Abstract
Objective
The mode of influence of the aromas of plant essential oils on human behaviour is largely unclear. This study was designed to assess the potential pharmacological relationships between absorbed 1,8-cineole following exposure to rosemary aroma, cognitive performance and mood.
Methods
Twenty healthy volunteers performed serial subtraction and visual information processing tasks in a cubicle diffused with the aroma of rosemary. Mood assessments were made pre and post testing, and venous blood was sampled at the end of the session. Pearson correlations were carried out between serum levels of 1,8-cineole, cognitive performance measures and change in mood scores.
Results
Here we show for the first time that performance on cognitive tasks is significantly related to concentration of absorbed 1,8-cineole following exposure to rosemary aroma, with improved performance at higher concentrations. Furthermore, these effects were found for speed and accuracy outcomes, indicating that the relationship is not describing a speed–accuracy trade off. The relationships between 1,8-cineole levels and mood were less pronounced, but did reveal a significant negative correlation between change in contentment and plasma 1,8-cineole levels.
Conclusion
These findings suggest that compounds absorbed from rosemary aroma affect cognition and subjective state independently through different neurochemical pathways.
Keywords: aroma, cholinergic, cineole, cognition, essential oil, mood, rosemary
Introduction
Putative effects of aromas on aspects of human behaviour can be traced back to ancient Greece, where the extracts of aromatic plants were used for cosmetic, religious and medical purposes. Today the popularity of aromas for pleasure, relaxation and in therapeutics is unabated and typified in the ever popular application of aromatherapy [Tisserand, 1993]. The essential oils used in aromatherapy are extracted from natural sources such as plant leaves, fruits, roots and barks. The unique relationships between plant essential oil aromas and any behavioural impact are potentially due to the complex molecular composition containing a range of alcohols, aldehydes, acids, phenols, esters, ketones and terpenes [Hopkins, 1996].
A small, but growing body of research has been carried out to investigate the possible influence of the aromas of essential oils on cognition and mood in the healthy population – see Herz for a review [Hertz, 2009]. Diego and colleagues found subjective mood and objective electroencephalogram (EEG) effects for lavender and rosemary as were predicted based on the aromas’ reputed properties [Diego et al. 1998]. However, whilst both aromas improved the speed of maths computations, only lavender increased accuracy. Moss and colleagues reported differential effects of lavender and rosemary on aspects of cognition, particularly working memory, but also that rosemary aroma led to an improvement in long-term memory compared with controls [Moss et al. 2003]. The potential for equivalence of the impact of herbal supplementation and aroma exposure was investigated by Moss and colleagues [Moss et al. 2010]. The authors report largely consistent effects for Salvia officinalis aroma but not Salvia lavandulaefolia aroma compared with the effects of oral administration of extracts of these herbs as detailed by Scholey and colleagues [Scholey et al. 2008].
Consideration of the mechanisms by which essential oil aromas might impact upon behaviour provides a number of possibilities. Given that the properties of aromas are to a great extent defined by folk wisdom rather than scientific evaluation, expectancy might be a reasonable candidate or at least a confounding variable worthy of addressing. Indeed, Moss and colleagues found a complex pattern of relationships between induced expectancies and aroma effects when investigating the influence of chamomile aroma on cognition and mood [Moss et al. 2006]. Their findings support to some extent those previously identified elsewhere for the impact of expectancy on physiological measures [Campenni et al. 2004], and of priming on relaxation effects under aroma conditions [Howard and Hughes, 2008]. Indeed the latter argue that expectancies and not aroma is the major factor underpinning observed psychophysiological effects. However, Wartik used EEG recording and reported that jasmine produced increased alpha-power in the frontal cortices, indicative of increased arousal and unlikely to be as a result of expectancy [Wartik, 1995]. Furthermore peppermint aroma seems capable of reliably producing small EEG and electromyogram or muscular conductance fluctuations during rapid eye movement and nonrapid eye movement sleep [Badia et al. 1990]. The authors suggest that such findings rule out the possible effects of expectancy.
A second potential mode of influence of aromas is the hedonic valence mechanism that describes the relationship between the pleasantness of an aroma, the associated effect on mood and the consequential impact on behaviour/performance [Baron and Bronfen, 1994]. In support of the proposition, Degel and Köster discuss data that run counter to predictions based on received wisdom, namely, the authors report improved mathematical performance for exposure to the ‘sedating’ aroma of lavender compared with the ‘stimulating’ aroma of jasmine [Degel and Köster, 1999]. By considering participants’ ratings of pleasantness for the two aromas, Degel and Köster identify that the more pleasant lavender was associated with better performance. However, the evidence in support of the hedonic valence mechanism can be difficult to disentangle from other possible explanations based on physiological processes. For example, Degel and Köster go on to consider how the improved performance could be equally well explained by the sedating effect of lavender reducing arousal in a stressful environment, and so improving performance in accordance with the Yerkes–Dodson law.
The mechanism of interest in the current study, and potentially more valuable regarding the usefulness of aroma as an intervention is the pharmacological mechanism outlined by Jellinek [Jellinek, 1997]. This describes how constituents of the essential oils may influence behaviour through the central nervous or endocrine systems. Volatile compounds (e.g. terpenes) may enter the blood stream by way of the nasal or lung mucosa. Terpenes are small organic molecules which can easily cross the blood–brain barrier and therefore may have direct effects in the brain by acting on receptor sites or enzyme systems. Such compounds have been detected in the blood of rodents exposed to the vapours of essentials oils. Jirovetz and colleagues demonstrated that serum levels of linalool and linalyl acetate in rats following lavender exposure were related to observed sedation [Jirovetz et al. 1990]. Kovar and colleagues assayed serum levels of 1,8-cineole, and recorded locomotor activity when rosemary oil was administered by inhalation or orally [Kovar et al. 1987]. The data showed that both the inhalation and oral administration of rosemary oil stimulated locomotor activity and that this was related to serum 1,8-cineole concentration. These findings cannot be explained by expectancy theory and suggest that more than simple stimulation of the olfactory organ is involved, with a direct pharmacological action on the central nervous system being implicated.
In vitro neuropharmacological research has also provided some interesting data that are pertinent here. Orhan and colleagues reported that extracts of rosemary displayed significant inhibitory effects on both acetylcholinesterase (AChE) and butyrylcholinesterase enzymes [Orhan et al. 2008]. The authors go on to identify that the major active component of the essential oil is 1,8-cineole, a terpene that has previously been identified as possessing anti- AChE activity [Perry et al. 2000, 2003; Savelev et al. 2003]. Such activity is suggestive of potential cognitive impact and indeed underpins the pharmacological activity of a number of dementia treatments [Orhan et al. 2008]. However, it should be stressed that any activity might be a consequence of the synergistic combinations of components present rather than a single compound. The possibility of such a pharmacological mechanism for rosemary aroma would provide supporting evidence to the concept that each individual aroma of an essential oil has its own unique pattern of influence on both cognition and mood as a result of the unique composition of volatile aromatic compounds. To investigate this further, the current study assayed serum for 1,8-cineole and correlated this with cognitive performance under conditions of rosemary aroma exposure.
Method
Design
The study used a correlational design to investigate a possible relationship between the plasma 1,8-cineole levels and cognitive performance and mood. Treatment was by way of exposure to the aroma of rosemary essential oil. Participants were randomly assigned to be exposed to the aroma in the cubicle for 4, 6, 8 or 10 min prior to completing the cognitive tests. This period was not kept constant so as to facilitate a range of levels of absorption of compounds to take place across the participant group. Total pre-test time was held constant across participants by asking them to wait outside the cubicle for 6, 4, 2 or 0 min dependent on their assigned exposure condition. The time spent completing the tasks did not differ between participants. All participants were blind as to the nature of the study, being informed that the study was investigating the relationship between mood and cognitive performance. On the occasions that participants commented on the aroma the experimenter explained that the aroma had ‘nothing to do with me’ and that it was ‘left over from a previous study’. When asked at the end of testing and prior to debriefing, none of the participants indicated that they felt that the aroma had affected them in any way. To assess any relationship between ‘pleasantness’ of the aroma (i.e. hedonic valence) and performance measures, subjective ratings were obtained from each participant at the end of testing as in previous studies [e.g. Moss et al. 2010].
Participants
Twenty healthy volunteers [12 women, mean age 23.2 years, standard deviation (SD) 3.2 and 8 men, mean age 22.6 years, SD 2.9] took part in the study. All volunteers completed a health screening questionnaire prior to participation. None were excluded from the study.
Testing cubicles
The testing cubicle measured 2.4 m long × 1.8 m wide × 2.4 m high and was maintained at a temperature of between 18°C and 22°C throughout the testing sessions. The door was kept closed except for participant access.
Aromas
‘Tisserand’ pure essential oil (Tisserand Aromatherapy, Newtown Road, Hove, Sussex, UK) of rosemary was used to produce the ambient aroma. Four drops of the oil were applied to a diffuser pad for a ‘Tisserand aroma stream’. The aroma stream was placed under the bench in the testing cubicle and was switched on for 5 min prior to the introduction of each participant.
Cognitive measures
Serial threes subtraction task
A starting number between 800 and 999 was displayed on the computer screen. The participant was asked to subtract three from this number and enter their answer using the key pad; they were then required to subtract three from this answer and enter it likewise. They were instructed to continue in the same way until the programme stopped after 2 min.
Serial sevens subtraction task
A starting number between 800 and 999 was displayed on the computer screen. The participant was asked to subtract seven from this number and enter their answer using the keypad, they were then required to subtract seven from this answer and enter it likewise. They were instructed to continue in the same way until the programme stopped after 2 min.
Rapid visual information processing task
Participants were presented with a continuous series of digits in the centre of the screen and they were asked to detect sequences of any three consecutive odd digits or any three consecutive even digits by pressing the space bar. The task stopped automatically after 3 min.
All tasks were drawn from the Computerized Mental Performance Assessment System battery developed by the Brain Performance and Nutrition Research Centre at Northumbria University. The tasks were selected for a number of reasons. Firstly they tap into a number of cognitive domains: working memory, executive function and sustained attention, and represent functions involved in a wide range of real world activities [Ritter et al. 2007]. Secondly these tasks have previously been demonstrated to be sensitive to the impact of herbal and dietary based interventions [e.g. Reay et al. 2006; Scholey et al. 2010], and they have been identified as possessing different degrees of cognitive demand based on participants’ self reports [Scholey et al. 2001; Scholey and Kennedy, 2002]. This was considered potentially important as some interventions have been shown to differentially affect task performance based on cognitive load [e.g. Kennedy et al. 2002].
Subjective mood measures
Mood was assessed using the Bond-Lader visual analogue scales [Bond and Lader, 1974]. The scales were developed for use in medical psychology and psychopharmacology research, with the original paper cited in more than 1000 peer reviewed publications including clinical trials worldwide. A critical review has described them as ‘A simple technique for measuring subjective experience. They have been established as valid and reliable in a range of clinical and research applications’ [McCormack et al. 1988, p. 1007]. The Bond–Lader mood scales have also been used widely in aroma and other herbal intervention studies where they have been demonstrated to be sensitive to changes in subjective state [e.g. Moss et al. 2003, 2006, 2008, 2010; Scholey et al. 2010; Kennedy et al. 2002]. The 16 visual analogue scales of the Bond-Lader assessment were combined as recommended by the authors to form three mood factors: ‘alert’, ‘calm’ and ‘content’.
Procedure
Ethical clearance was granted by the School of Psychology and Sport Sciences Ethics Committee. Recruitment took place 1 week prior to testing and at this point participants were told the aims of the study and given a time to attend the laboratory. All testing took place in the same cubicle between 9:00 am and 12:00 noon. Participants were asked to read a brief for the study and instructions for task completion prior to providing informed consent. A pre-test mood scale was completed prior to entering the aroma-infused cubicle. The cognitive tasks were completed followed by a second (post-test) mood scale. Finally, a sample of venous blood was taken by a trained phlebotomist. The participants were then debriefed, thanked for their participation and any questions answered.
Blood sampling and analysis
One 5 ml blood sample was taken per participant, by venous puncture into a serum gel monovette. All samples were immediately centrifuged at 3000g for 10 min at 20°C using an Allegra X-22 centrifuge (Beckman Coulter Ltd, High Wycombe, UK). The serum was then decanted into a 1.5 ml microtube and stored in a freezer at −80°C until all the samples were ready for analysis.
Standards
Terpinolene was used as an internal standard in the serum samples; it was added at a concentration of 2 ppm to all samples. A calibration set was also made up using standard 1,8-cineole and terpinolene at similar concentrations.
Extraction
The sample (0.9 ml) containing 2 ppm internal standard was loaded onto a 2.5 ml C8 solid phase extraction cartridge that had been activated using methanol and subsequently washed with distilled water. The sample was allowed to flow through at a slow rate to maximize residence time. After all of the sample had passed through the column it was washed with distilled water. Elution of adsorbed substances was achieved by washing through with 1 ml methanol. The eluted sample was placed in a gas chromatography sample vial ready for analysis.
Analysis
Analysis was carried out using a Thermo gas chromatograph fitted with a DSQ mass spectral detector (Thermo Fisher Scientific, Austin,Texas, USA) operating in single ion monitoring mode with splitless injection to maximize sensitivity (the principal ions had been determined in full scan mode with the prepared standards). The initial column temperature was 40°C, rising at a ramp rate of 5°C per min to 110°C followed by a second ramp of 20°C per min to 300°C.
Results
1,8-Cineole assay
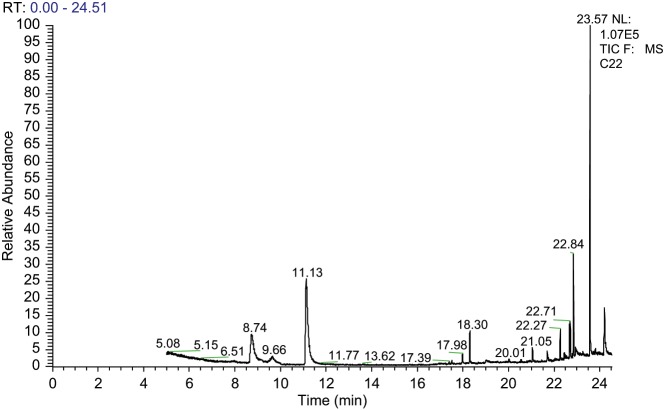
Quantification was achieved by comparing the peak area for 1,8-cineole with that of the internal standard and relating this to the concentration of the internal standard after applying a correction factor for the relative response factors of 1,8-cineole and the internal standard. The retention time for 1,8-cineole was 9.66 min and that of the internal standard, terpinolene, was 11.13 min (Figure 1). The desired graduation in serum 1,8-cineole concentration was achieved across the participant sample as evidenced by the significant correlation between pre-test exposure time and serum 1,8-cineole levels, r(18) = 0.727, p < 0.001.
Figure 1.
Chromatogram for a 5 ppm concentration standard. 1,8-Cineole comes out at 9.62 min and the internal standard, terpinolene, at 11.12 min.
Data were analysed using SPSS V.16. Pearson correlations were performed to determine the degree of relationship between plasma 1,8-cineole and the behavioural variables (cognition and mood).
Cognitive performance measures
Serial threes subtraction task
A positive linear relationship was found between serum 1,8-cineole concentration and the number of correct answers on the serial threes subtraction task: r(18) = 0.469, p = 0.037; r 2 = 0.22.
A negative linear relationship was found between the reaction time of participants on the serial threes subtraction task and the serum 1,8-cineole concentration: r(18) = −0.502, p = 0.024; r 2 = 0.25.
Serial sevens subtraction task
A positive linear relationship that approached but did not quite reach statistical significance was found between the number of correct answers on the serial sevens subtraction task and serum 1,8-cineole concentration: r(18) = 0.433, p = 0.056; r2 = 0.19.
A negative linear relationship was found between the reaction time of participants on the serial sevens subtraction task and serum 1,8-cineole concentration: r(18) = −0.466, p = 0.038; r 2 = 0.22.
Rapid visual information processing
No relationship was found between the number of correct responses on the rapid visual information processing (RVIP) task and serum 1,8-cineole concentration: r(18) = 0.117, p = 0.624; r 2 = 0.01.
A negative linear relationship was found between reaction time on the RVIP task and serum concentration of 1,8-cineole: r(18) = −0.446, p = 0.049; r 2 = 0.19.
Mood measures
To calculate the change in the three mood variables, pre-test values were subtracted from post-test values.
Alertness
No relationship was found between serum concentration of 1,8-cineole and pre to post change in alertness: r(18) = 0.069, p = 0.773; r 2 = 0.01.
Contentedness
A negative linear relationship was found between 1,8-cineole concentration and the change in contentedness: r(18) = −0.454, p = 0.044; r 2 = 0.21.
Calmness
No relationship was found between serum 1,8-cineole concentration and the change in calmness: r(18) = −0.268, p = 0.253; r 2 = 0.07.
Hedonic valence
No relationship was found between self-reported pleasantness of the aroma and any of the performance variables, p > 0.05 in each case.
Discussion
The results reported here support the proposal that 1,8-cineole would be detectable in the blood serum of healthy human volunteers following inhalation of the aroma of rosemary essential oil. Previously only demonstrated in animals [Jirovetz et al. 1990; Kovar et al. 1987], this study supports the suggestion that active compounds present in aromas may be absorbed through the nasal or lung mucosa and thus provide the potential for pharmacological activity as outlined by Jellinek [Jellinek, 1997]. The small size of these lipid soluble compounds facilitates passage across the blood–brain barrier [Boyle et al. 2005] and consequently they may produce effects at the neuronal level by either acting directly on receptor sites, or indirectly by impacting on enzyme activity. 1,8-Cineole is one of a number of volatile organic compounds present in the essential oil of rosemary. Typically between 35% and 45% by volume of rosemary essential oil, 1,8-cineole may possess direct pharmacological properties [Perry et al. 2000, 2003] or may serve as a suitable marker for the absorption of highly active compounds such as rosmarinic acid and ursolic acid that are present at much lower concentrations in rosemary essential oil. Orhan and colleagues [Orhan et al. 2008] reported powerful AChE and butyrylcholinesterase inhibition by rosmarinic acid extracted from rosemary essential oil, which as a whole was found to possess moderate inhibition of AChE in keeping with previous data [Perry et al. 1996]. Similarly ursolic acid is a potent inhibitor of AChE [Chung et al. 2001], which is found in rosemary essential oil [Huang et al. 1994]. Taken together these findings strongly support the contention that inhalation of rosemary essential oil would possess the potential to impact on cognitive performance in a positive manner by preventing the breakdown of acetylcholine and so perpetuating cholinergic stimulation. One further important consideration that should be taken into account here is the evidence provided by Savelev and colleagues that demonstrates the presence of synergistic and antagonistic relationships between compounds with AChE properties extracted from Salvia species [Savelev et al. 2003]. It seems highly likely if not inevitable that such interactions would exist between compounds extracted from rosemary and consequently that composition–impact relationships are not easy to predict.
In addition to the aforementioned effects on the cholinergic system, Machado and colleagues used an animal model to demonstrate that a hydroalcoholic extract of rosemary interacts with the monaminergic system in a similar manner to antidepressant drugs [Machado et al. 2009]. Additionally the authors report significant effects on the noradrenergic and dopaminergic systems. Such interactions are potentially the cause of mood effects reported in humans following exposure to rosemary aroma. When considered in the round a reasonable conclusion is that each plant species (and by virtue their essential oils) may have a distinct influence on cognition and mood as a result of the unique composition of volatile compounds present. Furthermore given that the biochemical composition of plants is highly dependent upon geographical location, growing conditions and indeed time of day [Sousa et al. 2010] the determination of standardized extracts is one that presents serious challenges for the progress of phytomedicine.
With regard to the behavioural effects of exposure to rosemary essential oil aroma, the results reported here support previous work indicating that rosemary aroma can influence cognitive performance and mood [Moss et al. 2003]. Here, serum levels of 1,8-cineole were correlated with the performance outcomes (number of correct responses and reaction times) for each task. Although it is wise to exercise caution when dealing with small samples, effect sizes (r 2) of between 0.1 and 0.3 (medium to large effects by Cohen’s definitions) [Cohen, 1992] were found for all cognitive task variables except the accuracy of the RVIP task for which no relationship was identified between 1,8-cineole and percentage correct hits. These effect sizes compare favourably to those found previously in our laboratory for the effect of rosemary aroma on cognition. Moss and colleagues found r 2 values of 0.06 and 0.07 for the aspects of long-term and working memory that were found to be enhanced by exposure to aroma compared with controls [Moss et al. 2003].
The tasks used in this study were selected to tap into different cognitive processes: the RVIP task provides an assessment of sustained attention and central executive function compared with the continuous working memory, arithmetic processing and central executive composition of the serial subtractions tasks. Furthermore, the opportunity was taken to observe effects on tasks with discreet differences in cognitive load; with the serial sevens task being considerably more demanding than the serial threes task [Scholey and Kennedy, 2002]. Comparison of the relative relationships between plasma 1,8-cineole and performance on the serial subtraction tasks indicates that 1,8-cineole (or other essential oil components as discussed above) had a greater impact on the task with a lower cognitive load. Analysis of the difference between the correlation coefficients shows a stronger relationship between 1,8-cineole for accuracy [t(15) = −3.27, p = 0.002] and speed [t(15) = −6.06, p < 0.001] for the serial threes task than for the serial sevens task. The finding that the strength of relationships differs between tasks implies that there may be an interaction with cognitive load, and that at some point task difficulty might be such that enhancement is not possible as a consequence of exposure to rosemary aroma. Alternatively, more of the active compounds might need to be absorbed to have an effect. Increased levels of absorption would also provide opportunity to observe the classic inverted U-shaped function that underpins pharmacological impacts on behavioural variables of all kinds [Yerks and Dodson, 1908]. No relationship was found for the RVIP task that has a low cognitive load. It would appear that a demand on memory processes is required for enhancement to be observed, and that attention-based resources of the kind involved in the RVIP task may not be available for improvement. This supports findings reported by Moss and colleagues when no effect was found for rosemary aroma on the accuracy of attention factor [Moss et al. 2003]. Importantly, consideration of the relationships between 1,8-cineole and reaction times on the subtraction tasks indicates that for both tasks the improved accuracy is not the consequence of a speed–accuracy trade off. Clearly there is scope for further investigation of the precise cognitive components that might be available for modulation by essential oils, with previous research largely focusing on tasks such as mathematical computations [e.g. Ludvigson and Rottman, 1989; Diego et al. 1998; Degel and Köster, 1999] or more global cognitive factors [e.g. Moss et al. 2003, 2008, 2010]. Cognitive psychology has much to say about the fractionation of memory systems [Baddeley, 1996] and it would be valuable to use such arguments to inform future studies of the effects of aromas to better understand exactly how any effects are underpinned by the cognitive architecture.
Table 1.
Means and standard deviations for serum 1,8-cineole concentrations, cognitive task performance, baseline and change in mood (a positive value indicates an increase for that dimension over the experimental session).
| Mean | SD | Skewness | Kurtosis | |
|---|---|---|---|---|
| 1,8-Cineole (mg/ml) | 0.105 | 0.024 | −0.29 | −0.48 |
| Threes correct responses | 23.4 | 11.8 | 0.55 | −0.03 |
| Threes RT (ms) | 5593.9 | 2846.8 | 1.11 | 0.18 |
| Sevens correct responses | 12.4 | 9.2 | 1.60 | 0.96 |
| Sevens RT (ms) | 8657.3 | 3790.8 | 0.51 | −0.67 |
| RVIP correct responses | 11.8 | 7.9 | 0.85 | −0.23 |
| RVIP RT (ms) | 524.1 | 82.5 | 0.53 | 1.35 |
| Alert baseline (mm) | 47.6 | 15.4 | −0.863 | 0.735 |
| Content baseline (mm) | 57.6 | 12.8 | −0.533 | −0.293 |
| Calm baseline (mm) | 48.6 | 19.0 | −0.439 | −0.967 |
| Alert change (mm) | 14.0 | 16.6 | 0.06 | −0.18 |
| Content change (mm) | 13.1 | 15.8 | 1.39 | 1.52 |
| Calm change (mm) | 8.7 | 21.1 | 0.47 | 1.13 |
RVIP, Rapid visual information processing task; SD, standard deviation.
Table 2.
Pearson correlation coefficients between all variables.
| Threes correct | Threes RT | Sevens correct | Sevens RT | RVIP correct | RVIP RT | Alert change | Content change | Calm change | |
|---|---|---|---|---|---|---|---|---|---|
| 1,8 Cineole | 0.469* | −0.502* | 0.433 | −0.466* | 0.117 | −0.446* | 0.069 | −0.454* | −0.268 |
| Threes correct | - | −0.864*** | 0.880*** | −0.466* | 0.327 | −0.167 | −0.323 | −0.565** | −0.264 |
| ThreesRT | - | - | −0.692*** | −0.819*** | −0.291 | 0.190 | 0.269 | 0.525* | 0.299 |
| Sevens correct | - | - | - | −0.801*** | 0.323 | −0.093 | −0.247 | −0.482* | −0.303 |
| Sevens RT | - | - | - | - | −0.106 | −.070 | 0.145 | 0.353 | 0.512* |
| RVIP correct | - | - | - | - | - | −0.161 | −0.043 | −0.115 | 0.224 |
| RVIP RT | - | - | - | - | - | - | 0.192 | 0.315 | −0.118 |
| Alert change | - | - | - | - | - | - | - | 0.449* | −0.009 |
| Content change | - | - | - | - | - | - | - | - | 0.295 |
Significant correlations are marked with asterisks *p < 0.05, **p < 0.01, ***p < 0.001.
RT, Reaction Time (milliseconds); RVIP, Rapid visual information processing task; SD, standard deviation.
The observation that speed for the RVIP task was also improved may indicate that 1,8-cineole has a general psychomotor effect that is independent of cognitive resources, or even an effect on the peripheral cholinergic system that controls movement in a manner similar to that of other cholinergic drugs [Salamone et al. 1986]. This suggestion of a general psychomotor effect links interestingly to findings reported by Schifferstein and colleagues [Schifferstein et al. 2011] who reported enhanced dancing activity for all odorants tested compared with controls, irrespective of purported properties. It may be parsimonious to suggest that the perception of smells produces a global psychomotor enhancement, but the evidence does suggest somewhat greater specificity. For example, Moss and colleagues report a slowing of response speed during exposure to ylang ylang, something not associated with peppermint in the same study, indicating differential effects of the two aromas [Moss et al. 2008]. An alternative possibility regarding the finding of improved reaction time on all three tasks might be that 1,8-cineole affects speed as a consequence of increased subjective alertness of participants. However, correlations performed here between 1,8-cineole and the subjective mood reports suggest otherwise, with no strong evidence present that change in subjective ratings of alertness bore any relationship to plasma 1,8-cineole levels. Only contentedness possessed a significant relationship with 1,8-cineole levels, and interestingly to some of the cognitive performance outcomes, leading to the intriguing proposal that positive mood can improve performance whereas aroused mood cannot. Previously, Moss and colleagues suggested that the impact of aromas on task performance was independent of subjective feelings [Moss et al. 2003]. Others [e.g. Warm et al. 1991; Itai et al. 2000] have also argued for the independence of effects of aromas on cognition and mood, proposing avenues of influence which are not related to psychological beliefs and expectations. Such proposals sit well with the pharmacological mechanisms described above. Whether the effects on mood found here and elsewhere are a consequence of interactions of compounds with the monoaminergic system is an intriguing possibility worthy of further investigation. The relationship between the Bond-Lader mood scales used here, and those of another widely used scale in environmental research, the Pleasure, Arousal, Dominance (PAD) scale has yet to be established. The PAD scale [Mehrabian and Russell, 1974] was developed at the same time as Bond and Lader were working on their scale, and has been described as ‘the premier measure in the area of environmental psychology for assessing the impact of the environment on people’ [Machleit and Eroglu, 2000, p. 102]. Given the considerable value of the two scales for assessing mood states it is perhaps surprising that they have not previously been explored in tandem. It would be of considerable interest to incorporate both scales in future work to identify the relationship between the two scales components. This would permit greater comparisons between existing and future work to be made. A further consideration regarding the use of self-report scales generally is that the relationship between self–report measures and physiological correlates of arousal tends to be inconsistent [e.g. Mikalsen et al. 2001]. Indeed self-reports have long been seen to depend upon the cognitive explanations available to the individual to interpret perceived changes in their state of arousal [Schachter and Singer, 1962]. For example, where physiological changes are expected, these tend to be under reported as changes in subjective state due to cognitive preparedness. In contrast where physiological changes are unexpected, changes in subjective state tend to be over reported due to the salience of the change in physiological arousal. This can be of particular concern in blind designs, or studies with potentially or directly misleading instructions of the kind often used for aromas, when the causes of changes in arousal may be difficult for participants to attribute. Such difficulties can produce problems for self-report measures and caution is advised when interpreting results. It is very important to recognize that the self-report mood scales used in this study are not seen as substitutes for or estimates of measures of physiological arousal, and that subjective alertness might not be dependent on changes in such measures as heart rate and blood pressure, or indeed any other measure of physiological arousal. However, the impact of aromas on the more subtle aspects of psychological mood state are still of interest – even when they appear not to be related to physiology or performance measures as here.
To further our understanding of the effects and mechanisms underpinning the behavioural impact of rosemary aroma, combined in vivo and in vitro studies need to be carried out to assess both pharmacological and behavioural properties of a single source plant strain. As well as AChE inhibition, receptor-binding properties should be investigated as previous research has shown herbal extracts to exhibit acetylcholine receptor activity, including nicotinic [Perry et al. 1996; Wake et al. 2000] and muscarinic [Wake et al. 2000] binding properties in human cerebral cortex tissue. If these assays are made in tandem with cognitive and mood assessments it would help confirm that rosemary possesses cholinergic properties, and that such properties underpin the cognitive effects reported following inhalation of rosemary aroma.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest (real or apparent) that may have a direct bearing on the subject matter of this article.
Contributor Information
Mark Moss, Brain, Performance and Nutrition Research Centre, Department of Psychology, School of Life Sciences, Northumbria University, Newcastle upon Tyne NE1 8ST, UK.
Lorraine Oliver, Brain, Performance and Nutrition Research Centre, Department of Psychology, School of Life Sciences, Northumbria University, Newcastle upon Tyne, UK.
References
- Baddeley A. (1996). The fractionation of working memory. Proc Natl Acad Sci U S A 93: 13468–13472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia P., Wesensten N., Lammers W., Culpepper J., Harsh J. (1990) Responsiveness to olfactory stimuli presented in sleep. Physiol Behav 48: 87–90 [DOI] [PubMed] [Google Scholar]
- Baron R.A., Bronfen M. I. (1994) A whiff of reality: empirical evidence concerning the effects of pleasant fragrances on work related behaviour. J Appl Soc Psychol 24: 1179–1203 [Google Scholar]
- Bond A., Lader M. (1974). The use of analogue scales in rating subjective feelings. Br J Med Psychol 47: 211–218 [Google Scholar]
- Boyle R.R., Mclean S., Brandon S., Wiggins N. (2005). Rapid absorption of dietary 1,8-cineole results in critical blood concentration of cineole and immediate cessation of eating in the common bushtail possum. J Chem Ecol 31: 2775–2790 [DOI] [PubMed] [Google Scholar]
- Campenni C.E., Crawley E.J., Meier M.F. (2004) Role of suggestion in odor-induced mood change. Psychol Rep 94: 1127–1136 [DOI] [PubMed] [Google Scholar]
- Chung Y.K., Heo H.J., Kim E.K., Kim H.K., Huh T.L., Lim Y.H., et al. (2001) Inhibitory effect of ursolic acid purified from Origanum majorana L. on the acetylcholinesterase. Mol Cells 11: 137–143 [PubMed] [Google Scholar]
- Cohen J. (1992) A power primer. Psychol Bull 112: 155–159 [DOI] [PubMed] [Google Scholar]
- Degel J., Köster E.P. (1999) Odors: implicit memory and performance effects. Chem Senses 24: 317–325 [DOI] [PubMed] [Google Scholar]
- Diego M.A., Jones N.A., Field T., Hernandez-Reif M., Schanberg S., Kuhn C., et al. (1998) Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int J Neurosci 9: 217–224 [DOI] [PubMed] [Google Scholar]
- Hertz R. (2009) Aromatherapy facts and fictions: a scientific analysis of olfactory effects on mood, physiology and behaviour. Int J Neurosci 119: 263–290 [DOI] [PubMed] [Google Scholar]
- Hopkins C. (1996) Principles of Aromatherapy. London: Thorsons [Google Scholar]
- Howard S., Hughes B. (2008) Expectancies, not aroma, explain impact of lavender aromatherapy on psychophysiological indices of relaxation in young women. Br J Health Psychol 13: 603–617 [DOI] [PubMed] [Google Scholar]
- Huang M.T., Ho C., Wang Z., Ferraro T., Lou Y., Stauber K., et al. (1994) Inhibition of skin tumorigenisis by rosemary and its constituents carnosol and ursolic acid. Cancer Res 54: 701–708 [PubMed] [Google Scholar]
- Itai T., Amayasu M., Kuribayaski N., Kawamura M., Okada A., Momose T., et al. (2000) Psychological effects of aromatherapy on chronic haemodialysis patients. Psychiatry Clin Neurosci 54: 393–397 [DOI] [PubMed] [Google Scholar]
- Jellinek J.S. (1997) Psychodynamic odour effects and their mechanisms. Cosmet Toilet 112: 61–71 [Google Scholar]
- Jirovetz J., Buchbauer G., Jager W., Raverdino V., Nikiforov A. (1990) Determination of lavender oil fragrance compounds in blood samples. Fresenius J Anal Chem 338: 922–923 [Google Scholar]
- Kennedy D.O., Scholey A.B., Wesnes K.A. (2002) Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng and a ginkgo/ginseng combination to healthy young adults. Physiol Behav 75: 739–751 [DOI] [PubMed] [Google Scholar]
- Kovar K.A., Gropper B., Friess D., Ammon H.T.P. (1987) Blood levels of 1,8-cineole and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Med 53: 315–319 [DOI] [PubMed] [Google Scholar]
- Ludvigson H.W., Rottman R. (1989) Effects of ambient odours of lavender and cloves on cognition, memory, affect and mood. Chem Senses 14: 525–536 [Google Scholar]
- Machado D., Bettio L., Cunha M., Capra J., Dalmarco J., Pizzolatti M., Rodrigues A. (2009) Antidepressant-like effect of the extract of Rosmarinus offinalis in mice: involvement of the monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry 33: 642–650 [DOI] [PubMed] [Google Scholar]
- Machleit K., Eroglu S.A. (2000) Describing and measuring emotional response to shopping experience. J Business Res 49: 101–111 [Google Scholar]
- McCormack H.M., Horne D.J.L., Sheather S. (1988) Clinical applications of visual analogue scales: a critical review. Psychol Med 18: 1007–1019 [DOI] [PubMed] [Google Scholar]
- Mehrabian A., Russell J.A. (1974) An Approach to Environmental Psychology. Cambridge, MA: The MIT Press [Google Scholar]
- Mikalsen A., Bertelsen B., Flaten M.A. (2001) Effects of caffeine, caffeine-associated stimuli, and caffeine-related information on physiological and psychological arousal. Psychopharmacology 157: 373–380 [DOI] [PubMed] [Google Scholar]
- Moss M., Cook J., Wesnes K., Duckett P. (2003) Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int J Neurosci 113: 15–38 [DOI] [PubMed] [Google Scholar]
- Moss M., Hewitt S., Moss L., Wesnes K. (2008). Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int J Neurosci 118: 59–77 [DOI] [PubMed] [Google Scholar]
- Moss M., Howarth R., Wilkinson L., Wesnes K. (2006) Expectancy and the aroma of Roman chamomile influence mood and cognition in healthy volunteers. Int J Aromather 16: 63–73 [Google Scholar]
- Moss L., Rouse M., Wesnes K., Moss M. (2010) Differential effects of the aromas of Salvia species on memory and mood. Hum Psychopharmacol Clin Exp 25: 388–396 [DOI] [PubMed] [Google Scholar]
- Orhan I., Aslan S., Kartal M., Sener B., Baser K.H.C. (2008) Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem 108: 663–668 [DOI] [PubMed] [Google Scholar]
- Perry E.K., Court G., Bidet N., Court J., Perry E. (1996) European herbs with cholinergic activities: potential in dementia therapy. Int J Geriatr Psychiatry 11: 1063–1069 [Google Scholar]
- Perry N.S.L., Houghton P., Theobald A., Jenner P., Perry E.K. (2000) In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituents terpenes. J Pharmacol 52: 895–902 [DOI] [PubMed] [Google Scholar]
- Perry N.S.L., Bollen C., Perry E.K., Bollard C. (2003) Salvia for dementia therapy, review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav 75: 651–658 [DOI] [PubMed] [Google Scholar]
- Reay J.L., Kennedy D.O., Scholey A.B. (2006) Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained mentally demanding tasks. J Psychopharmacol 20: 771–781 [DOI] [PubMed] [Google Scholar]
- Ritter F.E., Schoelles M., Klein L.C., Kase S.E. (2007) Modeling the range of performance on the serial subtraction task. In: Proceedings of the Eighth International Conference on Cognitive Modeling. Oxford, UK: Taylor & Francis, pp. 299–304 [Google Scholar]
- Salamone J. D., Lalies M.D., Channell S.L., Iversen S.D. (1986) Behavioural and pharmacological characterization of the mouth movements induced by muscarinic agonists in the rat. Psychopharmacology 88: 467–471 [DOI] [PubMed] [Google Scholar]
- Savelev S., Okello E., Perry N.S.L., Wilkins R.M., Perry E.K. (2003) Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol Biochem Behav 75: 661–668 [DOI] [PubMed] [Google Scholar]
- Schachter S., Singer J. (1962) Cognitive, social and physiological determinants of emotional state. Psychol Rev 69: 379–399 [DOI] [PubMed] [Google Scholar]
- Schifferstein H.N.J., Talke K.S.S., Oudshoorn D. (2011) Can ambient scent enhance the nightlife experience? Chemosens Percept 4: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey A.B., French S.J., Morris P.J., Kennedy D.O., Milne A., Haskell C.F. (2010) Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol 24: 1505–1514 [DOI] [PubMed] [Google Scholar]
- Scholey A.B., Harper S., Kennedy D.O. (2001) Cognitive demand and blood glucose. Physiol Behav 73: 585–592 [DOI] [PubMed] [Google Scholar]
- Scholey A.B., Kennedy D.O. (2002) Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax Ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol Clin Exp 17: 35–44 [DOI] [PubMed] [Google Scholar]
- Scholey A.B., Tildesley N.T.J., Ballard C.G., Wesnes K.A., Tasker A., et al. (2008) An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology 198:127–139 [DOI] [PubMed] [Google Scholar]
- Sousa E.O., Colares A.V., Rodrigues F.F.G., Campos A.R., Lima S.G., Costa J.G.M. (2010) Effect of collection time on essential oil composition of Lantana camara Linn (Verbenaceae) growing in Brazil northeastern. Rec Nat Prod 4: 31–37 [Google Scholar]
- Tisserand R. (1993) The Art of Aromatherapy. Essex: C.W. Daniel [Google Scholar]
- Wake G., Court J., Pickering A., Lewis R., Wilkins R., Perry E. (2000) CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J Ethnopharmacol 69: 105–114 [DOI] [PubMed] [Google Scholar]
- Warm J.S., Dember W.N., Parasuraman R. (1991) Effects of olfactory stimulation on performance and stress in a visual sustained attention task. J Soc Cosmet Chem 42: 199–210 [Google Scholar]
- Wartik N. (1995) Making sense of aromatherapy. Am Health 14: 73 [Google Scholar]
- Yerks R., Dodson J. (1908) The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol 18: 459–482 [Google Scholar]