Abstract
Objectives:
Strategies that focus on the reduction of oxidative stress and inflammation may have therapeutic benefit for the treatment of schizophrenia. This clinical trial sought to determine, in a double-blind study, whether epigallocatechin gallate (EGCG), a green tea extract, is a useful adjunct to maintenance antipsychotic medication.
Methods:
Adults with schizophrenia, schizoaffective disorder or bipolar disorder who were maintained on antipsychotic and other psychotropic medications were randomized to supplemental EGCG or placebo. Study participants completed clinical assessments and blood draws to evaluate supplemental treatment effects on psychiatric symptoms and plasma inflammatory markers.
Results:
A total of 34 participants (17 EGCG, 17 placebo) were randomized and 25 participants (14 EGCG, 11 placebo) completed the study. Both treatment groups showed significant reductions in psychotic, depressive and anxiety symptoms from baseline to end of treatment. However, EGCG did not significantly affect psychiatric symptoms or inflammatory markers, as compared with placebo. Adverse effects were mild and comparable between groups.
Conclusion:
There was no signal for a therapeutic effect of the green tea extract EGCG on psychiatric symptoms in this placebo-controlled pilot study.
Keywords: green tea, schizophrenia, depression, anxiety, nitric oxide synthase inhibitor, cytokines
Introduction
Current pharmacotherapeutic strategies to treat symptoms of schizophrenia are generally focused on blockade of the dopamine and serotonin receptors [Freedman, 2003; Muscatello et al. 2010; Catafau et al. 2011]. Although this treatment is effective for many patients with schizophrenia, residual symptoms frequently persist (e.g. affective flattening, anhedonia, attentional impairment), and present antipsychotic medications are associated with several adverse effects (e.g. weight gain and metabolic syndrome, somnolence, dyskinesia, liver toxicity). Thus, there is considerable interest in treatments for psychotic disorders that target pathways and novel pathophysiologic mechanisms other than those involving the dopaminergic or serotonergic systems.
The role of immune activation and inflammatory mediators are increasingly implicated as causal factors in schizophrenia [Kneeland and Fatemi, 2012; Severance et al. 2012] and as potential therapeutic targets [Muller and Schwarz, 2012]. In a comprehensive review, Leonard and colleagues concluded that in schizophrenia, there is a ‘chronic, low-grade inflammatory change associated with the active phase of schizophrenia and that effective treatment largely attenuates these changes’ [Leonard et al. 2012]. A number of strategies that focus on the reduction of oxidative stress and inflammation have been considered for the treatment of schizophrenia (e.g. folate [Hill et al. 2011], aspirin [Laan et al. 2010], long-chain polyunsaturated fatty acids [Das, 2004]). Green tea, a beverage that has been consumed for centuries, contains antioxidant polyphenols, most notably epigallocatechin-3-gallate (EGCG), that demonstrate inhibitory effects on nitric oxide synthase (NOS) and cytokine production [Ahmed et al. 2002; Singal et al. 2006]. Preclinical studies suggest that green tea extract may possibly benefit patients with schizophrenia. For example, green tea extract: (1) enhances learning and memory in aged rats [Kaur et al. 2008]; (2) causes antidepressant-like effects that are comparable to desipramine [Sattayasai et al. 2008]; (3) ameliorates lipopolysaccharide (LPS)-induced sickness behavior [Singal et al. 2006]; (4) induces anxiolytic effects [Vignes et al. 2006]; and (5) reduces reserpine-induced oxidative hepatic damage [Al-Bloushi et al. 2009]. As early as 2000 years ago, Chinese emperors made reference to the calming effects of green tea, but we are not aware of any clinical studies of EGCG’s psychotropic properties. To test the hypothesis that NOS inhibitors are anxiolytic and antipsychotic, we evaluated EGCG as an adjunct to antipsychotic medications in treatment refractory patients with schizophrenia. Bipolar patients who experience anxiety and psychotic symptoms similar to schizophrenic patients may benefit from the calming and antipsychotic effects of EGCG, and were also included in the study.
The objectives of this study were threefold: (1) to determine, in a double-blind study, whether EGCG is a useful adjunct to maintenance antipsychotic medication; (2) to evaluate effects of EGCG on mood in schizophrenic patients and bipolar patients; and (3) to determine ECGG effects on plasma inflammatory markers.
Materials and methods
Study design
This randomized, placebo-controlled, double-blind study of EGCG was conducted at the Portland VA Medical Center (PVAMC) from July 2005 through September 2008. The study had two phases: a 2-week, single-blind, placebo lead-in phase and an 8-week randomized, double-blind, placebo-controlled phase. The green tea extract capsules containing EGCG (theaflavin brand of enriched green tea extract [150 mg per capsule]; Nashai Biochemical, Nashville, TN) were over-encapsulated using Capsugel size AAel white opaque DBcaps® (Capsugel, Peapack, New Jersey) by the Research Pharmacy personnel at the PVAMC. The remaining space was filled with cellulose (microcrystalline cellulose NF (T-105); Hawkins). Placebo capsules were compounded using the same larger capsules, were filled with cellulose only, and had final fill weights that were equal to the active drug (i.e. EGCG) capsules. The PVAMC Research Pharmacy personnel were solely responsible for the compounding of the EGCG and placebo capsules.
After providing written informed consent, patients entered a 2-week, single-blind, placebo lead-in phase, during which they were instructed to take four capsules as two divided doses (two capsules two times per day) in addition to their prescribed medication. After completion of the placebo lead-in, patients who continued to meet study criteria were randomly assigned under double-blind conditions to receive an 8-week trial of four capsules of placebo or enriched green tea extract by mouth daily for 8 weeks.
All patients provided written informed consent before screening. The study was conducted in accordance with principles of Good Clinical Practice and was approved by the Institutional Review Board and regulatory agencies at the Portland VA Medical Center.
Participants
Men and women (≥18 years) with a primary Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Axis I diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder were eligible [American Psychiatric Association, 2000]. Patients had to be able to understand and sign the consent form. Women of childbearing age could not be pregnant or breastfeeding and had to agree to use contraception. Participants were excluded for any of the following reasons: Axis I diagnosis other than schizophrenia, schizoaffective disorder, or bipolar disorder; significant depressive symptoms (Hamilton Depression Rating Scale [HAM-D] ≥ 25); use of EGCG, either by regular consumption of green, white, or black tea, or use of diet pills or nutrition products containing EGCG; serious medical or neurological illness (based on physical exam, history, and laboratory tests); abuse of or addiction to alcohol or any illicit substances during the past six months; abuse of phencyclidine at any time; asthma (EGCG has been reported to exacerbate or even induce asthma [Shirai et al. 1994]); or acute exacerbation of psychosis sufficient to consider hospitalization and decrease competency to consent.
Concomitant medications
In this study green tea extract is proposed to serve as an adjunct to approved schizophrenia pharmacotherapy. As a result of this hypothesis, and the benign side-effect profile of green tea extract, we allowed participants to take their normally prescribed medications during this study. All medications being taken by each participant enrolled in this study were reviewed by the study psychiatrist.
Clinical assessments
Clinical assessments were performed at baseline (week 0) and after 4 and 8 weeks of treatment. The following measures were used to evaluate clinical efficacy:
Clinical Global Impression scale–Schizophrenia scale (CGI) [Guy, 1976; Conley and Buchanan, 1997]. The CGI was administered at baseline and week 10 only.
Positive and Negative Syndrome Scale (PANSS) [Kay et al. 1987]. PANSS scores were further analyzed using the subscales for general psychopathology symptoms (PANSS-G), positive symptoms (PANSS-P), and negative symptoms (PANSS-N).
HAM-D [Hamilton, 1960].
Hamilton Anxiety Scale (HAM-A) [Hamilton, 1959].
Safety and tolerability
Safety and tolerability were assessed with adverse events (AEs), physical assessments, laboratory measures (e.g. complete blood count [CBC], liver function tests, and fasting lipid profiles), and body mass index (BMI) measurements. Extrapyramidal side-effects (EPSs) were assessed using patient reports of EPS-related AEs, the Simpson Angus Scale (SAS) [Simpson and Angus, 1970], and the Abnormal Involuntary Movement Scale (AIMS) [Simpson and Angus, 1970].
Blood sampling and biomarker assays
To evaluate the relationship of psychiatric symptoms to markers of inflammation, blood samples were collected at baseline (following the completion of the placebo lead-in phase) and after 8 weeks of treatment with either EGCG or placebo. Blood was collected in BD Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ), and plasma was separated and stored at −80°C until assayed. Enzyme-linked immunosorbent assay (ELISA) kits were used to measure tumor necrosis factor-α (TNF-α; sensitivity 4 pg/ml), interferon-γ (IFN-γ; sensitivity 4 pg/ml), interleukin-10 (IL-10; sensitivity 2 pg/ml), and IL-9 (sensitivity 2 pg/ml). Patient samples were run in duplicate and assays were carried out according to manufacturer’s recommendations with minor modifications (Biolegend, San Diego, CA). Absorbance was measured at 450 nm using a BioRad Model 680 microplate reader (Bio-Rad Laboratories, Hercules, CA).
Statistical analysis
Demographic characteristics were compared between groups by Student’s two-sample t-test for continuous variables and χ2 test for categorical variables. Clinical efficacy was analyzed using two-way, repeated measures analysis of variance (ANOVA) for each psychiatric rating scale. Variables included group (EGCG versus placebo), time, and group × time. Bonferroni posttests were conducted, as appropriate. Differences in rating scale score changes between the randomized groups were evaluated using unpaired t-tests. Cytokine levels were analyzed using paired t-tests in order to compare week 0 (baseline) with week 10 (after 8 weeks of treatment with EGCG or placebo). Analyses were performed using Prism for Windows, version 4.03 (GraphPad Software, Inc., La Jolla, CA). The two-sided level of significance was set at 0.05.
Results
Baseline characteristics
A total of 42 patients were screened, 34 underwent randomization, and 25 completed 8 weeks of treatment (Supplementary Figure 1, all supplementary material can be found online with this article, http://tpp.sagepub.com). Supplementary Table 1 provides a summary of the demographic and patient characteristics. At baseline, the EGCG and placebo groups did not differ significantly on any of the demographic variables or psychiatric symptom rating scales. Concurrent psychiatric medications at baseline are reported in Supplementary Table 2.
Efficacy
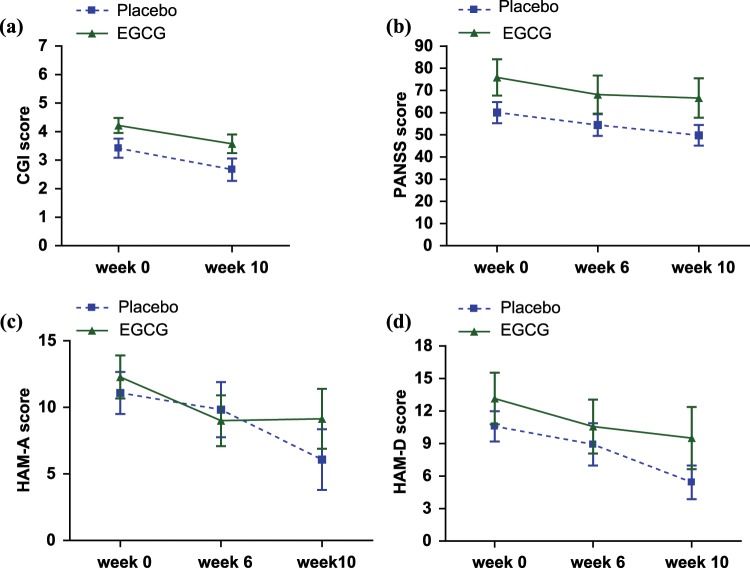
The CGI score improved significantly from baseline to week 10 (i.e. 8 weeks of EGCG treatment for the EGCG group) in both the EGCG and placebo groups (F = 15.46, p = 0.0006). There was a trend for a treatment effect (F = 3.90, p = 0.059); however, the interaction between treatment group and time did not reach statistical significance (Figure 1). The PANSS, HAM-A, and HAM-D scores also improved significantly from baseline to week 10 in both treatment groups (PANSS: F = 15.46, p < 0.0001; HAM-A: F = 6.50, p = 0.0032; HAM-D: F = 9.71, p = 0.0003), but the group and group × time interaction effects were not significant (Figure 1). Further, the EGCG and placebo groups did not significantly differ in the change from baseline to week 10 on any of the psychiatric measures, including the subscales for the PANSS (Table 1).
Figure 1.
Epigallocatechin-3-gallate (EGCG) versus placebo: clinical assessment results. Mean (± SEM) scores by group for all research participants completing 10 weeks of the study are shown. (a) Clinical Global Impressions (CGI). The CGI was administered at baseline (week 0) and at week 10 (after 8 weeks of double-blind, placebo-controlled treatment with EGCG or placebo). (b)–(d) Results from (b) the Positive and Negative Syndrome Scale (PANSS), (c) Hamilton Rating Scale-Anxiety (HAM-A), and (d) Hamilton Rating Scale-Depression (HAM-D). These measures were administered at baseline (week 0), week 6, and week 10.
Table 1.
Change in psychiatric rating scales from baseline to week 10 according to treatment group.
| Psychiatric rating scales | |||||||
|---|---|---|---|---|---|---|---|
| Drug treatment group | CGI | PANSS | PANSS-P | PANSS-N | PANSS-G | HAM-A | HAM-D |
| Placebo group mean ± SEM | −0.75 ± 0.22 | −10.25 ± 2.64 | −2.25 ± 0.86 | −2.33 ± 0.85 | −6.17 ± 1.61 | −5.00 ± 1.76 | −5.08 ± 1.25 |
| EGCG group mean ± SEM | −0.64 ± 0.27 | −9.29 ± 3.16 | −2.21 ± 0.72 | −2.43 ± 1.09 | −4.50 ± 1.67 | −3.14 ± 1.71 | −3.643 ± 1.84 |
| Group difference mean ± SEM | −0.11 ± 0.35 | −0.96 ± 4.20 | −0.04 ± 1.11 | 0.10 ± 1.42 | −1.67 ± 2.35 | −1.86 ± 2.46 | −1.44 ± 2.30 |
| (95% CI)a | (−0.84 to 0.62) | (−9.64 to 7.71) | (−2.33 to 2.27) | (−2.83 to 3.02) | (−6.51 to 3.18) | (−6.94 to 3.22) | (−6.19 to 3.31) |
From unpaired t-tests; all p-values > 0.05.
CGI, Clinical Global Impression scale–Schizophrenia scale; CI, confidence interval; EGCG, epigallocatechin gallate; HAM-A, Hamilton Anxiety Scale; HAM-D, Hamilton Depression Scale; PANSS, Positive and Negative Syndrome Scale (PANSS); PANSS-G, PANSS - general psychopathology symptoms; PANSS-N, PANSS - negative symptoms; PANSS-P, PANSS - positive symptoms.
Safety and tolerability
Three AEs were reported during the trial. One patient in the EGCG group experienced an exacerbation of bipolar depression and was discontinued from study medications at week 10, one patient in the placebo group reported tachycardia and was discontinued from study medications at week 10, and one patient in the placebo group developed an abdominal rash and was discontinued from the study prior to week 6 (Supplementary Figure 1). The EPS measures (SAS and AIMS) remained overall unchanged for both groups (data not shown); however, both measures showed levels already very low at baseline (Supplementary Table 1).
Biomarker assays
Cytokine levels were measured to determine whether treatment with EGCG was associated with alterations in the production of TNF-α, IFN-γ, IL-10, and IL-9. Cytokines from unstimulated blood samples were detected in 6/12 patients for the EGCG group and 3/9 patients for the placebo group. Consequently, the EGCG and placebo groups were combined to determine whether there was an association between changes in psychiatric symptoms and cytokine production from week 0 to week 10. Supplementary Table 3 shows nonsignificant reductions for TNF-α, IFN-γ, IL-10, and IL-9 (5.5%, 17.8%, 23.1%, and 22.3%, respectively).
Discussion
This 8-week, double-blind, prospective study of daily EGCG supplementation versus placebo in patients with schizophrenia, schizoaffective disorder, or bipolar disorder did not find significant differences in the efficacy or tolerability between the two treatments. Both EGCG and placebo groups showed significant decreases in psychiatric symptoms over time. The reduction in psychiatric symptomology was accompanied by nonsignificant decreases in the production of Th1, Th2, and Th9 cytokines.
It is well known that obesity is a significant contributor to inflammation [Stienstra et al. 2012]. According to the Centers for Disease Control and Prevention, an adult who has a BMI between 25 and 29.9 is considered overweight, and an adult who has a BMI of 30 or higher is considered obese (see http://www.cdc.gov/obesity/adult/defining.html). Based on these criteria, the mean BMIs for both the placebo and EGCG groups were greater than 32, placing them in the obese category. Consequently, the degree of inflammation in our sample may have contributed to the lack of statistically significant reductions in cytokine levels from baseline to week 10. Although we did not find significant treatment differences between EGCG and placebo groups, both groups showed significant reductions in psychotic, depressive, and anxiety symptoms, which were associated with reduced expression of cytokines.
Pharmacokinetics play a critical role in the clinical outcomes of drug therapy. Studies designed to investigate drug interactions with EGCG and its absorption show significant variability between subjects [e.g. Chow et al. 2006; reviewed in Colalto, 2010]. This variability suggests that pharmacogenetic factors may influence the pharmacokinetic mechanisms as well as the potential therapeutic effects of EGCG. Recently, a common polymorphism in the genetic code for catechol-O-methyltransferase (COMT) was investigated to assess the impact of COMT genotype on green tea catechin absorption and metabolism in humans. The authors reported that the COMT polymorphism [i.e. Val(158/108)Met] does not appear to have a significant influence on EGCG absorption and elimination [Miller and Schwarz, 2012]. More research studies are needed to better understand the pharmacokinetic mechanisms of EGCG.
Limitations of this study included a small sample size, broad diagnostic criteria, a single dose strength of EGCG, and no standardized use of antipsychotic medications. Standardizing the antipsychotic medications would have been optimal, but we proposed that EGCG would serve as an adjunct to schizophrenia pharmacotherapy. As a result of this hypothesis, we allowed subjects to take their normally prescribed medications during this study (Supplementary Table 2). In addition, although not statistically significant, patients in the EGCG group had more severe scores on all psychiatric rating scales. The increased baseline symptomology in the EGCG group may have hindered our ability to detect significant treatment group differences between the placebo and EGCG groups.
The lack of any signal for EGCG efficacy limits enthusiasm for its potential psychotropic properties; however, our sample size was less than the recommended 40–100 patients for drug augmentation studies in schizophrenia [Stern et al. 1997]. Consequently, the power to detect statistically significant clinical efficacy differences between EGCG and placebo groups was low, leaving the possibility of a type II error. However, we compared psychiatric symptom severity scores across time and had adequate power to detect significant within-group differences from baseline to week 10 on the CGI, PANSS, HAM-A, and HAM-D scores (Figure 1).
Future studies could examine whether EGCG is effective with narrower diagnostic categories (e.g. paranoid schizophrenia), during specific stages of illness (e.g. at initial onset of disease, during psychotic, depressive, or manic episodes), or following longer durations or higher doses of treatment [Niu et al. 2009; Noto et al. 2011]. In conclusion, the results of this first double-blind, placebo-controlled pilot study do not support the hypothesis that EGCG has antipsychotic or other psychotropic properties.
Acknowledgments
The authors thank S. Paul Berger, then staff psychiatrist, Mental Health and Clinical Neurosciences Division, Portland Veterans Affairs Medical Center for designing the study, writing the protocol, and obtaining funding for the project. The authors also thank the Research Pharmacy at the Portland Veterans Affairs Medical Center, in particular Vickie Vonderohe, Clara Chambers, Ursula Helmut, and Joshua Fryer for their work on the study. We are grateful to the study participants as well as to Murray Raskind (for manuscript review). All authors read and approved the final contents of the manuscript.
Footnotes
Funding: This work was supported by the Stanley Medical Research Institute (grant number 03T-471). J.M.L. (Supervisory Research Microbiologist) and M.H. (Staff Psychologist and Neuropsychologist) are supported by career development awards from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development. This material is the result of work supported with resources and the use of facilities at the Portland Veterans Affairs Medical Center, Portland, Oregon.
Conflict of interest statement: The authors declare that they have no conflicts of interests regarding the content of this research paper.
Contributor Information
Jennifer M. Loftis, Research and Development Service, Mental Health and Clinical Neurosciences Division, Portland VA Medical Center, R&D 16, 3710 SW US Veterans Hospital Road, Portland, OR 97239, USA
Clare J. Wilhelm, Research and Development Service, Mental Health and Clinical Neurosciences Division, Portland Veterans Affairs Medical Center, Portland, OR, USA; Department of Psychiatry, Oregon Health and Science University, Portland, OR, USA
Marilyn Huckans, Research and Development Service, Mental Health and Clinical Neurosciences Division, Portland Veterans Affairs Medical Center, Portland, OR, USA; Department of Psychiatry, Oregon Health and Science University, Portland, OR, USA.
References
- Ahmed S., Rahman A., Hasnain A. (2002) Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med 33: 1097–1105 [DOI] [PubMed] [Google Scholar]
- Al-Bloushi S., Safer A., Afzal M. (2009) Green Tea Modulates Reserpine Toxicity in Animal Models. J Toxicol Sci 34: 77–87 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). American Psychiatric Association [Google Scholar]
- Catafau A., Bullich S., Nucci G., et al. (2011) Contribution of SPECT measurements of D2 and 5-HT2A occupancy to the clinical development of the antipsychotic SB-773812. J Nucl Med 52: 526–534 [DOI] [PubMed] [Google Scholar]
- Chow H., Hakim I., Vining D., et al. (2006) Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol Biomarkers Prev 15: 2473–2476 [DOI] [PubMed] [Google Scholar]
- Colalto C. (2010) Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol Res 62: 207–227 [DOI] [PubMed] [Google Scholar]
- Conley R., Buchanan R. (1997) Evaluation of treatment-resistant schizophrenia. Schizophr Bull 23: 663–674 [DOI] [PubMed] [Google Scholar]
- Das U. (2004) Can perinatal supplementation of long-chain polyunsaturated fatty acids prevents schizophrenia in adult life? Med Sci Monit 10: HY33–HY37 [PubMed] [Google Scholar]
- Freedman R. (2003) Schizophrenia. N Engl J Med 349: 1738–1749 [DOI] [PubMed] [Google Scholar]
- Guy W. (1976) ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare [Google Scholar]
- Hamilton M. (1959) The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55 [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Shannahan K., Jasinski S., et al. (2011) Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res 127: 41–45 [DOI] [PubMed] [Google Scholar]
- Kaur T., Pathak C., Pandhi P. (2008) Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn 67: 25–30 [DOI] [PubMed] [Google Scholar]
- Kay S., Fiszbein A., Opler L. (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276 [DOI] [PubMed] [Google Scholar]
- Kneeland R., Fatemi S. (2012) Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan W., Grobbee D., Selten J. (2010) Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 71: 520–527 [DOI] [PubMed] [Google Scholar]
- Leonard B., Schwarz M., Myint A. (2012) The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol, in press. [DOI] [PubMed] [Google Scholar]
- Muller N., Schwarz M. (2012) Immunological treatment options for schizophrenia. Curr Pharm Biotechnol, in press. [DOI] [PubMed] [Google Scholar]
- Muscatello M., Bruno A., Pandolfo G., Micò U., Settineri S., Zoccali R. (2010) Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther 4: 187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu K., Hozawa A., Kuriyama S., et al. (2009) Green tea consumption is associated with depressive symptoms in the elderly. Am J Clin Nutr 90: 1615–1622 [DOI] [PubMed] [Google Scholar]
- Noto C., Gadelha A., Belanger S., et al. (2011) Association of biomarkers and depressive symptoms in schizophrenia. Neurosci Lett 505: 282–285 [DOI] [PubMed] [Google Scholar]
- Sattayasai J., Tiamkao S., Puapairoj P. (2008) Biphasic effects of morus alba leaves green tea extract on mice in chronic forced swimming model. Phytother Res 22: 487–492 [DOI] [PubMed] [Google Scholar]
- Severance E., Alaedini A., Yang S., et al. (2012) Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Sato A., Hara Y. (1994) Epigallocatechin gallate. The major causative agent of green tea-induced asthma. Chest 106: 1801–1805 [DOI] [PubMed] [Google Scholar]
- Simpson G., Angus J. (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11–19 [DOI] [PubMed] [Google Scholar]
- Singal A., Tirkey N., Pilkhwal S., Chopra (2006) Green tea (Camellia sinensis) extract ameliorates endotoxin induced sickness behavior and liver damage in rats. Phytother Res 20: 125–129 [DOI] [PubMed] [Google Scholar]
- Stern R., Schmeidler J., Davidson M. (1997) Limitations of controlled augmentation trials in schizophrenia. Biol Psychiatry 42: 138–143 [DOI] [PubMed] [Google Scholar]
- Stienstra R., Tack C., Kanneganti T. (2012) The inflammasome puts obesity in the danger zone. Cell Metab 15: 10–18 [DOI] [PubMed] [Google Scholar]
- Vignes M., Maurice T., Lante F., et al. (2006) Anxiolytic properties of green tea polyphenol (-)-epigallocatechin gallate (EGCG). Brain Res 1110: 102–115 [DOI] [PubMed] [Google Scholar]