11.
Results
Electroencephalogram abnormalities
EEG abnormalities can be epileptiform, defined as focal or generalized spikes (including spike—wave and polyspike discharges) or sharp waves, or nonepileptiform, defined as focal and/ or generalized slowing which may be mild, moderate or severe [Treves and Neufeld, 1996]. We identified 12 papers [Chung et al. 2002; Schuld et al. 2000; Boachie and McGinnity 1997; Freudenreich et al. 1997; Neufeld et al. 1996; Treves and Neufeld, 1996; Olesen et al. 1995; Risby et al. 1995; Haring et al. 1994; Welch et al. 1994; Gunther et al. 1993; Tiihonen et al. 1991] providing information on EEG changes in 565 patients studied. In total, 347 patients of the 565 had an abnormal EEG. The reported prevalence of EEG changes in people taking clozapine varied from 25% [Neufeld et al. 1996] via 53% [Freudenreich et al. 1997; Risby et al. 1995; Haring et al. 1994] to 100% (small populations) [Malow et al. 1994; Tiihonen et al. 1991]. These studies have been summarized in Table 1.
Table 1.
Summaries of reports on the prevalence of clozapine-associated electroencephalogram (EEG) abnormalities.
| Study | N | Clozapine dose mean (mg) [range] | Clozapine level mean (μg/1) | EEG abnormality prevalence (%) | EEG abnormality type | ||
|---|---|---|---|---|---|---|---|
| [Boachie and McGinnity, 1997] | 17 | 453.8 ± 238.5 | Not given | 35 | Nonspecific. | ||
| ‘Epileptogenic’. | |||||||
| [Chung et al. 2002] | 50 | 364.8 ±121.7 | Not given | Total | 62 | Slow wave. | |
| Spikes. | |||||||
| [100–625] | Minimal | 29 | FIRDA. | ||||
| Mild | 61 | ||||||
| Moderate | 10 | ||||||
| Slow wave | 58 | ||||||
| Spikes | 10 | ||||||
| FIRDAa | 8 | ||||||
| [Freudenreich et al. 1997] | 45 | 378 ± 167.91 | Total | 53 | Spike/sharp waves. | ||
| [64–900] | Slowing. | ||||||
| Spikes | 13 | ||||||
| Slowing: | |||||||
| 16 | (I)b 157 ± 75.9 | (I)c 94 ± 13.5 | (Group I) | 33 | |||
| 22 | (II) 421 ± 229.2 | (II) 248 ± 15.0 | (Group II) | 53 | |||
| 12 | (III) 594 ± 175.6 | (III) 391 ± 26.8 | (Group III) | 82 | |||
| [Gunther et al. 1993] | 283 | 215 ± 195 | Not given | Total | 61.5 | Slowing. | |
| Nonparoxysmal changes. | |||||||
| [6.25–1000] | Slowing | 47 | |||||
| NP | 43.8 | Paroxysmal changes. | |||||
| P | 12 | Sharp waves. | |||||
| Sharp | 30.4 | ||||||
| [Haring et al. 1994] | 29 | 319.8 ±183 | 161.3 ± 150 | 52 | General slowing. | ||
| [25–600] | Diffuse delta or localized theta/delta with intermittent sharp transients. | ||||||
| 14d | 266.8 ± 177.9 | 81.6 ± 64.6 | |||||
| 15 | 369.2 ± 179.4 | 235.7 ± 169.8 | |||||
| [Malow et al. 1994] | 10 | [250–900] | Not given | Total | 100 | Diffuse theta and delta slowing. | |
| Epileptiforme activity | 70 | Slow wave. | |||||
| Bilateral spike. | |||||||
| Polyspike. | |||||||
| [Neufeld et al. 1996] | 20 | 31.8 ± 20.7 [6.25–75] | Not given | 25 | General slowing: nonspecific, mild to moderate general slowing. | ||
| [Olesen et al. 1995] | 30 | 366.7 ± 149.3 | 469.9 ± 391.9 | 83 | Diffuse mixture of 5–7 Hz activity. | ||
| [Risby et al. 1995] | 15 | 388 ± 92.0 | Not given | 53.3 | Generalised theta slowing. | ||
| [250–525] | Moderate FIRDA. | ||||||
| Spike waves. | |||||||
| [Schuld et al. 2000] | 9 | 201 ± 88 | Not given | 78 | General slowing. | ||
| [100–350] | Spike/sharp waves. | ||||||
| [Silvestri et al. 1998] | 12 | Not given | Not given | 8 pts IED | Interictal epileptiform abnormalities (multifocal sharp waves, spikes, slowing). | ||
| [Tiihonen et al. 1991] | 16 | 512 ± Not given | Not given | Total | 100 | Paroxysmal delta/theta waves. | |
| [300–700] | PDT | 75 | |||||
| EP | 44 | Spike, spike and slow wave complexes, polyspikes. | |||||
| Theta | 25 | ||||||
| General theta slowing. | |||||||
| [Treves and Neufeld, 1996] | 11 | 300 (fixed dose) | Not given | 64 | General slowing. | ||
| Epileptic activity. | |||||||
| [Welch et al. 1994] | 35 | 571.4 ± 231.69 | Not given | 74 | Mainly mild to moderate slowing of dysrhythmic background. | ||
| [100–900] | |||||||
| Some paroxysmal discharge, spikes, slow sharp waves, polyspikes. | |||||||
| [White and Van Cott, 2007] | 2 |
|
1 = 577 (600 mg dose) | 100 | Theta slowing. | ||
| Case report | 2 = 468 | General spike and poly-spike with slow waves. | |||||
FIRDA, frontal intermittent rhythmic delta activity.
Dose range: Group I, 64–350 mg; Group II, 150–900 mg; Group III, 300–900 mg.
Patients assigned to one of three groups of clozapine serum levels:
Group I, 50–150 μg/l;
Group II, 200–300 μg/l;
Group III, 350–450 μg/l.
Patients divided into two groups:
Group 1 [n = 14], patients with no or minimal alterations;
Group 2 [n = 15], patients with clear alterations.
Epileptiform activity consisting of sharp waves, spikes, spike—wave complexes, bilateral and polyspike discharges PDT, profound disturbance of background activity and also paroxysmal episodes consisting of delta and theta waves; EP, epileptiform activity.
Although a spectrum of EEG abnormalities was observed in association with clozapine, the most common EEG abnormality was nonspecific generalized slowing [Chung et al. 2002; Schuld et al. 2000; Freudenreich et al. 1997; Treves and Neufeld, 1996; Haring et al. 1994; Welch et al. 1994] involving delta and theta waves (slow waves). Delta is the frequency range below 4 Hz, it is normally seen in deep sleep (slow wave sleep) in adults and is not usually seen in the awake adult. Theta is the frequency range from 4 to 8 Hz and can be observed in meditation and drowsy states. Theta waves are considered abnormal if they occur in excess in the awake adult [Alarcon et al. 2009]. Spike or sharp activity was present in a relatively smaller proportion.
The effect of clozapine dose on EEG
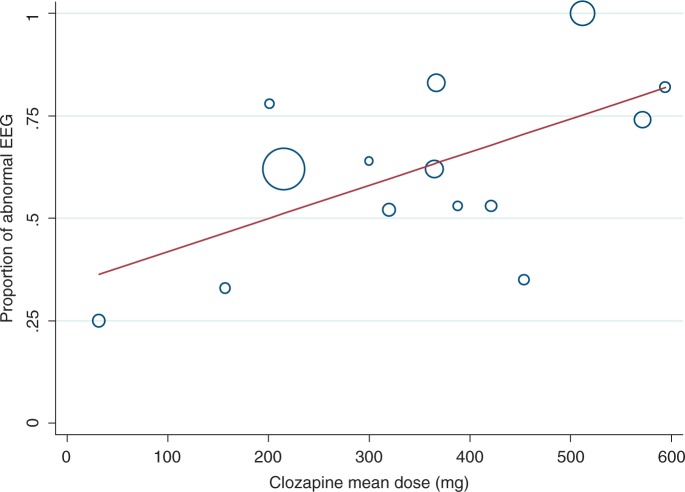
There was strong evidence of a dose-related effect on EEG, illustrated in the graph of proportion of patients with abnormal EEG versus clozapine mean dose (see Figure 1).
Figure 1.
Proportion of patients with abnormal electroencephalogram (EEG) versus clozapine mean dose.
Twelve studies contributed data to this weighted analysis; this enabled the size of each study to be taken into account, with larger studies carrying more weight which is proportional to the variance. One study [Freudenreich et al. 1997] included results for three subsets of patients based on different dose levels; these were included as three separate data points. The study by Malow and colleagues [Malow et al. 1994] was excluded, as it was unclear how they identified their 10 patients for EEG analysis from a subset of 40 patients. (All 10 patients displayed EEG abnormality.) Another study [Silvestri et al. 1998] was also excluded, as the clozapine doses used or levels attained were not given. The mean clozapine dose and standard deviation were not specified in the studies by Welch and associates [Welch et al. 1994] and Olesen and associates [Olesen et al. 1995]. These data were calculated using the individual doses given in both studies. The spectrum of EEG abnormalities from general slowing to spike/sharp waves was grouped together. The circumference of the circle is proportional to the weight of the study in the regression model.
The regression model indicated a significant relationship between mean dose and percentage of patients with an abnormal EEG. Each 100 mg increase in mean dose was associated with an 8% increase in percentage of patients with abnormal EEG (0.08, 95% confidence interval [CI] 0.01–0.15, p = 0.022). The regression model (mean clozapine dose) explained 39% of the variance between the study results (abnormal EEG).
A number of individual studies also found a positive correlation between the spectrum of EEG changes and mean daily clozapine dose [Chung et al. 2002; Treves and Neufeld, 1996; Gunther et al. 1993]. One study [Neufeld et al. 1996] highlighted that even low-dose clozapine in psychotic Parkinsonism caused EEG changes, albeit mild ones. Another study [Freudenreich et al. 1997] reported a contrasting relationship between clozapine dose/plasma levels and EEG spikes versus clozapine dose/plasma levels and EEG slowing. Spikes were seen at doses as low as 150 mg (plasma level 100 μg/l) and the authors concluded these were not related to clozapine dose or plasma level. Similar to Gunther and coworkers [Gunther et al. 1993], they did, however, find a positive relationship between EEG slowing and clozapine dose.
The effect of clozapine plasma level on EEG
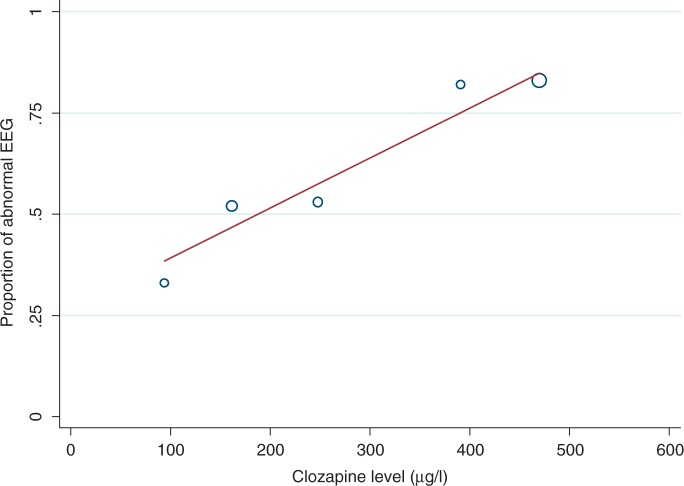
Studies investigating clozapine-induced EEG abnormalities and clozapine plasma levels are summarized in Table 2. Combining results from three studies [Freudenreich et al. 1997; Olesen et al. 1995; Haring et al. 1994], we found a positive relationship between clozapine plasma level and percentage of patients with abnormal EEG (see Figure 2). One study [Freudenreich et al. 1997] included results for three subsets of patients based on different dose levels, these were included as three separate data points. The mean clozapine level and standard deviation were not specified in the study by Olesen and associates [Olesen et al. 1995]. These data were calculated using the individual levels given in the study.
Table 2.
Summaries of reports on the prevalence of clozapine-induced electroencephalogram (EEG) abnormalities against clozapine level.
| Study | N | Clozapine level mean (μg/l) [range] | EEG abnormality prevalence (%) | EEG abnormality type | |
|---|---|---|---|---|---|
| [Antelo et al. 1994] | 2 | Pt 1 = 1376 | n/a | Pt 1 = Generalized, paroxysmal spike waves without focal pattern. | |
| Pt 2 = 820 | Pt 2 = EEG not obtained. | ||||
| [Freudenreich et al. 1997] | 45 | Total | 53 | Spike/sharp waves. | |
| Spikes | 13 | Slowing. | |||
| Slowing: | |||||
| 16 | (I)a 94 ± 13.5 | (Group I) | 33 | ||
| 22 | (II) 248 ± 15.0 | (Group II) | 53 | ||
| 12 | (III) 391 ± 26.8 | (Group III) | 82 | ||
| [Haring et al. 1994] | 29 | 161.3 ± 150 | 53 | General slowing. | |
| 14b | 81.6 ± 64.6 | Diffuse delta or | |||
| 15 | 235.7 ± 169.8 | localized theta/delta with intermittent sharp transients. | |||
| [Olesen et al. 1995] | 30 | 469.9 ± 391.9 | 83 | ||
| [230.9–615.4] | |||||
| [White and Van Cott, 2007] | 2 | 1 = 577 (600 mg dose) | 100 | Theta slowing. | |
| 2 = 468 | General spike and polyspike with slow waves. | ||||
Patients assigned to one of three groups of clozapine serum levels:
Group I, 50–150 μg/l; Group II, 200–300 μg/l; Group III, 350–450 μg/l.
Patients divided into two groups:
Group 1 (n = 14), patients with no or minimal alterations;
Group 2 (n = 15), patients with clear alterations.
Figure 2.
Proportion of patients with abnormal EEG versus clozapine plasma level.
The regression model indicated a significant relationship between clozapine level and percentage of patients with abnormal EEG. Each 100 μg/l increase in clozapine level was associated with a 12% increase in percentage of patients with abnormal EEG (0.12, 95% CI 0.03–0.21, p = 0.023).
Relationship between EEG changes and seizures
Most studies of clozapine-associated seizures have surmised that the occurrence of seizures is not necessarily predicted by changes in nonspecific EEGs [Chung et al. 2002; Treves and Neufeld, 1996; Risby et al. 1995; Haring et al. 1994; Gunther et al. 1993]. For example, Antelo and coworkers [Antelo et al. 1994] reported the case of a patient experiencing myoclonic jerks and ‘leg folding’. His EEG during the event showed generalized paroxysmal spikes suggestive of seizures; however, the EEG prior to the event was normal. In contrast, an uncontrolled study of a chronically ill group of treatment-refractory psychotic patients [Welch et al. 1994] revealed that the EEG was a sensitive indicator of liability to seizures. The authors stated that seizures were more likely to occur in patients displaying EEG abnormalities with paroxysmal spike/sharp wave discharges. Conversely, two studies [Treves and Neufeld, 1996; Risby et al. 1995], reported a positive association between the occurrence of clozapine-induced EEG abnormalities and a better clinical response to clozapine [Risby et al. 1995] with a shorter duration of psychotic symptoms [Treves and Neufeld, 1996].
Clozapine-associated seizures
We found data on a total of 6344 patients across 10 studies of whom 113 patients had seizures, at doses ranging between 150 and 600 mg. Seizure incidence ranged from 0.9% to 29% of treated patients [Boachie and McGinnity, 1997]. These studies have been summarized in Table 3. A number of studies detailed in the table have been excluded from the regression analysis of dose versus seizures. These consisted of the pre-marketing study by Devinsky and colleagues [Devinsky et al. 1991], which did not specify the number of people in each dose group, as well as two other studies [Silvestri et al. 1998; Malow et al. 1994] which were excluded for reasons mentioned previously (see the caption of Figure 1). In addition, Haller and Binder only disclosed 4 doses out of a possible 19, and so their study was also excluded from the regression analysis. Furthermore, Sajatovic and Meltzer failed to provide doses for all patients included in their sample, specifying the mean doses only for patients who had seizures. Case reports were not included in the analysis.
Table 3.
Occurrence of clozapine-induced seizures.
| Study | N | Clozapine dose (mg) mean [range] | Clozapine level mean (μg/l) | Seizure incidence (%) | Seizure type | |
|---|---|---|---|---|---|---|
| [Boachie and McGinnity, 1997] | 17 | 453.8 ± 238.5 | Not given | 29 | Temporal lobe. | |
| Complex partial. | ||||||
| [Chung et al. 2002] | 50 | 364.8 ± 121.7 | Not given | 4 | Mixed partial. | |
| [100–625] | Generalized tonic—clonic. | |||||
| [Devinsky et al. 1991] | 1418 | Low dose<300 | Not given | low 1.0 | 2.9 | Generalized tonic—clonic. |
| medium dose | medium 2.7 | |||||
| 300–600 | ||||||
| high dose ≥ 600 | high 4.4 | |||||
| [Freudenreich et al. 1997] | 50 | 378 ± 167.91 [64–900] | 6 (3 pts) | Myoclonic possibly mistaken for tonic—clonic. | ||
| 16 | (I)a 157 ± 75.9 | (I)b 94 ± 13.5 | (n= 1 dose 900 mg level 320 μg/l) | |||
| 22 | (II) 421 ± 229.2 | (II) 248 ± 15.0 | ||||
| 12 | (III) 594 ± 175.6 | (III) 391 ± 26.8 | ||||
| [Gouzoulis et al. 1991] Case report | 3 | [300–450] | Not given | 100 | Generalized epileptic seizure. | |
| [Gunther et al. 1993] | 283 | 215 ± 195 [6.25–1000] | Not given | 1.1 | Generalized tonic—clonic. | |
| [Haller and Binder, 1990] | 19 | [600–750] 75 mgc | Not given | 21 | Generalized tonic—clonic. | |
| [Liukkonen et al. 1992] | 127 | 496 ± 129d [300–700] | Not given | 9.4 | Mainly Generalized tonic—clonic (8 patients). | |
| [Malow et al. 1994] | 40 | [250–900] | Not given | 17.5 | Generalized tonic-clonic. Myoclonus. | |
| [Pacia and Devinsky, 1994] | 5629 | Not given | 1.3 | Generalized tonic—clonic. | ||
| 1302 | Low dose 0–299 | low 1.6e | ||||
| 3192 | medium dose 300–599 | med 0.9 | ||||
| 1135 | high dose ≥ 600 | high 1.9 | ||||
| [Sajatovic and Meltzer, 1996] | 148 | Generalized seizure | Not given | 7.4 | Generalized seizure. | |
| (n = 9) 575 ± 215f | ||||||
| [200–900] | ||||||
| Myoclonus only | Myoclonus only. | |||||
| (n=2) 413 ± 165 | ||||||
| [300–600] | ||||||
| Myoclonus followed by generalized seizure. | ||||||
| [Silvestri et al. 1998] | 12 | Not given | Not given | 67 | Generalized tonic—clonic. | |
| Generalized myoclonic. | ||||||
| Complex partial. | ||||||
| Simple partial. | ||||||
| [Tiihonen et al. 1991] | 16 | 512 ± not given | Not given | 6.25 (1 pt on 500 mg dose) | ||
| [300–700] | ||||||
| [Welch et al. 1994] | 35 | 571.4 ± 231.69 [100–900] | Not given | 20 | Complex partial tonic—clonic. Myoclonic. | |
| [Wilson and Claussen, 1994] | 100 | 323 ± 181g | Not given | 10 | Mainly tonic—clonic. | |
| 0–299 mg/day n = 6 | ||||||
| 300–599 mg/day | ||||||
| n=2 | ||||||
| 600–900 mg/day | ||||||
| n=2 [100–600] | ||||||
| [Wilson, 1992] | 37 | Month 1 = 315 ± 135 | Not given | 8 | Tonic—clonic (3 patients). | |
| Month 6 = 597 ± 156h | ||||||
Dose range: Group I, 64–350 mg; Group II, 150–900 mg; Group III, 300–900 mg.
Patients assigned to one of three groups of clozapine serum levels: Group I, 50–150 μg/l; Group II, 200–300 μg/l; Group III, 350–450 μg/l.
Pre-existing seizure disorder.
n = 12.
Seizure frequency for maximum daily dose of clozapine prior to first seizure.
n = 9 in mean daily clozapine dose causing generalized seizures 575 ± 215; n = 2 in mean daily clozapine dose causing myoclonus only 413 ± 165.
Seizures occurred in the 10 patients at this mean dose.
Mean dose used in regression analysis.
In all studies, there was a greater risk of clozapine-induced seizures than the 1% risk associated with conventional antipsychotics [Murphy and Delanty, 2000; Balen and Procyshyn, 1999; Wilson and Claussen, 1994; Liukkonen et al. 1992; Haller and Binder, 1990]. Premarketing studies disclosed by the manufacturer reported seizure occurrence at a crude rate of 3.5% over the course of a year [Wilson and Claussen, 1994]. Devinsky and co-authors [Devinsky et al. 1991] observed the seizure incidence increasing over time, with a cumulative seizure risk of 10% after 3.8 years of clozapine therapy. A US post-marketing investigation reported generalized tonic—clonic seizures in 1.3% of clozapine-treated patients (71 out of 5629) in the first 6 months after its release. A total of 24 (34%) of these 71 patients had recurrent seizures.
The majority of clozapine-induced seizures were of the generalized tonic—clonic type [Liukkonen et al. 1992; Devinsky et al. 1991]. Clozapine may also cause myoclonus (myoclonic jerks), at times occurring alone [Antelo et al. 1994; Berman et al. 1992; Gouzoulis et al. 1991] or preceding a generalized tonic—clonic seizure. This is discussed later.
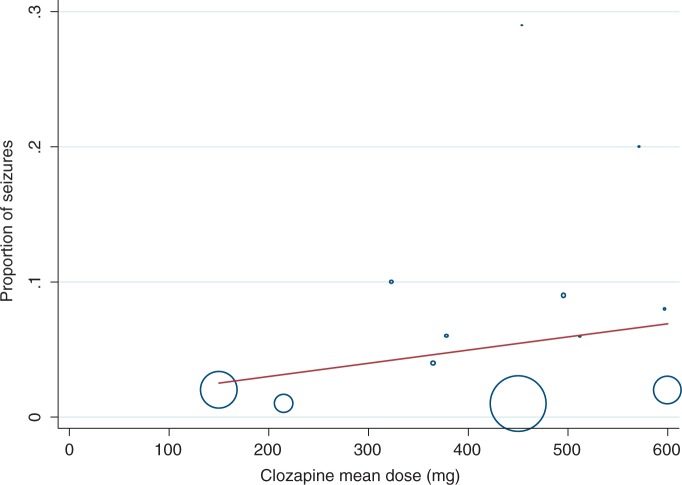
Relationship between dose and occurrence of seizures
We did not find a statistically significant relationship between mean clozapine dose and percentage of patients experiencing seizures (p = 0.353). Ten studies contributed data towards the regression analysis, illustrated in Figure 3. Patients in one study [Boachie and McGinnity, 1997] displayed a particularly high seizure incidence at doses of 200–400 mg (although these were patients with learning disability who have a higher propensity to seizures).
Figure 3.
Proportion of patients with seizures versus mean dose of clozapine.
There was wide variation across the studies with regards to an association of clozapine dose and seizures. In the main, clozapine dose was found to be closely correlated with seizure incidence: the higher the dose; the greater the risk of seizures, even though our regression analysis did not find this to be statistically significant. The majority of case studies reported clozapine-induced seizures in patients taking doses greater than 600 mg a day [Karper et al. 1992; Baker and Conley, 1991; Haller and Binder, 1990; Simpson and Cooper, 1978]. However, a post-marketing study [Pacia and Devinsky, 1994] did not find a dose-related risk for seizures. The low-dose group had a surprisingly high frequency of seizures. This was attributed to a number of factors; seizures unrelated to clozapine therapy, a pre-existing seizure disorder, organic brain injury or a combination of epileptogenic medication [Wilson and Claussen, 1994; Devinsky et al. 1991; Haller and Binder, 1990], and initiating clozapine on a more-rapid dose titration (12 days) contrary to manufacturer recommendations of 2–3 weeks [Wilson and Claussen, 1994; Devinsky et al. 1991].
One study found most seizures occurring soon after a clozapine dose increase (mean ± SD increase = 54 ± 26 mg/day) [Wilson and Claussen, 1994], although the authors suggested this was more likely to be related to an associated rapid increase in clozapine plasma levels rather than dose per se. Similarly Haller and Binder reported an increase in seizures following large dose increments (accidental increase of 350 mg and ingestion of an additional 1200 mg as a suicide attempt). Also, seizures are reported to be more common during the initiation phase (when doses are gradually increased) [Sajatovic and Meltzer, 1996; Pacia and Devinsky, 1994; Wilson and Claussen, 1994; Devinsky et al. 1991]: Pacia and Devinsky recorded the median time to develop seizures was 42 days for the entire group, similar to Sajatovic and Meltzer who reported that half of the seizures occurred within the first 34 days of clozapine treatment.
Relationship between clozapine plasma level and occurrence of seizures
Our review only found three case reports (four patients) reporting plasma level and seizure incidence. Relevant case reports are summarized in Table 4. There were not enough data to allow a metaregression analysis to be performed. Clozapine concentrations exceeding 1300 μg/l have been associated with an increased risk of seizures [Funderburg et al. 1994] particularly those of the tonic—clonic type. Another report [Simpson and Cooper, 1978] also found seizures occurring at levels above 1300 μg/l in two patients and proposed a safe therapeutic maximum clozapine level of 600 μg/l. Authors of all three reports emphasized the importance and usefulness of clozapine plasma level monitoring in the prevention of adverse effects related to raised concentrations.
Table 4.
Incidence of clozapine-induced seizures against plasma levels.
| Study | N | Clozapine level mean (μg/l) | Seizure incidence (%) | Seizure type | Notes |
|---|---|---|---|---|---|
| [DuMortier et al. 2001] | 1 | 2115 | n/a | Clonic | Valpromidec discontinued 7 days before seizure |
| [Funderburg et al. 1994] | 1 | 1300 | n/a | Tonic clonic followed by postictal confusion | Erythromycin added to stable dose of clozapined |
| [Simpson and Cooper, 1978] | 2 | Pt 1= 1313 | n/a | Generalized tonic clonic by both patients | Pt 1 = accidental overdose of 2 g |
| Pt 2 = 2194a | Pt 2 = accidental double dose given | ||||
| 2064b | |||||
First seizure.
Second seizure
A prodrug of valproic acid.
Enzyme inhibition likely to increase clozapine plasma levels.
Conversely, 3 out of 50 patients investigated by Freudenreich and associates had seizures with low clozapine levels. However, these patients had pre-existing seizure disorders, confirming the importance of obtaining a full clinical history [Freudenreich et al. 1997].
Neurotoxic adverse effects are associated with higher clozapine plasma levels and therapeutic drug monitoring has been advocated to ensure that clozapine levels are kept around the accepted therapeutic threshold. Such an approach is also likely to be valuable in assessing and monitoring the risk of clinical toxicity [Greenwood-Smith et al. 2003]; however, there is no clear, statistically significant evidence to support this suggestion.
Case reports
Clozapine-induced myoclonic seizures, myoclonic jerks, drop attacks, ‘leg folding’, stuttering and facial tics
Clozapine may cause myoclonus, at times occurring alone [Antelo et al. 1994; Berman et al. 1992; Gouzoulis et al. 1991] or preceding a generalized tonic-clonic seizure [Haddad and Sharma, 2007; Haberfellner, 2002; Sajatovic and Meltzer, 1996; Meltzer and Ranjan, 1994; Gouzoulis et al. 1993; Berman et al. 1992]. Myoclonus affects approximately 2% of clozapine-treated patients [Lieberman and Safferman, 1992]. It is a potentially serious motor phenomenon presenting as spontaneous brief jerking movements of the head, face, trunk, fingers or toes, alone or in clusters and can be epileptic or nonepileptic in nature [Sajatovic and Meltzer, 1996]. Myoclonic seizures tended to occur during the clozapine initiation phase [Taner et al. 1998] and at doses ranging between 150 mg [Haberfellner, 2002] and 500 mg [Gouzoulis et al. 1993]. Associated EEG abnormalities were observed and ranged from paroxysmal (epileptiform) patterns [Haberfellner, 2002; Gouzoulis et al. 1991] to generalized spike-wave complexes [Gouzoulis et al. 1993]. Sajatovic and Meltzer encountered an equal number of patients (2 out of 11) experiencing myoclonus alone and myoclonus followed by a generalized seizure. Thus the authors, along with others, postulated that myoclonus may be a harbinger of generalized seizures [Dhar et al. 2008; Haberfellner, 2002; Taner et al. 1998; Sajatovic and Meltzer, 1996; Antelo et al. 1994; Gouzoulis et al. 1993; Berman et al. 1992]. Three case studies reported clozapine-induced myoclonic jerks along with ‘drop attacks’ or ‘leg folding’ [Dhar et al. 2008; Antelo et al. 1994; Berman et al. 1992]. In all cases there was no loss of consciousness and the patient was well oriented to time. ‘Drop attacks’ in this context were thought to be atonic seizures, which are the result of sudden loss of muscle tone [Berman et al. 1992] or flexion tonic seizures [Antelo et al. 1994] resulting from muscle contraction rather than loss of muscle tone.
Eight cases of clozapine-induced stuttering have been reported [Hallahan et al. 2007; Lyall et al. 2007; Begum, 2005; Duggal et al. 2002; Supprian et al. 1999; Thomas et al. 1994]. These occurred at doses ranging between 125 mg [Thomas et al. 1994] and 700 mg [Supprian et al. 1999] with two authors suggesting a dose-dependent relationship [Hallahan et al. 2007; Thomas et al. 1994]. Three case reports associated clozapine-induced stuttering with seizure activity [Begum, 2005; Duggal et al. 2002; Supprian et al. 1999] and this view is supported by four cases where the use of valproate greatly improved speech difficulties [Lyall et al. 2007; Begum, 2005; Duggal et al. 2002; Supprian et al. 1999], including facial tics as reported by Begum. Three patients went on to have a generalized seizure [Hallahan et al. 2007; Duggal et al. 2002; Supprian et al. 1999].
Special cases: Cigarette smoking and Asian patients
Cigarette smoking reduces clozapine plasma levels by up to 50% and higher doses may be required in smokers than in nonsmokers.
Plasma level reduction may be even greater in those receiving valproate [Taylor et al. 2009a]. Tobacco smoke contains polycyclic aromatic hydrocarbons that induce liver enzymes, in particular CYP1A2 which in turn increases the metabolism of clozapine. This effect is particularly important when patients give up smoking; the enzyme activity lessens causing the clozapine plasma level to rise substantially, often requiring a reduction in dose. Nicotine replacement agents, however, have no effect on this process. Close monitoring of clozapine plasma levels is crucial, as seizures have occurred 8 weeks following smoking cessation in a clozapine responder [McCarthy, 1994]. The patient was also on fluoxetine, which can raise clozapine levels by 30–75% [Spina et al. 1998; Centorrino et al. 1994] however, the author observed seizure occurrence only after the smoking cessation.
Seizures were reported in two Asian patients on low-dose (200 mg) clozapine: in one case a male Chinese patient discontinued low-dose benzodiazepine (lorazepam), and a seizure occurred 40 hours after the last lorazepam dose [Lane et al. 1999]. The authors hypothesized that stopping the lorazepam may have unmasked the underlying seizure potential from clozapine. In the second case [Ravasia and Dickson, 1998] a tonic—clonic seizure was observed in a female Vietnamese patient whose clozapine plasma level was 1076 μg/l preseizure. Considering the lack of more familiar risk factors, the authors suggested the patient may have been a slow metabolizer of clozapine, and that race may be a risk factor for seizures on clozapine. Asians show higher plasma levels than Caucasians for a given clozapine dose [Ng et al. 2005].
A comparison of clozapine use in Korean and Caucasian patients found a greater change in the Brief Psychiatric Rating Scale (BPRS) scores in Korean patients while on significantly lower doses of clozapine [Matsuda et al. 1996]. It appears that lower maintenance doses of clozapine might be enough to treat Asian patients successfully but that seizures (which are usually associated with higher clozapine doses) might present at much lower clozapine doses.
The use of valproate for prophylaxis of clozapine-induced seizure
Valproate is an effective GABA-ergic antiepileptic drug (AED) [McElroy et al. 1989]. It has been widely regarded as the drug of choice for the treatment and prophylaxis of clozapine-induced seizures [Foster and Olajide, 2005; Iqbal et al. 2003; Miller, 2000; Littrell et al. 1995; Kando et al. 1994; Toth and Frankenburg, 1994; Liukkonen et al. 1992], and is the most commonly used AED for this indication. There are, however, very few studies prospectively examining the efficacy of valproate in preventing clozapine-related seizures.
Valproate has advantages over other AEDs: it has a broad spectrum of antiepileptic activity; it is effective in primary generalized seizures such as tonic—clonic, tonic, clonic, myoclonic (seizures and jerks) and both simple and complex absence seizures [McElroy et al. 1989]. Valproate has been used successfully in one case of clozapine-induced tonic—clonic seizure in a patient with treatment-resistant schizophrenia [Foster and Olajide, 2005]; the authors noted an improved outcome in treatment-resistant schizophrenia with the concomitant use of an antiepileptic/ mood-stabilizing agent.
Clozapine-associated myoclonic seizures seem to respond well to valproate. Two cases reporting myoclonic seizures with clozapine therapy described successful treatment with valproic acid [Taner et al. 1998]. This allowed the patients to continue with their effective clozapine treatment whilst remaining seizure-free. The authors of another case report [Meltzer and Ranjan, 1994] also advocate the use of valproic acid in the treatment of clozapine-induced myoclonic jerks. Meltzer and Ranjan suggested that it may be the serotonergic receptor blocking properties of clozapine that causes myoclonus, with valproic acid displaying an antimyoclonic effect.
It is the dual effect of valproate when added to clozapine treatment that is attractive to clinicians. It acts prophylactically against seizures and also has psychotropic properties; it acts as a mood stabilizer and as an antimanic agent [Brodtkorb and Mula, 2006]. This can add greatly to the potential therapeutic benefits for the patient. A retrospective study of 55 patients examined the safety of the concurrent clozapine and valproate [Kando et al. 1994]; valproate was used as a mood stabilizer in 25 of the patients, as seizure prophylaxis in 12 patients, and as an antiepileptic in 5 patients with a history of a seizure disorder. The combination of clozapine and valproate was found to be effective and well tolerated in 87% of the patients. No seizures occurred, nor were any blood dyscrasias reported. Another advantage of valproate is that it may be less likely to cause cognitive impairment in comparison with some of the older AEDs [McElroy et al. 1989].
Common adverse effects of valproate include dyspepsia, gastric irritation, nausea, increased appetite and weight gain (8–14 kg in up to 59% of patients) [Tranulis et al. 2006]. Many of these adverse effects are additive to those caused by clozapine. In one study [Kando et al. 1994], sedation was the most common adverse effect experienced by 34 patients (62%) and led to the discontinuation of valproate in 3 patients. Other adverse effects include hair loss with curly regrowth, more rarely anaemia and blood disorders leucopenia and pancytopenia [Langosch and Trimble, 2002]. A case study also reported an apparently increased risk of agranulocytosis and neutropenia with valproate used adjunctively with clozapine [Pantelis and Adesanya, 2001]. This was reversed when the valproate was stopped.
Valproate should not normally be used in women of child-bearing age because it is an established human teratogen; neural tube defects have been associated with valproate taken during the first trimester of pregnancy [McElroy et al. 1989]. If valproate cannot be avoided, then adequate contraception should be strongly recommended and prophylactic folic acid prescribed [National Institute for Clinical Excellence, 2006].
There are conflicting reports on the effect of valproate on clozapine metabolism. Two studies found a moderate increase in the clozapine level (39%, Centorrino et al. [1994], and 20%, Facciola et al. [1999]) after at least 1 week of steady dose treatment. In contrast, a case report [Conca et al. 2000] found that the clozapine plasma level was significantly decreased, suggesting an induction of clozapine metabolism by valproate. Similarly, a small study (n = 7) [Longo and Salzman, 1995] found a 15% decrease in clozapine plasma levels after the addition of valproate. The mechanism by which valproate might induce or inhibit the metabolism of clozapine is unclear. Facciola and colleagues surmised that the interaction might involve displacement of clozapine from plasma protein binding sites. The findings described above could be explained by the coexistence of two mechanisms of interaction (enzyme inhibition and protein binding displacement) leading to opposite changes in total clozapine levels [Facciola et al. 1999]. Perhaps more important is the very significant variation in measured plasma levels of clozapine in patients receiving constant dose clozapine [Palego et al. 2002] which may lead to the opposing findings described above.
Overall, valproate does not appear to cause any clinically significant change in the steady-state plasma levels of clozapine and norclozapine.
Other antiepileptic drugs for prophylaxis of clozapine-induced seizures
It has been suggested that AEDs can be divided into two categories based on their principal psychotropic properties [Brodtkorb and Mula, 2006]; AEDs can be classified as activating drugs which exert antidepressant properties by attenuating glutamatergic neurotransmission and as sedating drugs which enhance GABA-ergic neurotransmission, e.g. valproate. Valproate may not always be suitable for use in combination with clozapine because of certain adverse effects (weight gain and sedation) and so other AEDs may be preferable, according to their adverse effect/therapeutic profile.
Lamotrigine has also successfully been used in the prophylaxis and treatment of clozapine-induced generalized tonic-clonic seizures [Muzyk et al. 2010]. These authors noted that the myoclonic jerks experienced by the patient in their case report resolved with lamotrigine therapy. It is not associated with neural tube defects [Cunnington and Tennis, 2005]. It has a limited adverse effect profile and there are few pharmacodynamic interactions. Owing to its lack of effect on hepatic enzymes, there are also few pharmacokinetic interactions [Langosch and Trimble, 2002]. However, it should be noted that lamotrigine concentrations are decreased by high oestrogen levels in pregnancy and by oestrogen-containing oral contraceptives [de Haan et al. 2004].
Lamotrigine has mood-stabilizing (preventing depressive relapse) and antidepressant properties [Brodtkorb and Mula, 2006]: an advantage when an affective component is present. A meta-analysis [Tiihonen et al. 2009] and a case series [Dursun et al. 1999] suggested lamotrigine augmentation to be an effective treatment for patients with treatment-resistant or clozapine-resistant schizophrenia. Both authors suggested that the mechanism of action was an additive relationship between lamotrigine and clozapine in reducing glutamate neurotransmission.
Topiramate is a well-documented AED which is said by some to have a good safety profile [Navarro et al. 2001]. It can be given as monotherapy or as adjunctive treatment of generalized tonic—clonic seizures or partial seizures with or without secondary generalization [British Medical Association, 2010]. It has been suggested that it may be particularly beneficial in clozapine-induced weight gain as it can induce significant weight loss, with one patient losing 21 kg over 5 months whilst successfully being treated for myoclonic jerks [Dursun and Devarajan, 2000]. It may also have mood-stabilizing properties [Brodtkorb and Mula, 2006]. Topiramate has also been suggested as an adjunctive to antipsychotic medication, however there is doubt over its effectiveness as two case reports [Hofer et al. 2003; Millson et al. 2002] noted a worsening of psychosis after the addition of topiramate.
Gabapentin is a possible alternative for patients intolerant of valproate in clozapine-induced seizure prophylaxis [Landry, 2001]. It does not affect the pharmacokinetics of clozapine, as it is not metabolized by the liver [Tranulis et al. 2006]. It is usually well tolerated when used with other medicines and has a mild adverse effect profile [Usiskin et al. 2000]. It possesses anxiolytic properties [Brodtkorb and Mula, 2006] and its use has been recommended for adjunctive treatment in anxiety [Tranulis et al. 2006] and treatment [Landry, 2001], prophylaxis [Usiskin et al. 2000] of clozapine-induced seizures. However, in one case, the addition of gabapentin to clozapine was associated with an exacerbation of psychosis [Jablonowski et al. 2002].
Carbamazepine possesses a serious adverse effect in common with clozapine: agranulocytosis [Iqbal et al. 2003]. There is also a firmly established interaction between the two drugs. Carbamazepine induces the hepatic enzymes CYP3A4 and CYP1A2. This enzyme induction accelerates the metabolism of clozapine, decreasing clozapine plasma levels [Jerling et al. 1994].
Phenytoin appears to be effective for clozapinerelated tonic—clonic seizures. It is also hepatically metabolized and induces the hepatic enzyme CYP1A2, increasing the metabolism of clozapine, leading to lower clozapine levels [Lieberman and Safferman, 1992; Miller, 1991]. Phenytoin intoxication has been reported in a patient with clozapine-related seizures, after an intravenous phenytoin loading dose [Gandelman-Marton et al. 2008]. The authors suggested it was CYP2C9 inhibition by clozapine that may have caused the phenytoin intoxication. Adverse effects include thrombocytopenia, leucopenia, agranulocytosis [Toth and Frankenburg, 1994] and pancytopenia with or without bone marrow suppression [Pfizer, 2010].
Phenobarbital is also hepatically metabolized and induces the hepatic enzyme CYP1A2, stimulating the metabolism of clozapine, decreasing clozapine levels [Lieberman and Safferman, 1992].
Pregabalin is an anxiolytic antiepileptic which has shown recent promise in improving anxiety and mood in patients with schizophrenia treated with antipsychotics. Data are limited to 11 patients (5 receiving clozapine) but pregabalin might be a suitable choice of antiepileptic in those on clozapine with anxiety symptoms [Englisch et al. 2010].
Discussion
We found a relationship between both dose of clozapine and, in particular, plasma level and the proportion of patients shown to have abnormal EEG. Our analysis of published data did not, however, show a clear clozapine dose-related effect on occurrence of seizures. There were insufficient data to test the hypothesis that clozapine plasma levels are related to seizure incidence but observations in patients who stop smoking strongly suggest that it is the plasma level and not the dose that predicts seizure occurrence.
This lack of relationship between dose and seizures is somewhat to be expected, as although clozapine plasma levels are broadly related to dose, it is difficult precisely to predict plasma levels from dose alone because of the many influencing patient variables: clozapine plasma levels are lower in smokers, younger patients and in males, and higher in Asian patients. Inflammation and infection also influence plasma levels [Taylor et al. 2009a]. In addition, the fixed regression analysis we conducted was dominated by the large postmarketing naturalistic study which included 5629 patients [Pacia and Devinsky, 1994]. The mean doses used were not specified in the study and so a middle value for each dose range was assumed. This approximation greatly reduced the capacity to demonstrate a dose-related effect.
Owing to the paucity of useful data, we were unable to conduct a meta-regression analysis exploring the relationship between clozapine plasma level and occurrence of seizures. Studies examining this relationship are scarce and our review only found three case reports, which suggest only that there is very substantial risk of seizures with clozapine plasma levels exceeding 1300 μg/l.
Other limitations of our analysis include selection bias (the reporting only or mainly of cases), differences in reporting (case studies, case series, retrospective population studies, study duration), the variability between study populations, the absence of data on patient risk factors (seizure history, neurological abnormalities, smoking status, etc.), the dearth of confirmatory observations of seizure occurrence and type (some seizures were clearly reported by patients or relatives), the subsequent drop-out rates, and the previously mentioned imprecision in reporting of individual or mean doses.
Can we say when to use an antiepileptic?
Our regression model showed that seizure risk increases linearly with dose and that EEG abnormalities increase linearly with dose and plasma level and so there is no clear exponential rise in risk at any dose or level. Because results showed there was no dose or level at which risk increases at a greater rate, and as there is no safe dose or level at which seizures do not occur, we cannot make a recommendation on basis of risk of seizures except to keep the plasma level as low as possible. Dose, however, is affected by too many variables for a clear risk relationship to be established.
The plasma level for acute response to clozapine is in the range 200–504 μg/l [Taylor et al. 2009a]. In those not responding to clozapine, a plasma level target range of 350–500 μg/l has been suggested. When initiating clozapine, we suggest titrating slowly to 350 μg/l, as seizures are more common during the initiation phase [Pacia and Devinsky, 1994; Wilson and Claussen, 1994; Devinsky et al. 1991]. If there is no response, increase the dose to give a plasma level of 500 μg/l. Consideration should be given to introducing an AED if the clozapine plasma levels are above 500 μg/l, if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Antiepileptic therapy should also be considered at the beginning of clozapine treatment: in patients using other epileptogenic medication, patients with pre-existing seizure disorder and in patients with neurological abnormalities.
Can we say which antiepileptic should be used?
The antiepileptics of choice for the treatment and prophylaxis of clozapine-induced seizures are valproate and lamotrigine. Valproate has the most data to support its use and it is widely regarded as the drug of choice. It does not appear to cause any major changes in clozapine plasma levels, although data are contradictory. It has mood-stabilizing properties and acts as an antimanic agent, so potentially optimizing therapeutic benefit. In addition, it is effective over a broad spectrum of antiepileptic activity and it is generally well tolerated. Rare adverse effects in common with clozapine include agranulocytosis and neutropenia, and so close monitoring would be prudent. Valproate is teratogenic and is not recommended in women of child-bearing age. Baseline monitoring of liver function, full blood count, weight and height is essential, with follow ups at 6-monthly intervals (weight at 3-monthly intervals).
The clinical benefits of lamotrigine for the treatment and prophylaxis of clozapine-induced seizures are being increasingly recognized by clinicians; it possesses mood-stabilizing (at least in respect to depressive episodes) [Bowden et al. 2003] and acute antidepressant properties [Geddes et al. 2009] and has been shown to have a beneficial additive antipsychotic effect when added to clozapine therapy for schizophrenia [Tiihonen et al. 2009]. In addition, lamotrigine is not sedative and does not cause weight gain. It is a good alternative to valproate in females of child-bearing age, as it is not a major teratogen (although it may be associated with cleft palate) [Holmes et al. 2008]. A disadvantage to its use in the urgent treatment of clozapine-induced seizures is the gradual titration needed to achieve a therapeutic dose (up to 6 weeks).
Topiramate may also have a place in the treatment and prophylaxis of clozapine-induced seizures; it should perhaps be considered for those patients with significant clozapine-induced weight gain. However, the risks of worsening of psychosis should be noted. Pregabalin might be considered in patients with anxiety also requiring seizure prophylaxis.
Carbamazepine and phenytoin should be avoided in clozapine therapy due to serious adverse effects additive to those of clozapine, which could potentially lead to clozapine cessation. The manufacturers of clozapine advise against the concurrent use with carbamazepine because of the risk of bone marrow suppression. If concurrent use is unavoidable, higher clozapine doses may be required as the pharmacokinetic interaction could cause clinical deterioration in the patient. Phenobarbital is not ideal in combination with clozapine and its use should be avoided. It is a very sedative drug so the additive sedative effects could potentially be very debilitating.
Conclusion and recommendations
There is a strong relationship between clozapine dose and level and occurrence of clozapine-induced EEG abnormalities. However, a relationship between dose and occurrence of seizures was not found. We consider that clozapine level is likely to be the more reliable indicator of the potential for seizure to occur. There is a distinct lack of studies investigating the relationship between clozapine plasma levels and occurrence of seizures. Additional large-scale studies are required to establish with certainty the relationship between clozapine and seizures.
For seizure prophylaxis, there appears to be a strong argument for prescribing an AED after the occurrence of myoclonus, stuttering or speech difficulties, any type of seizure, epilepti-form changes on the EEG, and in those with added risk factors such as pre-existing seizure disorder or those with relevant neurological abnormalities, and also once the clozapine plasma level reaches or exceeds 500 μg/l. The AEDs of choice appear to be valproate for a schizoaffective illness, topiramate or lamotrigine for patients with clozapine-induced weight gain, and lamotrigine in clozapine-refractory schizophrenia.
When should an antiepileptic be prescribed?
In pre-existing seizure disorder or in patients with relevant neurological abnormalities.
With concurrent use of epileptogenic medication.
When clozapine plasma level exceeds 500 μg/l.
If stuttering or other speech difficulties occur.
If myoclonic jerks occur.
If EEG shows epileptiform changes.
Following any type of seizure.
In clozapine treatment-refractory schizophrenia, augment with lamotrigine.
Antiepileptic choice
Schizoaffective disorder or mood-related psychosis: valproate.
Clozapine-induced weight gain: lamotrigine or topiramate
Lack of response with clozapine: lamotrigine.
Acknowledgement
The authors wish to thank Victoria Cornelius for her statistical advice.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
None declared.
References
- Alarcon G., Nashef L., Cross H., Nightingale J., Richardson S. (2009) Epilepsy (Oxford Specialist Handbooks in Neurology), Oxford: Oxford University Press [Google Scholar]
- Antelo R.E., Stanilla J.K., Martin-Llonch N. (1994) Myoclonic seizures and “leg folding” phenomena with clozapine therapy: Report of two cases. Biol Psychiatry 36: 759–762 [DOI] [PubMed] [Google Scholar]
- Atkinson J.M., Douglas-Hall P., Fischetti C., Sparshatt A., Taylor D.M. (2007) Outcome following clozapine discontinuation: A retrospective analysis. J Clin Psychiatry 68: 1027–1030 [DOI] [PubMed] [Google Scholar]
- Baker R.W., Conley R.R. (1991) Seizures during clozapine therapy. Am J Psychiatry 148: 1265–1266 [DOI] [PubMed] [Google Scholar]
- Balen R.M., Procyshyn R.M. (1999) Valproic acid for seizure prophylaxis during clozapine therapy: Where's the evidence?. Int J Psychiatry Clin Pract 3: 249–251 [DOI] [PubMed] [Google Scholar]
- Begum M. (2005) Clozapine-induced stuttering, facial tics and myoclonic seizures: A case report. Aust NZ J Psychiatry 39: 202. [DOI] [PubMed] [Google Scholar]
- Berman H., Zalma A., DuRand C.J., Green A.I. (1992) Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry 53: 329–330 [PubMed] [Google Scholar]
- Boachie A., McGinnity M.G.A. (1997) Use of clozapine in a mental handicap hospital-report of the first 17 patients. Ir J Psychol Med 14: 16–19 [Google Scholar]
- Bowden C.L., Calabrese J.R., Sachs G., Yatham L.N., Asghar S.A., Hompland M., et al. (2003) A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry 60: 392–400 [DOI] [PubMed] [Google Scholar]
- British Medical Association. (2010) British National Formulary, 59th edn, London: BMJ Group and Pharmaceutical Press [Google Scholar]
- Brodtkorb E., Mula M. (2006) Optimizing therapy of seizures in adult patients with psychiatric comorbidity. Neurology 67(Suppl. 4): S39–S44 [DOI] [PubMed] [Google Scholar]
- Centorrino F., Baldessarini R.J., Kando J., Frankenburg F.R., Volpicelli S.A., Puopolo P.R., et al. (1994) Serum concentrations of clozapine and its major metabolites: Effects of cotreatment with fluoxetine or valproate. Am J Psychiatry 151: 123–125 [DOI] [PubMed] [Google Scholar]
- Chung S.J., Jeong S.H., Ahn Y.M., Kang U.G., Koo Y.J., Ha J.H., et al. (2002) A retrospective study of clozapine and electroencephalographic abnormalities in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 26: 139–144 [DOI] [PubMed] [Google Scholar]
- Conca A., Beraus W., Konig P., Waschgler R. (2000) A case of pharmacokinetic interference in comedication of clozapine and valproic acid. Pharmacopsychiatry 33: 234–235 [DOI] [PubMed] [Google Scholar]
- Cunnington M., Tennis P. (2005) Lamotrigine and the risk of malformations in pregnancy. Neurology 64: 955–960 [DOI] [PubMed] [Google Scholar]
- de Haan G.J., Edelbroek P., Segers J., Engelsman M., Lindhout D., Devile-Notschaele M., et al. (2004) Gestation-induced changes in lamotrigine pharmacokinetics: A monotherapy study. Neurology 63: 571–573 [DOI] [PubMed] [Google Scholar]
- Devinsky O., Honigfeld G., Patin J. (1991) Clozapine-related seizures. Neurology 41: 369–371 [DOI] [PubMed] [Google Scholar]
- Devinsky O., Pacia S.V. (1994) Seizures during clozapine therapy. J Clin Psychiatry 55(Suppl. B): 153–156 [PubMed] [Google Scholar]
- Dhar R., Singh T., Charvan B.S. (2008) Clozapine-induced drop-attack and myoclonus. German J Psychiatry 11: 29–31 [Google Scholar]
- Duggal H.S., Jagadheesan K., Nizamie S.H. (2002) Clozapine-induced stuttering and seizures. Am J Psychiatry 159: 315. [DOI] [PubMed] [Google Scholar]
- DuMortier G., Mahe V., Pons D., Zerrouk A., Januel D., DeGrassat K. (2001) Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci 13: 302–303 [DOI] [PubMed] [Google Scholar]
- Dursun S.M., Devarajan S. (2000) Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 45: 198. [PubMed] [Google Scholar]
- Dursun S.M., McIntosh D., Milliken H. (1999) Clozapine plus lamotrigine in treatment-resistant schizophrenia. Arch Gen Psychiatry 56: 950. [DOI] [PubMed] [Google Scholar]
- Englisch S., Esser A., Enning F., Hohmann S., Schanz H., Zink M. (2010) Augmentation with pregabalin in schizophrenia. J Clin Psychopharmacol 30: 437–440 [DOI] [PubMed] [Google Scholar]
- Facciola G., Avenoso A., Scordo M.G., Madia A.G., Ventimiglia A., Perucca E., et al. (1999) Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit 21: 341–345 [DOI] [PubMed] [Google Scholar]
- Foster R., Olajide D. (2005) A case of clozapine-induced tonic-clonic seizures managed with valproate: Implications for clinical care. J Psychopharmacol 19: 93–96 [DOI] [PubMed] [Google Scholar]
- Freudenreich O., Weiner R.D., McEvoy J.P. (1997) Clozapine-induced electroencephalogram changes as a function of clozapine serum levels. Biol Psychiatry 42: 132–137 [DOI] [PubMed] [Google Scholar]
- Funderburg L.G., Vertrees J.E., True J.E., Miller A.L. (1994) Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry 151: 1840–1841 [DOI] [PubMed] [Google Scholar]
- Gandelman-Marton R., Theitler J., Klein C., Rabey J.M. (2008) Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med 35: 407–409 [DOI] [PubMed] [Google Scholar]
- Geddes J.R., Calabrese J.R., Goodwin G.M. (2009) Lamotrigine for treatment of bipolar depression: Independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry 194: 4–9 [DOI] [PubMed] [Google Scholar]
- Gouzoulis E., Grunze H., von B.U. (1991) Myoclonic epileptic seizures during clozapine treatment: A report of three cases. Eur Arch Psychiatry Clin Neurosci 240: 370–372 [DOI] [PubMed] [Google Scholar]
- Gouzoulis E., Ozdaglar A., Kasper J. (1993) Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry 150: 1128a. [DOI] [PubMed] [Google Scholar]
- Greenwood-Smith C., Lubman D.I., Castle D.J. (2003) Serum clozapine levels: A review of their clinical utility. J Psychopharmacol 17: 234–238 [DOI] [PubMed] [Google Scholar]
- Gunther W., Baghai T., Naber D., Spatz R., Hippins H. (1993) EEG alterations and seizures during treatment with clozapine. A retrospective study of 283 patients. Pharmacopsychiatry 26: 69–74 [DOI] [PubMed] [Google Scholar]
- Haberfellner E.M. (2002) Myoclonic and generalized tonic clonic seizures during combined treatment with low doses of clozapine and haloperidol. Eur Psychiatry 17: 55. [DOI] [PubMed] [Google Scholar]
- Haddad P.M., Sharma S.G. (2007) Adverse effects of atypical antipsychotics: Differential risk and clinical implications. CNS Drugs 21: 911–936 [DOI] [PubMed] [Google Scholar]
- Hallahan B.P., Murray I.T., Doyle P.G. (2007) Clozapine induced stuttering. Ir J Psychol Med 24: 121. [DOI] [PubMed] [Google Scholar]
- Haller E., Binder R.L. (1990) Clozapine and seizures. Am J Psychiatry 147: 1069–1071 [DOI] [PubMed] [Google Scholar]
- Haring C., Neudorfer C., Schwitzer J., Hummer M., Saria A., Hinterhuber H., et al. (1994) EEG alterations in patients treated with clozapine in relation to plasma levels. Psychopharmacology 114: 97–100 [DOI] [PubMed] [Google Scholar]
- Hofer A., Fleischhacker W.W., Hummer M. (2003) Worsening of psychosis after replacement of adjunctive valproate with topiramate in a schizophrenia patient. J Clin Psychiatry 64: 1267–1268 [DOI] [PubMed] [Google Scholar]
- Holmes L.B., Baldwin E.J., Smith C.R., Habecker E., Glassman L., Wong S.L., et al. (2008) Increased frequency of isolated cleft palate in infants exposed to lamotrigine during pregnancy. Neurology 70: 2152–2158 [DOI] [PubMed] [Google Scholar]
- Iqbal M.M., Rahman A., Husain Z., Mahmud S.Z., Ryan W.G., Feldman J.M. (2003) Clozapine: A clinical review of adverse effects and management. Ann Clin Psychiatry 15: 33–48 [DOI] [PubMed] [Google Scholar]
- Jablonowski K., Margolese H.C., Chouinard G. (2002) Gabapentin-induced paradoxical exacerbation of psychosis in a patient with schizophrenia. Can J Psychiatry 47: 975–976 [DOI] [PubMed] [Google Scholar]
- Jerling M., Lindstrom L., Bondesson U., Bertilsson L. (1994) Fluvoxamine inhibition and carbamazepine induction of the metabolism of clozapine: Evidence from a therapeutic drug monitoring service. Ther Drug Monit 16: 368–374 [DOI] [PubMed] [Google Scholar]
- Kando J.C., Tohen M., Castillo J., Centorrino F. (1994) Concurrent use of clozapine and valproate in affective and psychotic disorders. J Clin Psychiatry 55: 255–257 [PubMed] [Google Scholar]
- Kane J.M. (1998) Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull 24: 62–67 [PubMed] [Google Scholar]
- Karper L.P., Salloway S.P., Seibyl J.P., Krystal J.H. (1992) Prolonged postictal encephalopathy in two patients with clozapine-induced seizures. J Neuropsychiatry Clin Neurosci 4: 454–457 [DOI] [PubMed] [Google Scholar]
- Landry P. (2001) Gabapentin for clozapine-related seizures. Am J Psychiatry 158: 1930–1931 [DOI] [PubMed] [Google Scholar]
- Lane H.Y., Su K.P., Chang W.H. (1999) Seizures after discontinuation of low-dose lorazepam from originally seizure-free clozapine regimen: Combined effects?. J Clin Psychiatry 60: 408–409 [DOI] [PubMed] [Google Scholar]
- Langosch J.M., Trimble M.R. (2002) Epilepsy, psychosis and clozapine. Human Psychopharmacol Clin Exp 17: 115–119 [DOI] [PubMed] [Google Scholar]
- Lieberman J.A., Safferman A.Z. (1992) Clinical profile of clozapine: Adverse reactions and agranulocytosis. Psychiatr Q 63: 51–70 [DOI] [PubMed] [Google Scholar]
- Littrell K.H., Johnson C.G., Schultz R.E. (1995) The pharmacological management of clozapine-related seizures. J Psychosoc Nurs Ment Health Serv 33: 42–43 [DOI] [PubMed] [Google Scholar]
- Liukkonen J., Koponen H.J., Nousiainen U. (1992) Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res 44: 107–112 [DOI] [PubMed] [Google Scholar]
- Longo L.P., Salzman C. (1995) Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry 152: 650. [DOI] [PubMed] [Google Scholar]
- Lyall M., Pryor A., Murray K. (2007) Clozapine and speech dysfluency: Two case reports. Psychiatr Bull 31: 16–18 [Google Scholar]
- Malow B.A., Reese K.B., Sato S., Bogard P.J., Malhotra A.K., Su T.P., et al. (1994) Spectrum of EEG abnormalities during clozapine treatment. Electronencephlography Clin Neurophysiol 91: 205–211 [DOI] [PubMed] [Google Scholar]
- Matsuda K.T., Cho M.C., Lin K.M., Smith M.W., Young A.S., Adams J.A. (1996) Clozapine dosage, serum levels, efficacy, and side-effect profiles: A comparison of Korean-American and Caucasian patients. Psychopharmacol Bull 32: 253–257 [PubMed] [Google Scholar]
- McCarthy R.H. (1994) Seizures following smoking cessation in a clozapine responder. Pharmacopsychiatry 27: 210–211 [DOI] [PubMed] [Google Scholar]
- McElroy S.L., Keck P.E., Jr, Pope H.G., Jr, Hudson J.I. (1989) Valproate in psychiatric disorders: Literature review and clinical guidelines. J Clin Psychiatry 50(Suppl): 23–29 [PubMed] [Google Scholar]
- Meltzer H.Y., Ranjan R. (1994) Valproic acid treatment of clozapine induced myoclonus. Am J Psychiatry 151: 1246–1247 [DOI] [PubMed] [Google Scholar]
- Miller D.D. (1991) Effect of phenytoin on plasma clozapine concentrations in two patients. J Clin Psychiatry 52: 23–25 [PubMed] [Google Scholar]
- Miller D.D. (2000) Review and management of clozapine side effects. J Clin Psychiatry 61: 14–17 [PubMed] [Google Scholar]
- Millson R.C., Owen J.A., Lorberg G.W., Tackaberry L. (2002) Topiramate for refractory schizophrenia. Am J Psychiatry 159: 675. [DOI] [PubMed] [Google Scholar]
- Murphy K., Delanty N. (2000) Drug induced seizures: General principles in assessment, management and prevention. CNS Drugs 14: 135–146 [Google Scholar]
- Muzyk A., Gala G., Kahn D.A. (2010) Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract 16: 125–128 [DOI] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence (2006) Bipolar disorder. The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. Clinical Guidance 38, available at http://www.nice.org.uk [Google Scholar]
- Navarro V., Pons A., Romero A., Bernardo M. (2001) Topiramate for clozapine-induced seizures. Am J Psychiatry 158: 968–969 [DOI] [PubMed] [Google Scholar]
- Neufeld M.Y., Rabey J.M., Orlov E., Korcyzn A. (1996) Electroencephalographic findings with low-dose clozapine treatment in psychotic Parkinsonian patients. Clin Neuropharmacol 19: 81–86 [DOI] [PubMed] [Google Scholar]
- Ng C.H., Chong S.A., Lambert T., Fan A., Hackett L.P., Mahendran R., et al. (2005) An inter-ethnic comparison study of clozapine dosage, clinical response and plasma levels. Int Clin Psychopharmacol 20: 163–168 [DOI] [PubMed] [Google Scholar]
- Olesen O.V., Thomsen K., Jensen P.N., Wulff C.H., Rasmussen N.A., Refshammer C., et al. (1995) Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: A cross-sectional study. Psychopharmacology (Berl) 117: 371–378 [DOI] [PubMed] [Google Scholar]
- Pacia S.V., Devinsky O. (1994) Clozapine-related seizures: Experience with 5,629 patients. Neurology 44: 2247–2249 [DOI] [PubMed] [Google Scholar]
- Palego L., Biondi L., Giannaccini G., Sarno N., Elmi S., Ciapparelli A., et al. (2002) Clozapine, norclozapine plasma levels, their sum and ratio in 50 psychotic patients: Influence of patient-related variables. Prog Neuropsychopharmacol Biol Psychiatry 26: 473–480 [DOI] [PubMed] [Google Scholar]
- Pantelis C., Adesanya A. (2001) Increased risk of neutropenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust NZ J Psychiatry 35: 544–545 [DOI] [PubMed] [Google Scholar]
- Pfizer (2010) Summary of Product Characteristics. Epanutin Capsules 25, 50, 100 and 300 mg.
- Ravasia S., Dickson R.A. (1998) Seizure on low-dose clozapine. Can J Psychiatry 43: 420. [PubMed] [Google Scholar]
- Risby E.D., Epstein C.M., Jewart R.D., Nguyen B.V., Morgan W.N., Risch S.G., et al. (1995) Clozapine-induced EEG abnormalities and clinical response to clozapine. J Neuropsychiatry Clin Neurosci 7: 466–470 [DOI] [PubMed] [Google Scholar]
- Sajatovic M., Meltzer H.Y. (1996) Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry 39: 367–370 [DOI] [PubMed] [Google Scholar]
- Schuld A., Kuhn M., Haack M., Kraus T., Hinxe-Selch D., Lechner C., et al. (2000) A comparison of the effects of clozapine and olanzapine on the EEG in patients with schizophrenia. Pharmacopsychiatry 33: 109–111 [DOI] [PubMed] [Google Scholar]
- Silvestri R.C., Bromfield E.B., Khoshbin S. (1998) Clozapine-induced seizures and EEG abnormalities in ambulatory psychiatric patients. Ann Pharmacother 32: 1147–1151 [DOI] [PubMed] [Google Scholar]
- Simpson G.M., Cooper T.A. (1978) Clozapine plasma levels and convulsions. Am J Psychiatry 135: 99–100 [DOI] [PubMed] [Google Scholar]
- Spina E., Avenoso A., Facciola G., Fabrazzo M., Monteleone P., Maj M., et al. (1998) Effect of fluoxetine on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenia. Int Clin Psychopharmacol 13: 141–145 [DOI] [PubMed] [Google Scholar]
- Supprian T., Retz W., Deckert J. (1999) Clozapine-induced stuttering: Epileptic brain activity?. Am J Psychiatry 156: 1663–1664 [DOI] [PubMed] [Google Scholar]
- Taner E., Cosar B., Isik E. (1998) Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract 2: 53–55 [DOI] [PubMed] [Google Scholar]
- Taylor D., Paton C., Kapur S. (2009a) The Maudsley Prescribing Guidelines, 10th edn, Informa Healthcare: London [Google Scholar]
- Taylor D.M., Douglas-Hall P., Olofinjana B., Whiskey E., Thomas A. (2009b) Reasons for discontinuing clozapine: Matched, case-control comparison with risperidone long-acting injection. Br J Psychiatry 194: 165–167 [DOI] [PubMed] [Google Scholar]
- Thomas P., Lalaux N., Vaiva G., Goudemand M. (1994) Dose-dependent stuttering and dystonia in a patient taking clozapine. Am J Psychiatry 151: 1096a. [DOI] [PubMed] [Google Scholar]
- Tiihonen J., Nousianen U., Hakola P., Leinonen E., Tuunainen A., Mervaala E., et al. (1991) EEG abnormalities associated with clozapine treatment. Am J Psychiatry 148: 1406. [DOI] [PubMed] [Google Scholar]
- Tiihonen J., Wahlbeck K., Kiviniemi V. (2009) The efficacy of lamotrigine in clozapine-resistant schizophrenia: A systematic review and meta-analysis. Schizophr Res 109: 10–14 [DOI] [PubMed] [Google Scholar]
- Toth P., Frankenburg F.R. (1994) Clozapine and seizures: A review. Can J Psychiatry 39: 236–238 [DOI] [PubMed] [Google Scholar]
- Tranulis C., Mouaffak F., Chouchana L., Stip E., Gourevitch R., Poirier M.F., et al. (2006) Somatic augmentation strategies in clozapine resistance—what facts?. Clin Neuropharmacol 29: 34–44 [DOI] [PubMed] [Google Scholar]
- Treves I.A., Neufeld M.Y. (1996) EEG abnormalities in clozapine-treated schizophrenic patients. Eur Neuropsychopharmacol 6: 93–94 [DOI] [PubMed] [Google Scholar]
- Usiskin S.I., Nicolson R.O.B., Lenane M., Rapoport J.L. (2000) Gabapentin prophylaxis of clozapine-induced seizures. Am J Psychiatry 157: 482–483 [DOI] [PubMed] [Google Scholar]
- Wahlbeck K., Cheine M., Essali A., Adams C. (1999) Evidence of clozapine's effectiveness in schizophrenia: A systematic review and meta-analysis of randomized trials. Am J Psychiatry 156: 990–999 [DOI] [PubMed] [Google Scholar]
- Welch J., Manschreck T., Redmond D. (1994) Clozapine-induced seizures and EEG changes. J Neuropsychiatry Clin Neurosci 6: 250–256 [DOI] [PubMed] [Google Scholar]
- White D.M., Van Cott A.C. (2007) Clozapine (Clozaril®), seizures, and EEG abnormalities. Am J Electroneurod T 47: 190–197 [PubMed] [Google Scholar]
- Wilson W.H. (1992) Clinical review of clozapine treatment in a state hospital. Hosp Community Psychiatry 43: 700–703 [DOI] [PubMed] [Google Scholar]
- Wilson W.H., Claussen A.M. (1994) Seizures associated with clozapine treatment in a state hospital. J Clin Psychiatry 55: 184–188 [PubMed] [Google Scholar]
- Wong J., Delva N. (2007) Clozapine-induced seizures: Recognition and treatment. Can J Psychiatry 52: 457–463 [DOI] [PubMed] [Google Scholar]