Abstract
Objective: We previously found psychotic depression (PSDEP) to have positively correlating plasma norepinephrine (NE) and vasopressin (AVP) concentrations. Since central noradrenergic activity and plasma NE concentration are highly correlated, this suggests an increased noradrenergic activation of the hypothalamus–pituitary–adrenal axis. We hypothesize the increased release of NE in PSDEP to be an associated mechanism.
Methods: To test this hypothesis we analyzed the relation between plasma NE and PSDEP in a comparison with non-psychotically depressed patients. Potentially confounding variables were, among others, melancholia and two better validated subcategories in the field of melancholia and endogenous depression, three global dimensions of psychopathology – Emotional Dysregulation, Retardation and Anxiety – smoking habit, and different types of psychotropic and particularly antidepressant treatment. The data from nine patients with PSDEP and 69 patients with non-PSDEP were reanalysed.
Results: Analysis of covariance controlling for the effects of tricyclic antidepressant treatment (≥100 mg) and smoking habit showed that PSDEP had an increased concentration of plasma NE. The previously found correlation between plasma NE and AVP was still present after correcting for the effects of confounding variables.
Conclusions: The results suggest an increased activity of the sympathetic nervous system in PSDEP that may act as a specific mechanism for increased vasopressinergic activation. This supports the view of PSDEP as a distinct subcategory of major depression.
Keywords: norepinephrine, psychotic depression, smoking, tricyclic antidepressant, vasopressin
Introduction
This study on norepinephrine (NE) in psychotic depression (PSDEP) is part of a series of investigations within the same patient sample that aimed to develop an improved differentiation of subcategories of depression, and to detect neurobiological markers of these subcategories and of depression at large. The neurobiological focus of these studies is on vasopressinergic mechanisms in depression [Goekoop et al. 2010] and its subcategories [Goekoop and Wiegant, 2009; Goekoop et al. 2011]. The present study tests if PSDEP is characterized by a specifically high noradrenergic activation next to the increased noradrenergic–vasopressinergic coupling, evidence of which has been found previously in a comparison with non-PSDEP [Goekoop et al. 2011]. We hypothesized the plasma concentration of NE to be increased as a mechanism associated with the positively correlating plasma vasopressin (AVP) and NE concentrations in PSDEP [Goekoop et al. 2011].
The potential role of increased release of NE next to the increased NE–AVP correlation in PSDEP may be seen in the context of the vasopressinergic mechanisms in animal models of depression [Aguilera et al. 2008; Landgraf, 2006] and noradrenergic mechanisms involved in the hypothalamus–pituitary–adrenal (HPA) axis. The role of NE in stimulating the HPA axis has been studied extensively [Al-Damluji, 1993]. In human subjects noradrenergic agents stimulate the release of adrenocorticotroph hormone (ACTH) via an α-1 receptor in the brain at the level of the paraventricular nucleus (PVN) of the hypothalamus, and not at the peripheral level of the pituitary [Al-Damluji, 1993]. Though such noradrenergic stimulation of the PVN in rats and mice involves the synthesis of both corticotropin-releasing hormone [Day et al. 1999] and AVP in the parvocellular neurons [Vacher et al. 2002], the resulting release of ACTH depends particularly on the release of AVP [Al-Damluji, 1993]. We hypothesize that the increased noradrenergic activation suggested by the correlating plasma NE and AVP concentrations in PSDEP involves a centrally increased release of plasma NE. The correlation between central and plasma NE [Esler et al. 1995; Kelly and Cooper, 1997; Ziegler et al. 1977] enabled us to test this hypothesis by comparing the plasma concentrations of NE in patients with PSDEP and non-PSDEP.
The investigation of PSDEP is full of potential pitfalls due to many potentially confounding factors. As will be discussed below, PSDEP may have many nonpsychotic phenotypical admixtures that can be categorically or dimensionally defined and may be inherent to the disorder or not. As far as the categorically defined ‘comorbidity’ is concerned, to improve the assessment of potentially relevant subcategories of depression we used the highly anxious retarded (HAR) subcategory and the subcategory with above-normal AVP concentration (ANA) that have been derived from melancholia and familial depression [de Winter et al. 2004; Goekoop et al. 2006; Goekoop and Wiegant, 2009], next to the melancholic subtype according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) [American Psychiatric Association, 1994]. In previous studies that only used DSM categories, one study found increased levels of plasma NE in a mixed group of patients with melancholia (n = 6) and PSDEP (n = 4) compared with a group of patients with nonmelancholia and non-PSDEP (n = 7) [Kelly and Cooper, 1998]. This corresponds with cerebrospinal fluid NE being increased in melancholia compared with normal controls [Wong et al. 2000]. Another study found no increase in plasma NE in four patients with PSDEP compared with 18 patients with non-PSDEP [Rothschild et al. 1987]. This negative finding may have been due to the low number of patients with PSDEP, but also due to lack of control for confounding variables.
As far as the multidimensional background of PSDEP is concerned, we used three global dimensions of psychopathology, called Emotional Dysregulation, Motivational Inhibition (Retardation) and Autonomic Dysregulation (Anxiety). These dimensions represent the nonmanic and nonpsychotic psychopathology of the six-dimensional global structure of psychopathology assessed by the semi-standardized interview of the Comprehensive Psychopathological Rating Scale (CPRS) [Goekoop et al. 1992]. Since Emotional Dysregulation correlates 0.95 with the Montgomery Asberg Depression Rating Scale (MADRS) [Montgomery and Asberg, 1979; Goekoop et al. 1994], we used this dimension as the general measure of severity of depression. The potential usefulness of this approach is predicted by several findings. A previous study combined dimensions of psychopathology and subcategories of depression, and found that plasma NE was related to the melancholic subcategory and to Retardation in a group of patients with depression (mostly melancholic or previously melancholic depression compared with normal control subjects) [Roy et al. 1985a]. NE was related to Anxiety in the melancholic subcategory. Two series of studies have shown that PSDEP can be conceived to represent the highest level of severity on a global dimension of psychopathology: in one series of studies PSDEP was found to relate to high Retardation, while melancholia was found to have an intermediate position on that same dimension of Retardation [Parker et al. 1999]. In another series of investigations PSDEP appeared to relate to a global hierarchic dimension of psychopathology that comprises depressive symptoms at the lowest level of the hierarchy, specific neurotic symptoms at a higher level and psychotic symptoms at the highest level [Surtees and Kendell, 1979]. The symptoms of the nonpsychotic part of this hierarchy correspond to the symptoms found in the hierarchic global dimension of Emotional Dysregulation [Goekoop and Zwinderman, 1994]. These findings support the usefulness of a multidimensional assessment of nonpsychotic psychopathology to control for relations with PSDEP that could be explained by relations with the nonpsychotic admixture of that subcategory.
We also searched for potentially confounding effects on the concentration of plasma NE of current antipsychotic, antidepressant and benzodiazepine treatment and their dosages, type of antidepressant drug, smoking habit, sex, age, duration of the disease and inpatient or outpatient treatment. Smoking may immediately affect peripheral sympathetic activity [Grassi et al. 1994], and long-term smoking may increase the NE concentration [Christensen and Jensen, 1995]. Long-term treatment with a tricyclic drug, at least 100 mg, has been found to increase plasma NE concentration [Veith et al. 1994], while plasma NE has been found to be nonsignificantly decreased in patients with major depressive disorder treated with selective serotonin reuptake inhibitors (SSRIs) [Barton et al. 2007]. As far as we know, this method of analysing the plasma NE concentration as a potential biomarker of PSDEP, controlling for several confounding effects, has not been used before. Since in our previous study [Goekoop et al. 2011] we did not analyse the effect of antidepressant drug type, we reanalysed the correlation between plasma NE and AVP in PSDEP. Antipsychotic drug dose was used as an additional confounder in these analyses as it correlates positively with the concentration of plasma AVP [Goekoop et al. 2006]. In summary, we hypothesize the plasma concentration of NE to be increased in PSDEP compared with non-PSDEP, and the correlation between plasma NE and plasma AVP still to be present when accounting for several potentially confounding effects.
Methods
Subjects
We reanalysed the data from the same patient sample in which we previously found support for the HAR and ANA subcategories of depression [Goekoop and Wiegant, 2009] and a general vasopressinergic theory of depression [Goekoop et al. 2010]. All patients fulfilled DSM-IV criteria [American Psychiatric Association, 1994] for major depression and scored at least 20 on the MADRS [Montgomery and Asberg, 1979]. The 78 patients with complete NE data were selected from 89 patients who were initially included, 9 with PSDEP and 69 with non-PSDEP. There were no significant clinical and demographic differences between these groups. Seventy-five patients had complete AVP data, 9 with PSDEP and 66 with non-PSDEP.
The treating psychiatrist of the inpatient clinic or outpatient clinic made the first diagnosis of major depression. Patients were included if this diagnosis was confirmed by an independent investigator (RFPdeW) using a semi-standardized interview for depressive DSM-IV diagnoses. We used the items of the CPRS [Goekoop et al. 1992] that correspond with the DSM items, and the severity score of at least 3 for each symptom to meet the cutoff criterion for clinical relevance. Patients with an organic disorder, or a primary psychotic disorder, or bipolar depression were excluded. Patients with depression and panic disorder were not included because they participated in a different research project. The Ethical Committee of the Leiden University Medical Centre approved the informed consent protocol. Written informed consent was obtained from all patients. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Global dimensions of psychopathology, depression severity and subcategories of depression
The global dimensions of Emotional Dysregulation, Motivational Inhibition (Retardation), and Autonomous Dysregulation (Anxiety) and the MADRS score were assessed by RFPdeW using the semi-standardized interview of the CPRS [Asberg et al. 1978]. The MADRS consists of nine items from the dimension of Emotional Dysregulation and one item of the dimension of Retardation.
The melancholic and psychotic subcategories according to the DSM-IV [American Psychiatric Association, 1994] were diagnosed by means of the semi-standardized interview based on the CPRS mentioned above. The melancholic item of ‘symptoms being worse in the morning’ was scored separately. The symptoms of the psychotic subcategory comprised mood-congruent and mood-incongruent psychotic features. The HAR subcategory was defined by combined above-median scores on the dimensions of Anxiety and Retardation [de Winter et al. 2004]. The ANA subcategory was defined by a plasma AVP concentration of at least 5.6 pg/ml [Goekoop et al. 2006].
Psychotropic treatment and smoking habit
Forty-nine of the 78 patients (62%) were using an antidepressant drug for at least 2 weeks, 10 patients (13%) used an antipsychotic drug, and 42 patients (57%) a benzodiazepine. Twenty patients (26%) were completely drug free. To analyse the effect of psychotropic drug dosage as covariate, currently accepted imipramine equivalent values of the dosages were computed [Moleman and Birkenhaeger, 1998]. Smoking habit of the 37 patients who smoked was quantified by the mean number of cigarettes on a daily basis during the last month.
Plasma norepinephrine and vasopressin
Within 7 days of the CPRS interview, blood samples were drawn on a single day between 09:00 and 9:30 and between 15:30 and 16:00 under standardized conditions. This implied that all patients refrained from ingesting alcohol and from undertaking strenuous physical exercise for 12 h before the study. Patients sat down 15 min before venipuncture. Smoking was not allowed for 30 min before venipuncture; eating and drinking were allowed ad libitum. Blood was collected in 10 ml vacutainer tubes and immediately stored at 4°C. Within 30 min plasma was separated and stored at −80°C.
Plasma NE concentration was assessed in the biochemical laboratory of the Endegeest Institute for mental illness. NE was extracted with aluminium oxide, and its concentration (pg/ml) was determined by means of high-performance liquid chromatography (HPLC) using electrochemical detection with dihydroxybenzylamine as an internal standard [Javidan and Cwik, 1996].
Plasma AVP-like immunoreactivity, further referred to as AVP, was determined as described previously [Van Londen et al. 1997] by radioimmunoassay using an antibody raised in a rabbit in the Rudolf Magnus Institute. The limit of detection [mean blank + 2 × standard deviation (SD) as criterion] was 0.5 pg/ml for plasma (extracted assay), and the intra- and inter-assay coefficients of variation (CV) were 9.9% and 15.9% respectively. All samples were processed and radioimmunoassayed in duplicate, in one and the same run. The performance of the assay was in the range of the values measured, Effective dose (ED)- 20, -50 and -80 being 0.5 pg/ml, 4 pg/ml and 32 pg/ml, respectively. The intra-assay CV was determined using samples taken from a plasma pool with an AVP concentration of around 4 pg/ml that were processed independently before radioimmunoassay. This is close to the cutoff point of 5.6 pg/ml for depression with above-normal plasma AVP [Goekoop et al. 2006].
For each patient, mean daytime plasma NE and AVP levels were computed from the morning and afternoon values. As plasma AVP was not normally distributed (Kolmogorov–Smirnov Z = 1.914; p = 0.001), we used log-transformed values (lnAVP) in linear correlation analyses. LnAVP values were normally distributed (Kolmogorov–Smirnov Z = 0.939; p = 0.341).
Data analysis
Chi-square and Student’s t-test were used to test differences between the PSDEP and non-PSDEP groups for inpatient versus outpatient treatment and duration of the current episode. The dependence of PSDEP on the three global dimensions of psychopathology was tested by separate logistic regression analyses and the dependence of PSDEP on the combination of these dimensions and the three subcategories of depression by multiple logistic regression analysis. The dependence of plasma NE on smoking habit, age, sex and psychotropic drug dosages or treatments, and three global dimensions of psychopathology and three nonpsychotic subcategories of depression was tested by analysis of covariance (ANCOVA). After a Student’s t-test to analyse the uncorrected relation between NE and PSDEP, we used ANCOVA to analyse the relation between plasma NE and PSDEP, using NE as a dependent variable, PSDEP, HAR depression and tricyclic drug treatment as fixed factors, and smoking habit and the three dimensions of psychopathology as covariates. ANCOVA was also used to analyse the correlation between NE as dependent variable and lnAVP as covariate in interaction with the subcategory of PSDEP, with smoking habit and tricyclic drug treatment as confounding variables. Partial correlations between NE and lnAVP were used within the subgroups of PSDEP and non-PSDEP and within the subgroups of melancholic PSDEP in all other patients, controlling for the effects of smoking habit, tricyclic treatment and antipsychotic drug dosage. Fisher’s z statistics were used to test the differences between these correlations.
The analyses were carried out with the Statistical Package for the Social Sciences (SPSS) V.18.0.
Results
Demographic and clinical data
The mean age of the 78 patients with complete NE data was 40.2 years (SD = 11.5 years; range 20–64 years); 52 patients (67%) were women. Mean severity according to the MADRS was 30 (SD = 6). The mean age of the nine patients with PSDEP was 44.6 years (SD = 12.5 years) and of the 69 patients with non-PSDEP it was 39.7 years (SD = 11.4 years). The melancholic subtype of depression according to DSM-IV criteria was present in 36 of the 78 patients (46%). Twenty-six patients (33%) had HAR depression. HAR was present in 22 of the 36 patients (61%) with melancholia, and melancholia was present in 22 of 26 patients (85%) with HAR depression. Seven of the nine psychotic patients (78%) had antidepressant treatment versus 42 of the 69 nonpsychotic patients (61%).
Seventy-five patients had complete NE and AVP data. Nine of these 75 patients (12%) had psychotic depression. Fourteen (19%) had ANA depression. Seven of the 25 patients with HAR (28%) had ANA depression, and HAR was present in 50% of the patients with ANA depression. ANA depression was present in 9 of the 34 patients (27%) with melancholia, and melancholia was present in 64% of patients with ANA depression.
Mean antipsychotic, antidepressant and benzodiazepine dosages, if the dosage was greater than 0, were 3.4 mg (SD = 2.8 mg) haloperidol equivalents, 157 mg (SD = 84 mg) imipramine equivalents, and 38 mg (SD = 41 mg) chlordiazepoxide equivalents. Ten patients (3 with PSDEP) used a tricyclic antidepressant, 20 patients (3 with PSDEP) used an SSRI and 16 patients used an serotonin–norepinephrine reuptake inhibitor (SNRI) (1 with PSDEP). Mean equivalent dosages for these three groups were 173 mg (SD = 82 mg), 160 mg (SD = 57 mg) and 157 (SD = 116 mg) respectively. Nine of the 10 patients with tricyclic treatment had a higher dose than 100 mg, which is about the mean dose minus twice the SD of the study that demonstrated the increasing effect on plasma NE [Veith et al. 1994]. The one patient with a too low dose had PSDEP and used 75 mg. We used the criterion of at least 100 mg for the selection of patients with a minimally adequate dose, hereafter called ‘tricyclic treatment’. The mean number of cigarettes smoked was 10 (SD =13). In the group of smokers (n = 37) the mean was 21 (SD = 12); 2 patients smoked one cigarette/day, and 35 patients at least five cigarettes/day. Thirty-two patients were inpatients (41%), 7 of whom had PSDEP (78% of all patients with PSDEP) (χ2 = 5.680; p = 0.017). The mean duration of the current episode was 6.6 months (SD = 6.7); Student’s t-test showed that the difference between the PSDEP group (5.3 months; SD = 2.4) and the non-PSDEP group (6.8 months; SD = 7.0) was statistically nonsignificant.
Psychotic depression and relations with other subcategories of depression and dimensions of psychopathology
In the whole group of 78 patients, 7 of the 9 patients with PSDEP (78%) also fulfilled the criteria for melancholic depression, and 5 had HAR depression (56%). Three of the nine patients with PSDEP had ANA depression (33%). From the three patients with ANA and PSDEP, two fulfilled the criteria for both HAR depression and melancholia, and one had only melancholia. Separate logistic regression analyses and multiple logistic regression analysis of the relation between PSDEP and the melancholic, HAR and ANA subcategories showed PSDEP to be statistically nonsignificantly related to the melancholic subtype (Wald = 3.722; p = 0.054), while the relations with the two other subcategories were clearly statistically nonsignificant. Separate logistic regression analyses of the relation between PSDEP and the global dimensions of psychopathology showed PSDEP to depend on Emotional Dysregulation (Wald = 8.559; p = 0.003) and Retardation (Wald = 4.015; p = 0.045). Multiple logistic regression with PSDEP as a dependent variable and the three global dimensions of psychopathology, Emotional Dysregulation, Retardation and Anxiety, as independent variables showed that PSDEP related only to Emotional Dysregulation (Wald = 8.559; p = 0.003). The addition to the regression model of the three subcategories of depression, melancholia, HAR and ANA depression, did not result in a relation with any of these subcategories and did not change the relation between PSDEP and the dimension of Emotional Dysregulation. If the MADRS was used instead of Emotional Dysregulation than the result was highly comparable (Wald = 8.472; p = 0.004).
In conclusion, Emotional Dysregulation was highly significantly related to PSDEP and was a better measure of inherent nonpsychotic psychopathology of PSDEP than the melancholic subtype or the dimension of Retardation. Emotional Dysregulation was therefore used in the subsequent analyses as covariate to test for the role of general severity of depression in the relation between PSDEP and NE.
Plasma norepinephrine concentration and relations with drug treatment and drug dosage, smoking habit, demographic data, dimensions of psychopathology and subcategories of depression, and duration of the disease and inpatient or outpatient treatment
Table 1 shows the plasma concentrations of NE in depression and its subcategories compared with their complementary subgroups of patients with depression. Student’s t-tests did not reveal a relation between NE and any of the nonpsychotic subcategories. ANCOVA without the subcategories as fixed factors showed that plasma NE concentration in the whole group of 78 patients was not related to the dosages of antipsychotic, benzodiazepine and antidepressant drugs (F= 0.042, p = 0.838; F = 0.042, p = 0.838; F = 0.0274, p = 0.602 respectively). However, the NE concentration appeared to depend significantly on smoking habit and tricyclic treatment. Smoking habit was negatively related to NE (F = 6.826, p = 0.011) and positively related to tricyclic treatment (F = 6.448; p = 0.013). The SSRI and SNRI treatments were not related. The addition of age and sex to the ANCOVA model resulted in a significant relation with age (F = 4.128, p = 0.046) that slightly reduced the strength of the relation with smoking habit (F = 6.653, p = 0.012), while no relation was found between NE and sex (F = 0.085, p = 0.771).
Table 1.
Mean concentration of plasma norepinephrine (pg/ml) and standard deviation in major depressive disorder, psychotic depression and three other subcategories.
| n | NE (SD) | p | |
|---|---|---|---|
| MDD | 78 | 505 (255) | |
| Psychotic | 9 | 657 (276) | 0.057 |
| Nonpsychotic | 69 | 485 (247) | |
| Melancholic | 36 | 546 (292) | 0.188 |
| Nonmelancholic | 42 | 470 (217) | |
| HAR | 26 | 530 (268) | 0.548 |
| Non-HAR | 52 | 493 (251) | |
| ANA | 14 | 543 (276) | 0.447 |
| Non-ANA | 61 | 486 (245) |
ANA, depression with above normal arginine vasopressin concentration; HAR, highly anxious retarded depression; MDD, major depressive disorder; melancholic, Diagnostic and Statistical Manual of Mental Disorders IV melancholia; NE, norepinephrine; SD, standard deviation. p Values of uncorrected Student’s t-tests; n = number of patients.
ANCOVA in the 75 patients with complete NE and AVP data, using NE as a dependent variable, the subcategories of melancholic, HAR and ANA depression as fixed factors, and smoking habit, age and tricyclic treatment, and the three nonpsychotic dimensions of Emotional Dysregulation, Retardation and Anxiety as covariates, showed that NE was still negatively related to smoking habit (F = 8.525, p = 0.0054) and positively related to tricyclic treatment (F = 10.146, p = 0.002), but no longer with age (F = 0.628, p = 0.431). The HAR subcategory was negatively related to plasma NE (F = 4.786, p = 0.032), and the dimensions of Retardation and Anxiety were each positively related to NE (F = 5.372, p = 0.024; F = 7.315, p = 0.009 respectively). The dimension of Emotional Dysregulation was not related to NE (F = 1.058, p = 0.307). Duration of present episode and inpatient or outpatient treatment were not related to NE.
In conclusion, smoking habit, tricyclic treatment and HAR depression were found to be related to plasma NE. The intensity of the dimension of Emotional Dysregulation that specifically relates to PSDEP was not related to plasma NE, while the dimensions of Retardation and Anxiety that were not related to PSDEP, related to NE. The dimension of Emotional Dysregulation could therefore be used to test if the severity of depression that is an inherent aspect of PSDEP is involved in the relation between NE and PSDEP. Smoking habit and tricyclic treatment were subsequently used as other potential confounders in the analyses involving NE and PSDEP, as were the other two global dimensions of psychopathology and the HAR subcategory.
Plasma norepinephrine concentration in psychotic depression compared with nonpsychotic depression
A separate Student’s t-test showed a statistically nonsignificant increase in NE in PSDEP (Table 1). ANCOVA in the 78 patients with complete NE data, using NE as a dependent variable, PSDEP (n = 9) versus non-PSDEP (n = 69) and tricyclic treatment as fixed factors, and smoking habit as covariate showed that PSDEP was positively related to NE (F = 4.207, p = 0.044), while smoking habit was negatively related (F = 8.203, p = 0.005) and tricyclic treatment was positively related (F = 5.682, p = 0.020). Addition of the three dimensions of psychopathology, Emotional Dysregulation, Retardation and Anxiety, to the ANCOVA model resulted in a weak increase in the strength of the dependence of NE on PSDEP (F = 4.429, p = 0.039), while the strength of the relation with tricyclic treatment increased (F = 7.799, p = 0.007) and that of the relation with smoking habit decreased (F = 6.417, p = 0.014). Compared with the results of the analysis of the previous subsection in the absence of PSDEP Emotional Dysregulation was now negatively related to NE (F = 5.270, p = 0.025), while Retardation and Anxiety were still positively related (F = 5.623, p = 0.020; F = 5.378, p = 0.023 respectively). If the interaction between PSDEP and Emotional Dysregulation was added to the ANCOVA model there was no evidence of a deviant relation between Emotional Dysregulation and plasma NE in PSDEP (F = 0.057, p = 0.955). Accounting for the high intensity of depression inherent to PSDEP will therefore have resulted in the detection of a general negative relation between Emotional Dysregulation and NE.
If the subcategory method was used to analyse for additional psychopathology instead of the multidimensional method, then the addition of the HAR subcategory to the ANCOVA model with smoking habit and tricyclic treatment as covariates resulted in a somewhat lower significance of the relation between NE and PSDEP (F = 4.018, p = 0.049), despite HAR in this analysis not being related to plasma NE (F = 0.001, p = 0.978). The use of the melancholic subcategory in the ANCOVA model instead of the HAR subcategory resulted in a loss of significance of the relation between NE and PSDEP (F = 2.928, p = 0.091) and a nonsignificant positive relation with the melancholic subcategory (F= 1.742, p = 0.191). This suggests that the relation between PSDEP and NE may be specific for patients with PSDEP and melancholia. This was supported by a similar ANCOVA using the subcategories of patients with melancholic PSDEP (n = 7) and all other patients (n = 71) as fixed factor (F = 5.294, p = 0.024). The addition of the three global dimensions of psychopathology as covariates to the ANCOVA model resulted in further strengthening of the relation between this subcategory and plasma NE concentration (F = 5.975, p = 0.017).
In conclusion, the concentration of plasma NE appeared to be increased in PSDEP compared with non-PSDEP. If the positive relations with Anxiety and Retardation were accounted for, then the intensity of Emotional Dysregulation appeared to be generally negatively related to plasma NE. This applied to patients with psychotic and nonpsychotic depression. The relation between PSDEP and NE was particularly present in patients with PSDEP and melancholia.
Correlation between plasma norepinephrine and vasopressin in psychotic depression
Figure 1 shows the uncorrected positive correlations between plasma NE and lnAVP in PSDEP and non-PSDEP. Partial correlations between NE and lnAVP were analysed in the subgroups of PSDEP (n = 9) and non-PSDEP (n = 69), and in the subgroups of melancholic PSDEP (N = 7) and all other patients (N = 71), controlling for the effects of smoking habit, tricyclic treatment and antipsychotic drug dosage. These analyses showed positive correlations in both psychotic groups (r = 0.729 and r = 0.718 respectively), and the absence of a correlation in the two patient control groups (r = 0.050 and r = 0.049). Fisher’s z test showed that these correlations differed significantly in both comparisons (z = 4.11, p < 0.01; z = 3.32; p < 0.01).
Figure 1.
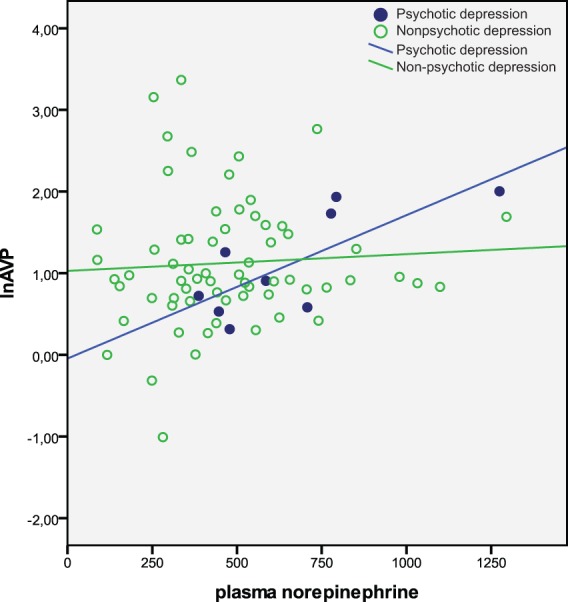
Relations between plasma norepinephrine and vasopressin (lnAVP).
Seventy-eight patients with depression were divided into psychotic (n = 9) and non-psychotic depression (n = 69). Lines represent uncorrected regression coefficients of the correlation between norepinephrine (pg/ml) and lnAVP.
Discussion
Increased noradrenergic activation in psychotic depression
This study confirmed the hypothesis that PSDEP is characterized by an increased concentration of plasma NE, and reconfirmed the correlation between plasma NE and AVP concentrations [Goekoop et al. 2011] while using a more complete set of confounders in the analyses. The correlation between central and plasma NE [Esler et al. 1995; Kelly and Cooper, 1997; Ziegler et al. 1977] suggests that in PSDEP a high central noradrenergic activation may induce a high noradrenergic–vasopressinergic activation. The role of NE and AVP in the activation of the HPA axis [Al-Damluji, 1993] suggests that an increased noradrenergic–vasopressinergic mechanism combined with the increased vasopressinergic activation of the HPA axis common to all depressive disorders [Goekoop et al. 2010] could explain the very high rate of dexamethasone nonsuppression that characterizes PSDEP [Nelson and Davis, 1997]. This hypothesis should be tested in future studies.
The role of confounders
The search for potential confounders appeared to be very useful in this study. As far as the subcategories of depression are concerned, neither melancholia according to the DSM-IV-TR nor the better validated HAR and ANA subcategories appeared to be significantly related to PSDEP, and only the HAR subcategory was (negatively) related to plasma NE. In contrast, all three global dimensions of the CPRS [Goekoop et al. 1992] selected for the study eventually appeared to be related to NE. The dimension of Emotional Dysregulation was negatively related, and the dimensions of Retardation and Anxiety positively related, as has been found previously [Roy et al. 1985b]. In separate analyses PSDEP appeared to be related to the dimension of (psychomotor) Retardation, evidence of which has also been found previously [Parker et al. 1999; Schatzberg and Rothschild, 1992], and with the dimension of Emotional Dysregulation. However, in multiple logistic regression analysis comparing PSDEP with non-PSDEP, PSDEP did not appear to be related to Retardation, which corresponds with a recent report [Keller et al. 2006], and only with the dimension of Emotional Dysregulation. The latter finding corresponds with the hierarchic structure that has repeatedly been found for the nonpsychotic symptoms of patients with a depression with psychotic features [Surtees and Kendell, 1979]. In fact, Emotional Dysregulation appeared only to be negatively related to NE after accounting for the effect of the high Emotional Dysregulation inherent to PSDEP. The use of the dimensions of Anxiety, Retardation and Emotional Dysregulation as covariates therefore enabled a rigorous test of our hypotheses. The finding of patients with melancholic PSDEP having the strongest relation with plasma NE concentration, while not being better characterized by correlating NE and AVP concentrations than the whole subcategory of PSDEP, may suggest a weakness of the diagnostic criteria for melancholic depression. The data warrant further investigation of the most specific nonpsychotic symptoms involved in PSDEP.
In all analyses, smoking habit and the use of tricyclic antidepressant therapy appeared to be highly significant confounders of plasma NE. The negative relation that we found with smoking habit does not seem to correspond to the increasing effect on NE of smoking one cigarette [Grassi et al. 1994] or chronic smoking [Christensen and Jensen, 1995; Christensen and Knudsen, 1998] in subjects without depression. However, a comparison of the upper level of the data (see Figure 2) suggests that the negative correlation that we found in patients with depression particularly pertains to high levels of NE in the range above ±320 pg/ml and not to the lower levels that were found in the investigation of subjects without depression [Christensen and Jensen, 1995; Christensen and Knudsen, 1998]. These data correspond with an antinoradrenergic effect of smoking in several conditions of high noradrenergic activation, like PSDEP and the use of tricyclic antidepressant treatment. The finding of high plasma NE during tricyclic antidepressant therapy corresponds with previous findings for desipramine in depression [Veith et al. 1994]. Although the number of patients with PSDEP on tricyclic treatment was very small, the effects of PSDEP and tricyclic treatment seemed to be additive. The absence of a negative effect of SSRI treatment in this study corresponds with the nonsignificant effect found previously in patients with depression [Barton et al. 2007].
Figure 2.
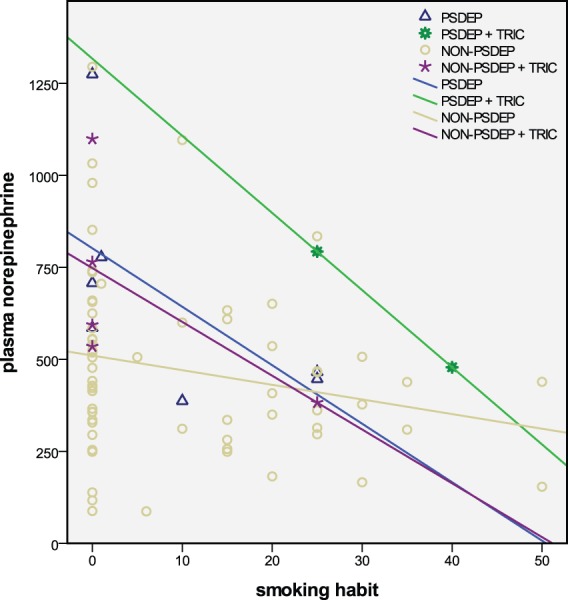
Relations between plasma norepinephrine and smoking habit.
Seventy-eight patients with depression were divided into psychotic without and with tricyclic treatment ≥100 mg (PSDEP, n = 7 and PSDEP + TRIC, n = 2), and non-psychotic without and with tricyclic treatment ≥100 mg (NON-PSDEP, n = 62 and NON-PSDEP + TRIC, n = 7). Lines represent regression coefficients of the correlation between norepinephrine (pg/ml) and smoking habit (number of cigarettes/day).
Potential causes of increased noradrenergic activation in psychotic depression
Part of the increased noradrenergic activation in PSDEP may be genetically determined [Keller et al. 2007], and another part may be related to traumatic experience [Gaudiano and Zimmerman, 2010]. The present study, however, does not permit one to analyse these etiological factors. The genetically increased release could be due to a reduced activity of catechol-O-methyl-transferase via a genetic polymorphism of this enzyme, which has been found to be associated with psychotic disorder combined with depression [McClay et al. 2006]. The increased correlation between NE and AVP could be due to an increased function of the excitatory α-1 receptor of the PVN [Al-Damluji, 1993]. Whether this would be due to a stress-induced sensitization mechanism analogous to the increase in NE transmission after a single administration of interleukin 1-α or amphetamine, in which sensitization of α-1 receptors for a stress condition may play a role [Jansen et al. 2003], is a matter for future studies. Since increased vasopressinergic activation may be a general mechanism in all depressive disorders, and subcategories are supposed to be characterized by specific vasopressinergic mechanisms [Goekoop et al. 2010], we assume that increased α-1 receptor-mediated noradrenergic activation of AVP release is the specific mechanism involved in PSDEP. As α-1 receptor-mediated noradrenergic activity also induces a reduction of the pre-pulse-inhibition and an increase of conditioned avoidance behaviour, both being targets in animal models for antipsychotic drug development [see Wadenberg et al. 2000], the same noradrenergic mechanism could be involved in the increased activation of the HPA axis and in the production of psychotic symptoms. As a consequence, pharmacological treatment involving a blockade [Wadenberg et al. 2000] or downregulation [Subhash et al. 2003] of the α-1 receptor could be a specifically efficacious component of pharmacological treatment of PSDEP.
The primary role supported by the present study for increased release of NE and increased α-1 receptor function in PSDEP implies that the meaning of previous findings in the field of noradrenergic and dopaminergic function should be reconsidered. Previous findings in PSDEP are a reduction of dopamine-beta-hydroxylase (DBH) activity and an increased concentration of plasma dopamine [Rothschild et al. 1987] compared with patients with non-PSDEP and normal controls. As far as dopamine release is concerned, evidence of a noradrenergic, α-1 mediated activation of that release from the ventral striatum has been found [Verheij and Cools, 2008]. Our present findings suggest that the previously found reduction of DBH activity in PSDEP could not be secondary to increased HPA axis function, as has been suggested [Cubells et al. 2002], but could actually depend more directly on the increased noradrenergic activation. Whether chronic downregulation of the synthesis of DBH occurs as an adaptation to a high tonic noradrenergic condition will have to be investigated in patients with PSDEP. Up to now only a state-dependent decrease in DBH activity has been found in children and adolescents with acute depression [Paclt et al. 2009].
Other potential mechanisms involved in the relation between noradrenergic activation and dimensions of Anxiety and Retardation
As far as the inhibitory presynaptic or even postsynaptic α-2 receptor function is considered, many studies in PSDEP [Duval et al. 2006] and in non-PSDEP [Ressler and Nemeroff, 1999; Siever and Davis, 1985] have reported a reduced clonidine-induced increase in growth hormone, corresponding to a reduced or downregulated α-2 receptor. Since this evidence of reduced α-2 receptor function has been found in non-PSDEP and PSDEP, and has been interpreted as a consequence of increased HPA axis activity in general, this reduction probably does not function as a specific and pathogenetic factor in PSDEP. The increased release of NE due to reduced inhibitory presynaptic α-2 function could correspond to increased cerebrospinal fluid or plasma NE and the large subgroup with melancholia compared with normal controls [Roy et al. 1985b; Wong et al. 2000], and with the positive relations between plasma NE and the dimensions of Retardation and Anxiety, which we found in the present study and have been found before in patients with depression and melancholia [Roy et al. 1985b]. These findings further stress the necessity to control for the confounding effects of these dimensions of psychopathology in studies of the relation between NE and PSDEP. Finally, a deficient negative feedback mechanism could also be involved in these changes. Since cortisol normally inhibits noradrenergic activation of the PVN via the glucocorticoid receptor [Kvetnansky et al. 1993; Pacak et al. 1995], hypofunction of this receptor could play a role, if premorbidly present and as a consequence of downregulation due to chronic stress [de Kloet et al. 1998; Raison and Miller, 2003].
Support for psychotic depression as a distinct subcategory of depression
Since the data suggest that increased release of NE in PSDEP is not just a state-dependent change, the specific relation between PSDEP and the temperament of low reward dependence (RD) after full remission of the depressive disorder, next to the temperament of high harm avoidance of all patients with depression [Goekoop and de Winter, 2011] may be seen as further support for the noradrenergic hypothesis of PSDEP, as the personality trait of RD has been found to be related to noradrenergic activity [Curtin et al. 1997; Garvey et al. 1996; Ham et al. 2005; Mitropoulou et al. 2003; Samochowiec et al. 2002; Yamano et al. 2008]. The low instead of high score on the RD dimension in PSDEP suggests that an inverted U-curve relationship could be involved between NE and RD.
The combined findings of relations between PSDEP and the dimension of Emotional Dysregulation, increased concentration of plasma NE, positively correlating NE and AVP concentrations, personality trait of low RD and the state-dependent reduction of cooperativeness support the hypothesis of PSDEP as a distinct psychotic subcategory of major depression [Schatzberg and Rothschild, 1992] that combines a dimension of Emotional Dysregulation with a psychotic dimension [Keller et al. 2007]. Further studies in these fields are warranted, particularly studies of the pathogenetic role of stress-induced variations of NE in patients at increased risk from the development of poor memory performance, unusual thought content and psychotic symptoms [Keller et al. 2006] and the effect of α-1 receptor blockade.
Limitations
The limitations of this study are the small number of patients with PSDEP, and the use of psychotropic drugs by all patients. The results need to be replicated in a larger patient sample. Since drug withdrawal of patients with PSDEP is ethically not very acceptable, the ideal of the recruitment of a large group of drug-free patients with PSDEP will not be easily attainable. The validity of the semi-standardized diagnostic method for major depression and its DSM-IV subcategories used in this study needs to be investigated by a comparison with one of the current (semi)standardized diagnostic interviews.
Footnotes
This study was supported by a grant from Wyeth.
The authors have no conflict of interest. The sponsoring company had no influence on any stage of the study from concept to report.
References
- Aguilera G., Subburaju S., Young S., Chen J. (2008) The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog Brain Res 170: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Damluji S. (1993) Adrenergic control of the secretion of anteriorpituitary hormones. Baillieres Clin Endocrinol Metab 7: 355–392 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Press [Google Scholar]
- Asberg M., Montgomery S.A., Perris C., Schalling D., Sedvall G. (1978) A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl 5–27 [DOI] [PubMed] [Google Scholar]
- Barton D.A., Dawood T., Lambert E.A., Esler M.D., Haikerwal D., Brenchley C., et al. (2007) Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens 25: 2117–2124 [DOI] [PubMed] [Google Scholar]
- Christensen N.J., Jensen E.W. (1995) Sympathoadrenal activity and psychosocial stress. The significance of aging, long-term smoking, and stress models. Ann N Y Acad Sci 771: 640–647 [DOI] [PubMed] [Google Scholar]
- Christensen N.J., Knudsen J.H. (1998) Peripheral catecholaminergic function evaluated by norepinephrine measurements in plasma, extracellular fluid, and lymphocytes, from nerve recordings and cellular responses. Adv Pharmacol 42: 540–544 [DOI] [PubMed] [Google Scholar]
- Cubells J.F., Price L.H., Meyers B.S., Anderson G.M., Zabetian C.P., Alexopoulos G.S., et al. (2002) Genotype-controlled analysis of plasma dopamine beta-hydroxylase activity in psychotic unipolar major depression. Biol Psychiatry 51: 358–364 [DOI] [PubMed] [Google Scholar]
- Curtin F., Walker J.P., Peyrin L., Soulier V., Badan M., Schulz P. (1997) Reward dependence is positively related to urinary monoamines in normal men. Biol Psychiatry 42: 275–281 [DOI] [PubMed] [Google Scholar]
- Day H.E., Campeau S., Watson S.J., Jr, Akil H. (1999) Expression of alpha(1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci 19: 10098–10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Vreugdenhil E., Oitzl M.S., Joels M. (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19: 269–301 [DOI] [PubMed] [Google Scholar]
- de Winter R.F., Zwinderman K.H., Goekoop J.G. (2004) Anxious-retarded depression: relation to family history of depression. Psychiatry Res 127: 111–119 [DOI] [PubMed] [Google Scholar]
- Duval F., Mokrani M.C., Monreal-Ortiz J.A., Fattah S., Champeval C., Schulz P., et al. (2006) Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology 31: 876–888 [DOI] [PubMed] [Google Scholar]
- Esler M.D., Lambert G.W., Ferrier C., Kaye D.M., Wallin B.G., Kalff V., et al. (1995) Central nervous system noradrenergic control of sympathetic outflow in normotensive and hypertensive humans. Clin Exp Hypertens 17: 409–423 [DOI] [PubMed] [Google Scholar]
- Garvey M.J., Noyes R., Jr., Cook B., Blum N. (1996) Preliminary confirmation of the proposed link between reward-dependence traits and norepinephrine. Psychiatry Res 65: 61–64 [DOI] [PubMed] [Google Scholar]
- Gaudiano B.A., Zimmerman M. (2010) Does comorbid posttraumatic stress disorder affect the severity and course of psychotic major depressive disorder? J Clin Psychiatry 71: 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekoop J.G., de Winter R., Wolterbeek R., Wiegant V. (2010) Support for two increased vasopressinergic activities in depression at large and the differential effect of antidepressant treatment. J Psychopharmacol 25: 1304–1312 [DOI] [PubMed] [Google Scholar]
- Goekoop J.G., de Winter R.F.P. (2011) Temperament and character in psychotic depression compared with other subcategories of depression and normal controls. Depress Res Treat 22 November [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekoop J.G., de Winter R.P., de Rijk R., Zwinderman K.H., Frankhuijzen-Sierevogel A., Wiegant V.M. (2006) Depression with above-normal plasma vasopressin: validation by relations with family history of depression and mixed anxiety and retardation. Psychiatry Res 141: 201–211 [DOI] [PubMed] [Google Scholar]
- Goekoop J.G., de Winter R.F., Wolterbeek R., van Kempen G.M., Wiegant V.M. (2011) Evidence of vasopressinergic-noradrenergic mechanisms in depression with above-normal plasma vasopressin concentration with and without psychotic features. J Psychopharmacol 25: 345–352 [DOI] [PubMed] [Google Scholar]
- Goekoop J.G., Hoeksema T., Knoppert-Van der Klein E.A., Klinkhamer R.A., Van Gaalen H.A., Van Londen L., et al. (1992) Multidimensional ordering of psychopathology. A factor-analytic study using the Comprehensive Psychopathological Rating Scale. Acta Psychiatr Scand 86: 306–312 [DOI] [PubMed] [Google Scholar]
- Goekoop J.G., Wiegant V.M. (2009) Support for two subcategories of depression with different vasopressinergic mechanisms in the field of melancholia. Curr Psychiatry Rev 5: 127–136 [Google Scholar]
- Goekoop J.G., Zwinderman A.H. (1994) Multidimensional hierarchic ordering of psychopathology. Rasch analysis in factor-analytic dimensions. Acta Psychiatr Scand 90: 399–404 [DOI] [PubMed] [Google Scholar]
- Goekoop J.G., Knoppert-Van der Klein E.A., Hoeksema T., Zwinderman H.A. (1994) Onderzoek met de CPRS in Nederlandse vertaling. Betrouwbaarheid, factorstructuur en intensiteitsbeoordeling. Tijdschrift voor Psychiatrie 36: 520–526 [Google Scholar]
- Grassi G., Seravalle G., Calhoun D.A., Bolla G.B., Giannattasio C., Marabini M., et al. (1994) Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 90: 248–253 [DOI] [PubMed] [Google Scholar]
- Ham B.J., Choi M.J., Lee H.J., Kang R.H., Lee M.S. (2005) Reward dependence is related to norepinephrine transporter T-182C gene polymorphism in a Korean population. Psychiatr Genet 15: 145–147 [DOI] [PubMed] [Google Scholar]
- Jansen A.S., Schmidt E.D., Voorn P., Tilders F.J. (2003) Substance induced plasticity in noradrenergic innervation of the paraventricular hypothalamic nucleus. Eur J Neurosci 17: 298–306 [DOI] [PubMed] [Google Scholar]
- Javidan S., Cwik M.J. (1996) Determination of catecholamines in human plasma by HPLC with electrochemical detection. J Liq Chromotogr R T 19: 1339–1348 [Google Scholar]
- Keller J., Gomez R.G., Kenna H.A., Poesner J., DeBattista C., Flores B., et al. (2006) Detecting psychotic major depression using psychiatric rating scales. J Psychiatr Res 40: 22–29 [DOI] [PubMed] [Google Scholar]
- Keller J., Schatzberg A.F., Maj M. (2007) Current issues in the classification of psychotic major depression. Schizophr Bull 33: 877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C.B., Cooper S.J. (1997) Plasma noradrenaline response to electroconvulsive therapy in depressive illness. Br J Psychiatry 171: 182–186 [DOI] [PubMed] [Google Scholar]
- Kelly C.B., Cooper S.J. (1998) Differences and variability in plasma noradrenaline between depressive and anxiety disorders. J Psychopharmacol 12: 161–167 [DOI] [PubMed] [Google Scholar]
- Kvetnansky R., Fukuhara K., Pacak K., Cizza G., Goldstein D.S., Kopin I.J. (1993) Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology 133: 1411–1419 [DOI] [PubMed] [Google Scholar]
- Landgraf R. (2006) The involvement of the vasopressin system in stress-related disorders. CNS Neurol Disord Drug Targets 5: 167–179 [DOI] [PubMed] [Google Scholar]
- McClay J.L., Fanous A., van den Oord E.J., Webb B.T., Walsh D., O’Neill F.A., et al. (2006) Catechol-O-methyltransferase and the clinical features of psychosis. Am J Med Genet B Neuropsychiatr Genet 141B: 935–938 [DOI] [PubMed] [Google Scholar]
- Mitropoulou V., Trestman R.L., New A.S., Flory J.D., Silverman J.M., Siever L.J. (2003) Neurobiologic function and temperament in subjects with personality disorders. CNS Spectr 8: 725–730 [DOI] [PubMed] [Google Scholar]
- Moleman P., Birkenhaeger T.K. (1998) Praktische psychofarmacologie. Houten: Bohn Stafleu Van Loghum [Google Scholar]
- Montgomery S.A., Asberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389 [DOI] [PubMed] [Google Scholar]
- Nelson J.C., Davis J.M. (1997) DST studies in psychotic depression: a meta-analysis. Am J Psychiatry 154: 1497–1503 [DOI] [PubMed] [Google Scholar]
- Pacak K., Palkovits M., Kopin I.J., Goldstein D.S. (1995) Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol 16: 89–150 [DOI] [PubMed] [Google Scholar]
- Paclt I., Koudelova J., Pacltova D., Kopeckova M. (2009) Dopamine beta hydroxylase (DBH) plasma activity in childhood mental disorders. Neuro Endocrinol Lett 30: 604–609 [PubMed] [Google Scholar]
- Parker G., Wilhelm K., Mitchell P., Roy K., Hadzi-Pavlovic D. (1999) Subtyping depression: testing algorithms and identification of a tiered model. J Nerv Ment Dis 187: 610–617 [DOI] [PubMed] [Google Scholar]
- Raison C.L., Miller A.H. (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160: 1554–1565 [DOI] [PubMed] [Google Scholar]
- Ressler K.J., Nemeroff C.B. (1999) Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry 46: 1219–1233 [DOI] [PubMed] [Google Scholar]
- Rothschild A.J., Schatzberg A.F., Langlais P.J., Lerbinger J.E., Miller M.M., Cole J.O. (1987) Psychotic and nonpsychotic depressions: I. Comparison of plasma catecholamines and cortisol measures. Psychiatry Res 20: 143–153 [DOI] [PubMed] [Google Scholar]
- Roy A., Pickar D., Linnoila M., Doran A.R., Ninan P., Paul S.M. (1985a) Cerebrospinal fluid monoamine and monoamine metabolite concentrations in melancholia. Psychiatry Res 15: 281–292 [DOI] [PubMed] [Google Scholar]
- Roy A., Pickar D., Linnoila M., Potter W.Z. (1985b) Plasma norepinephrine level in affective disorders. Relationship to melancholia. Arch Gen Psychiatry 42: 1181–1185 [DOI] [PubMed] [Google Scholar]
- Samochowiec J., Kucharska-Mazur J., Rybakowski F., Ostapowicz A., Horodnicki J., Rozpara M., et al. (2002) Norepinephrine transporter polymorphism and personality trait of reward dependence in male alcoholics. Pharmacopsychiatry 35:195–196 [DOI] [PubMed] [Google Scholar]
- Schatzberg A.F., Rothschild A.J. (1992) Psychotic (delusional) major depression: should it be included as a distinct syndrome in DSM-IV? Am J Psychiatry 149: 733–745 [DOI] [PubMed] [Google Scholar]
- Siever L.J., Davis K.L. (1985) Overview: toward a dysregulation hypothesis of depression. Am J Psychiatry 142: 1017–1031 [DOI] [PubMed] [Google Scholar]
- Subhash M.N., Nagaraja M.R., Sharada S., Vinod K.Y. (2003) Cortical alpha-adrenoceptor downregulation by tricyclic antidepressants in the rat brain. Neurochem Int 43: 603–609 [DOI] [PubMed] [Google Scholar]
- Surtees P.G., Kendell R.E. (1979) The hierarchy model of psychiatric symptomatology: an investigation based on present state examination ratings. Br J Psychiatry 135: 438–443 [DOI] [PubMed] [Google Scholar]
- Vacher C.M., Frétier P., Créminon C., Calas A., Hardin-Pouzet H. (2002) Activation by serotonin and noradrenaline of vasopressin and oxytocin expression in the mouse paraventricular and supraoptic nuclei. J Neurosci 22: 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Londen L., Goekoop J.G., van Kempen G.M., Frankhuijzen-Sierevogel A.C., Wiegant V.M., van der Velde E.A., et al. (1997) Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 17: 284–292 [DOI] [PubMed] [Google Scholar]
- Veith R.C., Lewis N., Linares O.A., Barnes R.F., Raskind M.A., Villacres E.C., et al. (1994) Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch Gen Psychiatry 51: 411–422 [DOI] [PubMed] [Google Scholar]
- Verheij M.M., Cools A.R. (2008) Twenty years of dopamine research: individual differences in the response of accumbal dopamine to environmental and pharmacological challenges. Eur J Pharmacol 585: 228–244 [DOI] [PubMed] [Google Scholar]
- Wadenberg M.L., Hertel P., Fernholm R., Hygge Blakeman K., Ahlenius S., Svensson T.H. (2000) Enhancement of antipsychotic-like effects by combined treatment with the alpha1-adrenoceptor antagonist prazosin and the dopamine D2 receptor antagonist raclopride in rats. J Neural Transm 107: 1229–1238 [DOI] [PubMed] [Google Scholar]
- Wong M.L., Kling M.A., Munson P.J., Listwak S., Licinio J., Prolo P., et al. (2000) Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A 97: 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano E., Isowa T., Nakano Y., Matsuda F., Hashimoto-Tamaoki T., Ohira H., et al. (2008) Association study between reward dependence temperament and a polymorphism in the phenylethanolamine N-methyltransferase gene in a Japanese female population. Compr Psychiatry 49: 503–507 [DOI] [PubMed] [Google Scholar]
- Ziegler M.G., Lake C.R., Wood J.H., Brooks B.R., Ebert M.H. (1977) Relationship between norepinephrine in blood and cerebrospinal fluid in the presence of a blood-cerebrospinal fluid barrier for norepinephrine. J Neurochem 28: 677–679 [DOI] [PubMed] [Google Scholar]


