Abstract
During the last two decades, a wealth of animal and human studies has implicated inflammation-derived oxidative stress and cytokine-dependent neurotoxicity in the progressive degeneration of the dopaminergic (DA) nigrostriatal pathway, the hallmark of Parkinson’s disease (PD). In this review, we discuss the various hypotheses regarding the role of microglia and other immune cells in PD pathogenesis and progression, the inflammatory mechanisms implicated in disease progression from pre-clinical and clinical studies, the recent evidence that systemic inflammation can trigger microglia activation in PD-relevant CNS regions, the synergism between gene products linked to parkinsonian phenotypes (α-synuclein, parkin, Nurr1, and RGS10) and neuroinflammation in promoting neurodegeneration of the nigrostriatal pathway, and the latest update on meta-analysis of epidemiological studies on the risk-lowering effects of anti-inflammatory drug regimens.
Keywords: microglia, Parkinson’s disease, neuroinflammation, neurodegeneration, peripheral immune cell, BBB
Introduction
Parkinson’s disease (PD), the second most common age-associated progressive neurodegenerative disorder is characterized by the loss of dopaminergic (DA) neurons, cytoplasmic inclusions of aggregated proteins (Lewy bodies), and neuroinflammation (Moore, 2005; McGeer and McGeer, 2008). The hallmarks of neuroinflammation are the presence of activated microglia and reactive astrocytes in the parenchyma of the CNS and increased production of cytokines, chemokines, prostaglandins, complement cascade proteins, and reactive oxygen and nitrogen species (ROS/RNS) which in some cases can results in disruption of the blood-brain barrier (BBB) and direct participation of the adaptive immune system (Ransohoff and Perry, 2009). The extent to which neuroinflammation and peripheral immune responses contribute to development of PD or modify its course is not known. It was once believed that the BBB prevented access of immune cells to the brain and, as a result, the immune system and the central nervous system (CNS) were relatively independent of each other. However, it has become clear that BBB permeability can be modulated and traffic of peripheral macrophages and leukocytes into the brain parenchyma is a normal process that must be tightly regulated to promote brain homeostasis and avoid neuronal demise (Ransohoff and Perry, 2009; Rezai-Zadeh et al., 2009). In fact, neuroimmune dysregulation has been postulated by many to underlie the chronic nature of neurodegenerative disease. Although no strong evidence exists to suggest neuroinflammation is the primary trigger that causes neurodegeneration, epidemiological and pre-clinical data suggest chronic neuroinflammation may be the “silent driver” of neuronal dysfunction during the pro-dromal/asymptomatic stage of age-associated neurodegenerative diseases (i.e. PD and AD).
I. Evidence of neuroinflammation in Parkinson’s disease (PD) and in animal models of nigral cell death
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and is characterized by the loss of dopamine (DA)-producing neurons in the ventral midbrain with cell bodies in the substantia nigra pars compacta (SNpc) that project to the striatum (nigrostriatal pathway), with a lesser effect on DA neurons in the ventral tegmental area (VTA) (Uhl et al., 1985; Moore et al., 2005). PD prevalence is age-associated, with approximately 1% of the population over 65-70 years of age, increasing to 4–5% in 85-year olds (Fahn, 2003). Epidemiological studies and pathological analyses demonstrate sporadic PD accounts for about 95% of patients (Tanner, 2003; Farrer, 2006); but familial forms of the disease linked to mutations in a restricted number of genes account for 4% of PD cases and these patients develop early-onset disease before the age of 50 (Mizuno et al., 2001; Van Den Eeden et al., 2003). Over the past decade, a definitive link has been demonstrated between mutations in specific genes and heritable forms of PD (for an in-depth review see (Farrer, 2006). Although some of these mutations can be found in higher frequency among certain ethnic populations, together they account for only a small percentage (perhaps up to 15%) of all PD cases. In short, the vast majority of cases are not due to monogenic disease and their etiology remains a major unanswered question.
The prevailing view on the causes of non-familial or idiopathic forms of PD is that multifactorial and genetic predispositions, environmental toxins, and aging are all likely to be important factors in disease initiation and progression (Nagatsu and Sawada, 2006). Because the single greatest risk factor for developing PD is age, cumulative oxidative damage in the CNS is thereby implicated as an important mechanism. However, nigral lesions in PD and aged individuals vary considerably and raise the possibility that aging and the disease process underlying PD may be occurring independently. At the cellular level, cumulative evidence supports an “oxidative stress hypothesis” for initiation of nigral dopamine neuron loss (for in-depth reviews see (Jenner and Olanow, 1996; Owen et al., 1996, 1997; Beal, 2005; Lin and Beal, 2006). Oxidative stress occurs when there is an intracellular accumulation of ROS/RNS due to reduced endogenous antioxidant capacity and/or overproduction of ROS within the cell. The brain is considered to be abnormally sensitive to oxidative damage in part because oxygen consumption by the brain constitutes 20% of the total oxygen consumption in the body; and the brain is enriched in the more easily peroxidizable fatty acids (20:4 and 22:6) while its anti-oxidant defenses (such as catalase, superoxide dismutase, glutathione, and glutathione peroxidase) are relatively sparse (Floyd, 1999). Within the midbrain, the SN appears to be among the most vulnerable regions primarily because it operates under a pro-oxidative state relative to other parts of the brain even in healthy individuals. In summary, increased oxidation of lipids, DNA and proteins and has been documented in the brains of PD patients in a large number of studies and support the oxidative stress hypothesis. Yet the evidence against sole dysfunction being caused by oxidative stress is the limited ability of antioxidants to afford robust DA neuroprotection and disease-modifying effects in the clinic (Olanow, 2007). Therefore, it’s been postulated for several years now that inflammation, oxidative stress, mitochondrial dysfunction, proteolytic stress, and axonal transport deficits all play important roles in almost every neurodegenerative disorder including PD. Seminal work by McGeer and colleagues over 20 years ago first identified significantly increased levels of HLA-DR-positive microglia in the brains of PD patients post-mortem (McGeer et al., 1988) and since then multiple studies have demonstrated that microglia are activated in PD and increased levels of pro-inflammatory mediators such as Tumor Necrosis Factor (TNF), interleukin-1beta (IL-1β), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (Cox-2) are found in the striatum and in the substantia nigra (reviewed in (Tansey et al., 2007).
II. The role of microglia and other immune cells in PD pathogenesis and progression
The brain’s innate immune response is quite heterogenous (reviewed by (Colton, 2009) and innate immune surveillance in the CNS is primarily performed by microglia, the monocyte-derived resident macrophages of the brain (Puntambekar, 2008; Tansey, 2008). Microglia play a homeostatic role in the CNS and respond to environmental stresses and immunological challenges by scavenging excess neurotoxins and removing dying cells and cellular debris (Nakamura, 2002; Orr et al., 2002; Ransohoff and Perry, 2009). An initial physical or pathogenic events in the CNS can trigger microglial expansion primarily through differentiation from progenitor cells or proliferation of brain-resident microglia and secondarily through recruitment of peripheral macrophages to the CNS as a result of increased permeability of the BBB (Ransohoff and Perry, 2009; Rezai-Zadeh et al., 2009). Studies performed in the last 15 years aimed at understanding how the brain resolves innate immune responses to injury or infection have shed light on the complex characteristics of the microglia phenotype in health and disease states (Colton, 2009). Interestingly, chronic inflammatory diseases, and therefore more than likely also the chronically inflamed environment of degenerating brain tissue, are believed to be characterized by a microglia phenotype that is a combination of classical activation states with alternative activation states (Colton, 2009). Because classically activated microglia produce prostaglandins, chemokines, cytokines, complement proteins, proteinases, and ROS and RNS including nitric oxide (NO), sustained production of these substances can have a deleterious effect on susceptible populations by enhancing oxidative stress and activating cell death pathways (McGeer and McGeer, 2004). Specifically, aging and/or repeated exposure to environmental toxins (including pesticides and particulate matter) may contribute to increased permeability of the BBB and increased likelihood of increased peripheral immune cell traffic into the brain. Although it is now widely believed that the microglia in the CNS are a long-lived population of tissue macrophages, how the resident populations of brain macrophages are maintained in homeostasis and during disease is still somewhat controversial (Ransohoff and Perry, 2009). Similarly, the immune reactions initiated by viruses and bacteria may compound with latent vulnerabilities which could then manifest into future immunologic challenges. Importantly, it should be noted that individuals with neurodegeneration are likely to have a compromised BBB and because of this fact the peripheral immune system may have an important role to play in limiting or accelerating progressive neuronal loss depending on the disease state.
At the molecular level, microglia activation may be triggered by protein aggregation and formation of inclusions arising from mutations (i.e., α-synuclein) or disruption of the ubiquitin-proteasome system, immunological challenges (bacterial or viral infections), or traumatic brain injury. For example, misfolded or aggregated proteins in Lewy Bodies (LBs) of diseased nigral DA neurons are likely to elicit a self-propelling cycle of microglial activation and increased production of inflammatory mediators in SN, thus providing a tertiary hit required for PD-associated dysfunction to spread to neighboring neurons (Zhang et al., 2005; Kim and Joh, 2006; Sulzer, 2007). Evidence in support of this idea comes from studies in mice in which overexpression of wild-type α-synuclein in neurons is associated with early microglia activation and release of α-synuclein from an α-synuclein-overexpressing dopaminergic neuron-like cell line triggers a cascade of inflammatory mediators that include TNF, IL1β, IL-6, COX-2 and iNOS (Su et al., 2007). In addition, α-synuclein and in particular the A30P, E46K and A53T α-synuclein mutations linked to familial PD have been reported to potently activate human microglia and the human monocytic cell line THP-1 to secrete high levels of IL-1β and TNF, exerting cytotoxicity on human SH-SY5Y neuroblastoma cells (Klegeris et al., 2006). α-synuclein may also have important roles in microglia in regulation of their activation state. Specifically, microglia from α-synuclein-deficient mice (Scna−/−) have been shown to display a reactive phenotype under basal culture conditions and a hyper-reactive phenotype (increased production of the pro-inflammatory cytokines TNF and IL-6) but impaired phagocytic behavior after stimulation compared with microglia from wild-type mice (Austin et al., 2006). Moreover, microglia from Scna−/− mice display significant morphologic differences (extremely large and ramified cells filled with vacuole-like structures) and increased levels of activation markers (CD68 and β1-integrin) compared with microglia from wild-type mice. The implication of these studies is that abnormal activation of microglia effector functions by dysfunctional DA neurons may enhance chronic neuroinflammation and gene products once believed to be of specific importance in DA neurons (α-synuclein) may also be involved in regulating microglial responses.
Microglia secrete multi-functional immunoregulatory proteins called cytokines, most notably TNF, IL-1 and IL-6 families, interferon gamma (IFNγ), and Transforming Growth Factor beta (TGF-β), all of which act in context-dependent ways to modulate inflammatory processes and the permeability of the BBB (Benveniste, 1992; Sedgwick et al., 2000; Whitton, 2007). Locally released cytokines and chemokines diffuse into the bloodstream, attract leukocytes to the site of inflammation, and upregulate the expression of cellular adhesion molecules to facilitate attachment and transmigration across post-capillary venules (Engelhardt, 2008). Cytokines as well as chemoattractant factors called chemokines can promote apoptosis of specific subsets of neurons, oligodendrocytes, and astrocytes and can cause damage to myelinated axons. Elevated levels of several cytokines and other inflammatory mediators have been reported in a wide range of neurodegenerative CNS disorders including PD. While there is no evidence to support a role for any cytokine in the direct triggering of any of these neurodegenerative conditions, cytokine-driven neuroinflammation and neurotoxicity has now been shown to modify disease progression in a number of pre-clinical models of these disorders.
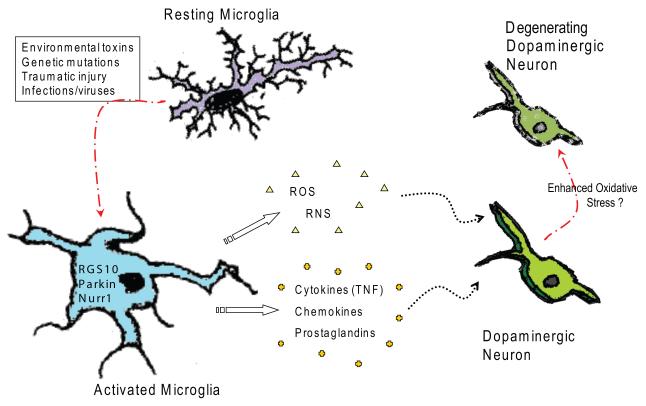
Microglial ‘priming’, in which activation precedes another stimulation/toxin, may be another mechanism by which neuroinflammation contributes to the death of dopaminergic neurons. Specifically, a recent study reported that a single paraquat exposure induced microglia activation, including induction of NADPH oxidase. If this activation was blocked with the anti-inflammatory drug minocycline, subsequent exposures to the herbicide failed to cause oxidative stress and neurodegeneration (Purisai et al., 2007). However, if microglia were first primed by pre-treatment with LPS, a single paraquat exposure became capable of triggering loss of DA neurons. Consistent with the importance of microglial-derived oxidant stress, mutant mice lacking functional NADPH oxidase were spared from neurodegeneration caused by repeated paraquat exposures (Purisai et al., 2007). LPS has been shown to sensitize animals to the toxic dopamine analog 6-hydroxydopamine (6-OHDA)-induced nigral degeneration. Specifically, a non-neurotoxic dose of LPS that induced microglia activation and secretion of cytokines, IL-1β in particular, was reported to predispose DA neurons to the degenerative effects of a subsequent low dose of 6-OHDA blockable by administration of an IL-1 receptor antagonist (Anakinra) (Koprich et al., 2008). Therefore, microglial priming may in part regulate microglial phenotype and shift microglial activities from neuroprotective to neurotoxic (i.e., from trophic factor production and phagocytosis to production of ROS/RNS, prostaglandins, cytokines, and chemokines) the outcome of which may hasten the death of vulnerable neuronal populations (Block and Hong, 2005; Mrak and Griffin, 2005; Ito et al., 2006; Kim and Joh, 2006; Nagatsu and Sawada, 2006; Sawada et al., 2006). Even in the absence of microglial priming, microglial activation during the sustained course of a disease results in respiratory bursts and sustained elevation of cytokines, chemokines, and prostaglandins that may act to compound neuronal dysfunction and aid in disease progression (Block and Hong, 2005; Minghetti, 2005; Minghetti et al., 2005; Nagatsu and Sawada, 2006; Wersinger and Sidhu, 2006; Zhang et al., 2006). Given the fact that recent PET imaging studies suggest microglia activation is not limited to end-stage PD but is likely to occur in parallel with DA neuron loss (Gerhard et al., 2006). Moderate increases in oxidative species generation due to sustained microglial activation in the nigral microenvironment could easily overwhelm the natural defenses of the remaining DA neurons by contributing to enhanced oxidative stress (Figure 1). Therefore, targeted inhibition of the glial reaction and inflammatory processes triggered by environmental toxins may represent an attractive therapeutic approach to delay progression of PD.
Figure 1. Potential initiating factors of microglia activation in the CNS that contribute to progressive loss of nigral dopaminergic neurons and development of Parkinson’s disease (PD).
A number of different stimuli can activate microglia in the CNS. If the initiating trigger is left unresolved and/or with additional insults, chronic microglia activation contributes to an enhanced inflammatory environment in the brain. Chronic neuroinflammation results in enhanced oxidative stress on and/or chronic activation of cell death pathways in vulnerable neuronal populations (i.e. nigral dopaminergic neurons) and accelerated degeneration.
The role of peripheral immune cell traffic into the brain and its relevance to PD has not been extensively explored to date but several studies implicating subsets of T cells are worth noting and deserve follow-up. Hirsch and colleagues recently reported that CD4+ and CD8+ populations of T cells are recruited to the SNpc of PD patients and in MPTP-intoxicated mice (Brochard et al., 2009) and mice lacking CD4+ cells are protected from MPTP-induced nigrostriatal degeneration. Nitrated α-synuclein may represent modified “self” epitopes that can recruit peripheral leukocytes in cervical lymph nodes in an MPTP mouse model and transfer of T cells from syngeneic donors immunized with nitrated α-synuclein worsens DA neuron loss after MPTP (Benner et al., 2008). Although it is clear that use of the MPTP neurotoxin can itself compromise BBB integrity depending on the dose used, a leaky BBB makes possible the recruitment of peripheral T cells in both the neurotoxin model and in PD but is unlikely to be sufficient for disease etiology. Nevertheless, data from recent in vivo studies suggested that CD4+/CD25− effector T cells can enhance microglia activation and neurotoxic activities in response to nitrated α-synuclein and CD4+/CD25+ regulatory T cells (Tregs) can inhibit microgliosis and induce microglia apoptosis (Reynolds et al., 2009). Taken together, these recent observations support the notion that adaptive immunity may be playing an as yet unappreciated role in PD and modulation of acquired immune responses could be potentially harnessed for therapeutic benefit in PD.
The question regarding the role of increased BBB permeability in etiology and progression of PD is still under investigation. New imaging technologies such as positron emission tomography (PET) scanning have made it possible to use [(11)C]-verapamil PET to study cerebrovascular P-glycoprotein (P-gp) pump efflux function as a measure of BBB permeability. While age-dependent and region-specific decreased P-gp function (consistent with increased BBB permeability) has been reported(Bartels et al., 2008) and some studies find decreased function in PD patients compared to controls (Kortekaas et al., 2005), others only see decreased function in advanced and not early PD patients (Bartels et al., 2008), suggesting that BBB breakdown is not causative of PD but may occur with increasing severity of disease. Not surprisingly, animal models of parkinsonism have also yielded variable results. 6-hydroxydopamine (6-OHDA)-lesioned hemimparkinsonian rats display increased leakage of fluorescently-labeled albumin or horseradish peroxidase from the vasculature into parenchyma of the ipsilateral SNpc and striatum and interestingly increased expression of P-gp and the angiogenesis marker β3-integrin which the authors interpreted to be a compensatory mechanism to the inflammation (Carvey et al., 2005). Using immunohistological markers to measure endothelial barrier antigens and extravasation of serum albumin into brain parenchyma, Cenci and colleagues reported defects in BBB in the basal ganglia in 6-OHDA/chronic L-DOPA-treated rats (Westin et al., 2006). More recently, using gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) in MRI studies Isacson and colleagues detected no BBB defects in MPTP-treated primates exhibiting L-DOPA-induced dyskinesia (Astradsson et al., 2009). In brief, the differences in study outcomes are likely to be a product of different experimental models, different neurotoxic insults, and different stage of clinical disease and more importantly use of different methodologies with very different sensitivities. PET imaging has also enabled investigators to detect and localize neuroinflammatory foci in the brains of patients with PD and the differences between them and control research subjects with radiolabeled isoquinolone [11C] (R)-PK11195 which binds selectively to a mitochondrial membrane translocator protein (TP)-18 in the peripheral benzodiazepine-binding site (PBBS) complex (Gerhard et al., 2006). Importantly, these proof-of-principle studies strongly suggest the occurrence of early and prolonged microglia activation in PD. These techniques need to be applied in the clinical setting to monitor the extent of neuroinflammation in patients’ brains at various stages of disease and/or in response to anti-inflammatory or other therapies to critically determine how these interventions are altering (or not) progression of disease. Given that the largest risk factor in idiopathic PD is age, one could envision that the increase in inflammatory load in the CNS that occurs as individuals age could increase BBB permeability to peripheral toxins and immune cells, thereby raising the vulnerability of the nigrostriatal pathway to degeneration and development of PD. But the more widely held belief is that disruption of the BBB and associated inflammation only occurs after a significant number of nigral DA neurons have degenerated and as such it is likely to contribute to progression of PD rather than be a major causative factor.
III. Rodent inflammation models of nigral cell death
Development of endotoxin-based in vivo models of nigrostriatal pathway degeneration demonstrates the susceptibility of nigral DA neurons to inflammatory stimuli. Approximately a decade ago, intranigral delivery of lipopolysaccharide (LPS) was first shown to induce an inflammatory reaction that activated microglia and induced selective and irreversible damage to nigral DA neurons while sparing serotonergic neurons (Castano et al., 1998). Since then, LPS-induced nigral DA neuron death has been shown to be independent of NO and inhibitable by dexamethasone (Castano et al., 2002). The intrinsic susceptibility of nigral DA neurons to inflammatory stimuli was directly shown by a critical experiment in which administration of alpha-methyl-p-tyrosine (alpha-MPT), an inhibitor of tyrosine hydroxylase, prevented LPS-induced nigral DA neuron loss (De Pablos et al., 2005). Additional inflammation-based models of nigral degeneration have been developed in recent years, including chronic low-dose intranigral LPS infusion in rats (Gao et al., 2002) or intrauterine exposure to LPS (Carvey et al., 2003), which induce delayed, chronic, and progressive loss of DA neurons in the adult SNpc or in the offspring, respectively. Further support that neuroinflammation resulting from peripheral inflammatory triggers can induce nigral degeneration and may play an important role in the progressive loss of DA neurons in sporadic PD has derived from a number of recent studies using intraperitoneal (i.p.) LPS as a trigger. First, Hong and colleagues reported that delayed and progressive nigral degeneration was detectable in C57Bl/6 male mice 7 months after a single i.p. injection of high-dose LPS (5 mg/kg) in all likelihood mediated by circulating TNF (Qin et al., 2007), while female C57Bl/6 mice appeared to be more resistant and required repeated high-dose LPS injections to display the nigral degeneration phenotype (Liu et al., 2008). Second, increased susceptibility to inflammation-induced nigral degeneration was observed in Parkin-deficient mice after 3 months of twice-weekly low-dose i.p. LPS (~0.5mg/kg) injections followed by a 3-month lag period which progressed significantly when injections were extended to 6 months (Frank-Cannon et al., 2008); the significance of these findings and their implication for Parkin function are discussed in more detailed below.
For example, inflammatory cytokines such as IL-1β have been show to promote upregulation of α-synuclein in neuronal cultures (Griffin et al., 2006), a mechanism which promotes protein aggregation and generation of LBs in the substantia nigra. Smeyne and colleagues have recently reported that intranasal administration of the neurotropic avian influenza virus H5N1 in C57BL/6J mice resulted in a short-lived infection in the peripheral nervous system that traveled into the CNS (Jang et al., 2009). In support of a link between neuroinflammation and proteinopathies, the neuropathological hallmarks of the infected regions included α-synuclein phosphorylation and aggregation and activation of microglia that persisted long after resolution of the infection. As predicted by multiple models, the chronic neuroinflammatory response was accompanied by a delayed loss of nigral dopaminergic neurons, suggesting that H5N1 or other neurotropic influenza viruses may be involved in the etiology of CNS proteinopathies and in particular of PD.
IV. Genes that modulate susceptibility to inflammation-induced degeneration
Recent evidence from genetic mouse models suggests that the outcome of neuroinflammatory responses in the midbrain SNpc may be influenced by the presence of abnormal α-synuclein. One recent such study demonstrated that nigral degeneration can result from synergistic interactions between α-synuclein and neuroinflammation. Specifically, a single intranigral injection of LPS in α-synuclein-null mice that overexpress wild-type human α-synuclein or A53T mutant α-synuclein was shown to significantly accelerate the loss of DA neurons and nitrated/oxidized α-synuclein was detected in nigral inclusions (Gao et al., 2008). Interestingly, inhibition of microglia-derived NO and superoxide afforded significant neuroprotection from the accelerating effects of the LPS injection, suggesting these mediators mechanistically link inflammation to aberrant α-synuclein signaling and may contribute to neurodegeneration in PD.
Similary, neuroinflammatory responses may be impacted by the function of certain gene products in microglia, some of which have thus far been thought to be important only in neurons. Human mutations resulting in reduced expression of Nurr1 are associated with late-onset familiarl PD (Le et al., 2003). Nurr1 (NR4A2) belongs to the receptor (NR)4 family of orphan nuclear receptors and is known to function as a constitutively active transcription factor by binding to target genes (Aarnisalo et al., 2002; Wang et al., 2003). Recent study demonstrated evidence that Nurr1 plays an essential role in both microglia and astrocytes as a single-dependent transcriptional repressor of genes that encode pro-inflammatory neurotoxic factors (Saijo et al., 2009). They have shown that Nurr1 recruit CoREST corepressor complexes to NFκB target genes and mediate the turnover of NFκB and restore activated gene expression to a basal state. Specially, loss of Nurr1 function in microglia and astrocytes of the SN results in exaggerated and prolonged inflammatory responses that accelerate the loss of dopaminergic neurons in response to LPS or overexpression of a mutant form of α-Synuclein (A30P) associated with familial PD suggesting that Nurr1 may play protective role in neurodegeneration by modulating extraneuronal cells.
Although loss-of-function mutations in the E3 ligase Parkin give rise to a rare form or autosomal recessive parkinsonism (Shimura et al., 2000), mice deficient in Parkin do not display nigral degeneration (Goldberg et al., 2003). Although disappointing at first, this important finding suggested the hypothesis that Parkin-deficient mice require an additional trigger to develop nigral degeneration. To test this possibility, Parkin-deficient mice (or wild-type mice of the same genetic background) were exposed to repeated intraperitoneal injections of LPS for specific periods of time. Although chronic administration of low-dose LPS triggered very similar neuroinflammatory and oxidative stress responses in the SNpc of both WT and Parkin-deficient mice, only the latter developed delayed and selective degeneration of dopaminergic neurons in substantia nigra pars compacta but not in VTA. These findings suggested that Parkin loss increases the vulnerability to inflammation-induced degeneration but did not identify the cell type responsible for the parkinsonian phenotype. Additional studies will need to establish whether Parkin loss in DA neurons changes their sensitivity to specific inflammatory mediators and/or the extent to which Parkin-deficient glia may respond aberrantly and compromise DA neuron survival.
Lastly, the Regulator of G-protein Signaling-10 (RGS10), a microglial-enriched GTPase that is a putative negative regulator of G-protein signaling, plays a protective role in the SNpc against the neurodegenerative effects of chronic peripheral inflammation via regulation of the microglial phenotype (Lee et al., 2008). RGS10-deficient mice displayed increased microglial burden in CNS, dysregulated inflammation-related gene expression in microglia and nigral DA neuron degeneration with repeated systemic administration of low-dose LPS injection. TNF, IL-1b and IL-6 secretions were much higher by LPS-stimulated microglia from RGS10 knockout mice than from wild-type mice. Mechanistically, in vitro study indicated that RGS10 functions to regulate microglia activation. Specifically, knockdown of RGS10 in the murine BV2 microglial cells resulted in dysregulated inflammation-related expression, increased production of inflammatory cytokines including TNF, and enhanced cytotoxic effect on MN9D dopaminergic cells. A better understanding of the function of Parkin, Nurr1, and RGS10 in microglia (Figure 1) may reveal new strategies for manipulating the levels or activities of these important regulators to block or delay the progressive loss of nigrostriatal DA neurons in PD. V.
V. Neuroprotection by anti-inflammatory drugs in animal and epidemiological studies
Early studies using inhibitors of iNOS provided evidence of their potential as neuroprotective agents in the treatment of Parkinson disease. Administration of MPTP(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) to mice induced a robust gliosis in the substantia nigra pars compacta associated with significant upregulation of iNOS and preceded or paralleled MPTP-induced dopaminergic neurodegeneration (Liberatore et al., 1999). In addition, mutant mice lacking the iNOS gene were significantly more resistant to MPTP than their wild-type littermates. Consistent with this finding, a study by the same group demonstrated that upregulation of the main ROS-producing enzyme, NADPH-oxidase, in SNpc of human PD coincided with the local production of ROS, microglial activation, and DA neuronal loss seen after MPTP injections (Wu et al., 2003). Mutant mice defective in NADPH-oxidase exhibited less SNpc DA neuronal loss and protein oxidation than their WT littermates after MPTP injections, suggesting that extracellular ROS are critical in inflammation-mediated DA neurotoxicity in the MPTP mouse model of nigral cell loss. Clearly, the clinical implication of these studies is that inhibition of microglial-derived toxic mediator production, may be protective in the progressive degeneration of nigral DA neurons in PD. For that reason, many labs and several biotech companies are actively pursuing identification of small molecules to target microglia activation as a therapeutic approach in neurodegenerative disease. Given the importance of microglial population for immune surveillance, neurotrophic factor production, and clearance of debris (perhaps including toxic oligomeric protein species), a global inhibition of microglia activation is likely to result in more collateral damage than therapeutic benefit.
Animal and epidemiologic studies suggested that non-steroidal anti-inflammatory drugs (NSAIDs) have neuroprotective properties by reducing general inflammation which suggest inflammatory mechanisms contribute to neurodegeneration. NSAIDs scavenge free oxygen radicals and inhibit prostaglandin production by cyclo-oxygenases 1 and 2 (COX1 and 2). The inducible form COX-2 in particular has been implicated in the pathogenesis of PD because it is upregulated in the nigral neurons of PD patients and in neurotoxin-induced models of nigral cell death and can participate in oxidation of dopamine (Teismann et al., 2003b; Teismann et al., 2003a; Tyurina et al., 2006; Chae et al., 2008). In pre-clinical models of nigral cell death, inhibition of cyclooxygenase (COX-2), decreased microglial activation and prevented the progressive degeneration in a retrograde lesion induced by 6-OHDA) (Sanchez-Pernaute et al., 2004). In that rat study, a selective inhibitor of the inducible form of cyclooxygenase (COX-2), celecoxib, was used to examine the protective effect on 6-OHDA-induced DA cell loss. COX-2 inhibition did not reduce the typical astroglial response in the striatum at any stage. Therefore, inhibition of COX-2 by celecoxib appears to be able to prevent or slow down DA cell degeneration (Sanchez-Pernaute et al., 2004). Ghosh et al. reported that activation of NF-κB is induced in vivo in the SNpc of MPTP-intoxicated mice and within the substantia nigra pars compacta of PD patients. A cell-permeable peptide corresponding to the NF-κB essential modifier (NEMO)-binding domain (NBD) of IκB kinase α(IKKα) or IKKβ was capable of inhibiting the induction of NF-κB activation and suppressing nigral microglial activation, which protected both the nigrostriatal axis and neurotransmitters, and improved motor functions in MPTP-intoxicated mice (Ghosh et al., 2007). Their study suggested that selective inhibition of NF-κB activation by NBD peptide may be of therapeutic benefit for PD patients.
Animal studies demonstrating anti-inflammatory drugs protected against progressive nigral DA neuron loss encouraged investigation of the association between NSAID use and PD risk in humans. For a detailed review of evidence relating to the protective effects of anti-inflammatory drugs on DA neurons in animal models of PD as well as epidemiological data exploring the effectiveness of NSAIDs in the prevention of PD see (Esposito et al., 2007) In brief, the most convincing and compelling evidence supporting the claim that inflammatory mechanisms are likely to contribute to PD risk comes from epidemiological studies (McGeer and McGeer, 1998a; Chen et al., 2003, 2005). Inverse association of NSAID use with risk of PD has been observed in 2 prospective studies for nonaspirin NSAIDs, as well as for aspirin (Abbott et al., 2003; Chen et al., 2003; Chen et al., 2005). Specifically, a large prospective study of hospital workers indicated that the incidence of idiopathic Parkinson’s disease (PD) in chronic users of over-the-counter NSAIDs which scavenge free oxygen radicals and inhibit COX activity was 46% lower than that of age-matched non-users (Chen et al., 2003). Similar findings were reported for chronic users of the non-selective COX inhibitor ibuprofen in a follow-up study involving a large (~180,000) cohort of U.S. men and women (Chen et al., 2005). However, other studies showed that the effect of NSAIDs in decreasing the risk of PD is limited or of no benefit (Hernan et al., 2006; Hancock et al., 2007). Hernan et al. reported that this inverse association was observed only for men but not for women, in whom non-aspirin NSAID use was associated with a higher risk of PD. Importantly, a recently published systematic review and meta-analysis of studies published between 1966 and 2008 does indicate that although NSAIDs as a class do not seem to modify risk of PD, ibuprofen may have a slight protective effect (Samii et al., 2009).
In summary, it is clear that multiple pre-clinical and epidemiological studies strongly suggest that levated levels of inflammatory mediators in the early stages of PD may contribute to the progressive loss of nigral DA neurons. Therefore, it follows that timely intervention with anti-inflammatory agents may likely exert some degree of neuroprotection. In fact, even if the greatest therapeutic benefit is expected from interventions that target the initial trigger(s) when they are administered during the prodromal phase or earlier, therapies that prevent neuronal death and dystrophy by targeting steps in the cascade downstream of the disease trigger may also provide disease modification in patients with mild-to-moderate clinical symptoms (Golde, 2009). However, most PD therapies are symptomatic or aimed at cell replacement (transplantation to replace the lost nigral DA neurons) and anti-inflammatory regimens have only been explored in patients with late-stage disease with understandably disappointing results. This, therefore, is the biggest challenge that investigators aimed at translating mechanism-based findings into therapies face today: the need to test the neuroprotective effects of drugs in a clinical setting where it may be too late (Golde, 2009). Nevertheless, more emphasis and priority needs to be given to identification of inflammatory mediators that drive dysfunction of the nigrostriatal pathway and promote death of nigral DA neurons. These studies should then be followed by aggressive target validation in pre-clinical models of PD with a clear path to clinical trials in humans perhaps involving patients with inherited mutations who are at higher risk for development of PD. Given that neuroinflammation is common to many neurodegenerative diseases, it is likely that targeting neuroinflammatory mediators that drive cell apoptotic death pathways (i.e. TNF) may reduce multiple diseases (Wyss-Coray, 2006; McGeer and McGeer, 2007; Saijo et al., 2009)
Summary and Conclusions
During the last two decades, a wealth of animal and human studies has convincingly implicated inflammation-derived oxidative stress and cytokine-dependent neurotoxicity in the progressive degeneration of the nigrostriatal pathway, the hallmark of idiopathic PD. Post-mortem analyses of brains from PD patients gave initial clues regarding the presence of inflammatory processes including activated microglia and accumulation of cytokines such as TNF, interleukin-1, and interferon gamma (IFNγ) in the substantia nigra but such end-point analyses made it impossible to establish how early the inflammatory process began and whether it played a protective or detrimental role in disease progression. In the last 5 years, PET imaging studies in live patients and age-matched healthy controls clearly demonstrate that PD patients have greater microglial burden not only in the basal ganglia and regions affected in early-to-mid-stages of PD but also in cortex and other brain regions which show later involvement. Therefore, the working model is that chronically elevated levels of cytokines serve to maintain activation of abundant numbers of microglia in the midbrain, potentiating prolonged central inflammatory responses which may increase the permeability of the BBB and increase peripheral immune cell traffic—all of which creates an environment of oxidative stress to further accelerate oxidative damage to DA neurons. Consistent with a role of inflammatory mechanisms in contributing to disease development and/or progression, chronic ibuprofen use does appear to confer a slight decrease in disease incidence. Therefore, tangible therapeutic benefit and disease-modifying effects in PD may be derived by timely delivery of therapies that prevent neuronal death and dystrophy by targeting inflammatory mediators that activate proximal cell-death pathways, molecular players that increase the sensitivity of neurons to injury induced by neuroinflammatory mediators, or pathways that regulate activation states of microglia.
Abbreviations
- CNS
central nervous system
- RNS/ROS
reactive nitrogen species/reactive oxygen species
- 6-OHDA
6-hydroxydopamine
- TNF
tumor necrosis factor
- IL-1β
interleukin-1beta
- IL-6
interleukin-6
- IFNγ
interferon gamma
- TGF-β
Transforming Growth Factor beta
- BBB
blood-brain barrier
- SNpc
substantia nigra pars compacta
- LPS
lipopolysaccharide
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
- DA
dopaminergic
- VTA
ventral tegmental area
- NO
nitric oxide
- LBs
Lewy bodies
- PET
Positron Emission Tomography
- alpha-MPT
alpha-methyl-p-tyrosine
- RGS10
Regulator of G-protein Signaling-10
- COX1
cyclo-oxygenase 1
- COX2
cyclo-oxygenase 2
- NSAIDs
nonsteroidal anti-inflammatory drugs
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- iNOS
inducible nitric oxide synthase
- NF-κB
nuclear factor kappa beta
- NBD
NF-κB essential modifier (NEMO)-binding domain
- IKKα
IκB Kinase α subunit
- IKKβ
IκB Kinase β subunit
References Cited
- Aarnisalo P, Kim CH, Lee JW, Perlmann T. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J Biol Chem. 2002;277:35118–35123. doi: 10.1074/jbc.M201707200. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Ross GW, White LR, Sanderson WT, Burchfiel CM, Kashon M, Sharp DS, Masaki KH, Curb JD, Petrovitch H. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: recent findings from the Honolulu-Asia Aging Study. J Neurol. 2003;250(Suppl 3):III30–39. doi: 10.1007/s00415-003-1306-7. [DOI] [PubMed] [Google Scholar]
- Astradsson A, Jenkins BG, Choi JK, Hallett PJ, Levesque MA, McDowell JS, Brownell AL, Spealman RD, Isacson O. The blood-brain barrier is intact after levodopa-induced dyskinesias in parkinsonian primates--evidence from in vivo neuroimaging studies. Neurobiol Dis. 2009;35:348–351. doi: 10.1016/j.nbd.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, van Oostrom JC, Portman A, Leenders KL. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm. 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson’s disease. Front Biosci. 2003;8:s826–837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci. 2005;22:1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. J Neurochem. 2002;81:150–157. doi: 10.1046/j.1471-4159.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- Chae SW, Kang BY, Hwang O, Choi HJ. Cyclooxygenase-2 is involved in oxidative damage and alpha-synuclein accumulation in dopaminergic cells. Neurosci Lett. 2008;436:205–209. doi: 10.1016/j.neulet.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk of Parkinson’s disease. Annals of Neurology. 2005;59:988–989. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of Microglial Activation in the Innate Immune Response in the Brain. J Neuroimmune Pharmacol. 2009 doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablos RM, Herrera AJ, Villaran RF, Cano J, Machado A. Dopamine-dependent neurotoxicity of lipopolysaccharide in substantia nigra. Faseb J. 2005;19:407–409. doi: 10.1096/fj.04-2153fje. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. The blood-central nervous system barriers actively control immune cell entry into the central nervous system. Curr Pharm Des. 2008;14:1555–1565. doi: 10.2174/138161208784705432. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Golde TE. The therapeutic importance of understanding mechanisms of neuronal cell death in neurodegenerative disease. Mol Neurodegener. 2009;4:8. doi: 10.1186/1750-1326-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. Smoking, caffeine, and nonsteroidal anti-inflammatory drugs in families with Parkinson disease. Arch Neurol. 2007;64:576–580. doi: 10.1001/archneur.64.4.576. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Logroscino G, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology. 2006;66:1097–1099. doi: 10.1212/01.wnl.0000204446.82823.28. [DOI] [PubMed] [Google Scholar]
- Ito S, Sawada M, Haneda M, Ishida Y, Isobe K. Amyloid-beta peptides induce several chemokine mRNA expressions in the primary microglia and Ra2 cell line via the PI3K/Akt and/or ERK pathway. Neurosci Res. 2006;56:294–299. doi: 10.1016/j.neures.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, Smeyne RJ. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009;106:14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47:S161–170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. alpha-Synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2006;20:2000–2008. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1 beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation. 2008;5:8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33:85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- Lee JK, McCoy MK, Harms AS, Ruhn KA, Gold SJ, Tansey MG. Regulator of G-protein signaling 10 promotes dopaminergic neuron survival via regulation of the microglial inflammatory response. J Neurosci. 2008;28:8517–8528. doi: 10.1523/JNEUROSCI.1806-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson B, Wu X, Qian L, Granholm AC, Crews FT, Hong JS. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29:864–870. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Brain Res Rev. 2005;48:251–256. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Hattori N, Kitada T, Matsumine H, Mori H, Shimura H, Kubo S, Kobayashi H, Asakawa S, Minoshima S, Shimizu N. Familial Parkinson’s disease. Alpha-synuclein and parkin. Adv Neurol. 2001;86:13–21. [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annual Reviews in Neuroscience. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s Disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Cellular and molecular mechanisms of Parkinson’s disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol. 2006;26:781–802. doi: 10.1007/s10571-006-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. Regulating factors for microglial activation. Biol Pharm Bull. 2002;25:945–953. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- Olanow CW. The pathogenesis of cell death in Parkinson’s disease - 2007. Mov Disord. 2007;22:S335–S342. doi: 10.1002/mds.21675. [DOI] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson’s disease. Prog Neurobiol. 2002;68:325–340. doi: 10.1016/s0301-0082(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Owen AD, Schapira AH, Jenner P, Marsden CD. Oxidative stress and Parkinson’s disease. Ann N Y Acad Sci. 1996;786:217–223. doi: 10.1111/j.1749-6632.1996.tb39064.x. [DOI] [PubMed] [Google Scholar]
- Owen AD, Schapira AH, Jenner P, Marsden CD. Indices of oxidative stress in Parkinson’s disease, Alzheimer’s disease and dementia with Lewy bodies. J Neural Transm Suppl. 1997;51:167–173. doi: 10.1007/978-3-7091-6846-2_14. [DOI] [PubMed] [Google Scholar]
- Puntambekar SS, Doose JM, Carson MJ. Microglia: A CNS-specific tissue macrophage. In: Lane TEC M, Bergmann C, Wyss-Coray T, editors. Central Nervous System Diseases and Inflammation. First Edition Springer; New York: 2008. pp. 1–12. [Google Scholar]
- Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25:392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol. 2009;182:4137–4149. doi: 10.4049/jimmunol.0803982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Gate D, Town T. CNS Infiltration of Peripheral Immune Cells: D-Day for Neurodegenerative Disease? J Neuroimmune Pharmacol. 2009 doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A, Etminan M, Wiens MO, Jafari S. NSAID Use and the Risk of Parkinson’s Disease: Systematic Review and Meta-Analysis of Observational Studies. Drugs Aging. 2009;26:769–779. doi: 10.2165/11316780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson’s disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm. 2006;(Suppl):373–381. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Riminton DS, Cyster JG, Korner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–113. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Tanner CM. Is the cause of Parkinson’s disease environmental or hereditary? Evidence from twin studies. Adv Neurol. 2003;91:133–142. [PubMed] [Google Scholar]
- Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MGaW-C T. Cytokines in CNS Inflammation and Disease. In: Lane TEC M, Bergmann C, Wyss-Coray T, editors. Central Nervous System Diseases and Inflammation. First Edition Springer; New York: 2008. pp. 59–106. [Google Scholar]
- Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S. COX-2 and neurodegeneration in Parkinson’s disease. Ann N Y Acad Sci. 2003a;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci U S A. 2003b;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Kapralov AA, Jiang J, Borisenko GG, Potapovich AI, Sorokin A, Kochanek PM, Graham SH, Schor NF, Kagan VE. Oxidation and cytotoxicity of 6-OHDA are mediated by reactive intermediates of COX-2 overexpressed in PC12 cells. Brain Res. 2006;1093:71–82. doi: 10.1016/j.brainres.2005.10.105. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hedreen JC, Price DL. Parkinson’s disease: loss of neurons from the ventral tegmental area contralateral to therapeutic surgical lesions. Neurology. 1985;35:1215–1218. doi: 10.1212/wnl.35.8.1215. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, Perlmann T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423:555–560. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. An inflammatory pathomechanism for Parkinson’s disease? Curr Med Chem. 2006;13:591–602. doi: 10.2174/092986706776055760. [DOI] [PubMed] [Google Scholar]
- Westin JE, Lindgren HS, Gardi J, Nyengaard JR, Brundin P, Mohapel P, Cenci MA. Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia. J Neurosci. 2006;26:9448–9461. doi: 10.1523/JNEUROSCI.0944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dawson VL, Dawson TM. Role of nitric oxide in Parkinson’s disease. Pharmacol Ther. 2006;109:33–41. doi: 10.1016/j.pharmthera.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. Faseb J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]