Abstract
Objective: Medication errors are a common cause of avoidable morbidity, and transfer between clinical settings is a known risk factor for such errors. Medicines reconciliation means there is no unintended discrepancy between the medication prescribed for a patient prior to admission and on admission. Our aim was to improve the quality of practice supporting medicines reconciliation at the point of admission to a psychiatric ward.
Methods: An audit-based quality improvement programme (QIP), using the proxy measure for medicines reconciliation of two or more sources of information being consulted about current medicines, and compared.
Results: At baseline audit, 42 Trusts submitted data for 1790 patients. At re-audit 16 months later, 43 Trusts submitted data for 2296 patients. While doctors were most commonly identified in Trust policies as having overall responsibility for medicines reconciliation, the task was most often undertaken by pharmacy staff, with most activity occurring within 24 h of admission. The proportion of patients in whom medicines reconciliation was possible was 71% at baseline and 79% at re-audit. In such patients, discrepancies were identified in 25% at baseline and 31% at re-audit; a small proportion of these discrepancies were clearly clinically significant.
Conclusions: This QIP achieved modest improvement in medicines reconciliation practice.
Keywords: xxxx, xxxx
Introduction
Medication error is recognized as a common cause of avoidable morbidity and mortality across all areas of healthcare [Dean Franklin et al. 2005]. In hospitalized patients, approximately 20% of clinical negligence claims are due to medication error [Audit Commission, 2001]. It is therefore a clinical priority to understand the causes of these errors and develop systems to minimize them.
Errors can happen at the point a medicine is prescribed, dispensed or administered. At each stage in the process the root cause may be a simple lapse in concentration, a problem with decision making or a knowledge deficit. The point of transfer between care settings, and in particular hospital admission, is a known period of high risk for prescribing errors [National Institute for Health and Clinical Excellence and National Patient Safety Agency, 2007]. Immediately prior to admission, a patient may be taking a combination of medicines, some of which may have been prescribed in primary care, some by a hospital specialist, and some may have been obtained without the need for a prescription. It is therefore unlikely that any single source will consistently provide accurate and reliable information about all medicines that are being taken. In the UK, a technical patient safety solution was issued jointly by the National Institute for Health and Clinical Excellence (NICE) and the National Patient Safety Agency (NPSA) in December 2007 with the requirement that the recommendations be implemented by December 2008 [National Institute for Health and Clinical Excellence and National Patient Safety Agency, 2007]. The safety solution focused on the importance of medicines reconciliation, the aim of which is to ensure that medicines prescribed on admission to hospital do not differ unintentionally from those that the patient was taking immediately prior to admission. It is recommended that this is achieved by: collecting information on current medication using the most recent and accurate sources of information to create a full and current list of medicines (for example, the primary care repeat-prescribing record supplemented by information from the patient and/or carer); checking or verifying this list against the current prescription chart in the hospital, ensuring any discrepancies are accounted for and actioned appropriately; and communicating, through appropriate documentation, any changes, omissions and discrepancies. Further recommendations contained in the patient safety solution are shown in Table 1.
Table 1.
National Institute for Health and Clinical Excellence and National Patient Safety Agency recommendations for medicines reconciliation [National Institute for Health and Clinical Excellence and National Patient Safety Agency, 2007].
| 1. All healthcare organisations that admit adult inpatients should put policies in place for medicines reconciliation on admission. This includes mental health units, and applies to elective and emergency admissions. |
| 2. In addition to specifying standardized systems for collecting and documenting information about current medications, policies for medicines reconciliation on admission should ensure that: |
| a. pharmacists are involved in medicines reconciliation as soon as possible after admission |
| b. the responsibilities of pharmacists and other staff in the medicines reconciliation process are clearly defined; these responsibilities may differ between clinical areas |
| c. strategies are incorporated to obtain information about medicines for people with communication difficulties. |
The Prescribing Observatory for Mental Health (POMH-UK) conducts quality improvement programmes (QIPs) that focus on different aspects of prescribing practice in mental health services in the UK. We report here on the findings from a QIP on medicines reconciliation in psychiatric inpatient settings.
Methods
POMH-UK invited all NHS Trusts in the UK providing specialist mental health services to participate in an audit-based quality improvement programme focusing on medicines reconciliation. Clinical and clinical audit staff from each Trust that agreed to take part were invited to attend a regional introductory workshop to discuss and review the aims, objectives and methods of the QIP. Comments and discussions at the workshops led to refinements of the audit methods and data collection tool.
Initially, a questionnaire was sent to each participating Trust. The following data were collected: whether the Trust had an approved (or draft) policy for medicines reconciliation that covered patients being admitted to hospital; whether the policy stated who (which group/groups of clinical staff) was responsible overall for ensuring medicines reconciliation was completed; and whether the policy specified the sources of information that should be used to determine which medicines in which doses the patient was taking prior to admission, the timeframe over which this should occur, and where in the patient’s clinical record this information should be documented.
At baseline (February 2009) an audit of clinical practice was conducted. A bespoke audit tool was supplied to each participating Trust with instructions that copies should be made available to allow clinical teams in acute adult, acute elderly and forensic wards to audit a minimum of five consecutive admissions each, working backwards from the end of February 2009. The instructions also specified that the data should be gathered after the patient had been admitted for at least 7 days. The following data were collected for each patient: age, sex, ethnicity, diagnostic grouping using International Classification of Diseases, 10th revision (ICD-10) codes, time of admission, whether the patient was detained under the Mental Health Act, and the clinical setting in which the patient was treated (acute adult, acute elderly, or forensic ward); and documented details of medicines prior to admission (prescribed and nonprescribed), and whether these were being taken.
For each patient, the clinical team were asked which (if any) sources of information about medication they had checked within 24 h, 3 days and 1 week of admission to hospital, and whether any of these sources identified a discrepancy (i.e. yielded information that was different from that obtained from the initial or primary source). Members of the clinical team were also asked whether a pharmacist and/or medicines management technician had been involved in medicines reconciliation and, if so, how long after admission this had taken place. The clinical records were then cross-checked to determine whether the actions taken by the clinical team were documented, providing a measure of whether what was written accurately reflected what was done. Finally, clinical teams were given the option of giving narrative accounts of any discrepancies found during the process of medicines reconciliation. The primary purpose of this additional data collection was to inform discussion within Trusts and individual clinical teams of the nature of medicines reconciliation errors locally, and not to generate national data that would be suitable for methodologically robust qualitative review.
Data were collected using SNAP (electronic survey software), and stored and analysed using SPSS.
Each Trust was subsequently provided with a customized audit report that contained: the national findings; their overall performance in relation to the standards benchmarked against other participating Trusts and the total national sample; and, finally, the performance of each clinical team in that Trust benchmarked against the Trust as a whole and the total national sample. Each participating Trust was identified by a numerical code known only to that Trust and POMH-UK. POMH-UK did not have access to the key to team codes. Trusts were also provided with customized slide sets to facilitate local dissemination of the audit findings, and an Excel file containing their own data for further local analysis if desired.
A re-audit of clinical practice, using the same data collection tool and methods as at baseline, was conducted 16 months later (June 2010).
Results
Questionnaire
A total of 45 Trusts submitted a completed questionnaire describing the status and content of their medicines reconciliation policy. Out of these, 21 Trusts had an approved stand-alone policy for medicines reconciliation, 4 had included medicines reconciliation as part of a policy that had a wider scope, 11 had a policy in draft form and the remaining 9 did not have a policy in any form. Of the 36 Trusts that had a policy, 32 stated who had overall responsibility for ensuring medicines reconciliation was undertaken: this was the admitting doctor (10 Trusts), pharmacist (6 Trusts), pharmacy/medicines management technician (5 Trusts), doctor from the clinical team providing care for the patient (2 Trusts), consultant psychiatrist (1 Trust), nurse (1 Trust) or other member of staff (7 Trusts). The sources of information that should be used to access accurate information about a patient’s medication were described in 35 policies and the timeframe over which this should be done in 30 (within 1 day in 10 Trusts, 2 days in 2 Trusts, 3 days in 8 Trusts, 7 days in 1 Trust, and other timeframes in 8 Trusts). There were 32 policies that stated where in a patient’s clinical record information pertaining to medicines reconciliation should be recorded. Only 10 (22%) policies could be considered comprehensive in that they covered all of the following: who was responsible, in what timeframe and where medicines reconciliation should be documented in the clinical records.
Audits of clinical practice
Patient samples
At baseline, 42 Trusts submitted data for 1790 patients under the care of 375 clinical teams. At re-audit, 43 Trusts submitted data for 2296 patients under the care of 455 clinical teams. Five Trusts that participated at baseline did not participate at re-audit and six Trusts participated for the first time at re-audit.
The characteristics of patients in the baseline and re-audit samples, including demographics, diagnostic groupings, Mental Health Act status and types of admitting service, are shown in Table 2. With respect to the time of admission, in the baseline audit, 44% were admitted between 9 a.m. and 5 p.m. Monday to Friday, 33% between 5 p.m. and 9 a.m. on weekday nights, and 16% at the weekend, between 5 p.m. Friday and 9 a.m. Monday. For the remaining 7%, the time of admission was unknown. The respective figures at re-audit were 45%, 33%, 17% and 5%.
Table 2.
Demographic and clinical characteristics of the patient samples at baseline (n = 1790) and re-audit (n = 2296).
| Key demographic characteristics | Acute adult |
Acute elderly |
Forensic |
||||
|---|---|---|---|---|---|---|---|
| Baseline n = 1055 | Re-audit n = 1338 | Baseline n = 614 | Re-audit n = 683 | Baseline n = 121 | Re-audit n = 275 | ||
| Sex n (%) | Male | 550 (52%) | 694 (52%) | 249 (41%) | 274 (40%) | 102 (84%) | 232 (84%) |
| Ethnicity n (%) | White/White British | 822 (78%) | 980 (73%) | 551 (90%) | 601 (88%) | 93 (13%) | 182 (66%) |
| Black/Black British | 74 (7%) | 128 (10%) | 14 (2%) | 12 (2%) | 16 (3%) | 52 (19%) | |
| Asian | 71 (7%) | 104 (7%) | 4 (1%) | 14 (2%) | 4 (3%) | 11 (4%) | |
| Mixed or other | 88 (8%) | 126 (9%) | 45 (7%) | 54 (8%) | 8 (7%) | 30 (11%) | |
| Age | Mean age in years (SD) | 42 (13.5) | 41 (13.6) | 77 (8.6) | 77 (8.9) | 37 (10.5) | 36 (13%) |
| Age bands n (%) | 16–25 years | 135 (13%) | 189 (14%) | 0 (0%) | 0 (0%) | 21 (17%) | 52 (19%) |
| 26–35 years | 248 (24%) | 310 (23%) | 2 (0%) | 2 (0%) | 36 (30%) | 94 (34%) | |
| 36–45 years | 262 (25%) | 329 (25%) | 0 (0%) | 1 (0%) | 38 (31%) | 70 (25%) | |
| 46–55 years | 220 (21%) | 280 (21%) | 3 (0%) | 3 (0%) | 20 (17%) | 45 (16%) | |
| 56–65 years | 155 (15%) | 179 (13%) | 28 (5%) | 33 (5%) | 6 (5%) | 9 (3%) | |
| 66 years and over | 35 (3%) | 52 (4%) | 581 (95%) | 644 (94%) | 0 (0%) | 5 (2%) | |
| Detained under Mental Health Act n (%) | Yes | 462 (44%) | 658 (49%) | 144 (23%) | 174 (25%) | 120 (99%) | 249 (90%) |
| ICD-10* n (%) | F00–F09 | 15 (1%) | 13 (1%) | 263 (43%) | 269 (39%) | 2 (2%) | 5 (2%) |
| F10–F19 | 57 (5%) | 86 (6%) | 5 (1%) | 18 (3%) | 4 (3%) | 13 (5%) | |
| F20–F29 | 464 (44%) | 555 (41%) | 75 (12%) | 67 (10%) | 77 (64%) | 168 (61%) | |
| F30–F39 | 336 (32%) | 386 (29%) | 190 (31%) | 224 (33%) | 12 (10%) | 26 (9%) | |
| Other diagnoses | 103 (10%) | 138 (10%) | 23 (4%) | 25 (4%) | 11 (9%) | 45 (16%) | |
| Diagnosis not yet reached | 80 (8%) | 160 (12%) | 58 (9%) | 80 (12%) | 15 (12%) | 18 (7%) | |
F00–F09 = organic, including symptomatic, mental disorders (e.g. dementia); F10–F19 = mental and behavioural disorders due to psychoactive substance misuse; F20–F29 = schizophrenia, schizotypal and delusional disorders; F30–F39 = mood (affective) disorders (e.g. bipolar affective disorder, recurrent depressive disorder).
ICD-10, International Classification of Diseases, 10th revision.
Clinical teams’ accounts of medicines reconciliation
The sources of information that were checked by members of the clinical team within 24 h, 3 days and 7 days of admission at baseline and re-audit are shown in Table 3. At baseline, within 7 days of admission, patients who were admitted to adult settings were more likely to have been asked about the medication they were taking (736/1055, 70%) than those admitted to elderly settings (209/614, 34%) (χ2 = 201.6, p < 0.001). Those patients in elderly settings (371/614, 60%) were more likely to have had particular sources of information checked compared with those in adult care settings: consultation with their GP (488/1055, 46%) (χ2 = 31.2, p < 0.001); examination of their medication (258/614, 42% versus 179/1055, 17%; χ2 = 126.0, p < 0.001); and enquiry of their carer (171/614, 28% versus 148/1055, 14%; χ2 = 61.3, p < 0.001) or residential or care home (97/614, 16% versus 29/1055, 3%; χ2 = 94.6, p < 0.001).
Table 3.
Sources of information about medicines, used during the reconciliation process, in acute adult inpatient settings at baseline and re-audit.
| Information source n (%) | Baseline (n = 1055) |
Re-audit (n = 1338) |
||||
|---|---|---|---|---|---|---|
| Number of days after admission | Number of days after admission | |||||
| 1 | 3 | 7 | 1 | 3 | 7 | |
| Patient asked | 677 (64%) | 722 (68%) | 736 (70%) | 784 (59%) | 863 (64%) | 893 (67%) |
| GP consulted | 327 (31%) | 455 (43%) | 488 (46%) | 532 (40%) | 762 (57%) | 854 (64%) |
| CMHC record consulted | 451 (43%) | 499 (47%) | 519 (49%) | 522 (39%) | 614 (46%) | 640 (48%) |
| Medication examined | 156 (15%) | 174 (16%) | 179 (17%) | 201 (15%) | 224 (17%) | 238 (18%) |
| Carer asked | 127 (12%) | 140 (13%) | 148 (14%) | 154 (12%) | 176 (13%) | 178 (13%) |
| Residential or care home asked | 27 (3%) | 27 (3%) | 29 (3%) | 25 (2%) | 32 (2%) | 32 (2%) |
| Other source used | 153 (15%) | 191 (18%) | 197 (18%) | 198 (15%) | 246 (18%) | 266 (20%) |
By the time of the re-audit, there had been an increase in the use of the GP records, within 7 days of admission, as a source of information about the medicines being taken in patients admitted to either adult (46% at baseline versus 64% at re-audit; χ2 = 73.9, p 70.001) or elderly wards (from 60% to 73%; χ2 = 22.4, p < 0.001). The use of other sources did not change significantly. All of the sources checked had yielded potentially clinically significant discrepancies. The findings showed that: primary care (GP) records were a source of information about drugs prescribed for physical illness; patients and carers were a source of information about medication that was actually being taken; and community mental health team records were a source of information about depot antipsychotic medication.
Regarding the staff members who undertook the task of medicines reconciliation, at baseline, pharmacy staff were involved with 1251 (70%) patients in the total sample: 467 patients (26%) within 1 day of admission, an additional 533 patients by 3 days (30%) and a further 251 within 7 days (14%). The respective figures at re-audit were 1902 (83%) for the total sample, and 749 (33%), 713 (31%) and 440 (19%) for the 1-, 3- and 7-day involvement. At baseline, 62% of all discrepancies identified in acute adult settings were found by pharmacy staff (pharmacist, pharmacy or medicines management technicians). This proportion increased to 80% at re-audit. With respect to the involvement of doctors in medicines reconciliation, 24% of discrepancies in acute adult settings at baseline were identified by doctors and at re-audit this proportion had fallen to 14%. Across the total sample, the two sources that, when consulted, were most likely to identify a discrepancy were the primary care record (14% at baseline and 17% at re-audit) and asking the patient (6% at baseline and 7% at re-audit).
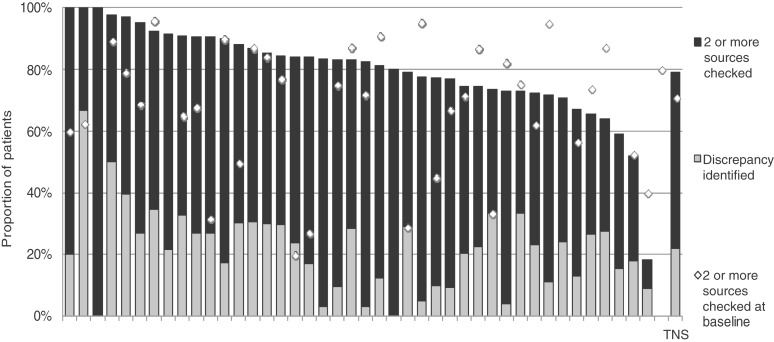
Medicines reconciliation can only be achieved when two or more sources of information are consulted about current medicines, and compared. The proportion of patients across all participating services in whom medicines reconciliation was possible because more than one source was checked is shown in Figure 1. Figure 1 also shows the proportion of such patients in whom a discrepancy was identified at re-audit (31%). The respective proportion at baseline audit had been 25%.
Figure 1.
The proportions of patients in each participating Trust for whom two or more sources of information about medicines being taken were checked (i.e. medicines reconciliation was possible) and the proportions for whom discrepancies were identified: re-audit. Each bar represents a different Trust. The white diamond indicates the proportion of patients for whom two or more sources of information about medication were checked in the baseline audit; the difference between the white diamond and the top of the black bar indicates change in practice between baseline and re-audit. TNS, total national sample.
Clinical practice with respect to checking two or more sources of information about a patient’s medicines was slightly better at re-audit in Trusts that had had a comprehensive medicines reconciliation policy in place at baseline than in those Trusts that had not, but this difference did not reach statistical significance (t = −0.021, DF = 36, p = 0.081).
Documentation of medicines reconciliation
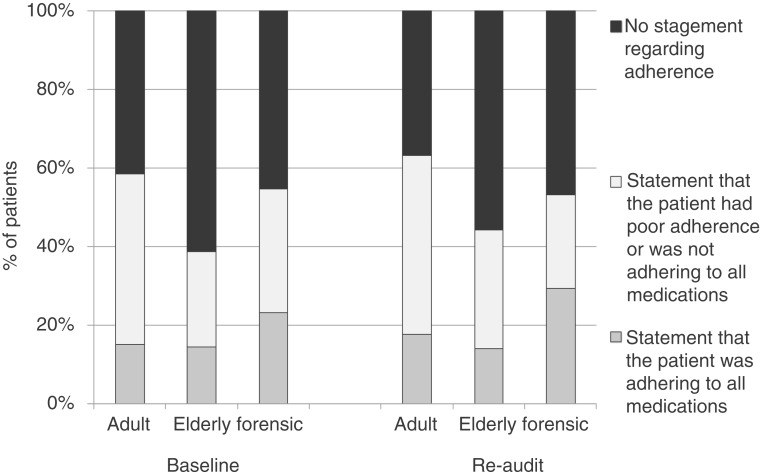
With respect to documentation of medication prescribed prior to admission at baseline, 122 (7%) of the total national sample of patients were not prescribed any medication, for 1089 (61%) the drug(s) and dose(s) were clearly recorded, for 176 (10%) the name of the drug(s) only was recorded, for 180 (10%) there was reference to medication but no drug name(s) or dose(s), and for 190 (11%) there was no reference to medication at all. The respective proportions at re-audit were very similar (6%, 65%, 10%, 10% and 10%). In those patients who were known to have been prescribed medication prior to admission, the proportions in which the drug name and dose were clearly documented were similar across adult, elderly and forensic services, being 73%, 77% and 89% at baseline, and 77%, 77% and 77% at re-audit. Documentation of an assessment of medication adherence is shown in Figure 2.
Figure 2.
Documentation of adherence to prescribed medication in acute adult, acute elderly and forensic services, for those in whom medication was prescribed, at baseline and re-audit. There was no documented statement about adherence to medication in 41% adults, 61% elderly and 45% forensic patients at baseline. At re-audit, these figures were 37%, 56% and 47%, respectively.
Documentation of the reconciliation process overall was generally good and accurately reflected the practice described above in over 80% of cases. Examples of the reconciliation discrepancies described by clinical teams are shown in Table 4. Note that these data are presented in a pragmatic way that is likely to be meaningful to practising clinicians. They were not subject to quantitative or qualitative analyses and no theoretical model was applied.
Table 4.
Examples of discrepancies identified during medicines reconciliation, and their potential to be clinically significant.
| Potential clinical significance | Examples of discrepancies identified |
|---|---|
| Likely to be a problem in the short term | Omitted medicines: LMWH, phenytoin, erythropoietin, methotrexate, goserelin |
| Wrong drug: aripiprazole prescribed in place of omeprazole, amisulpride in place of aripiprazole | |
| Wrong dose: lithium, warfarin, insulin, bumetanide | |
| Previously discontinued medicine prescribed at full treatment dose: methadone, clozapine | |
| Likely to be a problem in the medium term if left undetected | Omitted medicines: antihypertensives, vitamin B12, statins, inhalers, eye drops for glaucoma, depot antipsychotics |
| Previously discontinued medicines prescribed: furosemide, nicorandil | |
| Patients who brought in medicines prescribed for someone else had these medicines prescribed for them: ezetimibe | |
| Unclear but likely to be minor | Omitted medicines: hypnotics, creams, analgesics |
| Variations in timing and/or dose: antipsychotics, antidepressants |
LMWH, low molecular weight heparin.
Discussion
In this QIP, our proxy measure of medicines reconciliation practice was the proportion of newly admitted patients for whom two or more sources of information about the medicines they were taking immediately prior to hospital admission had been checked. We found that this proportion, representing all those patients for whom medicines reconciliation was possible, increased modestly between baseline (71%) and re-audit (79%). Most of the activity related to checking sources occurred in the first 24 h of admission to hospital, irrespective of the time of admission, and most of this activity was documented in patients’ clinical records. The sources of information most frequently consulted were the primary care medical records and patients themselves. The primary care record was the only source of information that was consulted significantly more frequently at re-audit, compared with baseline, and this source also yielded the highest proportion of discrepancies.
Medicines reconciliation as a source of medication error
Of the total national sample for whom two or more sources of information had been checked, discrepancies were identified in 25% at baseline, and 31% at re-audit. These figures are consistent with the published literature, in that discrepancies have been shown to be relatively common at the point of admission to hospital [Strunk et al. 2008; Morcos et al. 2002]. Discrepancies have also been commonly found at other clinical interfaces: between outpatient psychiatric and primary care prescribing records [Robinson, 2008; Clarke, 1993], and, more generally, between medication prescribed on hospital discharge and the primary care record or the medication the patient was actually taking [Glintborg et al. 2007; Morcos et al. 2002]. In describing the background to their technical patient safety solution focusing on medicines reconciliation, the NPSA revealed that over a period of 40 months, 7070 medication errors relating to either admission or discharge medication were received from NHS Trusts [National Patient Safety Agency, 2007]. Of these errors, 30 resulted in severe harm to the patient, and 2 were fatal. The relatively high prevalence of discrepancies in prescribed medicines that were found in our patient sample would seem consistent with these data. Further, despite our finding of medication discrepancies in one quarter of patients for whom medicines reconciliation was possible, only a very small proportion of such discrepancies had the potential for serious harm, and these tended to involve drugs prescribed for physical illness. Thus, only a very small proportion of such discrepancies would be considered clinically significant, at least in the short term, an outcome that, if routinely observed in clinical practice, might lead clinicians to conclude that medicines reconciliation takes too long and is not worth the effort [Clay et al. 2008]. However, there are no other reliable methods available that would ensure that potentially detrimental medication errors are avoided. Although it is possible to identify patients who may be at a higher risk of the consequences of a medicines reconciliation error (such as the elderly, or those with significant comorbid physical illness) the process of medicines reconciliation itself may identify physical health problems that may otherwise have been missed or overlooked.
Sources of information about medicines that were prescribed/taken
In a survey of hospital doctors, Clay and colleagues found that a major barrier to medicines reconciliation was patients being unclear about which medicines they take [Clay et al. 2008]. We found that the frequency with which the patient was asked, or other sources of information were checked, differed across the clinical settings included in the audits. For example, in acute adult settings patients were more likely to be asked directly about their medication, whereas in elderly settings, the primary care record was more likely to be consulted. These findings may be explained at least partially by the nature of the conditions that prompted hospital admission. A high proportion of the patients admitted to acute adult wards had a psychotic illness such as schizophrenia or mania. Such patients may be unreliable historians regarding the details of their medication regimen when acutely unwell, but as a minimum may be able to confirm levels of adherence in the period leading up to admission. In elderly settings, over one third of those admitted had a diagnosis of dementia and so may be uncertain about adherence to prescribed medicines or the details of administered medicines. For this latter group of patients, their carer or care home was often asked for this information.
Between baseline and re-audit there was a significant increase in the proportion of patients for whom their GP was contacted for information about their medication. This may reflect an increasing awareness within mental health settings of the importance of inquiring about, investigating and treating physical illness.
Nature of medication discrepancies
It is clear from the literature that the most common type of discrepancy identified during the process of medicines reconciliation is the omission of previously prescribed medication [de Winter et al. 2010; Robinson, 2008; Strunk et al. 2008; Morcos et al. 2002; Clarke, 1993]. The nature of the omitted medicines is related to the clinical setting, in that psychiatric teams may fail to prescribe medicines for physical illness [Clarke, 1993] and GPs may miss medicines for psychiatric illness [Robinson, 2008; Clarke, 1993]. Our findings were consistent with those of previous studies in that omissions were the most commonly reported discrepancy, and most commonly involved medicines for physical illness.
Further, reviews of published studies on medication adherence have concluded that within all healthcare settings poor adherence or nonadherence to prescribed medication is the rule rather than the exception, particularly immediately prior to admission to an adult acute psychiatric ward [Lacro et al. 2002; Cramer and Rosenheck, 1998]. In accordance with this, we found that fewer than 20% of patients admitted to such wards had documented evidence in their clinical record that medication was being taken as prescribed prior to admission. Thus, when prescribing at the time of admission, medicines that the patient has been taking at home may be missed, but it is also possible that medicines that have been prescribed but not taken may be re-instated. Both types of error can lead to harm, and clinically significant examples of both were reported in our audits. In most cases where the omission had the potential to cause significant harm, the drug had been initiated or continued by a physician or other hospital specialist. Examples of omitted drugs include erythropoietin, goserelin and methotrexate. Such drugs may not be documented in a patient’s primary care record; some primary care systems record only the drugs that are being prescribed by primary care, rather than by all clinicians involved in a patient’s care. With respect to re-instatement at full dose of medicines that were prescribed but not being taken prior to admission, the notable examples from our audits were methadone and clozapine. Tolerance to both of these drugs is lost relatively rapidly after they have been discontinued; a single high dose of methadone in an intolerant patient can be fatal while a full treatment dose of clozapine is likely to lead, as a minimum, to profound sedation and hypotension.
Who undertakes medicines reconciliation?
Where participating Trusts had an approved medicines reconciliation policy, doctors were the professional group most frequently cited as having the lead role in ensuring that reconciliation took place. Local reflection in some Trusts may have led to increased awareness of the responsibilities of doctors with respect to medicines reconciliation and the establishment of systems to facilitate the transfer of information between primary and secondary care. However, we found that activity related to medicines reconciliation was usually undertaken by pharmacy staff, and their relative contribution increased markedly between baseline and re-audit. This may partly reflect local investment in pharmacy staff, particularly medicines management technicians, to meet the recommendations made by NICE and the NPSA [National Institute for Health and Clinical Excellence and National Patient Safety Agency, 2007].
In a review of the literature, Karnon and colleagues concluded that a structured medicines reconciliation process that is pharmacist or nurse led reduces but does not eliminate errors [Karnon et al. 2009]. These authors also described an alternative system that involved clerical staff faxing requests for information about currently prescribed medicines to GPs. These different approaches have not been directly compared and so their relative efficacy, and advantages and disadvantages, including sustainability, are unknown. Further, it could be argued that processes that separate medicines reconciliation from the clinical history taking and formulation process that occur when a patient is admitted to hospital have the potential to de-skill clinical staff, particularly junior doctors.
Measuring medicines reconciliation practice
In the re-audit, medicines reconciliation was possible in about 80% of patients, in that two or more sources of information about the medication taken prior to admission had been checked. However, it is not possible to know the proportion of these patients for whom medicines were appropriately reconciled. For example, it is possible that not all relevant sources were checked for a given patient: a patient may be prescribed medication through a specialist hospital clinic or take herbal medicines supplied by family members. Conversely, according to our definition, medicines reconciliation was not possible in around one in five patients because only one or no source of information about the medicines being taken was checked. However, a legitimate exception to this may be a patient admitted from a care home where medicines were administered by the staff. In this example, examining the medication record sheet from the home as the only source of information would still provide an accurate account of the medication being administered.
Medicines reconciliation is different from medication review as the former process does not include an assessment of the clinical appropriateness of the medicines that are prescribed. It is simply matching the current prescription to the medication actually being taken immediately prior to admission. At the point of admission to hospital, both reconciliation and clinical review of the medication regimen are important. Where the latter results in a change in prescribed medication but the rationale has been poorly documentation, the apparent discrepancy may be misinterpreted as a reconciliation error.
Documentation of medicines reconciliation
By directly asking clinical teams about the actions taken to achieve medicines reconciliation in recently admitted patients, rather than seeking this information from the clinical records, we sought to gain a more accurate reflection of clinical practice. However, we found that a high proportion (80%) of this activity had been clearly documented. This suggests that in relation to the practice supporting medicines reconciliation, or, specifically, checking sources of information about medication and assessing medication adherence, audits of clinical records are likely to yield data that closely reflect clinical practice.
Acknowledgements
Acknowledgments are due to the participating Trusts and the NHS clinicians and administrators who collected the audit data. Thanks are also due to Janey Antoniou, Dr Michael Phelan and Krysia Zalewska for advice and support.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors declare no conflicts of interest in preparing this article.
References
- Audit Commission (2001) A Spoonful of Sugar – Medicines Management in NHS Hospitals, Audit Commission: London [Google Scholar]
- Clarke N.A. (1993) What the eye doesn’t see: drugs psychiatrists and GPs don’t know their patients are on. Psychiatric Bull 17: 469–470 [Google Scholar]
- Clay B.J., Halasyamani L., Stucky E.R., Greenwald J.L., Williams M.V. (2008) Results of a medication reconciliation survey from the 2006 society of hospital medicine national meeting. J Hosp Med 3: 465–472 [DOI] [PubMed] [Google Scholar]
- Cramer J.A., Rosenheck R. (1998) Compliance with medication regimens for mental and physical disorders. Psychiatr Serv 489: 196–201 [DOI] [PubMed] [Google Scholar]
- Dean Franklin B., Vincent C., Schachter M., Barber N. (2005) The incidence of prescribing errors in hospital inpatients. Drug Safety 28: 891–900 [DOI] [PubMed] [Google Scholar]
- de Winter S., Spriet I., Indevuyst C., Vanbrabant P., Desruelles D., Sabbe M., et al. (2010) Pharmacist- versus physician-aquired medication history: a prospective study at the emergency department. Qual Saf Health Care 19: 371–375 [DOI] [PubMed] [Google Scholar]
- Glintborg B., Andersen S.E., Dalhoff K. (2007) Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care 16: 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnon J., Campbell F., Czoski-Murray C. (2009) Model-based cost-effectiveness analysis on interventions aimed at preventing medication error at hospital admission (medicines reconciliation). J Eval Clin Pract 15: 299–306 [DOI] [PubMed] [Google Scholar]
- Lacro J.P., Dunn L.B., Dolder C.R., Leckband S.G., Jeste D.V. (2002) Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 159: 1653–1664 [DOI] [PubMed] [Google Scholar]
- Morcos S., Francis S.A., Duggan C. (2002) Where are the weakest links? A descriptive study of discrepancies in prescribing between primary and secondary sectors of mental health service provision. Psychiatric Bull 26: 371–374 [Google Scholar]
- National Institute for Health and Clinical Excellence and National Patient Safety Agency (2007) Technical Patient Safety Solutions for Medicines Reconciliation on Admission of Adults to Hospital. NICE Patient Safety Guidance 1. London: NICE. Available from http://www.nice.org.uk/PSG001.
- National Patient Safety Agency (2007) Background to technical patient safety solution. http://npsa.nhs.uk/corporate/news/guidance-to-improve-medicines-reconciliation/?locale=en, 12 December 2007.
- Robinson J. (2008) Discrepancies between GPs’ and psychiatrists’ medication records. Prog Neurol Psychiatry 12: 17–20 [Google Scholar]
- Strunk L.B., Matson A.W., Steinke D. (2008) Impact of a pharmacist on medication reconciliation on patient admission to a veterans affairs medical centre. Hospital Pharmacy 43: 643–649 [Google Scholar]