Abstract
Background: Amisulpride is a second-generation antipsychotic which has been proved to be effective in the control of both positive and negative symptoms of schizophrenia. In this study we aimed to determine metabolic, endocrinologic and cardiac effects of amisulpride commonly used in our clinical practice.
Methods: A total of 18 patients (11 males, 7 females) diagnosed with schizophrenia received amisulpride at the dosage of 800 mg/day and were followed up for 24 weeks. Positive and negative psychotic symptoms, extrapyramidal and sexual side effects, metabolic, endocrinologic and cardiac parameters were evaluated at regular intervals.
Results: Significant improvement in both positive and negative symptoms was observed in patients starting from the second week of treatment. Prolactin levels increased significantly both in men and women starting from the measurement on day 4. Prolactin elevation was significantly higher in women than in men. Increase in total cholesterol level became significant at week 24. No other significant difference was observed between weeks 1 and 24 regarding the other parameters.
Conclusions: The clinical data from the present study supports the fact that amisulpride is an effective and safe antipsychotic drug, but elevates prolactin levels in both sexes.
Keywords: amisulpride, efficacy, hormones, hyperprolactinemia, metabolic control, QT interval, side effects
Introduction
Schizophrenia is a chronic and disabling disorder with a lifetime prevalence of approximately 1% [American Psychiatric Association, 1994]. Antipsychotic treatment remains mandatory for patients with an established diagnosis of schizophrenia [Mortimer, 2003]. The introduction of the second-generation antipsychotics (SGAs) also known as ‘atypical antipsychotics’ over the last decade has brought a significant improvement in the treatment of schizophrenia, both in terms of improved tolerability and the spectrum of efficacy [Mortimer, 2004].
Amisulpride is a unique drug among SGAs. Whereas most of the other new generation antipsychotics have a high affinity for both dopamine and serotonin receptors, amisulpride is a selective dopamine receptor antagonist with high affinity to D3 and D2 receptors [Scatton et al. 1997]. It is well established that amisulpride is at least as effective in the control of positive symptoms as conventional antipsychotic drugs and other SGAs [Möller, 2000; Burns and Bale, 2001]. At low doses amisulpride preferentially blocks presynaptic dopamine autoreceptors [Schoemaker et al. 1997] which leads to an enhancement of dopamine transmission and may explain why amisulpride was found to be more effective than placebo and conventional antipsychotics for patients with predominantly negative symptoms [Leucht et al. 2002]. In a recent meta-analysis, Leucht and colleagues reported that amisulpride is more efficacious than conventional antipsychotics for overall efficacy [Leucht et al. 2009].
Antipsychotic medications have pronounced effects on hormone secretion which explain their endocrinologic side effects [Gründer et al. 1999]. Hyperprolactinemia is a commonly observed side effect of the conventional antipsychotics and some of the SGAs. These agents rely on their dopamine antagonistic properties to provide their antipsychotic effects. However, this also removes the brake on prolactin secretion, leading to hyperprolactinemia [Hummer and Huber, 2004]. Normal prolactin levels in women and men are below 530 mIU/L (25 ng/ml) and 424 mIU/L (20 ng/ml) respectively, with the more commonly used assays. Hyperprolactinemia is usually simply defined as a sustained level of prolactin above the laboratory upper level of normal [Peveler et al. 2008]. Marked prolactin excess, which is above 2120 mIU/L (100 ng/ml), is commonly associated with hypogonadism, galactorrhea and amenorrhea. Moderate prolactin excess, which is between 1000 and 1600 mIU/L (51 and 75 ng/ml), may be associated with oligomenorrhea. Mild prolactin excess, which is below 1000 mIU/L (50 ng/ml), is associated with decreased libido and infertility [Serri et al. 2003]. Two of the SGAs, risperidone and amisulpride, have a significant prolactin elevating property more like those of the traditional variety [Stanniland and Taylor, 2000]. Kopecek and colleagues stated that subjects who receive amisulpride doses as low as 50 mg/day have hyperprolactinemia in almost all cases that is significantly high (mean 113 ng/ml, 2400 mIU/L) and higher in females (160 ng/ml, 3400 mIU/L) than males (48ng/ml, 1000 mIU/L) [Kopecek et al. 2004]. Akkaya and colleagues reported hyperprolactinemia resulting in prolactin-secreting pituitary adenoma development with amisulpride treatment in three schizophrenic patients [Akkaya et al. 2009]. The alteration of growth hormone (GH) and thyroid-stimulating hormone (TSH) secretion by antipsychotics is less pronounced than the marked stimulation of prolactin release, and the effects of these changes on various body functions may be more subtle [Tuomisto and Männistö, 1985].
There is substantial evidence that certain SGAs are associated with clinically significant weight gain, increased risk for insulin resistance, hyperglycemia, type 2 diabetes mellitus and dyslipidemia compared with first-generation antipsychotics [Allison et al. 1999; Newcomer, 2005; Fleischhacker et al. 2008]. There are very few data available on metabolic effects of amisulpride. Recent reviews concluded that amisulpride is associated with minimal weight change, ranging between 0.2 and 1.4 kg over varying treatment durations [Russell and Mackell, 2001; Tschoner et al. 2007]. Amisulpride appears to have less risk of treatment-emergent dyslipidemia in comparison with olanzapine and clozapine [Newcomer, 2005; Rettenbacher et al. 2007]. In the study of Tschoner and colleagues, olanzapine and clozapine were found to constitute a high-risk group for metabolic dysregulation while amisulpride, quetiapine, risperidone and ziprasidone could be assigned to a non-high-risk group [Tschoner et al. 2009]. Subjects from the high-risk group displayed significant weight gain with concomitant increases in levels of insulin, total cholesterol, triglyceride (TG), low-density lipoprotein (LDL) and leptin. No significant changes were observed in the non-high-risk group.
Information on the cardiovascular safety and tolerance of antipsychotics is of significant clinical importance because antipsychotics can promote cardiac arrhythmias, which are anomalies implicated as a cause of sudden death among antipsychotic-treated patients [Ungvári, 1982; Brown and Kocsis, 1984; Dunne, 1994]. Rein and colleagues stated that electrocardiographic data of 341 patients in long- and short-term studies revealed no QTc prolongation with amisulpride [Rein et al. 2000]. In the study of Agelink and colleagues, the neuro-cardiac effects of four so-called atypical antipsychotics (amisulpride, olanzapine, clozapine and sertindole) were compared [Agelink et al. 2001]. An increase in mean resting heart rate was seen during treatment with all drugs but amisulpride. Electrocardiogram showed that all antipsychotics except for amisulpride tended to prolong mean QTc times, prolongation of which increases the risk of ventricular tachycardia. Although safe in regular doses, it is reported that amisulpride overdose is associated with QT prolongation and torsades de pointes [Lynch et al. 2008; Isbister et al. 2010].
In this study we aimed to determine endocrinologic, metabolic and cardiac effects of amisulpride, which is commonly used in our clinical practice.
Materials and methods
A total of 21 patients (12 males, 9 females) referred to the Psychiatry Department of Uludag University Medical Faculty were enrolled in the study and 18 of them (11 males, 7 females) completed the study. Patients were diagnosed with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [American Psychiatric Association, 2000]. All subjects gave written consent for the study which has full ethics approval by the local ethics committee.
Patients enrolled in the study were not on antipsychotic medication or had problems about efficacy or tolerability of their medication. Patients with any systemic or endocrinologic disorder, having a first-degree relative with cardiovascular disease or diabetes, alcohol/substance users, pregnant women, women using oral contraceptives and patients with severe abnormalities in blood tests and pretreatment hyperprolactinemia were excluded from the study. Patients on medication had a 10-day drug-free period if their clinical situation was suitable. Amisulpride was started as 200 mg/day and the dosage was increased to 400 mg/day on the day 3, to 600 mg/day on day 7 and to 800 mg/day at the end of week 2 of treatment. Patients were followed up for 24 weeks.
The patients were evaluated at baseline, week 2 and then every four weeks according to Brief Psychiatric Rating Scale (BPRS), Scale for the Assessment of Positive Symptoms (SAPS), Scale for the Assessment of Negative Symptoms (SANS), Simpson–Angus extrapyramidal side effects Scale (SAS) by the same psychiatrist experienced about scoring of the mentioned scales. Body mass index (BMI), electrocardiography, blood pressure, pulse rate and problems in sexual function were also evaluated in the same period. Blood samples were collected in the morning (08:00–10:00) after an overnight fast. Complete blood count, serum electrolyte assay, liver and renal function tests, thyroid function tests, serum lipid profile, lipoprotein a, apolipoprotein A1, apolipoprotein B1, leptin, adiponectin, sex hormones, adrenocorticotrophic hormone (ACTH), GH, cortisol, oral glucose tolerance test, insulin and HbA1c levels were determined at baseline, at week 12 and at week 24 of the treatment period. Prolactin levels were determined at baseline, day 4, day 7, week 2, week 3, week 4, week 6, week 8 and every four weeks afterwards.
All statistical analyses were performed with SPSS version 13.0. Paired data were analyzed using paired Student’s t-test and Wilcoxon signed rank test when data were not normally distributed. A p value <0.05 was considered as significant.
Results
Of the 21 patients enrolled, 18 completed the study. Two of the patients dropped out of the study due to lack of efficacy and one due to akathisia at the end of the first month. Ten patients were on antipsychotic medication and had a 10-day drug-free period before transferring to amisulpride. Patients received no drug other than amisulpride. All of the 18 patients tolerated the 800 mg/day dosage of amisulpride. Demographics and disease characteristics of the patients are given in Table 1. BPRS, SAPS and SANS scores differed significantly from baseline starting from week 2 and this difference became prominent at week 24 (Table 2). There was a significant increase in SAS score at week 4, but no significant difference was observed between baseline and the endpoint. At week 24 the mean SAS score was 0.08, which is less than 0.3, the upper limit of the normal range [Simpson and Angus, 1970] (Figure 1).
Table 1.
Baseline demographic and disease characteristics.
| Age (years) | 34.2 ± 9 |
| Duration of education (years) | 10.6 ± 3.9 |
| Sex (female/male) | 7 (39%) / 11 (61%) |
| Marital status (single/married) | 12 (67%) / 6 (33%) |
| Smokers/nonsmokers | 10 (56%) / 8 (44%) |
| Age onset of illness | 22.2 ± 4.4 |
| Duration of illness (years) | 11.9 ± 8.5 |
| Type of schizophrenia | |
| Paranoid | 14 (78%) |
| Disorganized | 2 (11%) |
| Undifferentiated | 2 (11%) |
Table 2.
BPRS total score, SAPS score and SANS score.
| Baseline | Week 2 | p * | Week 24 | p ** | |
|---|---|---|---|---|---|
| BPRS | 23.3 ± 8.8 | 18.2 ± 8.2 | <0.05 | 8.7 ± 6.1 | <0.05 |
| SAPS | 37.6 ± 22.5 | 25.2 ± 17.3 | <0.05 | 14.0 ± 17.0 | <0.05 |
| SANS | 52.2 ± 19.3 | 41.8 ± 21.6 | <0.05 | 22.2 ± 16.4 | <0.05 |
p value for baseline and week 2.
p value for baseline and week 24.
BPRS, Brief Psychiatric Rating Scale; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms.
Figure 1.
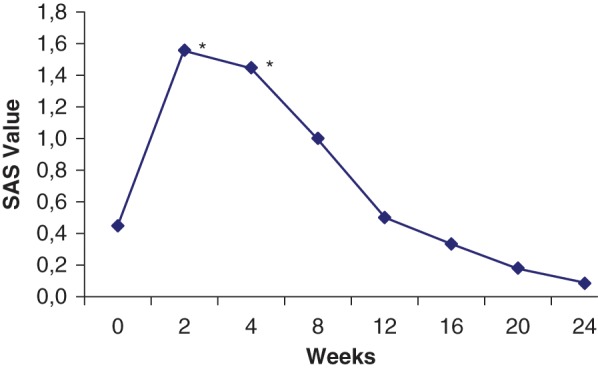
Simpson–Angus extrapyramidal side effects Scale (SAS) score change during the treatment period (*p < 0.05).
Prolactin levels increased significantly both in men and women starting from the measurement on day 4 of treatment. There was no significant difference between prolactin levels at weeks 2 and 24 (Figure 2). Prolactin elevation was significantly higher in women than in men starting from day 4. Baseline mean prolactin level was 30.2 ± 19.7 ng/ml for women and 21.3 ± 15.4 ng/ml for men. Prolactin levels increased up to 235.3 ± 68.7 ng/ml in women and 67.9 ± 21.3 ng/ml in men. Of the 18 patients one woman developed amenorrhea, two women developed menstrual irregularity, one women and one man developed decreased libido and anorgasmia. Sexual function could not be adequately evaluated in two of the patients with a disorganized type of schizophrenia.
Figure 2.
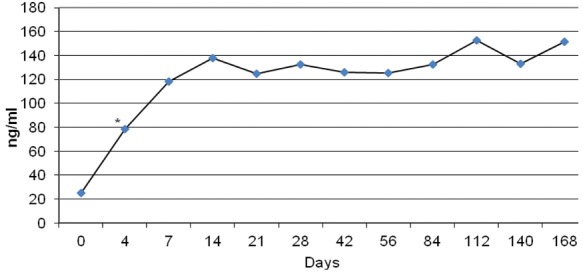
Prolactin level change during the treatment period (*p < 0.05 starting from day 4).
Figure 3.
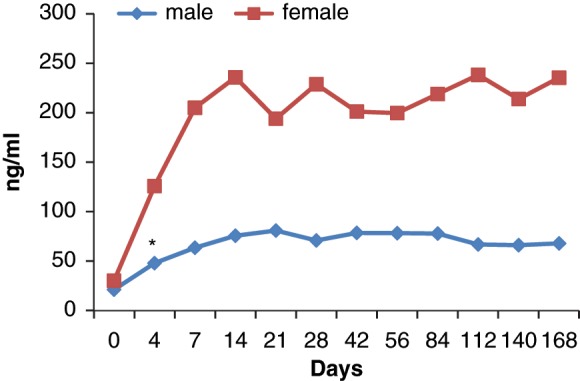
Prolactin level change during the treatment period in male and female patients (*p < 0.05).
There was no significant difference regarding BMI between the first and last visits. Amisulpride is associated with only a slight weight gain of approximately 0.8 kg within 24 weeks. Total cholesterol levels increased significantly compared with baseline (176.7 ± 35.8) at week 12 (206.8 ± 53.7) and week 24 (194.4 ± 47.6). LDL cholesterol levels increased significantly compared with baseline (106.4 ± 30.9) at week 12 (130.4 ± 43.8), but this significance did not continue up to week 24 (116.5 ± 39.7). No significant difference from baseline was determined regarding high-density lipoprotein (HDL) cholesterol, TG, apolipoprotein A1, apolipoprotein B1, lipoprotein a, leptin or adiponectin levels or atherogenic indices (total cholesterol / HDL cholesterol and LDL cholesterol / HDL cholesterol).
No significant difference from baseline was determined regarding thyroid function tests, sex hormones, ACTH, GH, cortisol, oral glucose tolerance test, insulin and HbA1c.
Except for prolactin and sex hormone levels, there was no significant difference between men and women when all of the other blood parameters were compared.
Electrocardiograms revealed no QT prolongation during the treatment period. Blood pressure and pulse rate measurements did not differ significantly from baseline.
Discussion
In this study investigating metabolic, endocrinologic and cardiac effects of amisulpride, it was found that amisulpride was an effective and safe drug except for the fact that it elevates prolactin levels markedly in both sexes.
The patients enrolled in the study were markedly symptomatic as shown by high BPRS, SAPS and SANS scores. All scale scores reduced significantly by week 2 of treatment and symptom improvement became prominent at week 24. This finding is in line with literature data suggesting that amisulpride is effective in the control of both positive and negative symptoms [Mortimer, 2009]. Early clinical response to amisulpride was observed at the second week of treatment in our study and this finding is in line with the meta-analysis of Agid and colleagues, who showed that a larger reduction of symptoms occurs during the first two weeks than during the second two weeks of amisulpride treatment [Agid et al. 2003]. Leucht and colleagues stated that clinical response to amisulpride showed the same time course pattern as that of the other antipsychotic drugs [Leucht et al. 2005].
The low incidence of extrapyramidal side effects assessed by SAS scores is in line with the findings of comparative trials [Carrière et al. 2000; Sechter et al. 2002; Mortimer et al. 2004]. It has been shown in animal studies [Schoemaker et al. 1997] and also in humans [Bressan et al. 2003] that amisulpride has selectivity for mesolimbic over striatal dopamine mechanisms. This selectivity probably explains why, similarly to other SGAs, amisulpride induces fewer extrapyramidal side effects.
Recently, much attention has been focused on the increased metabolic syndrome components among patients receiving antipsychotics, including weight gain, glucose intolerance, hyperglycemia, diabetes mellitus, hyperlipidemia and hypertension [Kabinoff et al. 2003]. In our study, mean values for BMI did not differ between baseline and endpoint. Amisulpride is associated with only a slight weight gain of approximately 0.8 kg within 24 weeks. This is comparable with the data of Leucht and colleagues who stated that mean weight gain with amisulpride (doses above 400 mg/day) is 1.27 kg in 6 months [Leucht et al. 2004]. Amisulpride is associated with little effect on weight gain in a recent meta-analysis [Leucht et al. 2009]. A review by Taylor and McAskill ranking atypical antipsychotic drugs according to their associated risk of weight gain, recorded the lowest risk with amisulpride [Taylor and McAskill, 2000].
There are very few data available on the other metabolic effects of amisulpride. Consensus guidelines, published in Belgium, on metabolic problems with atypical antipsychotics recommended that atypical antipsychotics with the lowest risk profile (amisulpride, aripiprazole and ziprasidone) be preferred, particularly in patients with other identified risk factors for metabolic complications [De Hert et al. 2006]. Peuskens and colleagues reported lower risk of weight gain and hyperglycemia associated with amisulpride treatment compared with olanzapine [Peuskens et al. 2007]. Supporting these findings, there were not any significant differences between serum levels of fasting and satiety glucose, insulin and HbA1c at baseline and week 24 in our study. Although increase in total cholesterol level became significant at the end of the study, LDL cholesterol, HDL cholesterol, atherogenic indices (total cholesterol / HDL cholesterol and LDL cholesterol / HDL cholesterol), TG, apolipoprotein A1, apolipoprotein B1, lipoprotein a, leptin and adiponectin levels did not differ between baseline and week 24 suggesting that amisulpride does not induce dyslipidemia.
Amisulpride is probably the antipsychotic with the most potential for maximum prolactin elevation. The Halifax data found 100% hyperprolactinemia with amisulpride [Bushe and Shaw, 2007]. According to prolactin data from the cohort of 178 patients receiving antipsychotic drugs, hyperprolactinemia was measured in 33.1% and was associated with all antipsychotics except clozapine. The highest prevalence rate was found in amisulpride (n = 20) 89% [Bushe et al. 2008]. A Greek cohort (n = 17) also found 100% prevalence of hyperprolactinemia for patients receiving amisulpride [Paparrigopoulos et al. 2007]. In a 6-week study with 433 female inpatients with mainly schizophrenic disorders, it was reported that amisulpride and risperidone increased prolactin significantly in 100% of patients, as early as after the first week of therapy [Svestka et al. 2007]. Many if not all of the data are of too short a duration to make definitive statements regarding the persistence of hyperprolactinemia during treatment with antipsychotics [Bushe et al. 2008]. However, there is a 5-year naturalistic study of risperidone that suggests that prolactin levels may decrease over time [Eberhard et al. 2007]. In the present study we observed a significant increase in prolactin levels both in women and men in concordance with the literature data. As shown in earlier studies [Kuruvilla et al. 1992; Melkersson et al. 2001; Jung et al. 2006; Bushe and Shaw, 2007], prolactin levels of women were significantly higher than those of men in our study. Prolactin levels continued to stay elevated over 24 weeks of treatment. Despite high prolactin levels, we observed few clinical symptoms associated with hyperprolactinemia. This raised the question whether these high levels of prolactin physiologically active or not. Hattori and Diver and colleagues stated that women who suffered from hyperprolactinemia consisting mainly of the trimeric form of prolactin (macroprolactin) neither showed any clinical symptoms nor suffered from reproductive dysregulation, despite elevated prolactin concentrations [Hattori, 1996; Diver et al. 2001]. It is thought that these polymeric forms of prolactin can be detected by the current prolactin assays [Biller et al. 1999], but that they are not necessarily physiologically active. We certainly cannot suggest that the high levels of prolactin in our study were due to macroprolactin as we did not measure the levels of macroprolactin.
There are few data about other endocrinologic effects of amisulpride in literature. Wetzel and colleagues reported that amisulpride elevated plasma TSH levels but did not affect LH and cortisol levels [Wetzel et al. 1994]. Gründer and colleagues stated that amisulpride treatment elevated TSH levels in both male and female patients and GH levels only in females [Gründer et al. 1999]. In our study, we assessed TSH, free T3, free T4, GH, ACTH, cortisol and sex hormones and found no significant difference in their levels with amisulpride treatment.
We found no QT prolongation or any other abnormality in electrocardiograms of patients during amisulpride treatment consistent with the findings of Rein and colleagues [Rein et al. 2000].
Our study is limited since it was an open-label study with a small sample size and did not have a control group. However, we think that the present study will contribute to the literature as there is only a very limited number of studies investigating endocrinologic, metabolic and cardiac effects of amisulpride.
In conclusion, the clinical data from the present study supports the fact that amisulpride indicates several advantages for long-term use. The results of long-term clinical trials concur in demonstrating its efficacy against both positive and negative symptoms of schizophrenia. Amisulpride has a relatively low propensity to induce EPS compared with conventional antipsychotics and is associated with a lower risk of metabolic syndrome and cardiac dysfunction than some of other SGAs. The principle side effects appear to be associated with hyperprolactinemia with much higher prolactin levels in women. However, these side effects were subtle in our patient group. To the best of the authors’ knowledge, this study is the first to investigate many metabolic, endocrinologic and cardiac effects of amisulpride together in a 24-week follow-up period. Future studies with larger samples will help us to understand clinical and biochemical aspects of this unique molecule further.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest related to this study.
References
- Agelink M.W., Majewski T., Wurthmann C., Lukas K., Ullrich H., Linka T., et al. (2001) Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol 21: 8–13 [DOI] [PubMed] [Google Scholar]
- Agid O., Kapur S., Arenovich T., Zipursky R.B. (2003) Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 60: 1228–1235 [DOI] [PubMed] [Google Scholar]
- Akkaya C., Kaya B., Kotan Z., Sarandol A., Ersoy C., Kirli S. (2009) Hyperprolactinemia and possibly related development of prolactinoma during amisulpride treatment; three cases. J Psychopharmacol 23: 723–726 [DOI] [PubMed] [Google Scholar]
- Allison D.B., Mentore J.L., Heo M., Chandler L.P., Cappelleri J.C., Infante M.C., et al. (1999) Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 156: 1686–1696 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (4th edn). Washington, DC: American Psychiatric Association [Google Scholar]
- Biller B.M., Luciano A., Crosignani P.G., Molitch M., Olive D., Rebar R., et al. (1999) Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med 44: 1075–1084 [PubMed] [Google Scholar]
- Bressan R.A., Erlandsson K., Jones H.M., Mulligan R., Flanagan R.J., Ell P.J., et al. (2003) Is regionally selective D2/D3 dopamine occupancy sufficient for atypical antipsychotic effect? An in vivo quantitative [123I]epidepride SPET study of amisulpride-treated patients. Am J Psychiatry 160: 1413–1420 [DOI] [PubMed] [Google Scholar]
- Brown R.P., Kocsis J.H. (1984) Sudden death and antipsychotic drugs. Hosp Community Psychiatry 35: 486–491 [DOI] [PubMed] [Google Scholar]
- Burns T., Bale R. (2001) Clinical advantages of amisulpride in the treatment of acute schizophrenia. J Int Med Res 29: 451–466 [DOI] [PubMed] [Google Scholar]
- Bushe C., Shaw M. (2007) Prevalence of hyperprolactinaemia in a naturalistic cohort of schizophrenia and bipolar outpatients during treatment with typical and atypical antipsychotics. J Psychopharmacol 21: 768–773 [DOI] [PubMed] [Google Scholar]
- Bushe C., Shaw M., Peveler R.C. (2008) A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol 22: 46–55 [DOI] [PubMed] [Google Scholar]
- Carrière P., Bonhomme D., Lempérière T. (2000) Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study (the Amisulpride Study Group). Eur Psychiatry 15: 321–329 [DOI] [PubMed] [Google Scholar]
- De Hert M., van Eyck D., De Nayer A. (2006) Metabolic abnormalities associated with second generation antipsychotics: fact or fiction? Development of guidelines for screening and monitoring. Int Clin Psychopharmacol 21: 11–15 [DOI] [PubMed] [Google Scholar]
- Diver M.J., Ewins D.L., Worth R.C., Bowles S., Ahlquist J.A., Fahie-Wilson M.N. (2001) An unusual form of big, big (macro) prolactin in a pregnant patient. Clin Chem 47: 346–348 [PubMed] [Google Scholar]
- Dunne F. (1994) Neuroleptics and the heart. Br J Cardiol 1: 364–369 [Google Scholar]
- Eberhard J., Lindström E., Holstad M., Levander S. (2007) Prolactin level during 5 years of risperidone treatment in patients with psychotic disorders. Acta Psychiatr Scand 115: 268–276 [DOI] [PubMed] [Google Scholar]
- Fleischhacker W.W., Cetkovich-Bakmas M., De Hert M., Hennekens C.H., Lambert M., et al. (2008) Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry 69: 514–519 [DOI] [PubMed] [Google Scholar]
- Gründer G., Wetzel H., Schlösser R., Anghelescu I., Hillert A., Lange K., et al. (1999) Neuroendocrine response to antipsychotics: effects of drug type and gender. Biol Psychiatry 45: 89–97 [DOI] [PubMed] [Google Scholar]
- Hattori N. (1996) The frequency of macroprolactinemia in pregnant women and the heterogeneity of its etiologies. J Clin Endocrinol Metab 81: 586–590 [DOI] [PubMed] [Google Scholar]
- Hummer M., Huber J. (2004) Hyperprolactinaemia and antipsychotic therapy in schizophrenia. Curr Med Res Opin 20: 189–197 [DOI] [PubMed] [Google Scholar]
- Isbister G.K., Balit C.R., Macleod D., Duffull S.B. (2010) Amisulpride overdose is frequently associated with QT prolongation and torsades de pointes. J Clin Psychopharmacol 30: 391–395 [DOI] [PubMed] [Google Scholar]
- Jung D.U., Conley R.R., Kelly D.L., Kim D.W., Yoon S.H., Jang J.H., et al. (2006) Prevalence of bone mineral density loss in Korean patients with schizophrenia: a cross-sectional study. J Clin Psychiatry 67: 1391–1396 [DOI] [PubMed] [Google Scholar]
- Kabinoff G.S., Toalson P.A., Healey K.M., McGuire H.C., Hay D.P. (2003) Metabolic issues with atypical antipsychotics in primary care: dispelling the myths. Prim Care Companion J Clin Psychiatry 5: 6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecek M., Bears M., Svarc J., Dockery C., Horacek J. (2004) Hyperprolactinemia after low dose of amisulpride Neuro Endocrinol Lett 25: 419–422 [PubMed] [Google Scholar]
- Kuruvilla A., Peedicayil J., Srikrishna G., Kuruvilla K., Kanagasabapathy A.S. (1992) A study of serum prolactin levels in schizophrenia: comparison of males and females. Clin Exp Pharmacol Physiol 19: 603–606 [DOI] [PubMed] [Google Scholar]
- Leucht S., Busch R., Hamann J., Kissling W., Kane J.M. (2005) Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 57: 1543–1549 [DOI] [PubMed] [Google Scholar]
- Leucht S., Corves C., Arbter D., Engel R.R., Li C., Davis J.M. (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373: 31–41 [DOI] [PubMed] [Google Scholar]
- Leucht S., Pitschel-Walz G., Engel R.R., Kissling W. (2002) Amisulpride, an unusual “atypical” antipsychotic: a meta-analysis of randomized controlled trials. Am J Psychiatry 159: 180–190 [DOI] [PubMed] [Google Scholar]
- Leucht S., Wagenpfeil S., Hamann J., Kissling W. (2004) Amisulpride is an “atypical” antipsychotic associated with low weight gain. Psychopharmacology (Berl) 173: 112–115 [DOI] [PubMed] [Google Scholar]
- Lynch M.J., Woods J., George N., Gerostamoulos D. (2008) Fatality due to amisulpride toxicity: a case report. Med Sci Law 48: 173–177 [DOI] [PubMed] [Google Scholar]
- Melkersson K.I., Hulting A.L., Rane A.J. (2001) Dose requirement and prolactin elevation of antipsychotics in male and female patients with schizophrenia or related psychoses. Br J Clin Pharmacol 51: 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller H.J. (2000) Amisulpride: a review of its efficacy in schizophrenia. Acta Psychiatr Scand Suppl 400: 17–22 [PubMed] [Google Scholar]
- Mortimer A., Martin S., Lôo H., Peuskens J. for the SOLIANOL Study Group (2004) A double-blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. Int Clin Psychopharmacol 19: 63–69 [DOI] [PubMed] [Google Scholar]
- Mortimer A.M. (2003) Antipsychotic treatment in schizophrenia: atypical options and NICE guidance. Eur Psychiatry 18: 209–219 [DOI] [PubMed] [Google Scholar]
- Mortimer A.M. (2004) Novel antipsychotics in schizophrenia. Expert Opin Investig Drugs 13: 315–329 [DOI] [PubMed] [Google Scholar]
- Mortimer A.M. (2009) Update on the management of symptoms in schizophrenia: focus on amisulpride. Neuropsychiatr Dis Treat 5: 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer J.W. (2005) Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19: 1–93 [DOI] [PubMed] [Google Scholar]
- Paparrigopoulos T., Liappas J., Tzavellas E., Mourikis I., Soldatos C. (2007) Amisulpride-induced hyperprolactinemia is reversible following discontinuation. Prog Neuropsychopharmacol Biol Psychiatry 31: 92–96 [DOI] [PubMed] [Google Scholar]
- Peuskens J., De Hert M., Mortimer A. for the SOLIANOL Study Group (2007) Metabolic control in patients with schizophrenia treated with amisulpride or olanzapine. Int Clin Psychopharmacol 22: 145–152 [DOI] [PubMed] [Google Scholar]
- Peveler R.C., Branford D., Citrome L., Fitzgerald P., Harvey P.W., Holt R.I., et al. (2008) Antipsychotics and hyperprolactinaemia: clinical recommendations. J Psychopharmacol 22: 98–103 [DOI] [PubMed] [Google Scholar]
- Rein W., Coulouvrat C., Dondey-Nouvel L. (2000) Safety profile of amisulpride in short- and long-term use. Acta Psychiatr Scand Suppl 400: 23–27 [DOI] [PubMed] [Google Scholar]
- Rettenbacher M.A., Hummer M., Hofer A., Baumgartner S., Ebenbichler C., Edlinger M., et al. (2007) Alterations of glucose metabolism during treatment with clozapine or amisulpride: results from a prospective 16-week study. J Psychopharmacol 21: 400–404 [DOI] [PubMed] [Google Scholar]
- Russell J.M., Mackell J.A. (2001) Bodyweight gain associated with atypical antipsychotics: epidemiology and therapeutic implications. CNS Drugs 15: 537–551 [DOI] [PubMed] [Google Scholar]
- Scatton B., Claustre Y., Cudennec A., Oblin A., Perrault G., Sanger D.J., et al. (1997) Amisulpride: from animal pharmacology to therapeutic action. Int Clin Psychopharmacol 2: 29–36 [DOI] [PubMed] [Google Scholar]
- Schoemaker H., Claustre Y., Fage D., Rouquier L., Chergui K., Curet O., et al. (1997) Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. J Pharmacol Exp Ther 280: 83–97 [PubMed] [Google Scholar]
- Sechter D., Peuskens J., Fleurot O., Rein W., Lecrubier Y. for the Amisulpride Study Group (2002) Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology 27: 1071–1081 [DOI] [PubMed] [Google Scholar]
- Serri O., Chik C.L., Ur E., Ezzat S. (2003) Diagnosis and management of hyperprolactinemia. CMAJ 169: 575–581 [PMC free article] [PubMed] [Google Scholar]
- Simpson G.M., Angus J.W. (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11–19 [DOI] [PubMed] [Google Scholar]
- Stanniland C., Taylor D. (2000) Tolerability of atypical antipsychotics. Drug Saf 22: 195–214 [DOI] [PubMed] [Google Scholar]
- Svestka J., Synek O., Tomanová J., Rodáková I., Cejpková A. (2007) Differences in the effect of second-generation antipsychotics on prolactinaemia: six weeks open-label trial in female in-patients. Neuro Endocrinol Lett 28: 881–888 [PubMed] [Google Scholar]
- Taylor D.M., McAskill R. (2000) Atypical antipsychotics and weight gain—a systematic review. Acta Psychiatr Scand 101: 416–432 [DOI] [PubMed] [Google Scholar]
- Tschoner A., Engl J., Laimer M., Kaser S., Rettenbacher M., Fleischhacker W.W., et al. (2007) Metabolic side effects of antipsychotic medication. Int J Clin Pract 61: 1356–1370 [DOI] [PubMed] [Google Scholar]
- Tschoner A., Engl J., Rettenbacher M., Edlinger M., Kaser S., Tatarczyk T., et al. (2009) Effects of six second generation antipsychotics on body weight and metabolism - risk assessment and results from a prospective study. Pharmacopsychiatry 42: 29–34 [DOI] [PubMed] [Google Scholar]
- Tuomisto J., Männistö P. (1985) Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev 37: 249–332 [PubMed] [Google Scholar]
- Ungvári G. (1982) Neuroleptic treatment and unexpected death. Fortschr Neurol Psychiatr 50: 267–273 [PubMed] [Google Scholar]
- Wetzel H., Wiesner J., Hiemke C., Benkert O. (1994) Acute antagonism of dopamine D2-like receptors by amisulpride: effects on hormone secretion in healthy volunteers. J Psychiatr Res 28: 461–473 [DOI] [PubMed] [Google Scholar]


