Abstract
Depression affects a significant proportion of the population, with 1-year and lifetime prevalence of 3–5% and 10–30% respectively. Full remission is achieved in only a third of patients following treatment with first-line antidepressant. There is a need for novel treatments for treatment-resistant depression (TRD). Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis has been described in patients with depression. There is persistent rise in the levels of cortisol (end product of the HPA axis) and impairment of the negative feedback inhibition mechanism of the HPA axis. Dysregulation of the HPA axis has been found to be linked to nonresponse to antidepressants and relapse following successful treatment. The efficacy of pharmacological agents that intervene with the mechanisms involved in dysregulation of cortisol synthesis and release are being explored in depression, particularly in TRD. Studies have been carried out with these drugs as augmenting agents for antidepressants or as monotherapy. The strongest evidence has come from studies using metyrapone, a cortisol synthesis inhibitor, and this has been described in detail in this review. The most robust evidence for its antidepressant efficacy in depression comes from a double-blind, randomized, placebo-controlled study of augmentation of serotonergic antidepressants with metyrapone. A 3-week augmentation of serotonergic antidepressants with 1 g metyrapone daily was shown to be superior to placebo in reducing the Montgomery–Asberg Depression Rating Scale by 50%, 5 weeks following initiation of treatment. The mechanism of the antidepressant action of metyrapone is not clear but the evidence for various potential mechanisms is discussed.
Keywords: antidepressant, antiglucocorticoid, depression, hypothalamic–pituitary–adrenal axis, metyrapone, treatment resistant
Introduction
Depressive illness affects a significant proportion of the population. It has been reported to have a 1-year prevalence of 3–5% [Hasin et al. 2005; Waraich et al. 2004] and a lifetime prevalence varying from 10 to 30% [Hasin et al. 2005; Waraich et al. 2004]. Depression is ranked by the World Health Organization as the third highest cause of disability across the world and it is projected to become the second by 2020 [Murray and Lopez, 1997; World Bank, 2004]. Furthermore depressive illness poses a significant financial burden to society: in 2000 depression in adults cost the UK £9 billion, including direct and indirect costs. Treatment of depression is not always effective. Only a third of patients achieve full remission after their first antidepressant treatment in naturalistic conditions [Rush et al. 2006]. More effective treatments are therefore required and to achieve this it is important to further understand the biology underpinning depressive illness.
A possible target for future treatment of depression is the hypothalamic–pituitary–adrenal (HPA) axis and the release of its major final hormone, cortisol. In this paper we review the evidence for the use of metyrapone, a cortisol synthesis inhibitor, for the treatment of treatment-resistant depression (TRD). Other reviews have examined the evidence of antiglucocorticoids in depressive illness (for instance (Gallagher et al., 2008)). To the authors knowledge this is the first review that focuses on the use of metyrapone in depressive illness.
Background
The hypothalamic–pituitary–adrenal axis
The HPA axis is a neuroendocrine system which incorporates the hypothalamus, the pituitary and the adrenal cortex. In addition to its role in the regulation of hormone secretion it is involved in responding to challenges to homeostasis, the regulation of the immune system, energy release and storage, sleep and sexual behaviour and emotions. It is under tight feedback control and is modulated by afferent connection from multiple brain areas, including the amygdala and hippocampus. In the hypothalamus, arginine vasopressin and corticotrophin-releasing hormone (CRH) are synthesized by parvocellular neurones of the paraventricular nuclei which project widely to the limbic system, brain stem and spinal cord and to the median eminence. Secretion into the hypothalamo-pituitary portal system of these two peptides regulates the secretion from the anterior lobe of the pituitary gland into the systemic circulation of adrenocorticotropic hormone (ACTH). ACTH, a polypeptide derived from the pro-opiomelanocortin precursor molecule, acts at the adrenal cortex to rapidly stimulate the biosynthesis of corticosteroid hormones such as cortisol from cholesterol.
Circulating cortisol acts at two types of receptor – type 1 mineralocorticoid receptors (MRs) and type 2 glucocorticoid receptors (GRs) [Herman et al. 1989a]. GRs have high affinity for dexamethasone. Regions of high GR mRNA levels include CA1, CA2 and dentate subregions of the hippocampus, paraventricular hypothalamus, lateral geniculate, lateral and medial amygdala, and cerebellum. Regions of high MR mRNA levels include all hippocampal pyramidal cell fields, dentate gyrus granule cell layer, lateral septum, medial and lateral amygdala, and to a lesser extent, cerebellum [Patel et al. 2000]. Cortisol diffuses through the cell membrane, binds to intracellular GRs and MRs and promotes their translocation to the nucleus. In response to stress, glucocorticoid levels rise, MR saturate and GR becomes the primary mediator of feedback inhibition of CRH (and the HPA axis) (Pariante and Miller, 2001, De Kloet et al., 1998). GR acts as a transcription factor to both positively and negatively regulate target genes. A decrease in glucocorticoid bioavailability might stem from decreased production of upstream glucocorticoid secretagogues including CRH and ACTH, this mechanism has been implicated in the pathogenesis of a range of neuropsychiatric diseases including atypical depression (Geracioti et al., 1997). Reduced glucocorticoid bioavailability may also be caused by a primary deficit in adrenal hormone production and/or release. Decreased glucocorticoid bioavailability might also result from alterations in 1) binding proteins, which have been identified for both cortisol and CRH (Rosner, 1991), 2) enzymes such as 11-β-hydroxysteroid dehydrogenase, which metabolize endogenous glucocorticoid hormones upon entry into the cell (Seckl and Walker, 2001), and 3) the multidrug resistance pump, which extrudes cortisol but not corticosterone from the cell (Karssen et al., 2001). Impairment at the level of the corticosteroid hormone receptors may also contribute to insufficient glucocorticoid signalling in depression (Pariante and Miller, 2001).
Dehydroepiandosterone (DHEA) is produced in the adrenal glands from cholesterol and, in its sulphated form, it is the most abundant circulating steroid in humans. It has antiglucocorticoid properties and thus the ratio of cortisol to DHEA has been used as a measure of functional hypercortisolaemia [Young et al. 2002].
The hypothalamic–pituitary–adrenal axis in depression
It has been repeatedly shown that there is dysregulation of the HPA axis in depression [Cowen, 2010; McAllister-Williams et al. 1998]. As early as the 1950s, reports of higher peripheral concentrations of cortisol in depression emerged, with levels typically normalizing as depressive symptoms remitted [Quarton et al. 1955]. There is evidence of a blunted ACTH response to CRH and of an increased cortisol response to ACTH in depression [Kellner et al. 1983]. The volume of pituitary and adrenal glands has also been shown to be increased in patients with depression [Kessing et al. 2011]. An increased cortisol/DHEA ratio is seen in adults and adolescents with depression and appears to be an indicator of poor prognosis [Markopoulou et al. 2009].
Studies have also shown altered feedback inhibition by corticosteroids as measured by the dexamethasone suppression test or the combined dexamethasone/CRH test [Heuser et al. 1994]. These tests measure the ability of the axis to suppress cortisol release in the presence of the synthetic steroid dexamethasone, a process reliant on the functional integrity of GRs.
Is the hypothalamic–pituitary–adrenal axis implicated in the pathogenesis and treatment of depression?
An aetiological role of HPA axis dysregulation in depression is supported by the findings that depression is common in patients with primary abnormalities of cortisol production, such as Cushing’s disease and that depression in these patients is most effectively treated by normalization of steroid levels [McFarland, 1963; Sonino et al. 1998]. Moreover, exogenous corticosteroid administration is associated with increased rates of depression, mood lability, cognitive impairment and psychosis [Hall et al. 1979; Rome and Braceland, 1952; Sprague et al. 1950; Wolkowitz et al. 1990a, 1990b]
Genes regulating HPA axis function contribute to the genetic vulnerability for depression. The heritability of the level of basal cortisol secretion is estimated to be 60% [Bartels et al. 2003]. HPA feedback disturbance has been observed in otherwise healthy people with a first-degree relative with an affective disorder [Holsboer et al. 1995]. The binding protein FKBP5 is an important modulator of the function of the GR and polymorphisms of genes encoding the GR and FKBP5 have been associated with variations in peripheral cortisol levels and have been implicated in the pathogenesis of stress-related disorders [Velders et al. 2011; Zimmermann et al. 2011].
HPA axis abnormalities appear to be associated not only with the pathogenesis of depression but also with poor outcome in patients with depression. For example, it has been found that dysregulation of the HPA axis is linked with an impaired response to antidepressants [Young et al. 2004; Zobel et al. 2001] and relapse following successful treatment [Appelhof et al. 2006; Aubry et al. 2007].
Chronic administration of selective serotonin reuptake inhibitors (SSRIs) has been shown to desensitize 5-hydroxytryptamine 1A (5-HT1A) autoreceptors on serotonergic neurones in the dorsal raphe nucleus (DRN) [de Montigny et al. 1990; Le Poul et al. 1995; Davidson and Stamford, 1998] and this allows levels of synaptic 5-HT in the forebrain to rise [Dawson et al. 2000; Gardier et al. 1996] where it can act on a range of 5-HT receptors, particularly postsynaptic 5-HT1A receptors, which has been argued to be critical for antidepressant response [Blier et al. 1990]. Corticosteroids also exert major effects on the expression of postsynaptic 5-HT1A receptors [Herman et al. 1989b]. For example, it is known that 5-HT1A receptor expression in the hippocampus is under tonic inhibition by adrenal steroids – the density of the receptors decreases in response to chronic stress or the administration of corticosteroids and increases after adrenalectomy [Grino et al. 1987; Guillaume et al. 1987]. Somatodendritic 5-HT1A autoreceptors in the DRN are also regulated by corticosteroids with reports in both animals and humans that repeated corticosteroid administration or stress decreases their functional activity [Fairchild et al. 2003; Laaris et al. 1997; McAllister-Williams et al. 2007; Young et al. 1994]. These effects of corticosteroids on somatodendritic and postsynaptic 5-HT1A receptors may potentially confound the effects of antidepressants, which may explain some of the findings of poor prognosis in patients with HPA axis dysregulation. This is supported by preclinical investigations. It has been shown in rats that flattening the corticosteroid rhythm, with an elevation of the nadir similar to that seen in patients with mood disorders [Deuschle et al. 1997; Wong et al. 2000], impairs the ability of SSRIs to elevate forebrain 5-HT [Gartside et al. 2003]. Conversely, the coadministration of a GR antagonist along with an SSRI is associated with higher forebrain 5-HT concentrations compared with an SSRI alone [Johnson et al. 2007]. This raises the distinct possibility of using drugs with an impact on the HPA axis to reduce some of the deleterious effects of HPA axis dysfunction and enhance the effectiveness of serotonergic antidepressants.
The hypothalamic–pituitary–adrenal axis as a target for the treatment of depression
Different strategies have been used to target the HPA axis in patients with depression. The treatment interventions include CRH receptor antagonists, GR antagonists and cortisol synthesis inhibitors. A Cochrane review in 2008 [Gallagher et al. 2008] summarized the findings of the clinical effect of antiglucocorticoid agents. Nine studies were included, of which three were in patients with psychotic major depression (pMDD), five in non-PMDD and one in patients with bipolar disorder (currently depressed; non-psychotic). Overall, when examining all trials together over all affective episodes, the mean change (weighted mean difference; baseline to endpoint) in Hamilton Depression Rating Scale (HDRS) scores indicated a significant difference in favour of treatment.
A number of small-scale clinical trials have examined the role of cortisol biosynthesis inhibitors such as ketoconazole, aminoglutethimide and metyrapone in the treatment of depression [Murphy, 1997; Murphy et al. 1998; O’Dwyer et al. 1995; Thakore and Dinan, 1995; Wolkowitz et al. 1993] and these are discussed in the section on ‘Metyrapone and treatment-resistant depression’ below.
Another strategy which has been used to target the HPA axis for the treatment of depression is the use of GR antagonists [e.g. RU486 (mifepristone), Org34517]. The mechanism of action of these drugs has not been fully elucidated, but it is speculated that they may act by enhancing MR function or by a rebound increase in GR function [Thomson and Craighead, 2008] suggesting the possibility that short-term treatment may exert persistent effects. Studies with RU486 suggest that it can reduce the psychiatric symptoms associated with Cushing’s disease [van der Lely et al. 1991]. There have also been a number of studies conducted using mifepristone in (non-Cushing’s) patients with pMDD. Positive findings in the initial open studies [Belanoff et al. 2001, 2002; Simpson et al. 2005] and randomized controlled trials [DeBattista et al. 2006; Flores et al. 2006] have been followed by a larger negative trial [Blasey et al. 2011], which used reduction in psychotic symptoms as the outcome measure. The authors argue that higher mifepristone doses may have led to a more robust response. An organon compound which acts as an antagonist at GRs has also been reported to have antidepressant properties in a poster and abstract but not in a full paper [Hoyberg et al. 2002].
CRH receptor antagonists have been developed and tested extensively in preclinical models to investigate their anxiolytic and antidepressant properties [Jones et al. 1998; van Gaalen et al. 2002; Zorrilla et al. 2002]. Most of the clinical trials done in this area are small scale and a definite role for these drugs can only be established after large-scale trials. A CRH receptor antagonist, R121919, was able to significantly reduce depression and anxiety scores in a cohort of 20 patients in an open-label clinical trial [Zobel et al. 2000]. Further studies showed that R121919 was effective in improving sleep and showed a good tolerability profile in patients with depression [Held et al. 2004; Kunzel et al. 2003, 2005; Zobel et al. 2000]. The increased liver enzymes seen in some patients after treatment [Zobel et al. 2000] has led to the discontinuation of development of this product.
Metyrapone and treatment of depression
Metyrapone characteristics
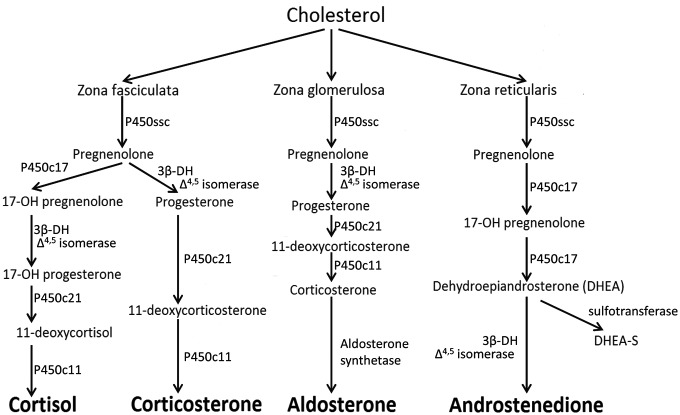
Metyrapone inhibits the enzyme 11-β hydroxylase that catalyses the conversion of 11-deoxycortisol to cortisol (see Figure 1). The attenuated HPA axis negative feedback (consequent on attenuated cortisol synthesis) after repeated metyrapone administration results in increased levels of ACTH, DHEA and 11-deoxycortisol levels, though with near normal plasma levels of cortisol [Jahn et al. 2004; Otte et al. 2007]. Metyrapone also inhibits 11β-HSD1 and the subsequent unopposed inactivation of cortisol by 11β-HSD2 results in an increase in the plasma cortisone:cortisol plasma ratio. Metyrapone also inhibits the production of aldosterone.
Figure 1.
Steroid synthesis pathway. Metyrapone acts by blocking the conversion of 11-deoxycortisol to cortisol by P450c11 (11β hydroxylase).
In humans, metyrapone is rapidly absorbed following oral administration. Blood levels peak 1 h after ingestion [eMC, 2010]. It has a half life of 20–26 min. Metyrapone’s main active metabolite –metyrapol – has a half life twice that of the parent compound. Metyrapone is excreted in the urine as metyrapone or as metyrapol [eMC, 2010].
Metyrapone is used in clinical practise as an aid for the differential diagnosis of ACTH-dependent Cushing’s syndrome and in the medical management of Cushing’s syndrome and aldosterone-induced oedema. Metyrapone is administered in doses varying from 250 mg to 6 g per day depending on the indication [Joint Formulary Committee, 2011].
Metyrapone is well tolerated. In a blinded study on patients with TRD, in which metyrapone was used alongside serotonergic antidepressants, only headaches and nausea were reported more frequently by participants in the metyrapone group compared with the placebo group [Jahn et al. 2004]. Other undesirable effects of metyrapone use include occasional vomiting, dizziness, sedation, hypotension and rarely hypoadrenalism, hirsuitism, allergic skin reactions and abdominal pain [eMC, 2010].
Metyrapone and treatment of treatment-resistant depression
There is limited evidence for the use of metyrapone in the treatment of depressive illness. Most of the evidence comes from three sources: preclinical studies, where the effect of metyrapone on animal models of depression is examined; studies on patients with Cushing’s syndrome and secondary depressive illness; and clinical studies of the effect of metyrapone in patients with depression.
The data from preclinical studies are based on studies of the effect of metyrapone treatment on the behaviour of rat models of depression or on the neurochemistry of the brain of rats. Healy and colleagues compared the effect of metyrapone with that of desipramine and placebo treatment in two rodent models of depression: the olfactory bulbectomized (OB) rat and the forced swim test (FST) [Healy et al. 1999]. In the OB rats, 14-day treatment with metyrapone (50 mg/kg) or desipramine attenuated OB-related hyperactivity. In the FST, administration of three doses of metyrapone or desipramine on rats between the first and second swim resulted in statistically significant reduction in immobility times during the second swim. In the same paper the authors reported a reduction in somatodendritic 5-HT1A sensitivity following 18-day treatment with metyrapone, offering a possible explanation for the ‘antidepressant’ effect of metyrapone observed in the OB and FST models of depression. Rogoz and colleagues investigated the effect of acute administration of metyrapone and imipramine on the FST in rats [Rogoz et al. 2003]. The greatest reduction in immobility time was seen with administration of metyrapone with imipramine rather than either drug alone. Furthermore the authors found that the ‘antidepressant’ effect (reduction in immobility time) of the combined metyrapone–imipramine treatment could be inhibited by using either a 5-HT1A antagonist (WAY 100635) or a D2/3 antagonist (sulpiride) but not when prazosin (α1 adrenergic receptor antagonist) was used. This indicates that metyrapone may directly or indirectly alter the sensitivity or the numbers of 5-HT1A or D2/3 receptor.
Since the late 1970s, metyrapone has been suggested for the treatment of psychiatric complications of Cushing’s disease. In 1979, Jeffcoate and colleagues reported the effect of metyrapone on 22 patients with depressive illness secondary to Cushing’s syndrome [Jeffcoate et al. 1979]. Metyrapone reduced cortisol levels to normal in all 22 patients. All patients with severe depression (5) achieved remission from their depressive symptoms but only 6 of the 13 patients with mild depression showed improvement. The antidepressant effect of metyrapone was seen within 2 months of normal cortisol levels being established.
Evidence supporting the use of metyrapone for TRD comes from studies of metyrapone as monotherapy or as an augmenting agent for antidepressants and are outlined in this order below. These studies are variable in quality and design. Murphy described a case report of a patient with TRD responding to metyrapone and aminoglutethimide who remained in remission for more than 2 years [Murphy, 1991]. Murphy reported that following initial withdrawal of all psychotropic medication, the patient received aminoglutethimide and cortisol (block replacement treatment) for 5 weeks of treatment followed by metyrapone (250 mg four times a day) with fludrocortisol for a further 8 weeks. In the same year Murphy and colleagues reported an open-label study of cortisol synthesis inhibitors, including metyrapone, of eight patients with TRD [Murphy et al. 1991]. Treatment lasted for up to 2 months and consisted of one or more of the steroid synthesis inhibitors (aminoglutethimide, ketoconazole and metyrapone). For six of the patients, remission lasted at least 5 months.
O’Dwyer and colleagues carried out a single-blind crossover study of 2 weeks’ treatment with metyrapone combined with replacement therapy with hydrocortisone compared with placebo in eight inpatients with depression and found a significant reduction in depression ratings in the active arm [O’Dwyer et al. 1995]. Metyrapone was titrated to achieve plasma cortisol levels within the normal range, starting from a dose of 500 mg four times a day and titrated to up to 1 g four times a day. Hydrocortisone was used at a physiological replacement dose of 7.5 mg four times a day. Six of the eight patients were medication free at the beginning of the trial whilst the other two were on antidepressant treatment (not specified), which was not altered for at least 4 weeks prior to the trial with metyrapone. The six patients who were not medicated at the beginning of the study were further investigated by Raven and colleagues, who reported a lack of a statistically significant reduction in cortisol levels at the end of a 2-week treatment with metyrapone and hydrocortisone [Raven et al. 1996]. The most significant effect of the metyrapone/hydrocortisone combination was an increase in the concentration of 11-deoxycortisol metabolites. These changes were seen within a week of initiation of the metyrapone/hydrocortisone treatment and are correlated with the improvement in depression rating scores (see below for further discussion). Iizuka and colleagues treated six patients with TRD (three with unipolar and three with bipolar depression) [Iizuka et al. 1996]; the treatment dose of metyrapone varied up to a maximum of 2 g per day and was used for 4 weeks. Three of the patients achieved full remission and one went into partial remission.
Murphy and colleagues reported on the use of aminoglutethimide, metyrapone and ketoconazole as monotherapy with hydrocortisone for 8 weeks in a study of 20 patients with TRD [Murphy et al. 1998]. Aminoglutethimide was given in a dose of 500–1750 mg daily, metyrapone 250–2000 mg daily and ketoconazole 400–1200 mg daily. Twenty milligrams of hydrocortisone was given at bedtime to ensure that there was no sudden drop in glucocorticoids and also to decrease the ACTH levels induced by activation of the HPA feedback mechanism in response to falling cortisol. All were started at the minimum dose, which was increased (by 250 mg increments for aminoglutethimide and metyrapone and by 200 mg for ketoconazole) every 4 days until the DHEA sulphate (DHEA-S) level fell below 3 nmol/liter, the HDRS fell below 15 or the patient developed troublesome side effects. If improvement was slow (no clear reduction in HDRS after 3 weeks and poor response in DHEA-S), one of the other agents was added. For the first six patients treated, aminoglutethimide was used as the first medication and metyrapone was added if required; subsequently, ketoconazole was the first medication used. A highly significant drop in the mean HDRS occurred at 8 weeks (p < 0.0001 using analysis of variance including all 20 patients) after which the mood scores remained steady. One patient failed to respond to aminoglutethimide plus metyrapone but did respond to a second course of ketoconazole plus metyrapone [Murphy et al. 1998]. Wolkowitz and colleagues also reported the antidepressant effect of ketoconazole as monotherapy in 10 patients with hypercortisolaemic depression but not in those with normal cortisol levels.
There has been one open-label trial and one placebo-controlled double-blind study of augmentation of serotonergic antidepressants with metyrapone. Rogoz and colleagues reported an open-label trial of augmentation of imipramine with metyrapone in patients with TRD. Patients commenced imipramine treatment for 6 weeks, followed by 6 weeks of the addition of metyrapone (250 mg twice daily) treatment [Rogoz et al. 2004]. Metyrapone augmentation significantly reduced the scores on the depression rating scales [HDRS (46%) and Beck Depression Inventory (39%)]. It was also found that metyrapone’s action was not related to an increase in plasma imipramine concentrations as the latter were unchanged. However, the most robust evidence for the use of metyrapone in depression comes from a placebo-controlled double-blind randomized trial by Jahn and colleagues [Jahn et al. 2004]. Sixty-three inpatients with depression received augmentation of nefazodone or fluvoxamine for 3 weeks with placebo or 1 g of metyrapone once daily. The group treated with metyrapone showed a significantly greater improvement compared with the placebo group (effect size of 0.6) using response (a decrease in HDRS score by 50%, 5 weeks post initiation of treatment) as the outcome measure. Unlike some of the previous studies [Murphy et al. 1991; O’Dwyer et al. 1995] described above, Jahn used a standard dose of metyrapone (rather than adjusting the dose according to cortisol levels). The change in morning plasma cortisol levels during metyrapone treatment did not reach statistical significance. Patients who showed an improvement in their HDRS score with metyrapone augmentation appeared to have higher levels of ACTH and 11-deoxycortisol compared with those whose condition did not respond, though the difference did not reach statistical significance.
Thus there is positive evidence for the use of metyrapone for the treatment of depression. However the exact mechanism of the antidepressant effect of metyrapone is not clear. Possible mechanisms include GR upregulation, alteration of the sensitivity of 5HT1A in the forebrain, activation of the MR or an antidepressant effect induced by other hormones in the steroid pathway. The absence of a significant reduction of cortisol in the Jahn study and the failure of another study [Raven et al. 1996] to show a correlation between response and cortisol levels argues against a simple reduction in plasma cortisol being the mechanism of action. However, these findings do not exclude the possibility that more subtle changes in cortisol dynamics caused by metyrapone (for instance a reduction in the trough levels) may underlie the therapeutic effect. A possible explanation for metyrapone’s antidepressant effect is the increase in concentration of precursors or hormones preceding cortisol in the metabolic pathway during metyrapone treatment. Metyrapone treatment leads to increases in plasma concentration of 11-deoxycortisol, ACTH and DHEA. Jahn and colleagues found that patients whose condition responded to metyrapone had higher ACTH and 11-deoxycortisol, though this did not reach statistical significance [Jahn et al. 2004]. Raven and colleagues also showed in a group of six patients with depression that an increase in urinary 11-deoxycortisol correlated with an improvement in Montgomery–Asberg Depression Scale score following treatment with metyrapone [Raven et al. 1996]. However, the changes in ACTH and 11-deoxycortisol may be simply a marker of the effect of metyrapone administration. Another explanation would be that metyrapone exerts its antidepressant effect by affecting aldosterone synthesis. Otte and colleagues found that patients with depression who had their serotonergic antidepressant augmented with a MR agonist (fludrocortisone) responded faster than those who had their medication augmented with a MR antagonist (spironolactone) or placebo [Otte et al. 2010]. However, it is difficult to assess metyrapone’s effect on MR receptors, since metyrapone has an ‘antimineralocorticoid’ effect by reducing aldosterone levels, but at the same time raises the levels of 11-deoxycortisol which is an MR agonist. A third explanation could be that Metyrapone exerts its effect by increasing the cortisol/corticosterone ratio (Raven et al., 1996), with cortisone having greater affinity for MR.
There remains uncertainty as to the optimal duration and frequency of metyrapone treatment. Jahn and colleagues administered metyrapone for 3 weeks only. At this stage it is unknown whether such a short duration of treatment is able to have a long-lasting effect, for example by leading to a ‘resetting’ of the HPA axis. The main outcome assessment of mood in the Jahn study was 5 weeks following the onset of treatment [Jahn et al. 2004]. Longer follow up as well as assessments of HPA axis function are required to address this issue.
Another aspect that needs further investigation is whether metyrapone can be used on its own or whether it is better used as an augmenting strategy for TRD. In the Jahn study, metyrapone was used to augment serotonergic antidepressants [Jahn et al. 2004]. The augmentative use of metyrapone is supported by preclinical evidence demonstrating that antiglucocorticoid treatments including GR antagonists [Johnson et al. 2007] and metyrapone [Rogoz et al. 2003] augments the effect of serotonergic medication. To date there are no double, randomized controlled trials of metyrapone monotherapy.
Discussion
HPA axis dysfunction is a promising therapeutic target for patients with depression, particularly those whose condition has not responded to conventional antidepressants alone. A single proof of principle randomized controlled trial of metyrapone augmentation of serotonergic antidepressants has been published so far. A larger replication study, the Antiglucocorticoid Augmentation of Antidepressants in Depression (ADD) study is currently underway in the North of England. This study involves metyrapone augmentation of serotonergic antidepressants in patients with refractory depression. Its results are eagerly awaited.
Footnotes
Funding: This work was supported by the Northumberland, Tyne and Wear Trust’s R&D committee.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Paul David Sigalas, Institution of Neurosciences – Academic Psychiatry, Campus for Ageing and Vitality, Westgate Road, Newcastle NE4 6BE, UK.
Himanshu Garg, Institution of Neurosciences – Academic Psychiatry, Newcastle, UK.
Stuart Watson, Institution of Neurosciences – Academic Psychiatry, Newcastle, UK.
Richard Hamish McAllister-Williams, Institution of Neurosciences – Academic Psychiatry, Newcastle, UK.
I. Nicol Ferrier, Institution of Neurosciences – Academic Psychiatry, Newcastle, UK.
References
- Appelhof B.C., Huyser J., Verweij M., Brouwer J.P., van Dyck R., Fliers E., et al. (2006) Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression). Biol Psychiatry 59: 696–701 [DOI] [PubMed] [Google Scholar]
- Aubry J.M., Gervasoni N., Osiek C., Perret G., Rossier M.F., Bertschy G., et al. (2007) The DEX/CRH neuroendocrine test and the prediction of depressive relapse in remitted depressed outpatients. J Psychiatr Res 41: 290–294 [DOI] [PubMed] [Google Scholar]
- Bartels M., Van den Berg M., Sluyter F., Boomsma D.I., de Geus E.J. (2003) Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology 28: 121–137 [DOI] [PubMed] [Google Scholar]
- Belanoff J.K., Flores B.H., Kalezhan M., Sund B., Schatzberg A.F. (2001) Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol 21: 516–521 [DOI] [PubMed] [Google Scholar]
- Belanoff J.K., Rothschild A.J., Cassidy F., DeBattista C., Baulieu E.E., Schold C., et al. (2002) An open label trial of C-1073 (mifepristone) for psychotic major depression. Biol Psychiatry 52: 386–392 [DOI] [PubMed] [Google Scholar]
- Blasey C.M., Block T.S., Belanoff J.K., Roe R.L. (2011) Efficacy and safety of mifepristone for the treatment of psychotic depression. J Clin Psychopharmacol 31: 436–440 [DOI] [PubMed] [Google Scholar]
- Blier P., de Montigny C., Chaput Y. (1990) A role for the serotonin system in the mechanism of action of antidepressant treatments: preclinical evidence. J Clin Psychiatry 51(Suppl.): 14–20 [PubMed] [Google Scholar]
- Cowen P.J. (2010) Not fade away: the HPA axis and depression. Psychol Med 40: 1–4 [DOI] [PubMed] [Google Scholar]
- Davidson C., Stamford J.A. (1998) Contrasting effects of chronic paroxetine on 5-HT1A control of dorsal raphe cell firing and 5-HT release. Neuroreport 9: 2535–2538 [DOI] [PubMed] [Google Scholar]
- Dawson L.A., Nguyen H.Q., Smith D.I., Schechter L.E. (2000) Effects of chronic fluoxetine treatment in the presence and absence of (+/−) pindolol: a microdialysis study. Br J Pharmacol 130: 797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBattista C., Belanoff J., Glass S., Khan A., Horne R.L., Blasey C., et al. (2006) Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol Psychiatry 60: 1343–1349 [DOI] [PubMed] [Google Scholar]
- de Montigny C., Chaput Y., Blier P. (1990) Modification of serotonergic neuron properties by long-term treatment with serotonin reuptake blockers. J Clin Psychiatry 51(Suppl. B): 4–8 [PubMed] [Google Scholar]
- Deuschle M., Schweiger U., Weber B., Gotthardt U., Korner A., Schmider J., et al. (1997) Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab 82: 234–238 [DOI] [PubMed] [Google Scholar]
- eMC (2010) Metopirone characteristics. Available at: http://www.medicines.org.uk/emc/document.aspx?documentid=126&docType=SPC (accessed 11 January 2011).
- Fairchild G., Leitch M.M., Ingram C.D. (2003) Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology 45: 925–934 [DOI] [PubMed] [Google Scholar]
- Flores B.H., Kenna H., Keller J., Solvason H.B., Schatzberg A.F. (2006) Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology 31: 628–636 [DOI] [PubMed] [Google Scholar]
- Gallagher P., Malik N., Newham J., Young A.H., Ferrier I.N., Mackin P. (2008) Antiglucocorticoid treatments for mood disorders. Cochrane Database Syst Rev (1): CD005168. [DOI] [PubMed] [Google Scholar]
- Gardier A.M., Malagie I., Trillat A.C., Jacquot C., Artigas F. (1996) Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam Clin Pharmacol 10: 16–27 [DOI] [PubMed] [Google Scholar]
- Gartside S.E., Leitch M.M., Young A.H. (2003) Altered glucocorticoid rhythm attenuates the ability of a chronic SSRI to elevate forebrain 5-HT: implications for the treatment of depression. Neuropsychopharmacology 28: 1572–1578 [DOI] [PubMed] [Google Scholar]
- Grino M., Guillaume V., Castanas E., Boudouresque F., Conte-Devolx B., Oliver C. (1987) Effect of passive immunization against corticotropin-releasing factor (CRF) on the postadrenalectomy changes of CRF binding sites in the rat anterior pituitary gland. Neuroendocrinology 45: 492–497 [DOI] [PubMed] [Google Scholar]
- Guillaume V., Conte-Devolx B., Szafarczyk A., Malaval F., Pares-Herbute N., Grino M., et al. (1987) The corticotropin-releasing factor release in rat hypophysial portal blood is mediated by brain catecholamines. Neuroendocrinology 46: 143–146 [DOI] [PubMed] [Google Scholar]
- Hall R.C., Popkin M.K., Stickney S.K., Gardner E.R. (1979) Presentation of the steroid psychoses. J Nerv Ment Dis 167: 229–236 [DOI] [PubMed] [Google Scholar]
- Hasin D.S., Goodwin R.D., Stinson F.S., Grant B.F. (2005) Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 62: 1097–1106 [DOI] [PubMed] [Google Scholar]
- Healy D.G., Harkin A., Cryan J.F., Kelly J.P., Leonard B.E. (1999) Metyrapone displays antidepressant-like properties in preclinical paradigms. Psychopharmacology (Berl) 145: 303–308 [DOI] [PubMed] [Google Scholar]
- Held K., Kunzel H., Ising M., Schmid D.A., Zobel A., Murck H., et al. (2004) Treatment with the CRH1-receptor-antagonist R121919 improves sleep-EEG in patients with depression. J Psychiatr Res 38: 129–136 [DOI] [PubMed] [Google Scholar]
- Herman J.P., Patel P.D., Akil H., Watson S.J. (1989a) Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol 3: 1886–1894 [DOI] [PubMed] [Google Scholar]
- Herman J.P., Schafer M.K., Young E.A., Thompson R., Douglass J., Akil H., et al. (1989b) Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci 9: 3072–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser I., Yassouridis A., Holsboer F. (1994) The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res 28: 341–356 [DOI] [PubMed] [Google Scholar]
- Holsboer F., Lauer C.J., Schreiber W., Krieg J.C. (1995) Altered hypothalamic–pituitary–adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology 62: 340–347 [DOI] [PubMed] [Google Scholar]
- Hoyberg O.J., Wik G., Mehtonen O.P., Peeters B.W.M.M., Sennef C. (2002) Org 34517, a selective glucocorticoid receptor antagonist with potent antidepressant activity: first clinical results. Int J Neuropsychopharmocol 5: S148 [Google Scholar]
- Iizuka H., Kishimoto A., Nakamura J., Mizukawa R. (1996) [Clinical effects of cortisol synthesis inhibition on treatment-resistant depression]. Nihon Shinkei Seishin Yakurigaku Zasshi 16: 33–36 [PubMed] [Google Scholar]
- Jahn H., Schick M., Kiefer F., Kellner M., Yassouridis A., Wiedemann K. (2004) Metyrapone as additive treatment in major depression: a double-blind and placebo-controlled trial. Arch Gen Psychiatry 61: 1235–1244 [DOI] [PubMed] [Google Scholar]
- Jeffcoate W.J., Silverstone J.T., Edwards C.R., Besser G.M. (1979) Psychiatric manifestations of Cushing’s syndrome: response to lowering of plasma cortisol. Q J Med 48: 465–472 [PubMed] [Google Scholar]
- Johnson D.A., Grant E.J., Ingram C.D., Gartside S.E. (2007) Glucocorticoid receptor antagonists hasten and augment neurochemical responses to a selective serotonin reuptake inhibitor antidepressant. Biol Psychiatry 62: 1228–1235 [DOI] [PubMed] [Google Scholar]
- Joint Formulary Committee (2011) British National Formulary, 61st ed. London: Pharmaceutical Press [Google Scholar]
- Jones D.N., Kortekaas R., Slade P.D., Middlemiss D.N., Hagan J.J. (1998) The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology (Berl) 138: 124–132 [DOI] [PubMed] [Google Scholar]
- Kellner C.H., Rubinow D.R., Gold P.W., Post R.M. (1983) Relationship of cortisol hypersecretion to brain CT scan alterations in depressed patients. Psychiatry Res 8: 191–197 [DOI] [PubMed] [Google Scholar]
- Kessing L.V., Willer I.S., Knorr U. (2011) Volume of the adrenal and pituitary glands in depression. Psychoneuroendocrinology 36: 19–27 [DOI] [PubMed] [Google Scholar]
- Kunzel H.E., Ising M., Zobel A.W., Nickel T., Ackl N., Sonntag A., et al. (2005) Treatment with a CRH-1-receptor antagonist (R121919) does not affect weight or plasma leptin concentration in patients with major depression. J Psychiatr Res 39: 173–177 [DOI] [PubMed] [Google Scholar]
- Kunzel H.E., Zobel A.W., Nickel T., Ackl N., Uhr M., Sonntag A., et al. (2003) Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res 37: 525–533 [DOI] [PubMed] [Google Scholar]
- Laaris N., Le Poul E., Hamon M., Lanfumey L. (1997) Stress-induced alterations of somatodendritic 5-HT1A autoreceptor sensitivity in the rat dorsal raphe nucleus – in vitro electrophysiological evidence. Fundam Clin Pharmacol 11: 206–214 [DOI] [PubMed] [Google Scholar]
- Le Poul E., Laaris N., Doucet E., Laporte A.M., Hamon M., Lanfumey L. (1995) Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol 352: 141–148 [DOI] [PubMed] [Google Scholar]
- Markopoulou K., Papadopoulos A., Juruena M.F., Poon L., Pariante C.M., Cleare A.J. (2009) The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology 34: 19–26 [DOI] [PubMed] [Google Scholar]
- McAllister-Williams R.H., Ferrier I.N., Young A.H. (1998) Mood and neuropsychological function in depression: the role of corticosteroids and serotonin. Psychol Med 28: 573–584 [DOI] [PubMed] [Google Scholar]
- McAllister-Williams R.H., Massey A.E., Fairchild G. (2007) Repeated cortisol administration attenuates the EEG response to buspirone in healthy volunteers: evidence for desensitization of the 5-HT1A autoreceptor. J Psychopharmacol 21: 826–832 [DOI] [PubMed] [Google Scholar]
- McFarland H. (1963) Addison’s disease and related psychoses. Compr Psychiatry 4: 90–95 [Google Scholar]
- Murphy B.E. (1991) Treatment of major depression with steroid suppressive drugs. J Steroid Biochem Mol Biol 39: 239–244 [DOI] [PubMed] [Google Scholar]
- Murphy B.E. (1997) Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology 22(Suppl. 1): S125–S132 [PubMed] [Google Scholar]
- Murphy B.E., Dhar V., Ghadirian A.M., Chouinard G., Keller R. (1991) Response to steroid suppression in major depression resistant to antidepressant therapy. J Clin Psychopharmacol 11: 121–126 [PubMed] [Google Scholar]
- Murphy B.E., Ghadirian A.M., Dhar V. (1998) Neuroendocrine responses to inhibitors of steroid biosynthesis in patients with major depression resistant to antidepressant therapy. Can J Psychiatry 43: 279–286 [DOI] [PubMed] [Google Scholar]
- Murray C.J., Lopez A.D. (1997) Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349: 1498–1504 [DOI] [PubMed] [Google Scholar]
- O’Dwyer A.M., Lightman S.L., Marks M.N., Checkley S.A. (1995) Treatment of major depression with metyrapone and hydrocortisone. J Affect Disord 33: 123–128 [DOI] [PubMed] [Google Scholar]
- Otte C., Hinkelmann K., Moritz S., Yassouridis A., Jahn H., Wiedemann K., et al. (2010) Modulation of the mineralocorticoid receptor as add-on treatment in depression: a randomized, double-blind, placebo-controlled proof-of-concept study. J Psychiatr Res 44: 339–346 [DOI] [PubMed] [Google Scholar]
- Otte C., Lenoci M., Metzler T., Yehuda R., Marmar C.R., Neylan T.C. (2007) Effects of metyrapone on hypothalamic–pituitary–adrenal axis and sleep in women with post-traumatic stress disorder. Biol Psychiatry 61: 952–956 [DOI] [PubMed] [Google Scholar]
- Patel P.D., Lopez J.F., Lyons D.M., Burke S., Wallace M., Schatzberg A.F. (2000) Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res 34: 383–392 [DOI] [PubMed] [Google Scholar]
- Quarton G.C., Clark L.D., Cobb S., Bauer W. (1955) Mental disturbances associated with ACTH and cortisone: a review of explanatory hypotheses. Medicine (Baltimore) 34(1): 13–50 [DOI] [PubMed] [Google Scholar]
- Raven P.W., O’Dwyer A.M., Taylor N.F., Checkley S.A. (1996) The relationship between the effects of metyrapone treatment on depressed mood and urinary steroid profiles. Psychoneuroendocrinology 21: 277–286 [DOI] [PubMed] [Google Scholar]
- Rogoz Z., Skuza G., Wojcikowski J., Daniel W.A. (2003) Effects of combined treatment with imipramine and metyrapone in the forced swimming test in rats. Behavioral and pharmacokinetic studies. Pol J Pharmacol 55: 993–999 [PubMed] [Google Scholar]
- Rogoz Z., Skuza G., Wojcikowski J., Daniel W.A., Wrobel A., Dudek D., et al. (2004) Effect of metyrapone supplementation on imipramine therapy in patients with treatment-resistant unipolar depression. Pol J Pharmacol 56: 849–855 [PubMed] [Google Scholar]
- Rome H.P., Braceland F.J. (1952) The psychological response to ACTH, cortisone, hydrocortisone, and related steroid substances. Am J Psychiatry 108: 641–651 [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., et al. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1917 [DOI] [PubMed] [Google Scholar]
- Simpson G.M., El Sheshai A., Loza N., Kingsbury S.J., Fayek M., Rady A., et al. (2005) An 8-week open-label trial of a 6-day course of mifepristone for the treatment of psychotic depression. J Clin Psychiatry 66: 598–602 [DOI] [PubMed] [Google Scholar]
- Sonino N., Fava G.A., Raffi A.R., Boscaro M., Fallo F. (1998) Clinical correlates of major depression in Cushing’s disease. Psychopathology 31: 302–306 [DOI] [PubMed] [Google Scholar]
- Sprague R.G., Power M.H., Mason H.L. (1950) Observations on the physiologic effects of cortisone and ACTH in man. Arch Intern Med (Chic) 85: 199–258 [DOI] [PubMed] [Google Scholar]
- Thakore J.H., Dinan T.G. (1995) Cortisol synthesis inhibition: a new treatment strategy for the clinical and endocrine manifestations of depression. Biol Psychiatry 37: 364–368 [DOI] [PubMed] [Google Scholar]
- Thomson F., Craighead M. (2008) Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochem Res 33: 691–707 [DOI] [PubMed] [Google Scholar]
- van der Lely A.J., Foeken K., van der Mast R.C., Lamberts S.W. (1991) Rapid reversal of acute psychosis in the Cushing syndrome with the cortisol-receptor antagonist mifepristone (RU 486). Ann Intern Med 114: 143–144 [DOI] [PubMed] [Google Scholar]
- van Gaalen M.M., Stenzel-Poore M.P., Holsboer F., Steckler T. (2002) Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci 15: 2007–2015 [DOI] [PubMed] [Google Scholar]
- Velders F.P., Kuningas M., Kumari M., Dekker M.J., Uitterlinden A.G., Kirschbaum C., et al. (2011) Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology 36: 1053–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waraich P., Goldner E.M., Somers J.M., Hsu L. (2004) Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry 49: 124–138 [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Reus V.I., Manfredi F., Ingbar J., Brizendine L., Weingartner H. (1993) Ketoconazole administration in hypercortisolemic depression. Am J Psychiatry 150: 810–812 [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Reus V.I., Weingartner H., Thompson K., Breier A., Doran A., et al. (1990a) Cognitive effects of corticosteroids. Am J Psychiatry 147: 1297–1303 [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Rubinow D., Doran A.R., Breier A., Berrettini W.H., Kling M.A., et al. (1990b) Prednisone effects on neurochemistry and behavior. Preliminary findings. Arch Gen Psychiatry 47: 963–968 [DOI] [PubMed] [Google Scholar]
- Wong M.L., Kling M.A., Munson P.J., Listwak S., Licinio J., Prolo P., et al. (2000) Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A 97: 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank (2004) The Global Burden of Disease. 2004 Update. Oxford: Oxford University Press [Google Scholar]
- Young A.H., Gallagher P., Porter R.J. (2002) Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiatry 159: 1237–1239 [DOI] [PubMed] [Google Scholar]
- Young A.H., Goodwin G.M., Dick H., Fink G. (1994) Effects of glucocorticoids on 5-HT1A presynaptic function in the mouse. Psychopharmacology (Berl) 114: 360–364 [DOI] [PubMed] [Google Scholar]
- Young E.A., Altemus M., Lopez J.F., Kocsis J.H., Schatzberg A.F., DeBattista C., et al. (2004) HPA axis activation in major depression and response to fluoxetine: a pilot study. Psychoneuroendocrinology 29: 1198–1204 [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Bruckl T., Nocon A., Pfister H., Binder E.B., Uhr M., et al. (2011) Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry 168: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A.W., Nickel T., Kunzel H.E., Ackl N., Sonntag A., Ising M., et al. (2000) Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 34: 171–181 [DOI] [PubMed] [Google Scholar]
- Zobel A.W., Nickel T., Sonntag A., Uhr M., Holsboer F., Ising M. (2001) Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. a prospective study. J Psychiatr Res 35: 83–94 [DOI] [PubMed] [Google Scholar]
- Zorrilla E.P., Valdez G.R., Nozulak J., Koob G.F., Markou A. (2002) Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res 952: 188–199 [DOI] [PubMed] [Google Scholar]