Abstract
Context
Although fluoroquinolones remain the most reliable urinary antimicrobial, resistance rates have increased and effective fluoroquinolone-sparing antimicrobials are needed.
Objective
To determine whether cefpodoxime is noninferior to ciprofloxacin for treatment of acute cystitis.
Design, Setting, and Patients
Randomized, double-blind trial of 300 women aged 18 to 55 years with acute uncomplicated cystitis comparing ciprofloxacin (n=150) with cefpodoxime (n=150); patients were from a student health center in Seattle, Washington, and a referral center in Miami, Florida. The study was conducted from 2005 to 2009 and outcomes were assessed at 5 to 9 days and 28 to 30 days after completion of therapy. Intent-to-treat and per-protocol analyses were performed; 15 women in the ciprofloxacin group and 17 women in the cefpodoxime group were lost to follow-up.
Interventions
Patients were given 250 mg of ciprofloxacin orally twice daily for 3 days or 100 mg of cefpodoxime proxetil orally twice daily for 3 days.
Main Outcome Measures
Overall clinical cure (defined as not requiring antimicrobial treatment during follow-up) at the 30-day follow-up visit. Secondary outcomes were clinical and microbiological cure at the first follow-up visit and vaginal Escherichia coli colonization at each follow-up visit. The hypothesis that cefpodoxime would be noninferior to ciprofloxacin by a 10% margin (ie, for the difference in the primary outcome for ciprofloxacin minus cefpodoxime, the upper limit of the confidence interval would be <10%) was formulated prior to data collection.
Results
The overall clinical cure rate at the 30-day visit with the intent-to-treat approach in which patients lost to follow-up were considered as having clinical cure was 93% (139/150) for ciprofloxacin compared with 82% (123/150) for cefpodoxime (difference of11%; 95% CI, 3% – 18%); and for the intent-to-treat approach in which patients lost to follow-up were considered as having not responded to treatment, the clinical cure rate was 83% (124/150) for ciprofloxacin compared with 71% (106/150) for cefpodoxime (difference of 12%; 95% CI, 3% – 21%). The microbiological cure rate was 96% (123/128) for ciprofloxacin compared with 81% (104/129) for cefpodoxime (difference of 15%; 95% CI, 8% – 23%). At first follow-up, 16% of women in the ciprofloxacin group compared with 40% of women in the cefpodoxime group had vaginal E coli colonization.
Conclusions
Among women with uncomplicated cystitis, a 3-day regimen of cefpodoxime compared with ciprofloxacin did not meet criteria for noninferiority for achieving clinical cure. These findings, along with concerns about possible adverse ecological effects associated with other broad-spectrum β-lactams, do not support the use of cefpodoxime as a first-line fluoroquinolone-sparing antimicrobial for acute uncomplicated cystitis.
Antimicrobial resistance among uropathogens causing uncomplicated cystitis has increased over the past decade and there is greater appreciation of the importance of the ecological adverse effects of antimicrobial therapy.1 Fluoroquinolones have high rates of efficacy, high rates of susceptibility among pathogens causing uncomplicated urinary tract infection (UTI), and minimal adverse drug reactions when used in a 3-day regimen as recommended. Indeed, recent surveys have found that fluoroquinolones are more commonly used than trimethoprim-sulfamethoxazole for uncomplicated UTI in women in the United States.2,3
However, increasing rates of fluoroquinolone-resistant Escherichia coli are being reported worldwide, including areas within the United States and Canada, even among young women with uncomplicated cystitis.4–7 To prevent further emergence of fluoroquinolone resistance, there are calls for restricting fluoroquinolones to those specific instances of uncomplicated cystitis when other UTI antimicrobials are not suitable.8 Although fluoroquinolones are highly efficacious and thus preferred in some circumstances, recently published guidelines from the Infectious Diseases Society of America for the treatment of uncomplicated cystitis recommend limiting their use because of the risk of propagating resistance and concerns about adverse ecological effects.1
Although most studies demonstrate that β-lactam antimicrobials are generally inferior in cure rates to trimethoprim-sulfamethoxazole and the fluoroquinolones for the treatment of cystitis,9–11 there is a paucity of data on the use of cefpodoxime proxetil, an oral third-generation cephalosporin, for the treatment of uncomplicated cystitis. Cefpodoxime, with its broad spectrum of antimicrobial activity, would provide a useful alternative to fluoroquinolones for the treatment of cystitis if demonstrated to be similar in efficacy to fluoroquinolones and without adverse ecological effects (such as the selection of drug-resistant organisms).12
Thus, we conducted a noninferiority trial of a 3-day course of cefpodoxime compared with a standard 3-day regimen of ciprofloxacin for the treatment of acute uncomplicated cystitis to assess whether cefpodoxime would have clinically acceptable efficacy and tolerance compared with ciprofloxacin. A noninferiority margin of 10% was considered to be clinically acceptable. We also evaluated the effects of both antimicrobials on vaginal E coli colonization because the inferior ability of β-lactam antimicrobials to eradicate E coli from the vagina has been postulated to explain their poorer clinical activity in the treatment of UTI.11 We also assessed the effects of antimicrobial resistance on efficacy.
METHODS
Study Population
The study was conducted at the Hall Health Primary Care Center, an outpatient clinic that offers care to students, faculty, and staff at the University of Washington and to the general public of Seattle, Washington, and at the Clinical Research Unit at the University of Miami, in Miami, Florida. Women were eligible if they were aged 18 to 55 years, in good general health, had acute cystitis as defined by typical symptoms (dysuria, frequency, and/or urgency) and pyuria (white blood cell count ≥8 cells/mm3), and received antimicrobial treatment. A positive urine culture was defined as 102 or more colony-forming units (CFU) per milliliter of a uropathogen.
Women were not eligible if they had diabetes mellitus, known anatomic abnormalities of the urinary tract, known allergy to the study drugs, exposure to an oral or parenteral antimicrobial (including prophylactic antimicrobials) in the last 2 weeks, or were pregnant, lactating, or not consistently using contraceptives. Race and ethnicity were self-reported on the enrollment questionnaire (options were defined by the investigators). We collect such data routinely in our studies because many of them are funded by the National Institutes of Health, which requires self-reporting of ethnicity. The human subjects review committees of the University of Washington and the University of Miami approved the study, and all patients gave written informed consent.
Study Procedures
Flyers and discussions with other local clinicians were the main methods of recruitment. Interested women were screened via a checklist of inclusion and exclusion criteria, and eligible patients signed written informed consent. At the initial visit, patients underwent a directed history and physical examination, an interview using a standardized study questionnaire, a midstream urine specimen collection to evaluate bacteriuria and pyuria, and a vaginal swab specimen collection to evaluate bacterial colonization.
Patients were randomized in a double-blind fashion to treatment with 250 mg of ciprofloxacin twice daily for 3 days or 100 mg of cefpodoxime proxetil twice daily for 3 days. Treatment assignments were generated by the study statistician using a computerized, random-number generator that was given to the pharmacy where the study medications were encapsulated in indistinguishable gelatin capsules, allocated according to treatment assignment, and packaged by study identifier number. Randomized treatments were assigned in blocks of 10 and clinic and laboratory personnel were kept naive to the treatment drug. Treatment assignment was unblinded only after the study had been completed and outcomes had been determined.
Patients were scheduled for follow-up visits at 5 to 9 days and 28 to 30 days after completion of therapy and were asked to return to the clinic if their cystitis symptoms did not resolve or if they had recurrent symptoms of acute cystitis. At each return visit, a questionnaire regarding UTI symptoms was administered and urine and vaginal specimens were collected using the same methods as in the initial visit. At symptomatic return visits, an antimicrobial treatment was instituted if patients met criteria for diagnosis of acute cystitis. If the infecting microbiological isolate at enrollment was susceptible, treatment was with trimethoprim-sulfamethoxazole, but if not, treatment was with nitrofurantoin. At the first follow-up visit (days 5–9), participants were asked how many study pills they had taken as a measure of compliance.
Laboratory Procedures
Methods for collecting urine and vaginal specimens and isolating, identifying, and quantifying urine and vaginal uropathogens have been previously described.13,14 Urine and vaginal samples were refrigerated and transported to the laboratory within 24 hours of collection. Standard urine culture and susceptibility testing was performed using the methods of the Clinical and Laboratory Standards Institute.15 Pyuria was assessed in undiluted urine using a hemocytometer. Vaginal cultures were considered positive for E coli if there was growth of 1 or greater on a semi-quantitative scale ranging from 1 to 4.
Outcome Measures
The primary study outcome was clinical cure at the 30-day follow-up visit (overall clinical cure). Women who did not require further antimicrobial treatment for acute cystitis during follow-up were defined as cured, whereas those who required further antimicrobial treatment were defined as not responding to treatment. In addition, the main outcome was stratified by patients’ prior UTI history.
Secondary study outcomes were clinical and microbiological cure at the first follow-up visit and vaginal E coli colonization at each follow-up visit. Microbiological cure was defined as having (in women who did not require treatment a second time) less than 105 CFU/mL of all uropathogens and at least a 10-fold decrease in colony count of the causative uropathogen compared with the urine culture at enrollment. In women who were treated a second time with an antimicrobial for persistent or recurrent UTI at or before the first follow-up visit, microbiological cure was defined as having less than 102 CFU/mL of a uropathogen at the time of the second treatment.
We evaluated associations between antimicrobial susceptibility of the initially infecting uropathogen and clinical and microbiological outcomes. Uropathogens included enteric gram-negative rods, Staphylococcus saprophyticus, enterococci, and group B streptococci. Enterococci and group B streptococci were considered causative uropathogens only if they occurred without other uropathogens or at quantities of 105 CFU/mL or greater. Coagulase-negative staphylococci, α-hemolytic streptococci, lactobacilli, diphtheroids, and mixed–grampositive flora were categorized as nonuropathogens.
Sample Size
Based on published cure rates of trimethoprim-sulfamethoxazole and ciprofloxacin, we assumed a reasonable and acceptable cure rate for the standard treatment group (ciprofloxacin) to be between 85% and 95%.11,16–19 Using a noninferiority margin of 10% for the experimental treatment (cefpodoxime), and setting significance at 5%, a sample size of 113 to 140 evaluable patients per treatment group would be necessary to achieve a level of power of 80% to 85%. The targeted enrollment was set at 300 women (150 in each study group) to aim for approximately 250 evaluable patients for comparison.
Statistical Analysis
An intent-to-treat approach was used to determine the sample for primary and secondary outcomes. All women who were enrolled and randomized were included in the main analysis. Patients without any follow-up visits were imputed as clinical cures given that we provided 24-hour availability of a study investigator for questions, immediate appointments to the study clinic during weekday hours, and no charge for clinic visits or antimicrobials. Additional analyses imputing those lost to follow-up as having not responded to treatment also were performed. For the secondary outcome of early microbiological cure, a per-protocol approach was used and women with positive urine cultures at enrollment and with follow-up urine culture information were evaluated.
A 2-sided 95% confidence interval was constructed regarding the difference in binomial outcomes between the 2 treatment groups for evaluation of the hypothesis of noninferiority of cefpodoxime. The difference was constructed using the outcome for the standard treatment minus the experimental treatment (ie, ciprofloxacin minus cefpodoxime). A value of less than 10% in the upper limit of this confidence interval would be interpreted as supporting the hypothesis that cefpodoxime is noninferior to ciprofloxacin. The hypothesis of noninferiority was also tested using the Wald statistic,20 using a 1-sided alternative hypothesis. A Kaplan-Meier curve depicting time to treatment nonresponse was constructed to display the timing of the nonresponses by treatment assignment using the per-protocol analysis sample. Data management and statistical analyses were performed using SAS version 9.2 (SAS Institute Inc).
RESULTS
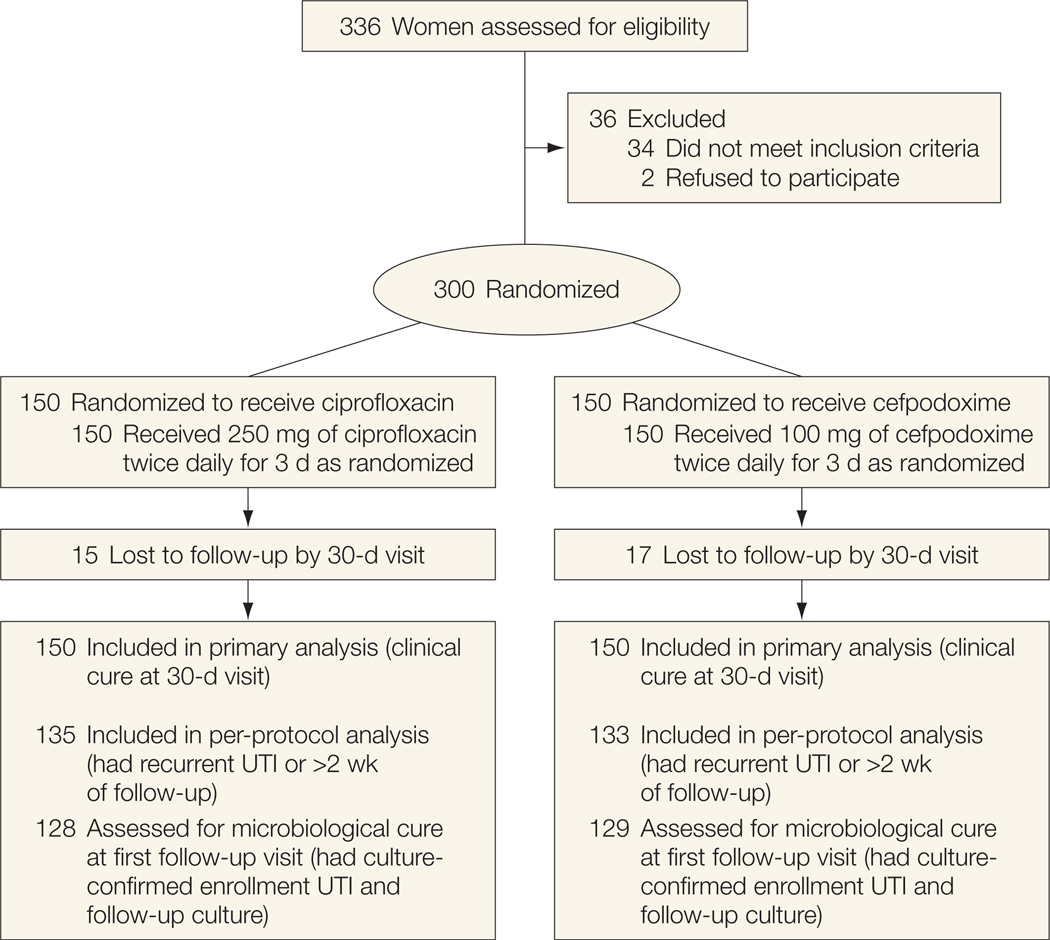
All patient enrollment and follow-up took place between 2005 and 2009. Three hundred women were enrolled and randomized to receive either ciprofloxacin (n=150) or cefpodoxime (n=150) (Figure 1). Two otherwise eligible individuals who were approached refused participation. Thirty-three women had negative cultures at enrollment, 17 in the ciprofloxacin group and 16 in the cefpodoxime group. Fifteen women in the ciprofloxacin group and 17 in the cefpodoxime group were lost to follow-up by the 30-day visit.
Figure 1.
Enrollment and Outcomes
Baseline characteristics were similar between the 2 study groups except that more women in the cefpodoxime group had previous UTIs and pyelonephritis and fewer had less than 105 CFU/mL of a uropathogen at enrollment (Table 1). The majority of enrollment UTIs were caused by E coli alone (75%) or in combination with another uropathogen (2%). The remaining enrollment UTIs were caused by S saprophyticus (3%) or by enterococci, Klebsiella species, Proteus mirabilis, or group B streptococci (1%–3% each). Overall, 4% of isolates (4% of E coli and 8% of non−E coli) were nonsusceptible to ciprofloxacin and 8% (4% of E coli and 36% of non–E coli) were nonsusceptible to cefpodoxime.
Table 1.
Description of Women at Enrollment
| No. (%) of Womena |
P Value |
|||
|---|---|---|---|---|
| Ciprofloxacin (n = 150) |
Cefpodoxime (n = 150) |
|||
| Age, mean (SD), y | 24 (6) | 23 (5) | .67 | |
| Never married | 129 (86) | 120 (80) | .17 | |
| Race | ||||
| White | 94 (63) | 103 (69) | .50 | |
| Asian | 39 (26) | 32 (21) | ||
| Black | 2 (1) | 4 (3) | ||
| Otherb | 15 (10) | 11 (7) | ||
| Ethnicity | ||||
| Hispanic | 6 (4) | 13 (9) | .10 | |
| History of UTI in lifetime | ||||
| Any | 85 (57) | 104 (69) | .02 | |
| ≥3 | 40 (27) | 62 (41) | .007 | |
| History of pyelonephritis | 6 (4) | 13 (9) | .09 | |
| UTI in past year | 31 (21) | 50 (33) | .02 | |
| Sexual activity during past month | ||||
| Sexually active | 141 (94) | 141 (94) | .81 | |
| Vaginal intercourse episodes, median (range) | 8 (0–40) | 8 (0–40) | .43 | |
| Spermicide exposurec | (n = 116) 23 (20) | (n = 113) 17 (15) | .39 | |
| Uropathogen colony count | ||||
| No growth | 16 (11) | 16 (11) | .42 | |
| 102 to <105 CFU/mL | 55 (37) | 45 (30) | ||
| ≥105 CFU/mL | 78 (52) | 89 (59) | ||
Abbreviations: CFU, colony-forming units; UTI, urinary tract infection.
Unless otherwise indicated.
Includes American Indians, Native Americans, and Alaskan Natives.
Includes use of diaphragm or spermicide-coated condom. If unknown for spermicide-coating, this was set to missing.
The number of E coli strains was 119 (79%) for ciprofloxacin compared with 114 (76%) for cefpodoxime (P = .49). The number of uropathogens susceptible to ciprofoxacin was 131 (96%) for ciprofloxacin compared with 129 (96%) for cefpodoxime (P = .98). The number of uropathogens susceptible to cefpodoxime was 125 (92%) for ciprofloxacin compared with 122 (91%) for cefpodoxime (P = .80).
Primary Outcome
The overall clinical cure rate with the intent-to-treat approach in which patients lost to follow-up were imputed as having clinical cure was 93% (139/150) for ciprofloxacin compared with 82% (123/150) for cefpodoxime (difference of 11%; 95% CI, 3% – 18%) (Table 2). Because the upper limit of the 95% confidence interval of the difference exceeded 10%, the results were inconsistent with a finding of noninferiority for cefpodoxime. The test of noninferiority was not statistically significant (P = .57). The per-protocol analysis yielded similar results with a clinical cure rate of 92% (124/135) in the ciprofloxacin group compared with 80% (106/133) in the cefpodoxime group (difference of 12%; 95% CI, 4%–20%). In an alternative intent-to-treat analysis in which patients who were lost to follow-up were considered to have not responded to treatment, the clinical cure rate was 83% (124/150) for ciprofloxacin compared with 71% (106/150) for cefpodoxime (difference of 12%; 95% CI, 3%–21%).
Table 2.
Treatment Outcomes by Study Groupa
| No./Total (%) | Difference (95% CI), % |
||
|---|---|---|---|
| Ciprofloxacin | Cefpodoxime | ||
| Primary outcomes | |||
| Overall clinical cureb | 139/150 (93) | 123/150 (82) | 11 (3 to 18) |
| Overall clinical curec | 124/150 (83) | 106/150 (71) | 12 (3 to 21) |
| Per-protocol analysis | 124/135 (92) | 106/133 (80) | 12 (4 to 20) |
| Secondary outcomes | |||
| Early clinical cured | 140/150 (93) | 132/150 (88) | 5 (−1 to 12) |
| Early microbiological cured | 123/128 (96) | 104/129 (81) | 15 (8 to 23) |
See text for definitions.
Intent-to-treat analysis, imputing values for patients without follow-up visits as having clinical cure.
Intent-to-treat analysis, imputing values for patients without follow-up visits as not having responded to treatment.
At first follow-up visit.
Among women who reported no previous UTI in the past year before enrollment, the overall clinical cure rate was 96% (113/118) for ciprofloxacin and 83% (83/100) for cefpodoxime (difference of 13%; 95% CI, 5%–21%). This magnitude of difference was not seen among women who reported 1 or more UTIs in the past year before enrollment (ciprofloxacin: 84% [26/31]; cefpodoxime: 80% [40/50]). Among women infected with strains that were susceptible to the study antibiotic, the overall clinical cure rates were 94% (117/125) for ciprofloxacin and 82% (97/119) for cefpodoxime (difference of 12%; 95% CI, 4%–20%). Among those infected with strains nonsusceptible to the treatment antibiotic, the overall clinical cure rate was 50% (3/6) for ciprofloxacin and 67% (8/12) for cefpodoxime.
Two women, 1 from each treatment group, were diagnosed with pyelonephritis at day 2 (ciprofloxacin) and day 28 (cefpodoxime) following enrollment. At enrollment, both had E coli susceptible to the assigned treatment drug. Information on the pyelonephritis strains was available only for the woman treated with cefpodoxime who had E coli susceptible to all antibiotics tested, including cefazolin, cefuroxime, and ceftriaxone; however, it was not tested against cefpodoxime.
Secondary Clinical Outcome
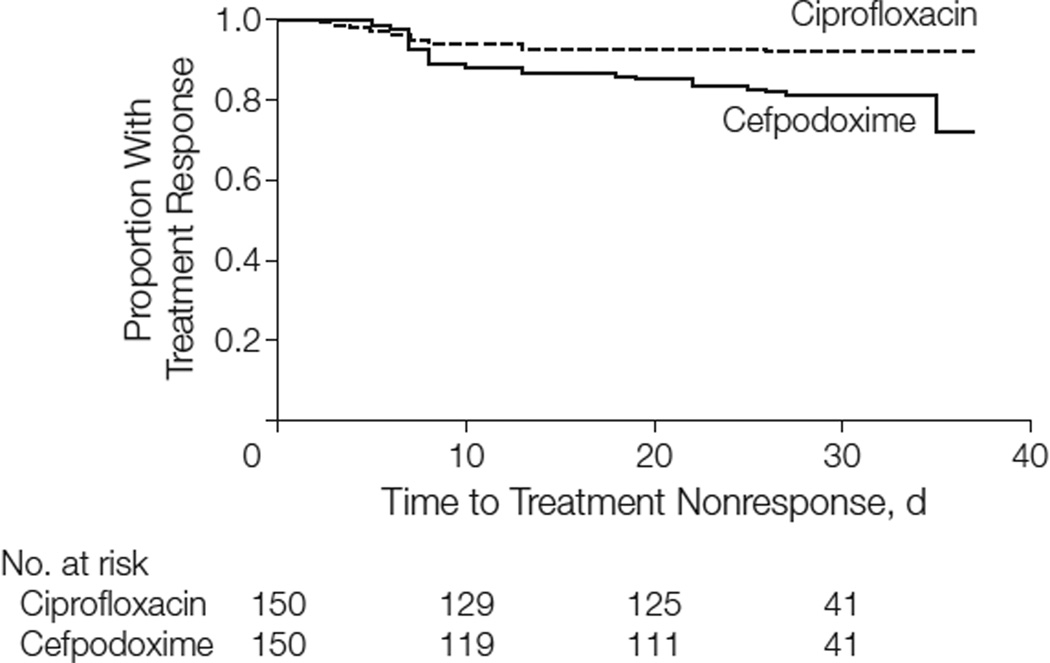
The clinical cure rate at the first follow-up visit (mean, 5 days after treatment) was 93% (140/150) for ciprofloxacin compared with 88% (132/150) for cefpodoxime (difference of 5%; 95% CI, −1% to 12%) (Table 2). The clinical cure rates over the duration of the study are shown in Figure 2, in which the Kaplan-Meier curves demonstrate the similarity in outcome during the first week of follow-up, after which the curves diverge due to further nonresponse to treatment in the cefpodoxime group.
Figure 2.
Time to Treatment Nonresponse in Women Treated With Ciprofloxacin vs Cefpodoxime
Among patients with available urine culture data, E coli was the causative uropathogen in 38% (3/8) of nonresponders to treatment for ciprofloxacin (5 had no growth) compared with 64% (16/25) for cefpodoxime (4 had no growth). Two women in the ciprofloxacin group with treatment nonresponse caused by E coli had a ciprofloxacin-resistant E coli strain at both enrollment and at the time of recurrent UTI and 1 had a susceptible strain at enrollment but a resistant strain causing the recurrent UTI. Thirteen of 16 women in the cefpodoxime group with treatment nonresponse caused by E coli had cefpodoxime-susceptible strains at enrollment and during the recurrent UTI, 2 had resistant strains at both enrollment and recurrent UTI, and 1 had a resistant strain at enrollment, but a susceptible strain during the recurrent UTI.
Secondary Microbiological Outcomes
The microbiological cure rate at the first follow-up visit (mean, 5 days after treatment) was 96% (123/128) for ciprofloxacin compared with 81% (104/129) for cefpodoxime (difference of 15%; 95% CI, 8%–23%). Among those women infected with strains that were susceptible to the study antibiotic, the microbiological cure rates were 97% (117/120) for ciprofloxacin and 81% (94/116) for cefpodoxime (difference of 16%; 95% CI, 9%–24%).
Vaginal E coli colonization was present at enrollment in 82% of women in both groups. By the first follow-up visit, however, 16% (21/132) of the women in the ciprofloxacin group compared with 40% (54/136) in the cefpodoxime group had vaginal E coli colonization. The difference persisted to a lesser extent at the 30-day follow-up visit (29% for ciprofloxacin vs 40% for cefpodoxime). The development of subsequent UTI did not correlate with the presence of vaginal E coli colonization at the first follow-up visit.
Tolerance and Adherence
Among women with follow-up, 99% of the ciprofloxacin group and 98% of the cefpodoxime group reported taking all 6 treatment doses. In response to an open-ended question, 20% of women in the ciprofloxacin group and 23% in the cefpodoxime group reported an adverse effect related to the study medication, whereas 30% and 27%, respectively, reported at least 1 adverse effect when asked about specific symptoms. The majority of adverse effects were nausea, diarrhea, headache, lightheadedness, or vaginal discomfort. Interruption of study medication due to adverse effects occurred in1%ofwomen in the ciprofloxacin group and 0% in the cefpodoxime group. Seven women in the ciprofloxacin group compared with 3 in the cefpodoxime group required treatment (primarily over-the-counter medication) for adverse effects.
COMMENT
The updated guideline on the management of uncomplicated UTI by the Infectious Diseases Society of America noted that the choice of a therapeutic agent should consider efficacy, risk of adverse effects, local resistance rates, propensity to cause adverse ecological effects,12 cost and availability of the drug, and clinician threshold for failure.1 The guideline panel also indicated that none of the antimicrobials currently available outweighs the others in terms of optimizing each of these factors for the treatment of acute cystitis.
The panel recommended that the fluoroquinolones, while highly efficacious, should be reserved for important uses other than acute cystitis, and thus should be considered alternative antimicrobials for acute cystitis. The main concern regarding fluoroquinolone use for acute cystitis is the possible promotion of fluoroquinolone resistance, not only among uropathogens but also other organisms causing more serious and difficult-to-treat infections at other sites. There also is concern about the association between fluoroquinolone use and increased rates of methicillin-resistant Staphylococcus aureus.12,21–23
This study is, to our knowledge, the first comparison of a 3-day regimen of ciprofloxacin with a 3-day regimen of cefpodoxime for the treatment of acute uncomplicated cystitis in women. Our study failed to demonstrate clinical noninferiority for 100 mg of cefpodoxime twice daily for 3 days compared with 250 mg of ciprofloxacin twice daily for 3 days (cure rate, 82% vs 93%, respectively) (difference of 11%; 95% CI, 3%– 18%). This finding was consistent with those in the per-protocol analysis and in microbiological cure and eradication of vaginal E coli colonization at the early follow-up visit. Study outcomes were similarly different when patients lost to follow-up were considered to have not responded to treatment. Ciprofloxacin’s clinical advantage was diminished in women with a history of UTI or infected with a nonsusceptible uropathogen.
Although extended-spectrum cephalosporins such as cefpodoxime have good activity against UTI pathogens, the expected efficacy with β-lactam drugs has in general been low compared with trimethoprim-sulfamethoxazole or fluoroquinolones.9–11 We have speculated that the lower clinical response with β-lactams compared with other first-line antimicrobials may be due to their poorer activity in eradicating the uropathogen from the vaginal flora.11,24 In this regard, cefpodoxime demonstrated significantly poorer activity than ciprofloxacin in eradicating E coli from the vaginal flora in this study.
In a smaller published trial of cefpodoxime for treatment for uncomplicated cystitis, cefpodoxime yielded high rates of clinical and microbiological cure similar to the comparator agent, trimethoprim-sulfamethoxazole.18
In addition to its poor performance compared with ciprofloxacin, another concern about cefpodoxime for uncomplicated cystitis is whether this agent promotes emergence of gram-negative extended-spectrum β-lactamase (ESBL) resistance. Although we are not aware of data suggesting that cefpodoxime is associated with ESBL resistance, parenteral broad-spectrum cephalosporins have been associated with promotion of ESBL resistance among gram-negative bacteria.12 None of the 16 E coli strains causing recurrent UTI among women treated with cefpodoxime appeared to be ESBL strains by susceptibility patterns.
This study adheres to the guidelines for clinical trials designed to test noninferiority recently published by the Consolidated Standards of Reporting Trials (CONSORT) group.25 Our study is one of the few double-blind randomized trials comparing antimicrobial drugs for treatment of acute uncomplicated cystitis. Strengths of this study include its double-blind study design, large sample size, well-defined study population, low dropout rate, and high rate of medication adherence. The participants in our trial were primarily a highly compliant, white student population and thus our findings may not be generalizable to settings in which these characteristics differ.
We chose to compare 3-day regimens of ciprofloxacin and cefpodoxime for the treatment of acute uncomplicated cystitis because of the need for safe and effective fluoroquinolone-sparing antimicrobials, the broad-spectrum coverage of cefpodoxime, and the absence of a large trial comparing 3-day regimens of cefpodoxime with current standard therapy. Both the intention-to-treat and per-protocol analyses produced consistent results showing that, among women with acute uncomplicated cystitis, a 3-day regimen of cefpodoxime compared with ciprofloxacin did not meet criteria for noninferiority in achieving clinical cure.
Our findings in this study and concerns about possible adverse ecological effects that have been associated with parenteral broad-spectrum cephalosporins do not support the use of cefpodoxime as a first-line fluoroquinolone-sparing antimicrobial for acute uncomplicated cystitis. As recommended in recently published guidelines, nitrofurantoin, trimethoprim-sulfamethoxazole (except in areas where the resistance prevalence is known to be high), fosfomycin, and pivmecillinam (not available in the United States) should be considered before fluoroquinolones and β-lactams such as cefpodoxime for patients with uncomplicated cystitis.1
Acknowledgments
Funding/Support: This work was supported by grants P01 DK053369 and SCOR P50 DK64540 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Role of the Sponsors: The funding source did not play any role in the design and conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Hooton had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hooton, Roberts, Stapleton.
Acquisition of data: Hooton, Stapleton.
Analysis and interpretation of data: Hooton, Roberts, Stapleton.
Drafting of the manuscript: Hooton, Roberts, Stapleton.
Critical revision of the manuscript for important intellectual content: Hooton, Roberts, Stapleton.
Statistical analysis: Roberts.
Obtained funding: Hooton, Stapleton.
Administrative, technical, or material support: Hooton, Stapleton.
Study supervision: Hooton, Stapleton.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Hooton reported that he has been a consultant for Pinnacle Pharmaceuticals, Pfizer Inc, and Alita Pharmaceuticals. No other author reported disclosures.
Previous Presentations: Presented in part at the 47th Annual Meeting of the Infectious Diseases Society of America; October 29-November 1, 2009; Philadelphia, Pennsylvania.
Additional Contributions: We greatly appreciate the involvement of Walter E. Stamm, MD (deceased) in discussions leading to the conception, conduct, and completion of this study. We are greatly indebted to D. C. Dugdale, MD, medical director, and the staff at Hall Health Primary Care Center (University of Washington, Seattle) for assistance with study enrollment; Niki Deshaw, MA, and Ellen Cassen, ARNP, for participant enrollment and follow-up; Marsha Cox, BS, and Sheila Manuguid, BS, for laboratory assistance at the University of Washington, Seattle; Wisvline Labrousse, PhD, ARNP, for participant enrollment and follow-up; and Nadege Atis, BS, for laboratory assistance at the University of Miami, Miami, Florida. All of these individuals were salaried employees of their respective institutions and no one received additional compensation for their efforts in this study.
REFERENCES
- 1.Gupta K, Hooton TM, Naber KG, et al. Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 2.Kallen AJ, Welch HG, Sirovich BE. Current antibiotic therapy for isolated urinary tract infections in women. Arch Intern Med. 2006;166(6):635–639. doi: 10.1001/archinte.166.6.635. [DOI] [PubMed] [Google Scholar]
- 3.Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med. 2002;162(1):41–47. doi: 10.1001/archinte.162.1.41. [DOI] [PubMed] [Google Scholar]
- 4.Olson RP, Harrell LJ, Kaye KS. Antibiotic resistance in urinary isolates of Escherichia coli from college women with urinary tract infections. Antimicrob Agents Chemother. 2009;53(3):1285–1286. doi: 10.1128/AAC.01188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta K. Emerging antibiotic resistance in urinary tract pathogens. Infect Dis Clin North Am. 2003;17(2):243–259. doi: 10.1016/s0891-5520(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 6.Goettsch W, van Pelt W, Nagelkerke N, et al. Increasing resistance to fluoroquinolones in Escherichia coli from urinary tract infections in the Netherlands. J Antimicrob Chemother. 2000;46:223–228. doi: 10.1093/jac/46.2.223. [DOI] [PubMed] [Google Scholar]
- 7.Karlowsky JA, Lagacé-Wiens PRS, Simner PJ, et al. Antimicrobial resistance in urinary tract pathogens in Canada from 2007 to 2009: CANWARD Surveillance Study. Antimicrob Agents Chemother. 2011;55(7):3169–3175. doi: 10.1128/AAC.00066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooton TM, Besser R, Foxman B, Fritsche TR, Nicolle LE. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin Infect Dis. 2004;39(1):75–80. doi: 10.1086/422145. [DOI] [PubMed] [Google Scholar]
- 9.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE Infectious Diseases Society of America (IDSA) Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin Infect Dis. 1999;29(4):745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 10.Nicolle LE, Madsen KS, Debeeck GO, et al. Three days of pivmecillinam or norfloxacin for treatment of acute uncomplicated urinary infection in women. Scand J Infect Dis. 2002;34(7):487–492. doi: 10.1080/00365540110080728. [DOI] [PubMed] [Google Scholar]
- 11.Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA. 2005;293(8):949–955. doi: 10.1001/jama.293.8.949. [DOI] [PubMed] [Google Scholar]
- 12.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38(suppl 4):S341–S345. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]
- 13.Counts GW, Stamm WE, McKevitt M, Running K, Holmes KK, Turck M. Treatment of cystitis in women with a single dose of trimethoprim-sulfamethoxazole. Rev Infect Dis. 1982;4(2):484–490. doi: 10.1093/clinids/4.2.484. [DOI] [PubMed] [Google Scholar]
- 14.Pabich WL, Fihn SD, Stamm WE, Scholes D, Boyko EJ, Gupta K. Prevalence and determinants of vaginal flora alterations in postmenopausal women. J Infect Dis. 2003;188(7):1054–1058. doi: 10.1086/378203. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (CLSI) M100-S15 (M100) 2005: performance standards for antimicrobial susceptibility testing 15th informational supplement. [Accessed February 1, 2005]; http://www.clsi.org. [Google Scholar]
- 16.Iravani A, Klimberg I, Briefer C, Munera C, Kowalsky SF, Echols RM. A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection. J Antimicrob Chemother. 1999;43(suppl A):67–75. [PubMed] [Google Scholar]
- 17.Richard GA, Mathew CP, Kirstein JM, Orchard D, Yang JY. Single-dose fluoroquinolone therapy of acute uncomplicated urinary tract infection in women: results from a randomized, double-blind, multicenter trial comparing single-dose to 3-day fluoroquinolone regimens. Urology. 2002;59(3):334–339. doi: 10.1016/s0090-4295(01)01562-x. [DOI] [PubMed] [Google Scholar]
- 18.Kavatha D, Giamarellou H, Alexiou Z, et al. Cefpodoxime-proxetil versus trimethoprim-sulfamethoxazole for short-term therapy of uncomplicated acute cystitis in women. Antimicrob Agents Chemother. 2003;47(3):897–900. doi: 10.1128/AAC.47.3.897-900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naber KG, Allin DM, Clarysse L, et al. Gatifloxacin 400 mg as a single shot or 200 mg once daily for 3 days is as effective as ciprofloxacin 250 mg twice daily for the treatment of patients with uncomplicated urinary tract infections. Int J Antimicrob Agents. 2004;23(6):596–605. doi: 10.1016/j.ijantimicag.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Chow SC, Shao J, Wang H. Sample Size Calculations in Clinical Research. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 21.MacDougall C, Powell JP, Johnson CK, Edmond MB, Polk RE. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin Infect Dis. 2005;41(4):435–440. doi: 10.1086/432056. [DOI] [PubMed] [Google Scholar]
- 22.Charbonneau P, Parienti JJ, Thibon P, et al. French Fluoroquinolone Free (3F) Study Group. Fluoroquinolone use and methicillin-resistant Staphylococcus aureus isolation rates in hospitalized patients: a quasi experimental study. Clin Infect Dis. 2006;42(6):778–784. doi: 10.1086/500319. [DOI] [PubMed] [Google Scholar]
- 23.Rogues AM, Dumartin C, Amadéo B, et al. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infect Control Hosp Epidemiol. 2007;28(12):1389–1395. doi: 10.1086/523280. [DOI] [PubMed] [Google Scholar]
- 24.Hooton TM, Stamm WE. The vaginal flora and UTIs. In: Mobley HLT, Warren JW, editors. UTIs: Molecular Pathogenesis and Clinical Management. Washington, DC: ASM Press; 1996. p. 67. [Google Scholar]
- 25.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ CONSORT Group. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]