Abstract
Objective: Brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF) and leptin have been hypothesized to be involved in the neurobiology of depression. The aim of this study was to investigate BDNF, VEGF and leptin levels in patients with severe melancholic depression.
Methods: A total of 40 drug-free patients with major depressive disorder (MDD) with melancholic features and 40 healthy controls were included in the study. Demographic information, psychiatric evaluation and physical examination were documented for both groups. Serum BDNF, VEGF levels were determined by enzyme-linked immunosorbent assay and leptin with radioimmunoassay methods. The Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale were applied to the patients.
Results: There were no significant differences in serum BDNF, VEGF and leptin levels between the patient and control groups. There was a negative correlation between BDNF levels and the number of depressive episodes. It was noted that VEGF levels decreased with increasing severity of depression.
Conclusions: These findings suggest that BDNF levels might be associated with the recurrence of depression and VEGF levels might be a determinant of the severity of depression.
Keywords: brain-derived neurotrophic factor, depression, leptin, vascular endothelial growth factor
Introduction
Major depressive disorder (MDD) is a devastating disease that afflicts approximately 8% of men and 15% of women [Kessler et al. 1994]. Approximately 25–30% of depressed patients are classified as ‘melancholic type’ [Rush and Weissenburger, 1994]. Clinical research has demonstrated that melancholic type-depressed patients are less likely to respond to placebo therapy, supporting the hypothesis of a biological foundation and the need for suitable pharmacotherapy [Peselow et al. 1992].
The word ‘neuroplasticity’ denotes the capacity of the brain to adapt continually to the demands placed on it by experience and major depression may be related to impairments of structural plasticity [Fuchs et al. 2004]. Neurotrophic factors are critical regulators of the formation and plasticity of neuronal networks. Recently, the involvement of neurotrophic factors, particularly brain-derived neurotrophic factor (BDNF), have been under intense investigation regarding their possible role in the pathophysiology of mood disorders and its antidepressant effects [Castren et al. 2007]. BDNF is a small dimeric neuroprotective protein and a member of the neurotrophin family, which is widely expressed in the mammalian adult brain [Hofer et al. 1990]. Its normal physiological role is to encourage the outgrowth of dendrites from nerve endings, and to help stabilize connections between neurons [Hartmann et al. 2001]. According to the neurotrophin hypothesis of depression, BDNF is of major importance because it modulates the plasticity, inhibits cell death cascades and increases cell survival proteins that are responsible for the proliferation and maintenance of central nervous system neurons [Yulug et al. 2009].
Some animal models of depression showed that both acute and chronic stress decreased expression of BDNF [Angelucci et al. 2000; Roceri et al. 2002], and direct administration of BDNF into specific brain regions has been shown to mimic antidepressant effects [Siuciak et al. 1997; Hoshaw et al. 2005; Shirayama et al. 2002]. However, some authors reported that mutant mouse lines with low or no detectable BDNF expression did not exhibit depressive-like behaviour [Chan et al. 2006; Chourbaji et al. 2004; MacQueen et al. 2001; Saarelainen et al. 2003]. Pan and colleagues demonstrated that BDNF crosses the blood–brain barrier (BBB), which suggests that serum BDNF levels may reflect BDNF levels of the brain [Pan et al. 1998]. It was found that brain and serum BDNF levels undergo similar changes during maturation and ageing, and there is a positive correlation between serum and cortical BDNF levels [Karege et al. 2002]. Data about serum BDNF concentrations in major depression are conflicting, some authors reported levels as decreased [Karege et al. 2002, 2005; Shimizu et al. 2003; Aydemir et al. 2006; Monteleone et al. 2008], and others as unchanged [Ziegenhorn et al. 2007], compared with the healthy controls. Serum levels of BDNF have been found to be 200-fold higher than plasma levels [Rosenfeld et al. 1995]. This difference could reflect the amount of BDNF stored in platelets. Accordingly, it was suggested that BDNF levels of the plasma may reflect circulating levels since platelets are not seriously damaged during the separation of plasma [Lee et al. 2007]. However, studies that investigated plasma BDNF levels of depressed patients also yielded conflicting results as decreased, unchanged or increased levels [Grassi-Oliveira et al. 2008; Karege et al. 2005; Lee et al. 2007; Piccinni et al. 2008; Lee and Kim, 2009; Bocchio-Chiavetto et al. 2010; Serra-Millàs et al. 2011].
The vascular endothelial growth factor (VEGF) is an angiogenic cytokine able to induce vasopermeability in many types of tissues, including the BBB, and to facilitate the neurogenesis and proliferation of neurons in the adult hippocampus. Recent evidence indicates that VEGF can act as a neuroprotective factor in the adult brain, inhibiting apoptosis and inducing growth of the associated vascular–neuronal networks. VEGF influences synaptic plasticity in hippocampus-dependent processes, such as learning and memory, and modulates synaptic transmission [Ventriglia et al. 2009]. Exposure to unpredictable stress decreases the expression of VEGF in the hippocampus [Heine et al. 2005].
The hormone leptin encoded by the obese (ob) gene is predominantly synthesized by adipocytes and circulates in the plasma in amounts proportional to the body fat content [Zhang et al. 1994; Maffei et al. 1995]. It was first identified by its ability to regulate food intake and body weight through its actions in the hypothalamus. However, recent studies have shown that the neuronal actions of leptin are not confined to the hypothalamus. There is accumulating evidence that leptin plays an important part in regulating neuroendocrine function, in addition to conveying the status of energy stores to the central nervous system. In the hippocampus, under conditions where N-methyl-D-aspartate (NMDA) receptors are activated, leptin acts as a potential cognitive enhancer as it facilitates synaptic plasticity by selective enhancement of NMDA responses. Regarding its functions, leptin emerges to play a novel role in the regulation of mood and emotion. On the basis of the finding of low circulating leptin levels in animal models of depression, it was hypothesized that leptin insufficiency may underlie depression-like behavioural deficits [Lu et al. 2006]. Furthermore, systemic leptin administration was found to reverse the depressive state [Lu et al. 2006; Kim et al. 2006; Hirano et al. 2007]. Available information about the role of leptin signalling in human depression is limited and controversial. Leptin levels were reported as decreased [Kraus et al. 2001; Jow et al. 2006, Yang et al. 2007], and as unchanged [Deuschle et al. 1996; Moosa et al. 2003; Kauffman et al. 2005], in patients with depression. There are also studies reporting that leptin levels were increased only in women with depression [Antonijevic et al. 1998; Rubin et al. 2002; Esel et al. 2005; Pasco et al. 2008].
The relationship between stress and depression is complex, and subjects who receive a depression diagnosis are likely to represent heterogeneous populations of phenocopies with a varying contribution from stress exposure [Kendler et al. 2001]. Depression type is not indicated in the studies investigating BDNF, VEGF and leptin in MDD. However, there is growing evidence that depressive disorders encompass a group of disorders represented by differences in pathophysiological mechanisms and the identification of distinct endophenotypes for MDD will improve understanding of the disease [Antonijevic, 2006]. In this study we created a homogeneous group with patients diagnosed as severe melancholic depression in which biological factors are of major importance. In these patients, we aimed to determine serum BDNF, VEGF and leptin levels, which are all related to a neurotrophic hypothesis of depression and compare them with healthy controls.
Methods
Subjects
The study included 40 MDD patients with melancholic features (18–65 years of age) evaluated by a semi-structured psychiatric examination. The patients were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders [American Psychiatric Association, 1994]. Patients with an Axis I disorder other than MDD, alcohol/substance users, patients with any systemic or endocrinological disorder, pregnant women, women using oral contraceptives and patients with severe abnormalities in blood tests were excluded from the study. The patients had been drug-free for at least 3 months. Healthy controls (n = 40) were recruited from the hospital–university staff and were also assessed by a semi-structured psychiatric interview. Informed consent was obtained from all of the participants. The study had local ethic committee approval.
Complete blood count, serum electrolyte assay, liver and thyroid function tests, several hormone assays and electrocardiography were performed on all participants after an overnight fast between 8:00 and 10:00 a.m. following a general physical examination.
The Hamilton Depression Rating Scale (HDRS) and Hamilton Anxiety Rating Scale were applied to patients to evaluate the severity of depression and anxiety.
Sample preparation and analysis
Blood was withdrawn from the antecubital vein in the fasting state. Blood samples were drawn into heparin-coated, ethylenediaminetetraacetic acid-containing and nonadditive tubes and were processed in the laboratory immediately after collection. Complete blood count, serum electrolyte assay, liver function tests, thyroid function tests, cortisol, adrenocorticotropic hormone, growth hormone, sex hormones, prolactin, insulin and serum lipid profile were determined on the same day that the blood was collected. Serum samples obtained for determination of BDNF, VEGF and leptin were kept at −80oC until the analyses. The time range for collecting the samples was about 6 months. BDNF (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) and VEGF (Invitrogen, Camarillo, CA, USA) levels were determined by enzyme-linked immunosorbent assay kits. Leptin levels were determined by a radioimmunoassay method (Linco Research, St. Charles, MO, USA). BDNF and VEGF levels were given as pg/ml and leptin levels were given as ng/ml.
Statistical analysis
All statistical analyses were performed with SPSS version 13.0. Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as frequency. The Shapiro-Wilk test was used as normality test. Continuous variables were compared using the Student’s t-test and Mann–Whitney test when the data were not normally distributed. Categorical variables were compared using Pearson’s chi-square test and Fisher’s exact test. Correlations between variables were tested using Pearson and Spearman correlation coefficients. A p-value < 0.05 was considered as significant. Bonferroni correction was used for multiple comparisons.
Results
Demographic findings and laboratory results of study groups are given in Table 1. Clinical characteristics of MDD patients are given in Table 2. There were no significant differences in age, sex distribution and body mass indices (BMI) between the study groups. In the MDD group 14 patients (35%) had their first depressive episode and never used antidepressants; whereas 20 patients (50%) had their second and 6 patients (15%) had their third depressive episodes.
Table 1.
Demographic findings and laboratory results of patients with major depressive disorder and the control group.
| Major depressive disorder group (n = 40) | Control group (n = 40) | p-value | ||
|---|---|---|---|---|
| Age (years) | 35 ± 8 | 34 ± 8 | t = 0.614 | 0.541 |
| Body mass index (kg/m2) | 23.5 ± 4.2 | 25.2 ± 5.3 | t = -1.626 | 0.108* |
| Sex (female/male) | 32/8 | 32/8 | NS$ | |
| Smokers/nonsmokers | 12/28 | 15/25 | NS$ | |
| Total cholesterol (mmol/l) | 4.7 ± 1.1 | 4.9 ± 1.1 | U = 676.50 | NS* |
| LDL-cholesterol (mmol/l) | 2.9 ± 0.8 | 3.0 ± 0.8 | U = 712.50 | NS* |
| VLDL-cholesterol (mg/dl) | 20.1 ± 12.2 | 23.0 ± 10.9 | U = 628.00 | NS* |
| HDL-cholesterol (mmol/l) | 1.3 ± 0.3 | 1.3 ± 0.3 | U = 771.00 | NS* |
| Triglyceride (mmol/l) | 1.14 ± 0.7 | 1.3 ± 0.6 | U = 628.00 | NS* |
| Cortisol (nmol/l) | 328.3 ± 212.8 | 338.3 ± 115.8 | U = 718.00 | NS* |
| Insulin (ng/ml) | 7.8 ± 4.3 | 8.3 ± 7.1 | U = 766.00 | NS* |
| BDNF (pg/ml) | 1577 ± 510 | 1624 ± 329 | U = 729.00 | NS* |
| VEGF (pg/ml) | 300 ± 158 | 358 ± 217 | U = 678.00 | NS* |
| Leptin (µg/l) | 12.9 ± 11.2 | 11.6 ± 9.0 | U = 679.50 | NS* |
Student’s t-test for independent groups. $Chi-square test. Data are expressed as mean ± standard deviation. A p-value < 0.05 was considered significant. BDNF, brain-derived neurotrophic factor; HDL, high density lipoprotein; LDL, low density lipoprotein; NS, not significant; VEGF, vascular endothelial growth factor; VLDL, very low density lipoprotein.
Table 2.
Clinical characteristics of major depressive disorder patients.
| Duration of episode (months) | 5.2 ± 4.6 |
| Beginning age of depression (years) | 28.2 ± 7.2 |
| Number of depressive episodes | 1.8 ± 0.7 |
| Hamilton Depression Rating Scale | 31.1 ± 3.2 |
| Hamilton Anxiety Rating Scale | 26.2 ± 5.9 |
| Suicidal/nonsuicidal | 6/34 |
| Family history (+)/family history (-) | 15/25 |
There was no significant difference between patient and control groups in terms of serum levels of BDNF, VEGF and leptin. There was also no difference between these parameters when compared according to sex. There was no correlation between BDNF and VEGF levels in terms of age and BMI in both groups. There was no statistical difference between BDNF levels of suicidal depressive patients, nonsuicidal depressive patients and controls. The number of depressive episodes and BDNF levels were found to be negatively correlated (r = -0.390, p = 0.017) (Figure 1). VEGF levels were negatively correlated with HDRS scores (r = -0.326, p = 0.043) in the patient group.
Figure 1.
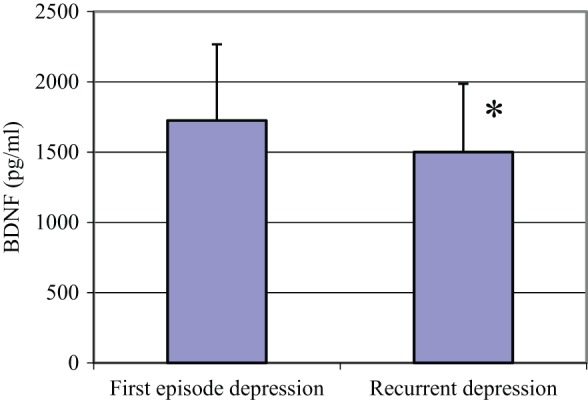
Comparison of serum BDNF levels in first episode and recurrent depressive patients.
*p < 0.05. BDNF, brain-derived neurotrophic factor.
Serum leptin levels correlated positively with BMI in both groups (r = 0.416, p = 0.009). Leptin levels were significantly higher in women in the control group (p = 0.030), but no such difference was observed in the patient group. Leptin levels correlated positively with triglyceride, very low density lipoprotein and insulin levels in the patient group (p < 0.01); no such relation was present in control group. There was no difference between cortisol levels in patient and control groups and leptin was not found to be correlated with cortisol. There was no correlation between leptin levels and HDRS scores, number of depressive episodes or suicidality in the patient group.
Discussion
In the present study, there were no significant differences between melancholic depressive patient and control groups in terms of serum BDNF, VEGF and leptin levels. However, BDNF levels were found to be negatively correlated with the number of depressive episodes. In addition, VEGF levels were found to be negatively correlated with increased severity of depression.
To our knowledge this study is the first to have investigated serum BDNF, VEGF and leptin levels in purely melancholic depressive patients. In parallel with our findings, Basterzi and colleagues and Ziegenhorn and colleagues reported that serum BDNF levels were not significantly different between depressive patients and controls [Basterzi et al. 2009; Ziegenhorn et al. 2007]. Furthermore, Kim and colleagues and Lee and Kim investigated BDNF levels in the plasma of depressive patients and also found unchanged levels [Kim et al. 2007; Lee and Kim, 2008]. We had difficulties in comparing our results with those of the other studies since subtypes of depression were not defined or evaluated as separate groups in almost all of the studies.
We found that patients with recurrent depressive episodes have lower BDNF serum levels compared with patients with a single episode and healthy controls. This finding is in line with the study of Dell’Osso and colleagues who stated that patients who were suffering from a recurrent episode had significantly lower levels of plasma BDNF [Dell’Osso et al. 2010]. Kauer-Sant’Anna and colleagues have shown that bipolar patients later in the course of their illness have greater decrements in BDNF compared with those earlier in the illness, suggesting a possible cumulative deficit in BDNF after multiple episodes [Kauer-Sant’Anna et al. 2009]. Furthermore, there are several studies indicating that BDNF levels correlate negatively with increased severity of depression [Karege et al. 2002; Shimizu et al. 2003; Gonul et al. 2005; Dell’Osso et al. 2010; Bus et al. 2011]. However, we also assessed the severity of depression with the use of HDRS and did not find any relation between the severity of depression and BDNF levels. This finding was in line with the study of Lee and colleagues [Lee et al. 2007].
The number of studies investigating the association of VEGF with depression is limited. Kahl and colleagues found increased concentrations of VEGF in nonmedicated depressive patients with borderline personality disorder in comparison with healthy controls [Kahl et al. 2009]. Iga and colleagues had previously suggested that a higher expression of VEGF mRNA in the peripheral leucocytes might be associated with the depressive state [Iga et al. 2007]. Takebayashi and colleagues reported that plasma VEGF levels were increased significantly in MDD patients compared with matched controls [Takebayashi et al. 2010]. However, patients were taking psychotrophic agents in the last two studies. In parallel with our findings Dome and colleagues and Ventriglia and colleagues did not find any significant differences in serum VEGF levels between the MDD patients and healthy controls [Dome et al. 2008; Ventriglia et al. 2009]. In a recent animal study, Elfving and colleagues reported that VEGF levels were significantly decreased in the hippocampus and frontal cortex of a genetic depression rat model [Elfving et al. 2010]; however, no such difference was observed in serum levels of VEGF. Although these results suggest that VEGF is not a peripheral marker of depression, we observed that VEGF levels were negatively correlated with HDRS scores. To our knowledge, this is the first study suggesting a relation between serum levels of VEGF and severity of depression.
In the present study, in line with several studies [Deuschle et al. 1996; Moosa et al. 2003; Kauffman et al. 2005], there was no significant difference in serum leptin levels between the MDD patients and controls. No relation was found between leptin levels and severity of depression, suicidality and recurrence of depressive episodes. We think that our data about leptin levels are noteworthy considering that the study had a very homogeneous subgroup of depression with a predominantly biological etiology.
It is surprising that there was no significant difference between serum cortisol levels of patients and controls as approximately 50% of depressed patients exhibit dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, which results in sustained elevations in cortisol levels [Gold and Chrousos, 2002; Swaab et al. 2005]. Elevation of basal cortisol levels [Belanoff et al. 2001], and nonsuppression on the dexamethasone suppression test [Nelson and Davis, 1997], were most clearly evident in psychotic depression. None of our patients had any psychotic symptoms. Moreover, hypercortisolaemia has not been a stable finding in all studies and a dysfunction of the HPA axis has been proposed as an alternative hypothesis [Peeters et al. 2004]. Peeters and colleagues suggested that erratic cortisol secretion may be a more characteristic feature of HPA-axis dysregulation than hypercortisolism, especially in outpatient populations [Peeters et al. 2004]. Assies and colleagues found no difference between the cortisol levels of depressed patients and controls and indicated that dehydroepiandrosterone-sulphate may be a more important indicator of depression [Assies et al. 2004]. Michopoulos and colleagues found that depressive patients had normal cortisol blood levels and there was no significant difference between melancholic and nonmelancholic depressive patients [Michopoulos et al. 2008]. Glucocorticoids play a critical role in mediating stress-induced downregulation of BDNF in the hippocampus [Schmidt and Duman, 2007]. Thus, our finding that there is no significant difference between serum BDNF levels of patients and controls is concordant with the finding that there is also no significant difference between the cortisol levels of the two groups.
One of the limitations of the study was the absence of a control group composed of other types of MDDs. One might consider the selection of serum instead of plasma as the specimen for BDNF as a limitation, however, as mentioned above, data about BDNF levels in serum or plasma of depressive patients are conflicting. Furthermore, it was reported that plasma BDNF has shown high interindividual variability [Piccinni et al. 2009]. Lommatzsch and colleagues found that plasma BDNF levels decreased significantly with increasing age, weight or cholesterol, whereas platelet levels (an important source of serum BDNF) did not [Lommatzsch et al. 2005]. Furthermore, there is evidence for the stability of BDNF levels in platelets or serum [Trajkovska et al. 2007], whereas in plasma, it circulates for less than 1 h [Kishino et al. 2001; Poduslo and Curran, 1996]. Another limitation of the study is that we did not consider the phases of menstrual cycle in female subjects. We know that leptin and BDNF levels especially differ according to hormonal changes. Another limitation is that we measured leptin only once so that we could not observe changes in its diurnal rhythm and pulsatility in depressive patients. Further studies with larger samples are required to investigate biological markers in homogeneous MDD groups.
This study showed that there are no significant differences in BDNF, VEGF and leptin levels in MDD patients with melancholic features compared with those of healthy controls. We think that this finding is significant as we studied with a diagnostically homogeneous group of patients. BDNF may be related to the recurrence of depressive episodes as its level decreased with remitting depression. VEGF may be a determinant of the severity of depression as its levels decreased with the increasing HDRS. Further investigations aiming to identify the role and putative function of neurotrophins in the pathogenesis of depressive disorders and their peripheral indicators in the blood are necessary for new diagnostic and therapeutic options. Neurotrophic factor levels may be a guide in the assessment of suicidality, severity and recurrence of depression and, accordingly, in the development of therapeutic interventions. Furthermore, treatment regimens with the direct or adjunctive addition of these neurotrophins may be indicated in the future.
Footnotes
The study was supported by the foundation of Uludag University (2008/37). This research received no specific grant from any funding agency in the public, commercial, or not- for-profit sectors.
The authors declare no conflicts of interest in preparing this article.
References
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition). Washington, DC: American Psychiatric Association [Google Scholar]
- Angelucci F., Aloe L., Vasquez P.J., Mathe A.A. (2000) Mapping the differences in the brain of brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in animal model of depression. Neuroreport 11: 1369–1373 [DOI] [PubMed] [Google Scholar]
- Antonijevic I.A. (2006) Depressive disorders – is it time to endorse different pathophysiologies? Psychoneuroendocrinology 31: 1–15 [DOI] [PubMed] [Google Scholar]
- Antonijevic I.A., Murck H., Frieboes R.M., Horn R., Brabant G., Steiger A. (1998) Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res 32: 403–410 [DOI] [PubMed] [Google Scholar]
- Assies J., Visser I., Nicolson N.A., Eggelte T.A., Wekking E.M., Huyser J., et al. (2004) Elevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: preliminary findings. Psychiatry Res 128: 117–122 [DOI] [PubMed] [Google Scholar]
- Aydemir C., Yalcin E.S., Aksaray S., Kisa C., Yildirim S.G., Uzbay T., et al. (2006) Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry 30: 1256–1260 [DOI] [PubMed] [Google Scholar]
- Basterzi A.D., Yazici K., Aslan E., Delialioglu N., Tasdelen B., Tot Acar S., et al. (2009) Effects of fluoxetine and venlafaxine on serum brain derived neurotrophic factor levels in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 33: 281–285 [DOI] [PubMed] [Google Scholar]
- Belanoff J.K., Kalehzan M., Sund B., Fleming Ficek S.K., Schatzberg A.F. (2001) Cortisol activity and cognitive changes in psychotic major depression. Am J Psychiatry 158: 1612–1616 [DOI] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L., Bagnardi V., Zanardini R., Molteni R., Nielsen M.G., Placentino A., et al. (2010) Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry 11: 763–773 [DOI] [PubMed] [Google Scholar]
- Bus B.A., Tendolkar I., Franke B., de Graaf J., Heijer M.D., Buitelaar J.K., et al. (2011) Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry DOI:10.3109/15622975.2010.545187 10.3109/15622975.2010.545187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E., Voikar V., Rantamaki T. (2007) Role of neurotrophic factors in depression. Curr Opin Pharmacol 7: 18–21 [DOI] [PubMed] [Google Scholar]
- Chan J.P., Unger T.J., Byrnes J., Rios M. (2006) Examination of behavioral deficits triggered by targeting BDNF in fetal or postnatal brains of mice. Neuroscience 142: 49–58 [DOI] [PubMed] [Google Scholar]
- Chourbaji S., Hellweg R., Brandis D., Zörner B., Zacher C., Lang U.E., et al. (2004) Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res 121: 28–36 [DOI] [PubMed] [Google Scholar]
- Dell’Osso L., Del Debbio A., Veltri A., Bianchi C., Roncaglia I., Carlini M. (2010) Associations between brain-derived neurotrophic factor plasma levels and severity of illness, recurrence and symptoms in depressed patients. Neuropsychobiology 62: 207–212 [DOI] [PubMed] [Google Scholar]
- Deuschle M., Blum W.F., Englaro P., Schwerger U., Weber B., Pflaum C.D., et al. (1996) Plasma leptin in depressed patients and healthy controls. Horm Metab Res 28: 714–717 [DOI] [PubMed] [Google Scholar]
- Dome P., Teleki Z., Rihmer Z., Peter L., Dobos J., Kenessey I., et al. (2008) Circulating endothelial progenitor cells and depression: a possible novel link between heart and soul. Mol Psychiatry 14: 523–531 [DOI] [PubMed] [Google Scholar]
- Elfving B., Plougmann P.H., Wegener G. (2010) Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci Lett 474: 13–16 [DOI] [PubMed] [Google Scholar]
- Esel E., Ozsoy S., Tutus A., Sofuoglu S., Kartalci S., Bayram F., et al. (2005) Effects of antidepressant treatment and of gender on serum leptin levels in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 29: 565–570 [DOI] [PubMed] [Google Scholar]
- Fuchs E., Czeh B., Kole M.H.P., Michaelis T., Lucassen P.L. (2004) Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol 14: 481–490 [DOI] [PubMed] [Google Scholar]
- Gold P.W., Chrousos G.P. (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs. low CRH/NE states. Mol Psychiatry 7: 254–275 [DOI] [PubMed] [Google Scholar]
- Gonul A.S., Akdeniz F., Taneli F., Ozlem D., Eker C., Vahip S. (2005) Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci 255: 381–386 [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R., Stein L.M., Lopes R.P., Teixeira A.L., Bauer M.E. (2008) Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression – a preliminary report. Biol Psychiatr 64: 281–285 [DOI] [PubMed] [Google Scholar]
- Hartmann M., Heumann R., Lessmann V. (2001) Synaptic secretion of BDNF after high frequency stimulation of glutamatergic synapses. EMBO J 20: 5887–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine V.M., Zareno J., Malsam S., Joels M., Lucassen P.J. (2005) Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 21: 1304–1314 [DOI] [PubMed] [Google Scholar]
- Hirano S., Miyata S., Kamei J. (2007) Antidepressant-like effect of leptin in streptozotocin-induced diabetic mice. Pharmacol Biochem Behav 86: 27–31 [DOI] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S.R., Hohn A., Leibrock J., Barde Y.A. (1990) Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J 9: 2459–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw B.A., Malberg J.E., Lucki I. (2005) Central administration of IGF-1 and BDNF leads to long-lasting antidepressant-like effects. Brain Res 1037: 204–208 [DOI] [PubMed] [Google Scholar]
- Iga J., Ueno S., Yamauchi K., Numata S., Tayoshi-Shibuya S., Kinouchi S., et al. (2007) Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 31: 658–663 [DOI] [PubMed] [Google Scholar]
- Jow G.M., Yang T.T., Chen C.L. (2006) Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord 90: 21–27 [DOI] [PubMed] [Google Scholar]
- Kahl K.G., Bens S., Ziegler K., Rudolf S., Kordon A., Dibbelt L., et al. (2009) Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology 34: 353–357 [DOI] [PubMed] [Google Scholar]
- Karege F., Bandolfi G., Gervasoni N., Schwald M., Aubry J.M., Bertschy G. (2005) Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 57: 1068–1072 [DOI] [PubMed] [Google Scholar]
- Karege F., Perret G., Bandolfi G., Schwald M., Bertschy G., Aubry J.M. (2002) Decreased serum brain-derived neurotrophic factor levels in major depressive patients. Psychiatry Res 109: 143–148 [DOI] [PubMed] [Google Scholar]
- Kauer-Sant’Anna M., Kapczinski F., Andreazza A.C., Bond D.J., Lam R.W., Young L.T., et al. (2009) Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol 12: 447–458 [DOI] [PubMed] [Google Scholar]
- Kauffman R.P., Castracane V.D., White D.L., Baldock S.D., Owens R. (2005) Impact of selective serotonin reuptake inhibitor citalopram on insulin sensitivity, leptin and basal cortisol secretion in depressed and non-depressed euglycemic women of reproductive age. Gynecol Endocrinol 21: 129–137 [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Thornton L.M., Gardner C.O. (2001) Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. Am J Psychiatry 158: 582–586 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., McGonagle K.A., Zhao S., Nelson C.B., Hughes M., Eshleman S. (1994) Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51: 8–19 [DOI] [PubMed] [Google Scholar]
- Kim C.S., Huang T.Y., Garza J., Ramos F., Frazer A., Liu F., et al. (2006) Leptin induces antidepressant-like behavioral effects and activates specific signal transduction pathways in the hippocampus and amygdala of mice. Neuropsychopharmacology 31: 237–238 [Google Scholar]
- Kim Y.K., Lee H.P., Won S.D., Park E.Y., Lee H.Y., Lee B.H., et al. (2007) Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry 31: 78–85 [DOI] [PubMed] [Google Scholar]
- Kishino A., Katayama N., Ishige Y., Yamamoto Y., Ogo H., Tatsuno T., et al. (2001) Analysis of effects and pharmacokinetics of subcutaneously administered BDNF. Neuroreport 12: 1067–1072 [DOI] [PubMed] [Google Scholar]
- Kraus T., Haack M., Schuld A., Hinze-Selch D., Polmacher T. (2001) Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology 73: 243–247 [DOI] [PubMed] [Google Scholar]
- Lee B.H., Kim H., Park S.H., Kim Y.K. (2007) Decreased plasma BDNF level in depressive patients. J Affect Disord 101: 239–244 [DOI] [PubMed] [Google Scholar]
- Lee B.H., Kim Y.K. (2009) Reduced platelet BDNF level in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 33: 849–853 [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Kim Y.K. (2008) Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology 57: 194–199 [DOI] [PubMed] [Google Scholar]
- Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., et al. (2005) The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging 26: 115–123 [DOI] [PubMed] [Google Scholar]
- Lu X.Y., Kim C.S., Frazer A., Zhang W. (2006) Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A 103: 1593–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G.M., Ramakrishnan K., Croll S.D., Siuciak J.A., Yu G., Young L.T., et al. (2001) Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci 115: 1145–1153 [DOI] [PubMed] [Google Scholar]
- Maffei M., Halaas J., Ravussin E., Pratley R.E., Lee G.H., Zhang Y., et al. (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Michopoulos I., Zervas I.M., Pantelis C., Tsaltas E., Papakosta V.M., Boufidou F., et al. (2008) Neuropsychological and hypothalamic-pituitary-axis function in female patients with melancholic and non-melancholic depression. Eur Arch Psychiatry Clin Neurosci 258: 217–225 [DOI] [PubMed] [Google Scholar]
- Monteleone P., Serritella C., Martiadis V., Maj M. (2008) Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord 10: 95–100 [DOI] [PubMed] [Google Scholar]
- Moosa M.Y.H., Panz V.R., Jeenah F.Y., Joffe B.I. (2003) African women with depression. The effect of imipramine and fluoxetine on body mass index and leptin secretion. J Clin Psychopharmacol 23: 549–552 [DOI] [PubMed] [Google Scholar]
- Nelson J., Davis J.M. (1997) DST studies in psychotic depression: a meta-analysis. Am J Psychiatry 154: 1497–1503 [DOI] [PubMed] [Google Scholar]
- Newton S.S., Collier E.F., Hunsberger J., Adams D., Terwilliger R., Selvanayagam E., et al. (2003) Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci 23:10841–10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. (1998) Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37: 1553–1561 [DOI] [PubMed] [Google Scholar]
- Pasco J.A., Jacka F.N., Williams L.J., Henry M.J., Nicholson G.C., Kotowicz M.A., et al. (2008) Leptin in depressed women: cross-sectional and longitudinal data from an epidemiologic study. J Affect Disord 107: 221–225 [DOI] [PubMed] [Google Scholar]
- Peeters F., Nicolson N.A., Berkhof J. (2004) Levels and variability of daily life cortisol secretion in major depression. Psychiatry Res 126: 1–13 [DOI] [PubMed] [Google Scholar]
- Peselow E.D., Sanfilipo M.P., Difiglia C., Fieve R.R. (1992) Melancholic/endogeneous depression and response to somatic treatment and placebo. Am J Psychiatry 149: 1324–1334 [DOI] [PubMed] [Google Scholar]
- Piccinni A., Del Debbio A., Medda P., Bianchi C., Roncaglia I., Veltri A., et al. (2009) Plasma brain-derived neurotrophic factor in treatment-resistant depressed patients receiving electroconvulsive therapy. Eur Neuropsychopharmacol 19: 349–355 [DOI] [PubMed] [Google Scholar]
- Piccinni A., Marazziti D., Catena M., Domenici L., Del Debbio A., Bianchi C., et al. (2008) Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord 105: 279–283 [DOI] [PubMed] [Google Scholar]
- Poduslo J.F., Curran G.L. (1996) Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res 36: 280–286 [DOI] [PubMed] [Google Scholar]
- Roceri M., Hendriks W., Ricagni G., Ellenbroek B.A., Riva M.A. (2002) Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry 7: 609–616 [DOI] [PubMed] [Google Scholar]
- Rosenfeld R.D., Zeni L., Haniu M., Talvenheimo J., Radka S.F., Bennett L., et al. (1995) Purification and identification of brain-derived neurotrophic factor from human serum. Protein Expr Purif 6: 465–471 [DOI] [PubMed] [Google Scholar]
- Rubin R.T., Rhodes M.E., Czambel R.K. (2002) Sexual diergism of baseline plasma leptin and leptin supression by arginine vasopressin in major depressives and matched controls. Psychiatry Res 113: 255–268 [DOI] [PubMed] [Google Scholar]
- Rush A.J., Weissenburger J.E. (1994) Melancholic symptom features and DSM-IV. Am J Psychiatry 151: 489–498 [DOI] [PubMed] [Google Scholar]
- Saarelainen T., Hendolin P., Lucas G., Koponen E., Sairanen M., MacDonald E., et al. (2003) Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23: 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.D., Duman R.S. (2007) The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 18: 391–418 [DOI] [PubMed] [Google Scholar]
- Serra-Millàs M., López-Vílchez I., Navarro V., Galán A.M., Escolar G., Penadés R., et al. (2011) Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology (Berl) DOI: 10.1007/s00213-011-2180-0 10.1007/s00213-011-2180-0 [DOI] [PubMed] [Google Scholar]
- Shimizu E., Hashimoto K., Okamura N., Koike K., Komatsu N. (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54: 70–75 [DOI] [PubMed] [Google Scholar]
- Shirayama Y., Chen A.C., Nakagawa S., Russell D.S., Duman R.S. (2002) Brain-derived neurotophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22: 3251–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak J.A., Lewis D.R., Wiegand S.J., Lindsay R.M. (1997) Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav 56: 131–137 [DOI] [PubMed] [Google Scholar]
- Swaab D.F., Bao A.M., Lucassen P.J. (2005) The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4: 141–194 [DOI] [PubMed] [Google Scholar]
- Takebayashi M., Hashimoto R., Hisaoka K., Tsuchioka M., Kunugi H. (2010) Plasma levels of vascular endothelial growth factor and fibroblast growth factor 2 in patients with major depressive disorders. J Neural Transm 117: 1119–1122 [DOI] [PubMed] [Google Scholar]
- Trajkovska V., Marcussen A.B., Vinberg M., Hartvig P., Aznar S., Knudsen G.M. (2007) Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res Bull 73: 143–149 [DOI] [PubMed] [Google Scholar]
- Ventriglia M., Zanardini R., Pedrini L., Placentino A., Nielsen M.G., Gennarelli M., et al. (2009) VEGF serum levels in depressed patients during SSRI antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry 33: 146–149 [DOI] [PubMed] [Google Scholar]
- Yang K., Xie G., Zhang Z., Wang C., Li W., Zhou W., et al. (2007) Levels of serum interleukin (IL)-6, IL-1β, tumour necrosis factor-α and leptin and their correlation in depression. Aust N Z J Psychiatry 41: 266–273 [DOI] [PubMed] [Google Scholar]
- Yulug B., Ozan E., Gonul A.S., Kilic E. (2009) Brain-derived neurotrophic factor, stres and depression: a minireview. Brain Res Bull 78: 267–269 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432 [DOI] [PubMed] [Google Scholar]
- Ziegenhorn A.A., Herbruggen O.S., Danker-Hopfe H., Malbranc M., Hartung H.D., Anders D., et al. (2007) Serum neurotrophins – a study on the time course influencing factors in a large old age sample. Neurobiol Aging 28: 1436–1445 [DOI] [PubMed] [Google Scholar]


