Abstract
Objective: To examine the tolerability of the recommended initiation doses for once-monthly injectable paliperidone palmitate in patients who have recently been diagnosed with schizophrenia and for whom high doses may pose tolerability concerns.
Methods: A post hoc analysis from a 13-week double-blind study of patients with schizophrenia randomized 1:1:1:1 to placebo or paliperidone palmitate at 25, 100, or 150 mg equivalents (mg eq) of paliperidone (corresponding to 39, 156, or 234 mg respectively). This analysis focused on the recently diagnosed subgroup (≤5 years; N = 146) who received the recommended initiation dosage of paliperidone palmitate [150 mg eq on day 1 (n = 109) followed by 100 mg eq on day 8 (n = 39)] or placebo (n = 37). Adverse events (AEs), reported in ≥2% of patients receiving paliperidone palmitate during days 1–7 or ≥5% during days 8–36, and in a higher percentage of patients receiving paliperidone palmitate than placebo, were identified. AE relative risks (RRs) and 95% confidence intervals (CIs) were determined. A RR was considered potentially significant when its 95% CI did not include 1.
Results: Overall, day 1–7 AE rates were 37.6% (41 of 109) and 29.7% (11 of 37) with paliperidone palmitate and placebo respectively; injection site pain (5.5% versus 2.7%, RR 2.0; 95% CI 0.25 to 16.37), agitation (4.6% versus 2.7%; RR 1.7; 95% CI 0.21 to 14.06), and headache (3.7% versus 0.0%; RR 3.1; 95% CI 0.17 to 56.41) met the ≥2% criteria. Day 8–36 AE rates were 41.0% (16 of 39) and 37.8% (14 of 37) with paliperidone palmitate and placebo respectively; anxiety (5.1% versus 0.0%; RR 4.8; 95% CI 0.24 to 95.76) met the ≥5% criteria.
Key limitations were that some patients may have been ill for a significant time before formal diagnosis and that the number of patients is low in this subgroup, limiting the ability to detect statistical significance for AE RRs.
Conclusions: Paliperidone palmitate initiation doses (150 mg eq day 1, 100 mg eq day 8) were tolerated in this subgroup of patients who were recently diagnosed with schizophrenia, with no unexpected findings. Although the same size was small, these data identified AEs that may be encountered during the week and month after initiation dosing. These findings may assist clinicians when paliperidone palmitate is considered an appropriate treatment choice for these patients.
Keywords: paliperidone palmitate, recent diagnosis, schizophrenia, tolerability, treatment
Introduction
Population studies of schizophrenia have shown occurrence rates ranging from 0.1 to 0.4 per 1000 persons per year [World Health Organization, 2007; Mueser and McGurk, 2004]. Schizophrenia is a disabling and progressive disease spanning the life course from premorbid, prodromal, to deterioration and chronic or residual stages. Of these four clinical stages of schizophrenia, the emergence of frank psychosis typically presents between the ages of 16 and 30 years with the majority of patients in the ‘deterioration stage’ experiencing progressive functional decline with each successive relapse [Lieberman et al. 2008, 2001]. The deterioration experienced by those with schizophrenia appears to be most pronounced within the first 5 years of disease onset [Tandon et al. 2009; Lieberman et al. 2001; McGlashan and Fenton, 1993; Bleuler, 1972]. Thus, it is generally accepted that the first 5–10 years of illness is a critical period for effective intervention [Francey et al. 2010; McGorry et al. 2008, 2007; Kelly et al. 2005; Marshall et al. 2005; Harrigan et al. 2003].
Several studies have found that earlier diagnosis and initiation of effective and well-tolerated treatment of schizophrenia is associated with greater clinical responsiveness and better long-term outcome, including a lower risk for recurrence [Weiden et al. 2009; Barnes et al. 2008; Perkins et al. 2005; Schooler et al. 2005; Wyatt, 1991], as well as possibly mitigating disease progression [Lieberman et al. 2001]. While patients with schizophrenia may have a robust response to treatment, those with a recent diagnosis may tolerate antipsychotic treatment less well with particular susceptibilities to extrapyramidal symptoms (EPS), weight gain, prolactin-related effects, and sedation [Salimi et al. 2009; Alvarez-Jimenez et al. 2008; Tschoner et al. 2007; Kelly et al. 2005; Llorca et al. 2002; Allison and Casey, 2001; Muench and Carey, 2001]. This higher sensitivity to adverse events (AEs) coupled with poor adherence to treatment are believed to be major contributors to relapse and the substantial deterioration that occurs early in the course of this chronic disease [Gilmer et al. 2004; Valenstein et al. 2004; Menzin et al. 2003; Coldham et al. 2002; Robinson et al. 1999]. In those at risk for poor adherence to daily therapy, the use of a long-acting injectable agent, if well tolerated, may be particularly beneficial. It has been suggested that long-acting injectable antipsychotics are a particularly appropriate treatment option in recently diagnosed patients in whom optimal therapeutic outcomes may be compromised by early treatment discontinuation and/or poor treatment adherence [Chue and Emsley, 2007; Keith and Kane, 2003]. Data from studies in patients with first episode [Kim et al. 2008] and recent onset psychosis [Emsley et al. 2008; Parellada et al. 2005] indicate that treatment with long-acting injectable antipsychotic agents may improve outcomes in patients with early disease symptoms.
Paliperidone palmitate is a long-acting, once-monthly (following two initiation doses given 1 week apart) injectable, atypical, antipsychotic for the treatment of adults with schizophrenia. It is the palmitate ester of paliperidone, which is also formulated for daily oral administration as paliperidone extended release (ER). The dosage of paliperidone palmitate may be expressed as milligram equivalents (mg eq) of the pharmacologically active fraction, paliperidone (Table 1).
Table 1.
Corresponding dose expression equivalents of paliperidone and paliperidone palmitate.
| Paliperidone (active fraction) (mg eq) | Paliperidone palmitate (mg) |
|---|---|
| 150 | 234 |
| 100 | 156 |
| 75 | 117 |
| 50 | 78 |
| 25 | 39 |
The efficacy and tolerability of paliperidone palmitate for the acute and maintenance treatment of schizophrenia has been studied in several controlled clinical studies using various dosing regimens [Gopal et al. 2010; Hough et al. 2010, 2009; Nasrallah, et al. 2010; Pandina et al. 2010]. A recently completed phase 3 acute treatment trial was the first placebo-controlled study to assess paliperidone palmitate administered at the recommended day 1 dose of 150 mg eq (234 mg) by deltoid injection. Patients then received 25, 100, or 150 mg eq (39, 156, or 234 mg respectively) on day 8 and monthly thereafter (deltoid or gluteal). In this study, paliperidone palmitate was associated with significant improvements in symptomatology with no unexpected tolerability findings in adults with symptomatic schizophrenia, at all doses tested [Pandina et al. 2010]. A post hoc analysis of this trial examined the recently diagnosed subgroup to assess the effects associated with the initiation doses [150 mg eq (234 mg) on day 1 and 100 mg eq (156 mg) on day 8], which may pose a tolerability concern when managing patients early in the course of their illness.
Methods
Design
This was a post hoc analysis of a 13-week double-blind, randomized, placebo-controlled phase 3 acute treatment trial conducted from March 2007 to March 2008 at 72 centers in eight countries in the United States, Europe, and Asia. Adult patients with a Positive and Negative Syndrome Scale (PANSS) total score of 70–120 (inclusive) at screening and 60–120 (inclusive) at double-blind baseline were eligible for study enrollment. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and consistent with Good Clinical Practices. Further details of the design are reported by Pandina and colleagues [Pandina et al. 2010].
Study medications
The study consisted of a screening period of up to 7 days for washout of disallowed psychotropic medications followed by a 13-week double-blind treatment period. Eligible patients were randomly assigned (1:1:1:1) to fixed doses of paliperidone palmitate 25, 100, or 150 mg eq (39, 156, or 234 mg respectively) or placebo, based on a computer-generated randomization schedule balanced by using permuted blocks of treatments and stratified by center. On day 1, all patients received a deltoid injection of paliperidone palmitate 150 mg eq (234 mg) or matching placebo; on day 8, and then on days 36 and 64, patients received their assigned treatment according to the randomization schedule, injected in the deltoid or the gluteal muscle at the discretion of the investigator. It should be noted that this differs slightly from the currently recommended initiation regimen of paliperidone palmitate that consists of 150 mg eq (234 mg) on day 1 and 100 mg eq (156 mg) on day 8, both given in the deltoid muscle, with gluteal muscle injection being an option for doses administered after day 8.
Patients were hospitalized from day 1 (first injection) until at least after the second injection of study drug on day 8. Antipsychotics, except the study drug, were prohibited during the double-blind treatment period. Antiparkinsonian medications (if EPS emerged or worsened during the study), and oral benzodiazepines (for agitation, anxiety, or sleep difficulties) at the permitted maximum daily doses, were allowed.
Tolerability and efficacy assessments
Tolerability was evaluated based on treatment-emergent AEs and related study discontinuations. Efficacy was evaluated using PANSS total scores, Clinical Global Impressions – Severity (CGI-S) ratings, and Personal and Social Performance (PSP) scores that were assessed at baseline and days 4 (PANSS and CGI-S only), 8, 22, 36, 64, and 92 (or study endpoint).
Analysis sets and statistical evaluations
All measures were assessed in the intent-to-treat (ITT) analysis set, defined as all randomized patients who took at least one dose of double-blind study medication and had both the baseline and at least one post-baseline efficacy assessment. This post hoc analysis of tolerability and efficacy focused on patients with ‘recently diagnosed’ (≤5 years) schizophrenia who received placebo or paliperidone palmitate at the recommended initiation dose of 150 mg eq on day 1 and 100 mg eq on day 8 (234 and 156 mg respectively) followed by 100 mg eq (156 mg) once monthly (days 36 and 64). This group will hereafter be referred to as the 150/100 mg eq arm. Data from the overall study population were provided as a reference where appropriate [Pandina et al. 2010].
Frequencies, percentages, and descriptive statistics were used to summarize demographic and clinical characteristics as well as tolerability and efficacy variables. AEs reported during days 1–7 were summarized for those reported in ≥2% of patients receiving paliperidone palmitate (included all three paliperidone palmitate treatment arms) and in a higher percentage of patients receiving paliperidone palmitate than placebo. At day 8, those assigned to paliperidone palmitate received their assigned fixed dose with approximately one-third being assigned to the 100 mg eq (156 mg) treatment arm. Because of this substantially lower total number of patients, AEs reported during days 8–36 were summarized for those reported in ≥5% of patients receiving paliperidone palmitate and in a higher percentage of patients receiving paliperidone palmitate than placebo.
Changes in weight and reports of prolactin-related and movement disorder-related events that occurred over the entire study period were summarized. AEs are presented in two panels – incidence by treatment group and relative risk (RR) with 95% confidence intervals (CIs) of an event in the active group relative to the placebo group. A RR was considered potentially significant when its 95% CI did not include 1. For AEs with an incidence of zero in one group, a correction of 0.5 was used in the logit estimator in calculating the RR. No adjustment was made for multiplicity.
An analysis of covariance (ANCOVA) model with effects of treatment, country, and baseline value without adjustment for multiple comparisons assessed between-group changes for continuous measures. The last-observation-carried-forward (LOCF) approach was utilized. Effect sizes (treatment versus placebo) were calculated using Cohen’s d based on the change from baseline in least-squares (LS) mean PANSS total score, mean CGI-S score, and mean PSP score at endpoint.
Results
Randomization, completion, and characteristics
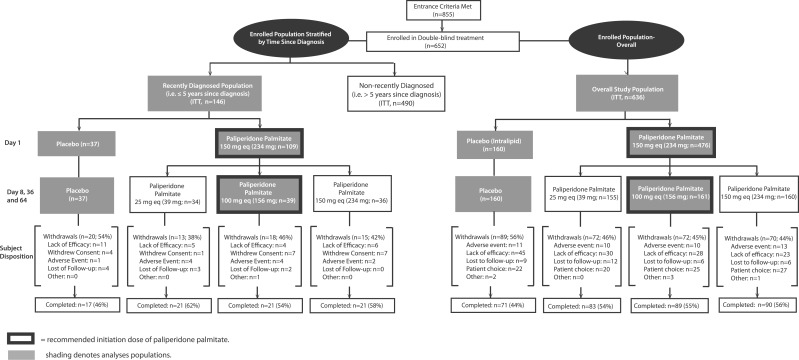
Of 855 patients screened, 652 (76.3%) were randomized, and 636 (476 assigned to paliperidone palmitate and 160 to placebo) comprised the ITT overall study population analysis set. In the ITT analysis set, 146 were diagnosed within the prior 5 years and were classified as the recently diagnosed subgroup (Figure 1).
Figure 1.
Subject randomization and completion in intent-to-treat (ITT) study populations: overall population and recently diagnosed subgroup. In the recently diagnosed subgroup, discontinuation rates due to adverse events were 10.3% (4 of 39) with paliperidone palmitate 150/100 mg eq and 2.7% (1 of 37) with placebo. Corresponding rates in the overall study population were 6.2% (10 of 161) and 6.9% (11 of 160) respectively.
Baseline demographics and disease characteristics of the ITT recently diagnosed and overall study populations show that the recently diagnosed was a younger subgroup of the population, with a slightly later age at diagnosis, somewhat different racial distribution, but similar symptomatology (Table 2). The mean (SD) time since diagnosis in the recently diagnosed group was 2.9 (1.3) years; 38.4% (n = 56) were ≤2 years since diagnosis; 26.0% (n = 38) were >2 to 3 years since diagnosis; 20.5% (n = 30) were >3 to 4 years since diagnosis; and 15.1% (n = 22) were >4 to 5 years since diagnosis. In this group, the mean (SD) age at baseline was 31.2 (9.6) years; 34.9% (n = 51) were ≤25 years of age; 36.3% (n = 53) were >25 to 35 years of age; 28.1% (n = 41) were >35 to 65 years of age, and 0.7% (n = 1) were >65 years of age at baseline.
Table 2.
Baseline demographics of the recently diagnosed (≤5 years since diagnosis) subgroup and overall study population.
| Parameter | Recently diagnosed subgroup, ITT n = 146 | Overall study population, ITT n = 636 |
|---|---|---|
| Gender, n (%) | ||
| Male | 100 (68) | 427 (67) |
| Female | 46 (32) | 209 (33) |
| Age (years) | ||
| Mean (SD) | 31.2 (9.6) | 39.4 (10.6) |
| Mean age at diagnosis (SD) | 28.3 (9.7) | 25.4 (8.2) |
| Race, n (%) | ||
| Caucasian | 95 (65) | 343 (54) |
| Black | 30 (21) | 192 (30) |
| Asian | 21 (14) | 92 (14) |
| Other | 0 (0) | 9 (1) |
| Type of schizophrenia, n (%) | ||
| Paranoid | 127 (87) | 558 (88) |
| Disorganized | 1 (<1) | 17 (3) |
| Catatonic | 2 (1) | 2 (<1) |
| Undifferentiated | 14 (10) | 54 (8) |
| Residual | 2 (1) | 5 (1) |
| PANSS total score, mean (SD) | 87.3 (10.5) | 87.1 (11.2) |
| CGI-S, n (%) | n = 146 | n = 635 |
| Mild | 1 (<1) | 16 (3) |
| Moderate | 82 (56) | 307 (48) |
| Marked | 56 (38) | 275 (43) |
| Severe | 7 (5) | 37 (6) |
CGI-S, Clinical Global Impressions – Severity; ITT, intent to treat; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation.
In the recently diagnosed subgroup, discontinuation rates due to AEs were 10.3% (4 of 39) with paliperidone palmitate 150/100 mg eq (234/156 mg) and 2.7% (1 of 37) with placebo. These rates in the overall study population were 6.2% (10 of 161) and 6.9% (11 of 160) respectively. AEs leading to discontinuation in the four recently diagnosed patients receiving paliperidone palmitate were: insomnia, psychotic disorder, paranoid type schizophrenia, suicidal ideation, dry mouth, toothache, injection site swelling, and musculoskeletal stiffness (n = 1 for each AE). AEs leading to discontinuation in the one recently diagnosed patient receiving placebo were schizophrenia and akathisia.
Tolerability during days 1–7
In the recently diagnosed subgroup, 37.6% (41 of 109) reported AEs during the week following the day 1 injection of paliperidone palmitate 150 mg eq (234 mg) and 29.7% (11 of 37) after receipt of placebo. In the overall study population, these rates were 38.0% (181 of 476) and 43.1% (69 of 160) respectively.
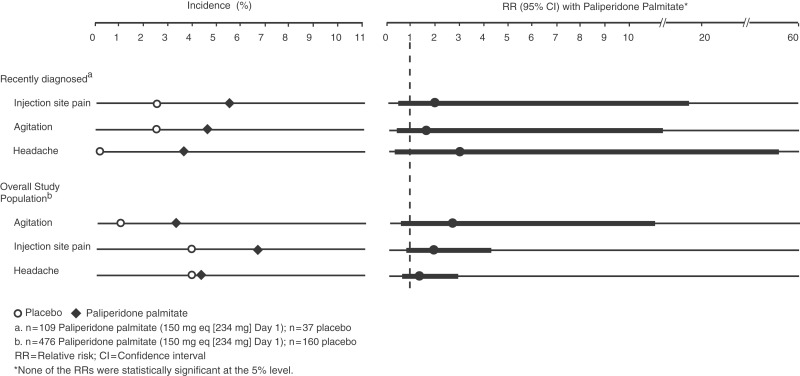
AEs reported by ≥2% of recently diagnosed patients receiving paliperidone palmitate and in a higher percentage of patients receiving paliperidone palmitate than placebo were injection site pain (5.5% versus 2.7%; RR 2.0; 95% CI 0.25 to 16.37), agitation (4.6% versus 2.7%; RR 1.7; 95% CI 0.21 to 14.06), and headache (3.7% versus 0.0%; RR 3.1; 95% CI 0.17 to 56.41) (Figure 2). RRs were not statistically significant as determined by 95% CIs. In the overall study population the same AEs met these criteria: injection site pain (paliperidone palmitate versus placebo 6.7% versus 3.8%; RR 1.8; 95% CI 0.76 to 4.21) and headache (4.0% versus 3.8%; RR 1.1; 95% CI 0.43 to 2.62), and agitation (3.2% versus 1.3%; RR 2.5; 95% CI 0.58 to 10.90). RRs were not statistically significant as determined by 95% CIs.
Figure 2.
Days 1–7: adverse events in ≥2% of patients receiving paliperidone palmitate and in a higher percentage of patients receiving paliperidone palmitate than placebo. Events that met these criteria during the week following the first injection in the recently diagnosed subgroup were injection site pain, agitation, and headache; relative risks were not statistically significant as determined by the 95% confidence intervals. The same events met these criteria in the overall study population.
In the recently diagnosed subgroup, a broad range of AEs were reported in one or two patients receiving active treatment and none receiving placebo (n = 2 for toothache, vomiting, and myalgia; n = 1 for aggression, schizophrenia, dry mouth, upper abdominal pain, nausea, dizziness, exertional dizziness, lethargy, Parkinsonism, sedation, asthenia, fatigue, back pain, muscle tightness, musculoskeletal stiffness, pain in extremity, hyperhidrosis, furuncle, sialodentitis, viral infection, eye rolling, increased blood amylase, weight increase, sinus bradycardia, galactorrhea, and nasal congestion).
Tolerability during days 8–36
In the recently diagnosed subgroup, 41.0% (16 of 39) reported AEs in the month following the day 8 injection of paliperidone palmitate 100 mg eq (156 mg), and 37.8% (14 of 37) after placebo. In the overall study population, these rates were 38.5% (62 of 161) and 41.3% (66 of 160) respectively.
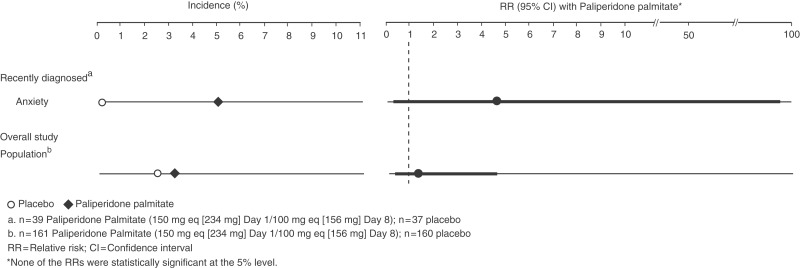
One AE, anxiety, was reported by ≥5% of recently diagnosed patients receiving paliperidone palmitate and in a higher percentage of patients receiving paliperidone palmitate than placebo (5.1% versus 0.0%; RR 4.8; 95% CI 0.24 to 95.76); the RR was not statistically significant (Figure 3). No AE, including anxiety (3.1% versus 2.5%; RR 1.2; 95% CI 0.34 to 4.54; p > 0.05), met the criteria in the overall study population; however, data are shown in Figure 3.
Figure 3.
Days 8–36: adverse events in ≥5% of patients receiving paliperidone palmitate and in a higher percentage of patients receiving paliperidone palmitate than placebo. In the recently diagnosed subgroup, anxiety met the criteria during the first month following the second injection; the relative risk was not statistically significant as determined by the 95% confidence interval. No events met these criteria in the overall study population.
Weight, movement disorders, and prolactin during entire study
In the recently diagnosed subgroup, the LS mean (SEM) weight change over the entire study period was 1.4 kg (0.76) in the paliperidone palmitate 150/100 mg eq (234/156 mg) group and 0.0 kg (0.81) in the placebo group (p = 0.157 for difference in LS means). In the overall study population, the mean weight change was 0.7 kg (0.36), and –0.3 kg (0.37) respectively (p = 0.028 for difference in LS means).
In the recently diagnosed subgroup, movement disorder-related events were reported over the entire study period by 10.3% (4 of 39) in the paliperidone palmitate group and by 8.1% (3 of 37) in the placebo group (RR 1.3; 95% CI 0.30 to 5.27; p > 0.05). In the overall study population, the respective rates were: 9.3% (15 of 161) and 8.1% (13 of 160) (RR 1.2; 95% CI 0.56 to 2.33; p > 0.05).
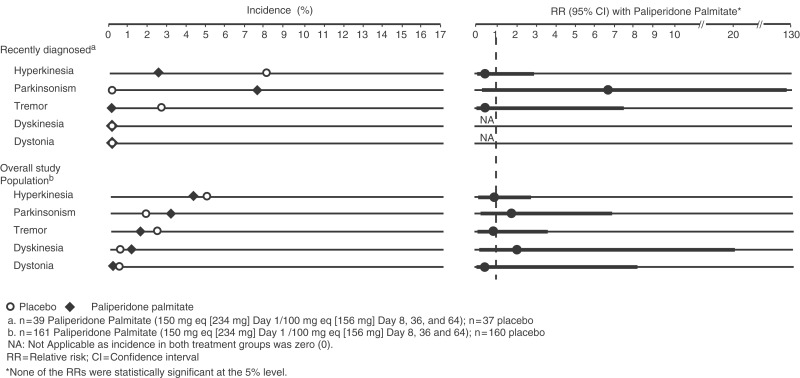
In the recently diagnosed subgroup, the most common movement disorder-related event during the entire study period was Parkinsonism in the paliperidone palmitate group and hyperkinesia in the placebo group, with a similar pattern noted in the overall study population. Individual movement disorder-related event incidence rates and RRs with 95% CIs that occurred during the study are illustrated in Figure 4. The RRs were not statistically significant as determined by the 95% CIs.
Figure 4.
Movement disorder-related adverse events over entire study. Parkinsonism was the most common movement disorder-related event during the entire study period in the recently diagnosed subgroup given paliperidone palmitate, with hyperkinesia being the most common movement disorder-related event in those given placebo. As with other events, the relative risks were not statistically significant as determined by the 95% confidence intervals.
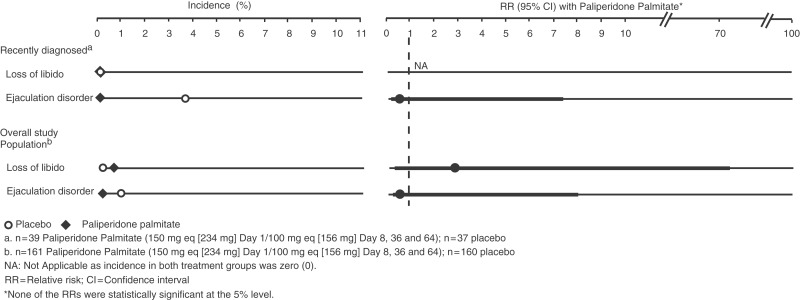
In the recently diagnosed subgroup, one male placebo-treated patient reported ejaculation disorder. In the overall study population, there was an additional report of one female patient in the paliperidone palmitate group who reported loss of libido (Figure 5).
Figure 5.
Prolactin-related adverse events over entire study. One male patient with recently diagnosed schizophrenia treated with placebo reported ejaculation disorder during the entire study period. In the overall study population, there was one additional report of a female patient treated with paliperidone palmitate who reported a loss of libido.
Efficacy during entire study
There was a significant improvement from baseline in PANSS total score at endpoint in recently diagnosed patients who received paliperidone palmitate 150/100 mg eq (234/156 mg) compared with those who received placebo (Table 3). The effect size (versus placebo) based on the LS mean score change was –0.7 (95% CI –1.16 to –0.23; p = 0.0031) in the recently diagnosed subgroup; it was –0.5 (95% CI –0.69 to –0.25; p < 0.0001) in the overall study population.
Table 3.
PANSS, CGI-S, and PSP mean baseline, mean changes from baseline to endpoint and effect sizes: paliperidone palmitate versus placebo (95% confidence interval, p-value).
| Parameter | Recently diagnosed subgroup |
Overall study population |
||
|---|---|---|---|---|
| Paliperidone palmitate 150/100 mg eq (234/156 mg) (n = 39) | Placebo (n = 37) | Paliperidone palmitate 150/100 mg eq (234/156 mg) (n = 161) | Placebo (n = 160) | |
| PANSS total | ||||
| Baseline, mean (SD) | 88.1 (10.89) | 85.2 (10.39) | 86.2 (10.77) | 86.8 (10.31) |
| Change from baseline at endpoint, mean (SD) | –16.5 (20.43) | –2.2 (20.91) | –11.6 (17.63) | –2.9 (19.26) |
| Effect size at endpoint | –0.7 (–1.16, –0.23) p = 0.0031 | –0.5 (–0.69, –0.25) p < 0.0001 | ||
| CGI-S score | ||||
| Baseline, mean (SD) | 4.4 (0.60) | 4.4 (0.65) | 4.4 (0.63) | 4.6 (0.63) |
| Change from baseline at endpoint, mean (SD) | –0.8 (1.21) | –0.3 (1.29) | –0.6 (1.13) | –0.3 (1.13) |
| Effect size at endpoint | –0.4 (–0.88, 0.03) p = 0.0675 | –0.3 (–0.48, –0.04) p = 0.0192 | ||
| PSP total | ||||
| Baseline, mean (SD) | 49.9 (12.40) | 51.4 (11.84) | 50.2 (12.78) | 49.7 (12.33) |
| Change from baseline at endpoint, mean (SD) | 9.2 (13.68) | 2.9 (17.46) | 6.1 (13.59) | 1.7 (15.60) |
| Effect size at endpoint | 0.4 (–0.05, 0.86) p = 0.835 | 0.3 (0.08, 0.52) p = 0.0085 | ||
Effect size at endpoint was for paliperidone palmitate versus placebo. The type of effect size is Cohen’s d; p-value is from two-sided Z test. CGI-S, Clinical Global Impressions – Severity; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance; SD, standard deviation.
In the recently diagnosed subgroup, effect sizes for improvements in CGI-S and PSP with paliperidone palmitate compared with placebo were similar to those observed in the overall study population, but they were not statistically significant in this subgroup (Table 3). In the overall study population (with much larger sample sizes), these effect sizes were statistically significant.
Discussion
The primary objective of this subgroup analysis was to assess the tolerability associated with the initiation doses of paliperidone palmitate in this potentially sensitive patient population. The recommended initiation dosing for paliperidone palmitate requires use of the higher doses given 1 week apart (150 mg eq on day 1 and 100 mg eq on day 8; 234 and 156 mg respectively) in the deltoid muscle, and is followed by once-monthly injections of 25–150 mg eq (39–234 mg). Published data show lower initial doses administered in the gluteal muscle can lead to subtherapeutic plasma levels and poor longer-term response in schizophrenia [Gopal et al. 2010; Nasrallah et al. 2010]. Nonetheless, the recommended initial dosing may raise tolerability concerns for clinicians, particularly when managing patients early in the course of their illness where relatively low doses of antipsychotics are commonly preferred [McGorry and Group IEPAW, 2005; Schooler et al. 2005]. Thus, data presented here examined this issue.
In this analysis, paliperidone palmitate at 150 mg eq on day 1 and 100 mg eq on day 8 (234 and 156 mg respectively) was tolerated without any new or unexpected AEs in patients recently diagnosed with schizophrenia. However, during the week following the initial injection of 150 mg eq (234 mg) paliperidone palmitate, the total AE rate was higher compared with the rate observed with placebo (37.6% versus 29.7%). This differed from that in the overall study population (38.0% and 43.1%, respectively). Of note, the differential in the total AE rate during the first week was due to a lower rate in the placebo arm of the recently diagnosed subgroup. While there is not an obvious interpretation of this placebo arm observation, in general the data do suggest that the recently diagnosed represent a subgroup likely to report AEs with active treatment. Further, discontinuations due to AEs occurred in four of 39 patients in the paliperidone palmitate group and one of 37 in the placebo group. These findings are consistent with the significant body of literature showing that patients with schizophrenia are more sensitive to adverse drug effects in the first few years of illness [Francey et al. 2010; Alvarez-Jimenez et al. 2008; Tschoner et al. 2007; Kelly et al. 2005; Llorca et al. 2002; Allison and Casey, 2001; Muench and Carey, 2001]. Specifically, published reports suggest that various EPS, weight gain, prolactin-related effects, and sedation are more frequent and problematic in patients with early illness [Kelly et al. 2005; Bobes et al. 2003; Merlo et al. 2002; Woods et al. 2002; Gupta et al. 2001; Masi et al. 2001; Sanger et al. 1999; Wudarsky et al. 1999]. In our dataset, the most common events during the first week were injection site pain, agitation, and headache in both the recently diagnosed subgroup as well as the overall study population, with similar RRs. Therefore, the most commonly reported AEs with paliperidone palmitate were not those that led to the higher rate of total events with treatment in the recently diagnosed. Further, no specific AE or class of AEs was identified as a particular concern in this subgroup after the initial injection of 150 mg eq (234 mg) paliperidone palmitate. Instead, there was a broad range of events reported in one or two patients with active treatment and in no patients receiving placebo, contributing to the higher observed rate.
During the month following the day 8 injection of 100 mg eq (156 mg), the total AE rate was somewhat comparable with paliperidone palmitate and placebo in the recently diagnosed subgroup (41.0% and 37.8%), and similar to that observed in the overall study population (38.5% and 41.3%). Thus, the higher rate of total AEs noted in the first week with active treatment did not appear to continue during the following month.
As stated above, EPS, weight gain, prolactin-related effects, and sedation have been identified as areas of concern with antipsychotic treatment in early illness patients. Further, it is relevant to note that these tolerability issues have also been associated with nonadherence, a particular concern in these patients [Kelly et al. 2005]. Since related AE rates were low in this dataset, they were further examined over the entire study period (versus just the first 36 days). Among the specific types of EPS, Parkinsonism was the most commonly reported in the recently diagnosed patients receiving paliperidone palmitate 150/100 mg eq (234/156 mg), and hyperkinesia in those receiving placebo. The literature is somewhat mixed on which types of EPS the early illness patients are likely to experience, with reports suggesting akathisia, dystonia, and Parkinsonism [Kelly et al. 2005; Janno et al. 2004; Seretti et al. 2004; Kasper, 1999]. Hence, the Parkinsonism finding was not unexpected for these patients, and consistent with the known tolerability profile for the paliperidone molecule [Canuso et al. 2010; Nasrallah et al. 2010; Pandina et al. 2010; Hough et al. 2009; Davidson et al. 2007; Kane et al. 2007; Kramer et al. 2007; Marder et al. 2007]. Further, the RR for anxiety was 4.8 (95% CI 0.24 to 95.76) in the recently diagnosed subgroup during the month after the day 8 initiation dose. Importantly, it cannot be ruled out that some reports of anxiety may actually be associated with akathisia. Thus, the reports of Parkinsonism, and possibly anxiety, in this subgroup analysis may raise the consideration of using lower initiation doses or lower subsequent monthly doses in patients with recently diagnosed schizophrenia. Relevant to this issue, it is important to note that lower initiation doses of paliperidone palmitate have been associated with subtherapeutic plasma levels [Gopal et al. 2010]. Further, this study was designed so that patients who received the recommended initiation doses of paliperidone palmitate then received 100 mg eq (156 mg) monthly dosing during the 13-week trial. However, since subsequent monthly dosing with paliperidone palmitate may range from 25 to 150 mg eq (39–234 mg), doses lower than 100 mg eq (156 mg) may be appropriate for some patients.
There were few reports of potentially prolactin-related effects in this dataset, and none reported in more patients receiving active treatment than placebo, except for the one report of galactorrhea in the paliperidone palmitate group compared with none in the placebo group. Also, the data on weight (mean weight gain and reports of weight gain) and sedation, observed in this dataset, did not suggest a substantial susceptibility in the recently diagnosed subgroup compared with the overall study population during this 13-week study period.
The original study was not designed to answer the question posed here, and as such, several limitations must be considered. First, it is important to note that this was a subgroup analysis, and the low number of patients limited the ability to identify, differentiate, or make conclusions regarding the risk of infrequent or rare AEs. Second, the 5-year cutoff as a definition for a recent diagnosis relied on historical information which may not be accurate, and many patients are ill for a significant period of time before receiving a formal diagnosis. In fact, identifying the onset of schizophrenia is often challenging because of the range of defining events [Tandon et al. 2009]. Nonetheless, it is generally accepted that the first 5–10 years of illness is a critical period for effective intervention [Francey et al. 2010; McGorry et al. 2008, 2007; Kelly et al. 2005; Marshall et al. 2005; Harrigan et al. 2003]. The 5-year cutoff used here should have captured a population enriched for this stage of the illness. However, a first episode population may have shown a greater level of intolerance.
Of note, the data presented here focused on the first 36 days of treatment to examine the tolerability specifically associated with the initiation doses of paliperidone palmitate (150 mg eq on day 1 and 100 mg eq on day 8; 234 and 156 mg respectively) in this sensitive patient population. It is important to remember that this study protocol permitted clinicians to administer the second initiation dose in either the gluteal or deltoid muscle, which differs somewhat from the recommended regimen that both initiation doses be administered in the deltoid muscle. In addition, longer-term tolerability is an important issue which could not be addressed in this 13-week study. Longer-term data have been reported elsewhere for broader patient populations [Hough et al. 2009], and an initial analysis was reported for those recently diagnosed [Alphs et al. 2009].
In this dataset, measures of symptomatology suggest that the recently diagnosed subgroup is quite responsive to treatment with paliperidone palmitate, without oral supplementation. The PANSS effect sizes for treatment versus placebo were similar in this subgroup to those observed in the overall study population, although they did not reach statistical significance in the former group for CGI and PSP (partly because of the small number of patients). The responsiveness of symptoms to treatment has been published in reports regarding first-episode patients [Ucok et al. 2004; Robinson et al. 1999]. Our finding confirms that tolerability with medications, not lack of efficacy, is an area of primary concern when managing these patients with early illness.
Current knowledge suggests that early detection and a shorter duration of untreated psychosis are key factors to optimizing outcome in patients with schizophrenia [Francey et al. 2010; Ucok et al. 2004; McGlashan et al. 2001; Falloon et al. 1996]. Thus, early comprehensive psychosocial interventions and antipsychotic medications, when clinically indicated, are typical standards of care for these patients [Francey et al. 2010; Kelly et al. 2005; Lieberman et al 2001]. While challenges to this dogma of early antipsychotic use have been raised [Francey et al. 2010], treatment is generally required for many patients with early illness and evident psychosis. While these patients are often responsive to the efficacy benefits of pharmacological agents, tolerability and adherence to treatment remain key areas of concern [Kelly et al. 2005; Fleischhacker et al. 1994]. Although none of the AEs identified in the first 36 days after initiating paliperidone palmitate treatment had a statistically significant RR as determined by 95% CIs, the small number of patients limited our ability to detect significance. Nonetheless, these data do identify AEs that clinicians may encounter when managing these patients. Thus, the findings presented here may help guide clinicians when paliperidone palmitate is considered an appropriate treatment of choice for these patients.
Acknowledgements
Editorial support was provided by Susan Ruffalo, PharmD, MedWrite, Inc., Newport Coast, California. The authors would like to acknowledge the contributions of J. Thomas Haskins, PhD of Johnson & Johnson PRD, Titusville, New Jersey in the development of these analyses. All authors provided assistance and direction in the data collection and analysis of this study, and in the preparation of the manuscript. These data were presented at the American Psychiatric Association Annual Meeting, 22–26 May 2010. This study is registered at ClinicalTrials.gov (NCT00590577).
Funding
This research was funded by Ortho-McNeil Janssen Scientific Affairs, Titusville, New Jersey, USA.
Conflicts of interest statement
Drs Alphs, Fu, Bossie, and Sliwa are employees of Ortho-McNeil Janssen Scientific Affairs, Titusville, New Jersey, and Dr Ma is an employee of Johnson and Johnson Pharmaceutical Research and Development (PRD), Titusville, New Jersey.
References
- Allison D.B., Casey D.E. (2001) Antipsychotic-induced weight gain: A review of the literature. J Clin Psychiatry 62(Suppl. 7): 22–31 [PubMed] [Google Scholar]
- Alphs L., Bossie C., Sliwa J.K., Ma Y.-W., Haskins J.T. (2009) Paliperidone palmitate: Clinical response in subjects with schizophrenia with recent diagnosis vs longer-time since diagnosis. American Psychiatric Association [Poster NR6-027] 16–21 May, San Francisco, CA [Google Scholar]
- Alvarez-Jimenez M., Gonzalez-Blanch C., Crespo-Facorro B., Hetrick S., Rodriguez-Sanchez J.M., Perez-Iglesias R., et al. (2008) Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: A systematic critical reappraisal. CNS Drugs 22: 547–562 [DOI] [PubMed] [Google Scholar]
- Barnes T.R., Leeson V.C., Mutsatsa S.H., Watt H.C., Hutton S.B., Joyce E.M. (2008) Duration of untreated psychosis and social function: 1-year follow-up study of first-episode schizophrenia. Br J Psychiatry 193: 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler M. (1972) The Schizophrenic Disorders: Long-term Patient and Family Studies London: Yale University Press [Google Scholar]
- Bobes J., Gilbert J., Ciudad A., Alvarez E., Canas F., Carrasco J.-L., et al. (2003) Safety and effectiveness of olanzapine versus conventional antipsychotics in the acute treatment of first-episode schizophrenic inpatients. Prog Neuropsychopharmacol Biol Psychiatry 27: 473–481 [DOI] [PubMed] [Google Scholar]
- Canuso C.M., Bossie C.A., Amatniek J., Turkoz I., Pandina G., Cornblatt B. (2010) Paliperidone extended-release tablets in patients with recently diagnosed schizophrenia. Early Interv Psychiatry 4: 64–78 [DOI] [PubMed] [Google Scholar]
- Chue P., Emsley R. (2007) Long-acting formulations of atypical antipsychotics: Time to reconsider when to introduce depot antipsychotics. CNS Drugs 21: 441–448 [DOI] [PubMed] [Google Scholar]
- Coldham E.L., Addington J., Addington D. (2002) Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr Scand 106: 286–290 [DOI] [PubMed] [Google Scholar]
- Davidson M., Emsley R., Kramer M., Ford L., Pan G., Lim P., et al. (2007) Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): Results of a 6-week, randomized, placebo-controlled study. Schizophr Res 93(1–3): 117–130 [DOI] [PubMed] [Google Scholar]
- Emsley R., Medori R., Koen L., Oosthuizen P.P., Niehaus D., Rabinowitz J. (2008) Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: A preliminary study. J Clin Psychopharmacol 28: 210–213 [DOI] [PubMed] [Google Scholar]
- Falloon I.R., Kydd R.R., Coverdale J.H., Laidlaw T.M. (1996) Early detection and intervention for initial episodes of schizophrenia. Schizophr Bull 22: 271–282 [DOI] [PubMed] [Google Scholar]
- Fleischhacker W.W., Meise U., Gunther V., Kurz M. (1994) Compliance with antipsychotic drug treatment: Influence of side effects. Acta Psychiatr Scand 89(Suppl.): 11–15 [PubMed] [Google Scholar]
- Francey S.M., Nelson B., Thompson A., Parker A.G., Kerr M., Macneil C. (2010) Who needs antipsychotic medication in the earliest stages of psychosis? A reconsideration of benefits, risks, neurology and ethics in the era of early intervention. Schizophr Res 119: 1–10 [DOI] [PubMed] [Google Scholar]
- Gilmer T.P., Dolder C.R., Lacro J.P., Folsom D.P., Lindamer L., Garcia P., et al. (2004) Adherence to treatment with antipsychotic medication and healthcare costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 161: 692–699 [DOI] [PubMed] [Google Scholar]
- Gopal S., Gassmann-Mayer C., Palumbo J., Samtani M.N., Shlwach R., Alphs L. (2010) Practical guidance for dosing and switching paliperidone palmitate treatment in patients with schizophrenia. Curr Med Res Opin 26: 377–387 [DOI] [PubMed] [Google Scholar]
- Gupta S., Frank B., Madhusodanan S. (2001) Risperidone-associated galactorrhea in a male teenager. J Am Acad Child Adolesc Psychiatry 40: 504–505 [DOI] [PubMed] [Google Scholar]
- Harrigan S.M., McGorry P.D., Krstev H. (2003) Does treatment delay in first-episode psychosis really matter? Psychol Med 33: 97–110 [DOI] [PubMed] [Google Scholar]
- Hough D., Gopal S., Vijapurkar U., Lim P., Morozova M., Eerdekens M. (2010) Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled study. Schizophr Res 116: 107–117 [DOI] [PubMed] [Google Scholar]
- Hough D., Lindenmayer J.P., Gopal S., Melkote R., Lim P., Herben V., et al. (2009) Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33: 1022–1031 [DOI] [PubMed] [Google Scholar]
- Janno S., Holi M., Tuisku K., Wahlbeck K. (2004) Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am J Psychiatry 161: 160–163 [DOI] [PubMed] [Google Scholar]
- Kane J., Canas F., Kramer M., Ford L., Gassmann-Mayer C., Lim P., et al. (2007) Treatment of schizophrenia with paliperidone extended-release tablets: A 6-week placebo-controlled trial. Schizophr Res 90: 147–161 [DOI] [PubMed] [Google Scholar]
- Kasper S. (1999) First-episode schizophrenia: the importance of early intervention and subjective tolerability. J Clin Psychiatry 60(Suppl. 23): 5–9 [PubMed] [Google Scholar]
- Keith S.J., Kane J. (2003) Partial compliance and patient consequences in schizophrenia: Our patients can do better. J Clin Psychiatry 64: 1308–1315 [DOI] [PubMed] [Google Scholar]
- Kelly D.L., Conley R.R., Carpenter W.T. (2005) First-episode schizophrenia: A focus on pharmacological treatment and safety considerations. Drugs 65: 1113–1138 [DOI] [PubMed] [Google Scholar]
- Kim B., Lee S.H., Choi T.K., Suh S.Y., Kim Y.W., et al. (2008) Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry 32: 1231–1235 [DOI] [PubMed] [Google Scholar]
- Kramer M., Simpson G., Maciulis V., Kushner S., Vijapurkar U., Lim P., et al. (2007) Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: A randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 27: 6–14 [DOI] [PubMed] [Google Scholar]
- Lieberman J.A., Drake R.E., Sederer L.I., Belger A., Keefer R., Perkins D., et al. (2008) Science and recovery in schizophrenia. Psychiatr Serv 59: 487–496 [DOI] [PubMed] [Google Scholar]
- Lieberman J.A., Perkins D., Belger A., Chakos M., Jarskog F., Boteva K., et al. (2001) The early stages of schizophrenia: Speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry 50: 884–897 [DOI] [PubMed] [Google Scholar]
- Llorca P.M., Chereau I., Bayle F.J., Lancon C. (2002) Tardive dyskinesias and antipsychotics: A review. Eur Psychiatry 17: 129–138 [DOI] [PubMed] [Google Scholar]
- Marder S.R., Kramer M., Ford L., Eerdekens E., Lim P., Eerdekens M., et al. (2007) Efficacy and safety of paliperidone extended-release tablets: Results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry 62: 1363–1370 [DOI] [PubMed] [Google Scholar]
- Marshall M., Lewis S., Lockwood A., Drake R., Jones P., Croudace T. (2005) Association between duration of untreated psychosis and in cohorts of first-episode outcome patients – a systematic review. Arch Gen Psychiatry 62: 975–983 [DOI] [PubMed] [Google Scholar]
- Masi G., Cosenza A., Mucci M. (2001) Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. J Child Adolesc Psychopharmacol 11: 389–394 [DOI] [PubMed] [Google Scholar]
- McGlashan T.H., Fenton W.S. (1993) Subtype progression and pathophysiologic deterioration in early schizophrenia. Schizophr Bull 19: 71–84 [DOI] [PubMed] [Google Scholar]
- McGlashan T.H., Miller T.J., Woods S.W. (2001) Pre-onset detection and intervention research in schizophrenia psychoses: Current estimates of benefit and risk. Schizophr Bull 27: 563–570 [DOI] [PubMed] [Google Scholar]
- McGorry P. (2005) and Group IEPAW International clinical practice guidelines for early psychosis. Br J Psychiatry 187(Suppl. 48): S120–S124 [DOI] [PubMed] [Google Scholar]
- McGorry P.D., Killackey E., Yung A.R. (2007) Early intervention in psychotic disorders: Detection and treatment of the first episode and the critical early stages. Med J Aust 187(7 Suppl.): S8–S10 [DOI] [PubMed] [Google Scholar]
- McGorry P.D., Killackey E., Yung A.R. (2008) Early intervention in psychosis: Concepts, evidence and future directions. World Psychiatry 7: 148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzin J., Boulanger L., Friedman M., Mackell J., Lloyd J.R. (2003) Treatment adherence associated with conventional and atypical antipsychotics in a large state Medicaid program. Psychiatr Serv 54: 719–723 [DOI] [PubMed] [Google Scholar]
- Merlo M.C.G., Hofer H., Gekle W., Berger G., Ventura J., Panhuber I., et al. (2002) Risperidone, 2 mg/day vs 4 mg/day, in first-episode, acutely psychotic patients: Treatment efficacy and effects on fine motor functioning. J Clin Psychiatry 63: 885–991 [DOI] [PubMed] [Google Scholar]
- Muench J., Carey M. (2001) Diabetes mellitus associated with atypical antipsychotic medications: New case report and review of the literature. J Am Board Fam Pract 14: 278–282 [PubMed] [Google Scholar]
- Mueser K.T., McGurk S.R. (2004) Schizophrenia. Lancet 363: 2063–2072 [DOI] [PubMed] [Google Scholar]
- Nasrallah H., Gopal S., Gassmann-Mayer C., Quiroz J.A., Lim P., Eerdekens M., et al. (2010) A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 35: 2072–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandina G., Lindenmayer J.P., Lull J., Lim P., Gogal S., Herben V., et al. (2010) A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol 30: 235–244 [DOI] [PubMed] [Google Scholar]
- Parellada E., Andrezina R., Milanova V., Glue P., Masiak M., St John Turner M., et al. (2005) Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol 19: 5–14 [DOI] [PubMed] [Google Scholar]
- Perkins D.O., Gu H., Boteva K., Liberman J.A. (2005) Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: A critical review and meta-analysis. Am J Psychiatry 162: 1785–1804 [DOI] [PubMed] [Google Scholar]
- Robinson D., Woerner M.G., Alvir J.M., Bilder R., Goldman R., Geisler S., et al. (1999) Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 56: 241–247 [DOI] [PubMed] [Google Scholar]
- Salimi K., Jarskog L.F., Lieberman J.A. (2009) Antipsychotic drugs for first-episode schizophrenia: A comparative review. CNS Drugs 23: 837–855 [DOI] [PubMed] [Google Scholar]
- Sanger T.M., Lieberman J.A., Tohen M., Grundy S., Beasley C., Jr, Tollefson G.D. (1999) Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry 156: 79–87 [DOI] [PubMed] [Google Scholar]
- Schooler N., Rabinowitz J., Davidson M., Emsley R., Harvey P.D., Kopala L., et al. (2005) Risperidone and haloperidol in first-episode psychosis: A long-term randomized trial. Am J Psychiatry 162: 947–953 [DOI] [PubMed] [Google Scholar]
- Seretti A., De Ronchi D., Lorenzi C., Berardi D. (2004) New antipsychotics and schizophrenia: A review of efficacy and side effects. Curr Med Chem 11: 343–358 [DOI] [PubMed] [Google Scholar]
- Tandon R., Nasrallah H.A., Keshavan M.S. (2009) Schizophrenia, ‘just the facts’ 4: Clinical features and conceptualization. Schizophr Res 110: 1–23 [DOI] [PubMed] [Google Scholar]
- Tschoner A., Eng J., Laimer M., Kaser S., Rettenbacher M., Fleischhacker W.W., et al. (2007) Metabolic side effects of antipsychotic medication. Int J Clin Pract 61: 1356–1370 [DOI] [PubMed] [Google Scholar]
- Ucok A., Polar A., Genc A., Caku S., Turan N. (2004) Duration of untreated psychosis may predict acute treatment response in first-episode schizophrenia. J Psychiatr Res 38: 163–168 [DOI] [PubMed] [Google Scholar]
- Valenstein M., Blow F.C., Copeland L.A., McCarthy J.R., Zeber J.E., Gillon L., et al. (2004) Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull 30: 255–264 [DOI] [PubMed] [Google Scholar]
- Weiden P.J., Schooler N.R., Weedon J.C., Elmouchtari A., Sunakawa A., Goldfinger S.M. (2009) A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: Initial adherence outcome. J Clin Psychiatry 70: 1397–1406 [DOI] [PubMed] [Google Scholar]
- Woods S.W., Martin A., Spector S.G., McGlashan T.H. (2002) Effects of development on olanzapine-associated adverse events. J Am Acad Child Adolesc Psychiatry 41: 439–446 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2007) Mental Health and Substance Abuse. Facts and Figures. Schizophrenia: Youth’s Greatest Disabler. Available at: http://www.searo.who.int/en/section1174/section1199/Section 1567_6755.htm (accessed May 2011)
- Wudarsky M., Nicolson R., Hamberger S.D., Spechler L., Gochman P., Bedwell J., et al. (1999) Elevated prolactin in pediatric patients on typical and atypical antipsychotics. J Child Adolesc Psychopharmacol 9: 239–245 [DOI] [PubMed] [Google Scholar]
- Wyatt R.J. (1991) Early intervention with neuroleptics may decrease the long-term morbidity of schizophrenia. Schizophr Res 5: 201–202 [DOI] [PubMed] [Google Scholar]