Abstract
Emerging evidence suggests sex and apolipoprotein E (APOE) genotype separately modify outcomes after intracerebral hemorrhage (ICH). We test the hypothesis that an interaction exists between sex and APOE polymorphism in modifying outcomes after ICH and is altered by administration of exogenous apoE-mimetic peptide. To define the effects of sex and APOE polymorphism in ICH, we created collagenase-induced ICH in male and female APOETR mice (targeted replacement mice homozygous for APOE3 or APOE4 alleles; n=12/group) and assessed performance on Rotarod (RR) and Morris water maze (MWM). To evaluate hematoma formation, we used hematoxylin and eosin staining at 24 h after injury (n=8/group). Using separate cohorts (n=12/group), apoE-mimetic peptide (COG1410 at 2 mg/kg) was administered after ICH, and mice were assessed by RR and MWM. Female mice outperformed male mice via RR and MWM by over 190% improvement through 7 days (RR) and 32 days (MWM) of testing after ICH (p<0.01). Female APOE3TR mice demonstrated improved function compared with all other groups (p<0.05) without any difference in hematoma volume at 24 h after injury in any group. Administration of a therapeutic apoE-mimetic peptide improved RR latencies through 7 days after ICH in male and female APOE4TR mice and MWM latencies over days 28–32 after ICH in male APOE4TR mice (p<0.05). Sex and APOE polymorphism influence functional outcomes in our murine model of ICH. Moreover, administration of exogenous apoE-mimetic peptide after injury differentially modifies the interaction between sex and APOE polymorphism.
Keywords: Apolipoprotein E, Sex differences, Murine, Intracerebral hemorrhage, Female
Introduction
Primary intracerebral hemorrhage (ICH), or hemorrhagic stroke, is a devastating and relatively common disease afflicting as many as 50,000 people annually in the USA alone, and accounting for up to 20% of all strokes in this country. Despite improvement in other areas of acute CNS injury, ICH remains associated with poor outcome, and remains without proven therapy. Approximately 40 to 50% of afflicted patients will die within 30 days, and little improvement has been made in the mortality rate or associated morbidity over the last 20 years [1]. One reason for the paucity of validated therapeutic interventions is the lack of fundamental knowledge regarding the pathophysiologic mechanisms that initiate hematoma formation and the resulting cascade of secondary inflammation. Furthermore, emerging evidence suggests sex-specific outcomes after acute CNS injury. It is increasingly recognized that sex may affect incidence [2], clinical outcome [3, 4], and quality of life after ICH in humans [5]. Furthermore, these clinical observations are supported by preclinical models [6–8].
It is reasonable to predict that genetic variations may be partially responsible for this functional effect after injury beyond that provided by sex steroids. For example, presence of an APOE4 allele is associated with poor prognosis in a variety of acute and chronic neurological diseases, including ICH [9], and the presence of an APOE4 allele is an independent risk factor for developing ICH in humans [10, 11]. Furthermore, there is increasing evidence supporting its role in downregulating endogenous inflammatory responses in an isoform-specific fashion, which is consistent with its known immunomodulatory properties [12]. This isoform-specific effect of apoE on neuroinflammation and acute injury responses may be particularly relevant in modifying outcomes after ICH [13], and clinical studies have implicated the presence of an APOE4 allele with poor outcome in this setting [9, 14–16].
The purpose of this study was to begin to explore the interaction between APOE genetic background and sex in a preclinical model of ICH. As promising therapeutics may have sex-specific efficacy, our further aim was to determine if COG1410 [17], an apoE-mimetic peptide, improved recovery and function after injury as a function of sex and APOE genotype.
Methods
Experimental Groups
All animal procedures were designed to minimize animal discomfort and numbers, conformed to international guidelines on the use of animals, and were approved by the Duke University Institutional Animal Care and Use Committee.
Group 1
To assess the interaction of APOE genotype and sex, we induced ICH in randomized cohorts of 10- to 12-week-old APOE3TR and APOE4TR male and female mice (n=12/ group). All female mice were injured in the estrus stage (peak estrogen) of their estrous cycle. Mice then performed Rotarod (RR) testing over days 1–7 after injury and Morris water maze (MWM) testing over days 28–32 after injury.
Group 2
To determine the influence of hemorrhage volume, we compared volumetric measurements of lesion volume at 24 h after ICH in 10- to 12-week-old APOE3TR and APOE4TR male and female mice (n=6/group).
Group 3
To assess the potential of an exogenous apoE-mimetic peptide to differentially influence sex-specific recovery, we randomized 10- to 12-week-old APOE3TR and APOE4TR male and female mice (n=12/group) to receive doses of COG1410 or vehicle. All female mice were injured in the estrus stage (peak estrogen) of their estrous cycle. After induction of ICH, these mice were intravenously (IV) injected via the tail vein with 2 mg/kg of COG1410 in 100 µl NS or 100 µl of sterile NS vehicle at 1 h after injury and then daily for 7 days in a blinded fashion. This dose and treatment regimen was determined from pharmacokinetic data [17] and from prior experiments in ICH [18]. Mice then performed RR testing over days 1–7 after injury and MWM testing over days 28–32 after injury.
APOETR Animals
APOETR mice were created by gene targeting of a human APOE3 or APOE4 genomic construct into E14TG2a embryonic stem cells derived from 129P2/OlaHsd mice [19]. The targeted embryonic stem cells were injected into C57BL6/J blastocytes, and the resulting chimeras were bred to wild-type C57BL6/J mice and backcrossed to C57BL6/J mice for eight generations. The colony was maintained by homozygous matings, and genotypes were confirmed prior to each experiment. Blinding to genotype was maintained throughout the experiments.
Preparation and Administration of COG1410
Peptides were synthesized by NeoMPS (San Diego, CA) to a purity of 95%. COG1410 is acetyl-AS-Aib-LRKLAib-KRLL-amide, which is derived from apoE residues 138–149 with Aib (amino isobutyric acid) substitutions at positions 140 and 145 [17, 20, 21]. For all in vivo experiments, COG1410 was dissolved in sterile normal saline (NS) immediately prior to use, and blinding to treatment group was maintained throughout the experiments.
Intracerebral Hemorrhage Model
Our murine injury model [22] was adapted from a previously described model of ICH in rats [23]. Briefly, after anesthesia induction with 4.6% isoflurane, mice underwent tracheal intubated and mechanical ventilated with 1.6% isoflurane in 30% O2/70% N2. Rectal temperature was maintained at 37±0.5°C by underbody warming system. The animal’s head was secured in a stereotactic frame, local anesthetic injected, and the scalp incised. After exposure of the skull, a burr hole was created 2 mm left lateral to bregma, and a 0.5-µl syringe needle (Hamilton, Reno, NV, USA) was advanced to a depth of 3 mm from cortex. Type IV-S Clostridial collagenase (Sigma, St. Louis, MO, USA) was injected into the left basal ganglia over 5 min (0.1 U in 0.4 µl NS) with the needle being held motionless for an additional 5 min. After slowly withdrawing the needle, the incision was closed, and animals were allowed to recover spontaneous ventilation with subsequent extubation. Following recovery in a warm non-stimulating environment, mice were allowed free access to food and water.
Assessment of Estrous Cycle
Based on described protocol [24], female mice were restrained in a polycarbonate mouse restrainer. The tip of plastic pipette, filled with normal saline (~10 µl), was placed into the vagina. The vagina was flushed gently five times with same saline solution. The final flush in pipette tip was collected. A volume of 10 µl of saline solution allows collecting sufficient material for observation of vaginal cytology. Final flush containing vaginal fluid was placed on a glass slide. Unstained material was observed under light microscope with a ×10 objective. Determination of the estrous cycle phase was based on the proportion among the three cell types (leukocytes, cornified epithelial cells, and nucleated epithelial cells) present.
Rotarod Testing
An automated RR (Ugo Basile, Comerio, Italy) was used to assess vestibulomotor function [25]. On the day prior to hemorrhage induction, mice underwent two consecutive conditioning trials at a set rotational speed (16 revolutions/ min) for 60 s followed by three additional trials with an accelerating rotational speed. The average time to fall from the rotating cylinder in the latter three trials was recorded as baseline latency. After injury, mice underwent consecutive daily testing with three trials of accelerating rotational speed (inter-trial interval of 15 min) on days 1, 3, 5, and 7. The average latency to fall from the rod was recorded. Mice unable to grasp the rotating rod were given a latency value of 0 s. RR testing was performed by an examiner blinded to genotype, sex, and treatment assignment.
Morris Water Maze Testing
The MWM [26] was used to assess the effects on spatial learning and memory. Prior to injury, mice were trained on the visible platform (1 day; platform flagged, located in a different quadrant for each trial to minimize quadrant habituation, and no extra-maze visual cues) and hidden platform (4 days; platform submerged in western quadrant for all trials with several extra-maze visual cues) versions of the MWM task to habituate the mice to handling and to swimming, as well as to teach them the goal of the task, which was to escape from the water by climbing onto a platform. After injury, performance was evaluated in a black aluminum pool (105 cm in diameter and 60 cm in depth) filled with water opacified with powdered milk containing a platform (7.5 cm in diameter) submerged 1 cm below the water surface (25–27°C). The maze was kept in a room dedicated to behavioral testing with light and sound maintained consistently throughout training and testing. Such habituation and pre-training has been shown to decrease stress, which negatively impacts the performance of mice on the MWM task [27]. Pre- and post-injury testing followed the same protocol. Each testing day consisted of four trials per day with an inter-trial interval of 20–30 min. For each trial, mice were placed into the pool facing the perimeter and were allowed to search for the platform for a maximum of 90 s. If they were unable to locate the platform within the allotted time, they were guided to it and remained on the platform for 10 s before being returned to their heated home cage. Mice were started in one of four different quadrants for each trial with starting quadrants randomly defined each day. Latency to find the platform and swimming speed were recorded by a computerized video tracking system (KeilSoft LLC, Chapel Hill, NC). On the final day of hidden platform testing, a probe trial was conducted to evaluate retention capabilities. The escape platform was removed from the pool and the mouse was released into the maze at a point diagonally opposite from the previous location of the platform (i.e., the eastern quadrant). The time spent searching all four and the number of crossings into the western quadrant were recorded. Post-injury MWM visible and hidden platform testing were conducted 4 weeks after injury with a probe trial following the last trial. Water maze testing was performed by an examiner blinded to genotype, sex, and treatment assignment.
Hemorrhage Volume
Mice were euthanized, and brains were removed and frozen at −20°C. Coronal sections of 20 µm thickness were taken at 320 µm intervals over the rostral-caudal extent of the lesion. Sections were stained with hematoxylin and eosin (H&E), and lesion volume measured by digitally sampling stained sections with an image analyzer (M2 Turnkey System, Imaging Research, Inc., St. Catharines, Ontario, Canada). Lesion volumes (in millimeters [3]) were computed as running sums of lesion area multiplied by the known interval (i.e., 320 µm) between sections over the extent of the lesion expressed as an orthogonal projection.
Statistical Analysis
Between group hemorrhage volume, RR latencies, and MWM latencies were compared with two-way repeated-measures analysis of variance (ANOVA) with day as the repeated variable. The F values were calculated, and if the probability distribution of F with the appropriate degrees of freedom suggested a significant group effect, pairwise testing was performed between groups using Scheffe’s post hoc method to correct for multiple comparisons. Student’s t test was used to compare two group comparisons where appropriate. Statistical significance was assumed with p<0.05. All values were expressed as mean±standard deviation and were performed on JMP (v7.0.1, SAS, Cary, NC).
Results
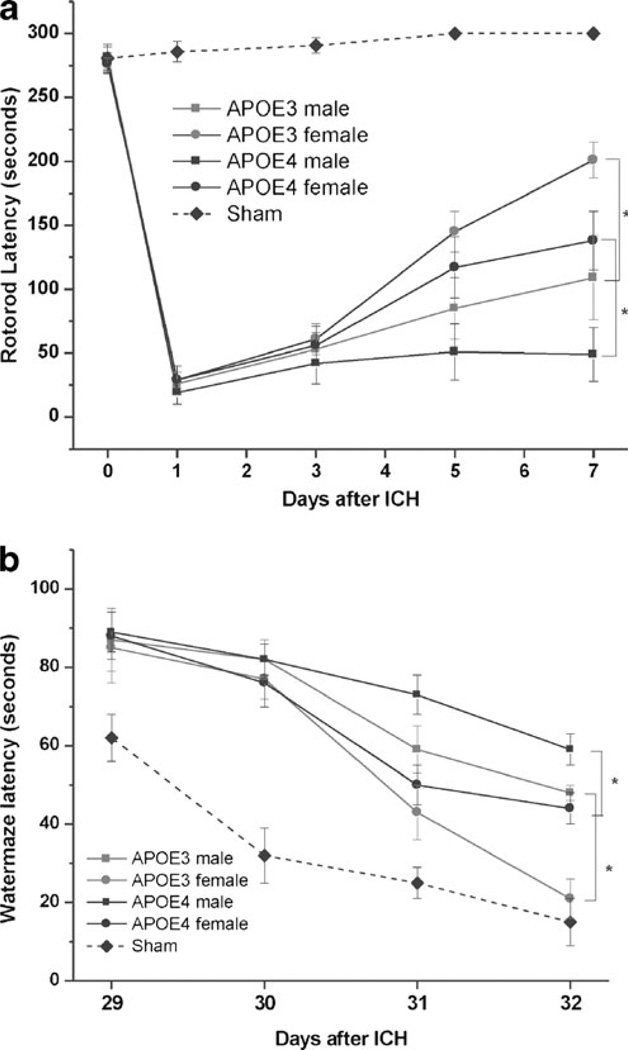
To evaluate the effect of sex and APOE genotype on the rate of recovery after ICH in mice, we performed RR testing over the first 7 days after injury with ICH. No injured animals were excluded from functional testing. However, 1 APOE3TR female mouse, one APOE3TR male mice, two APOE4TR female mice, and four APOE4TR male mice died within 24 h after ICH; no mice died after this timepoint. When grouped by sex alone, female mice demonstrated longer RR latencies over the 7 days of testing when compared with their male counterparts (day 7, 124.3±6.1 vs. 65 ±5.2 s; t test, p<0.01). When stratified by APOE genotype, female APOETR animals demonstrated faster rates of recovery when compared with the male genotype counterpart (ANOVA, p<0.01; Fig. 1a). APOE3TR female mice demonstrated longer RR latencies over the testing period compared with all other groups (ANOVA, p<0.05). Finally, female APOE4TR and male APOE3TR mice having similar recovery patterns, which was significantly improved when compared with APOE4TR male mice.
Fig. 1.
RR (a) and MWM (b) latencies in male and female APOETR mice after injury with ICH. Female APOE3TR animals improve to a greater degree than other groups of animals with male APOE4TR animals demonstrating the poorest short- and long-term neurobehavio-ral outcomes. Reference sham animals are denoted by the dotted line
To determine if these patterns of vestibulomotor recovery were associated with long-term neurocognitive outcome measures, we examined the performance of the same cohorts of mice in the MWM at 4 weeks after injury with ICH. Similar to our findings with RR testing, female mice outperformed their male counterparts at day 32 after ICH (27.2±7.1 vs. 53.7± 4.2 s; t test, p<0.05) despite similar swimming speeds. Furthermore, APOE3TR female mice outperformed all other groups, and females outperformed their male counterparts for each transgenic type (ANOVA, p<0.05; Fig. 1b). Finally, this improved functional recovery was not associated with a difference in hematoma size between the four groups as measured by H&E at 24 h after injury with ICH (APOE3TR males vs. APOE3TR females vs. APOE4TR males vs. APOE4TR females, 14.36±9.2 vs. 15.12±8.7 vs. 13.81±8.9 vs. 14.53± 8.6 mm [3]; ANOVA, p>0.10)
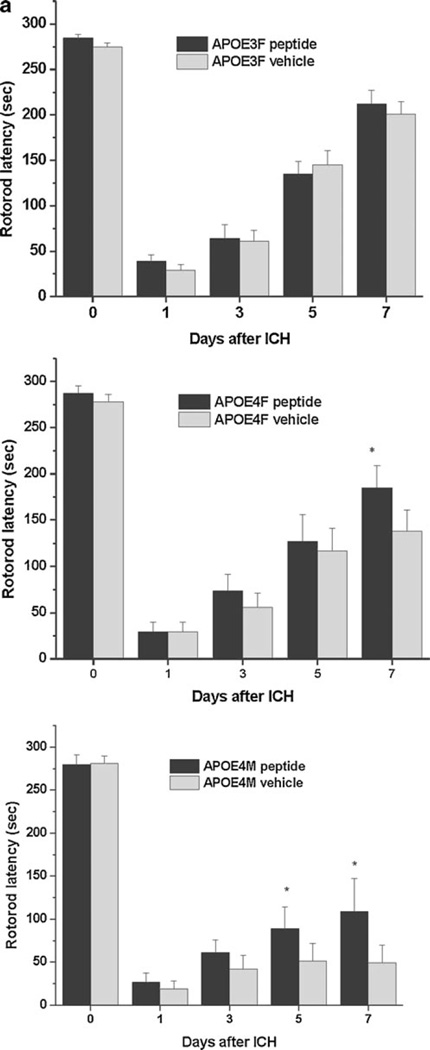
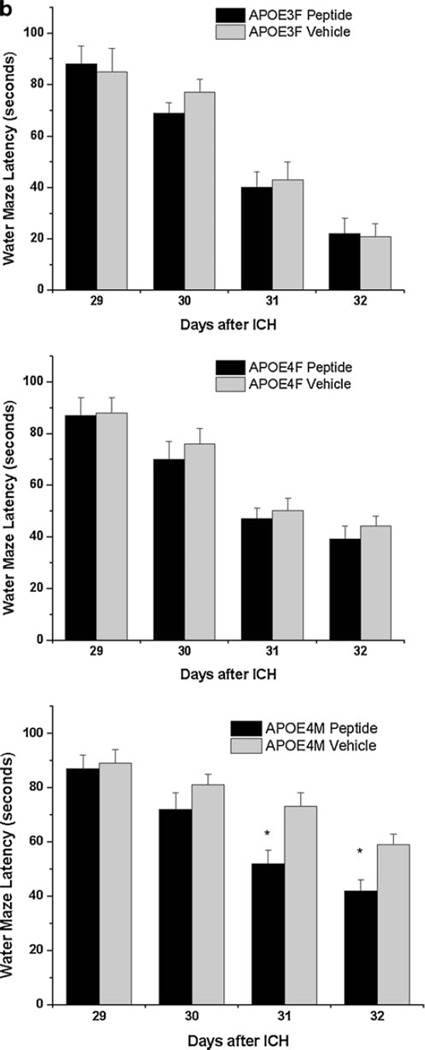
To evaluate the effect of the apoE-mimetic peptide, COG1410, within this sex-based paradigm, we assessed female APOE3TR and APOE4TR and male APOE4TR mice given either vehicle or COG1410 (2 mg/kg) IV initiated at 1 h after injury and then daily for 7 days. The timing of initial administration and dose of 2 mg/kg of COG1410 was chosen based on prior experiments [18]. No injured animals were excluded from functional testing. However, 1 APOE4TR female mice, 1 COG1410-treated APOE4TR female mice, 5 APOE4TR male mice, and 2 COG1410-treated APOE4TR male mice died within 24 h after ICH; no mice died after this time point. Although APOE3TR females did not demonstrate any improvement after administration of COG1410 when compared with their vehicle-treated counterparts, APOE4TR males and females both showed increased latencies when given COG1410 (Fig. 2a) compared with their vehicle-treated counterparts. This improvement was durable over the 7 days of testing in the APOE4TR males (ANOVA, p<0.05) but evident only on day 7 (final) of testing in APOE4TR female mice (38.7± 5.4 vs. 44.0±4.1 s; t test; p<0.05). However, APOE4TR females given COG1410 began to perform similarly to APOE3TR females by post-injury day 7 (201.21±13.91 vs. 184.67±24.01 s; t test, p>0.10). Similarly, APOE4TR males given COG1410 began to demonstrate statistically equivalent RR latencies to their vehicle-treated, APOE4TR female counterparts (137.83±23.24 vs. 108.96±38.52 s; t test, p>0.10) by post-injury day 7.
Fig. 2.
RR (a) and MWM (b) latencies in male and female APOETR mice after injury with ICH. Treatment with COG1410 improved the RR and MWM performance in female and male APOE4TR animals
In order to assess the effects of COG1410 on long-term cognitive function after ICH as a function of sex and APOE genotype, we next evaluated the APOETR males and females from the same cohort of animals used for RR testing above in the MWM at days 28–30 after injury. In this experiment, we demonstrated that, although APOE3TR females did not demonstrate any improvement after administration of COG1410 when compared with their vehicle-treated counterparts, they outperformed all other groups (Fig. 2b; ANOVA, p<0.05). There was no statistically significant difference between COG1410-treated female APOE4TR animals, their vehicle-treated counterparts, and the COG1410-treated male APOE4TR animals. However, these three groups outperformed vehicle-treated APOE4TR male mice over the 4 days of testing (ANOVA, p<0.05).
Discussion
Based on the results of this study, the effects of sex on recovery after ICH are at least partially accounted for by APOE genotype. Further in the current study, we confirm prior observations that presence of the human APOE4 allele is associated with poor outcome and that recovery can be influenced by the administration of COG1410, an exogenous apoE-mimetic peptide, in our murine model of ICH [13, 18].
These findings are consistent with prior data demonstrating a palliative effect of COG1410 in preclinical models of ICH, and a pharmacogenomic interaction between COG1410 and APOE genotype [13]. It appears that humanized APOE3/3 genotype confers some level of neuroprotection and results in improved recovery after ICH in mice, as it does in the human condition [9]. Furthermore, it has been previously observed that COG1410 crosses the blood–brain barrier in normal and injured brain [17], and use of COG1410 results in improved function after injury in an isoform-specific manner. The selection of the APOETR genotypes used in these experiments was based on these findings that male APOE3TR mice did not demonstrate improved recovery after administration of COG1410 in our model [13]. Also, the current findings build on our previous work by demonstrating an effect of APOE genotype and mimetic peptide on long-term neurocognitive outcome in this disease entity. There is substantial evidence that APOE genotype and COG1410 also modify outcomes in a number of preclinical models of acute brain injury [28, 29].
Although the mechanism(s) by which sex influences outcomes following ICH remains incompletely defined, it may be possible to gain some insight by extrapolating what is known about sex and ischemic stroke. Sex differences in stroke risk and outcome have been observed in humans and a variety of experimental models, although sex steroids only partially account for these differences [30]. Recently sex-specific mechanisms of ischemia and apoptosis in the brain have been found [31, 32, 33, 34]. Understanding sex-specific mechanisms of injury after cerebral ischemia may be useful when designing similar studies for ICH. Although preclinical models have provided mechanistic insight in other areas of acute CNS injury, the examination of sex effect in models of ICH is currently limited. Nakamura et al. [8] found that female mice demonstrate more rapid and durable recovery after injury as compared with their male counterparts. Addition of estradiol to male mice improved their outcomes but not to the level of females, suggesting that sex hormones are not the sole reason for the observed differences in recovery.
Our current findings reinforce the notion that a sex influences outcomes after ICH. The bulk of published data recognizes that that male sex predisposes individuals to developing ICH [2]. Furthermore, female sex may offer mechanisms for improved recovery after injury. Interestingly, sex may influence the impact of select clinical variables on outcome (e.g., a rapid decline in mean arterial pressure after ICH is associated with mortality in men but not women) [35]. Diringer et al. [3] found female sex to be an independent predictor of in-hospital survival following supratentorial ICH. A more recent study corroborated these findings by determining that male sex was an independent predictor of 30-day and 3-year mortality after all subtypes of primary ICH [4]. In a northern European population of patients with ICH, 1- and 5-year mortality risks (HR 0.92; 95% CI, 0.86 to 0.99) were lower for women compared with men after adjustment for age and previous admissions for cardiovascular diseases or diabetes mellitus [36]. Furthermore, quality of life after injury appears to be improved in females when compared with their male counterparts [5]. Recently a large meta-analysis [37] found five studies that provided data on 1-month case fatality in men and women separately [38–42]. In Melbourne, Australia case fatality was higher in women than in men [42], whereas the other regions reported similar case fatalities for men and women. Most interesting was the apparent lack of data from a US population with the most recent publication reflecting data collected in the mid-1990s that did not specifically address sex differences [43, 44].
Interestingly, our results suggest an interaction between sex and APOE polymorphism. This is consistent with recent studies demonstrating that the effects of APOE polymorphisms may be sex-specific. For example, a recent study has demonstrated that the presence of an APOE4 allele is associated with enhanced macrophage inflammatory responses in male, but not female mice [45]. Furthermore, two recent clinical reports demonstrate that presence of the APOE4 allele is associated with poor outcomes in men, but not women, following stroke [46] and traumatic brain injury [47]. Interestingly, administration of COG1410 did not appear to confer additional neuroprotection beyond that inherent in the female APOE3TR animals, but was most effective in APOE4TR males. A pharmacogenomic interaction between the therapeutic apoE-based peptides and background APOE genotype has been noted previously [12, 48, 49]; in the current model it would be interesting to speculate that the exogenous administration of the apoE-based therapeutic selectively overcame the neuroimmune dysregulation present in the APOE4TR animals.
There are several limitations about this study that should be addressed. First, although APOE polymorphism appears to modify the effect of sex on recovery after ICH, the exact mechanism remains unclear. Emerging evidence suggests that COG1410, related apoE-mimetic peptides, and the apoE holoprotein may exert their effect through binding to the SET protein, also known as inhibitor #2 of protein phosphatase 2A [50]. Further, prior work demonstrates the cellular effects of this protein based on the intact apoE holoprotein [51]. However, we again demonstrate the effectiveness of COG1410 in this model and indeed suggest the need to measure sex effects in humans to guide clinical trial design.
Conclusions
In summary, we find that male sex and APOE4/4 genotype are associated with poor outcomes in a murine model of ICH. Moreover, we find an interaction between these variables such that APOE4/4 genotype has the most detrimental effects in male animals. To address the clinical relevance of these findings, the possibility of APOE-sex interactions should be explored in clinical populations following ICH. We also confirm the therapeutic potential of COG1410, an apoE-based peptide, in our model of ICH.
Acknowledgments
This project was funded by grants from the American Heart Association Scientist Development Grant and Foundation for Anesthesia Education and Research Mentored Research Training Grant (MLJ). Dr. Laskowitz has previously served as a consultant for Cognosci, Inc. Dr. Vitek is Chief Executive Officer of Cognosci, Inc. COG1410 was provided by Cognosci, Inc.
Contributor Information
Beilei Lei, Multidisciplinary Neuroprotection Laboratories, Duke University, 3094, Durham, NC, USA; Department of Anesthesiology, Duke University, Durham, USA.
Brian Mace, Department of Medicine (Geriatrics), Duke University, Durham, USA; Geriatric Research, Education, and Clinical Center, Durham Veteran’s Affairs, Durham, NC, USA.
Steven T. Bellows, Department of Medicine (Neurology), Duke University, Durham, USA
Patrick M. Sullivan, Department of Medicine (Geriatrics), Duke University, Durham, USA Geriatric Research, Education, and Clinical Center, Durham Veteran’s Affairs, Durham, NC, USA.
Michael P. Vitek, Cognosci, Inc, Research Triangle Park, Durham, NC, USA
Daniel T. Laskowitz, Multidisciplinary Neuroprotection Laboratories, Duke University, 3094, Durham, NC, USA Department of Anesthesiology, Duke University, Durham, USA; Department of Medicine (Neurology), Duke University, Durham, USA; Department of Neurobiology, Duke University, Durham, NC, USA.
Michael L. James, Email: michael.james@duke.edu, Multidisciplinary Neuroprotection Laboratories, Duke University, 3094, Durham, NC, USA; Department of Anesthesiology, Duke University, Durham, USA; Department of Medicine (Neurology), Duke University, Durham, USA.
References
- 1.Broderick JP, Adams HP, Jr, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 2.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 3.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29:1352–1357. doi: 10.1161/01.str.29.7.1352. [DOI] [PubMed] [Google Scholar]
- 4.Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40:3567–3573. doi: 10.1161/STROKEAHA.109.556324. [DOI] [PubMed] [Google Scholar]
- 5.Cadilhac DA, Dewey HM, Vos T, Carter R, Thrift AG. The health loss from ischemic stroke and intracerebral hemorrhage: evidence from the North East Melbourne Stroke Incidence Study (NEMESIS) Health Qual Life Outcomes. 2010;8:49. doi: 10.1186/1477-7525-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attella MJ, Nattinville A, Stein DG. Hormonal state affects recovery from frontal cortex lesions in adult female rats. Behav Neural Biol. 1987;48:352–367. doi: 10.1016/s0163-1047(87)90918-6. [DOI] [PubMed] [Google Scholar]
- 7.Roof RL, Zhang Q, Glasier MM, Stein DG. Gender-specific impairment on Morris water maze task after entorhinal cortex lesion. Behav Brain Res. 1993;57:47–51. doi: 10.1016/0166-4328(93)90060-4. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24:487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 9.James ML, Blessing R, Bennett E, Laskowitz DT. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis. 2009;18:144–149. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo D, Kaushal R, Chakraborty R, et al. Association of apolipoprotein E4 and haplotypes of the apolipoprotein E gene with lobar intracerebral hemorrhage. Stroke. 2005;36:1874–1879. doi: 10.1161/01.STR.0000177891.15082.b9. [DOI] [PubMed] [Google Scholar]
- 11.Tzourio C, Arima H, Harrap S, et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology. 2008;70:1322–1328. doi: 10.1212/01.wnl.0000308819.43401.87. [DOI] [PubMed] [Google Scholar]
- 12.Laskowitz DT, Vitek MP. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–969. doi: 10.2217/14622416.8.8.959. [DOI] [PubMed] [Google Scholar]
- 13.James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT. Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke. 2009;40:632–639. doi: 10.1161/STROKEAHA.108.530402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberts MJ, Graffagnino C, McClenny C, et al. ApoE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346:575. doi: 10.1016/s0140-6736(95)91411-0. [DOI] [PubMed] [Google Scholar]
- 15.McCarron MO, Hoffmann KL, DeLong DM, Gray L, Saunders AM, Alberts MJ. Intracerebral hemorrhage outcome: apolipoprotein E genotype, hematoma, and edema volumes. Neurology. 1999;53:2176–2179. doi: 10.1212/wnl.53.9.2176. [DOI] [PubMed] [Google Scholar]
- 16.McCarron MO, Weir CJ, Muir KW, et al. Effect of apolipoprotein E genotype on in-hospital mortality following intracerebral haemorrhage. Acta Neurol Scand. 2003;107:106–109. doi: 10.1034/j.1600-0404.2003.01365.x. [DOI] [PubMed] [Google Scholar]
- 17.Laskowitz DT, McKenna SE, Song P, et al. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J Neurotrauma. 2007;24:1093–1107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- 18.Laskowitz DT, Lei B, Dawson HN, et al. The apoE-mimetic peptide, COG1410, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit Care. 2012;16:316–326. doi: 10.1007/s12028-011-9641-5. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan PM, Mezdour H, Aratani Y, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 20.Laskowitz DT, Thekdi AD, Thekdi SD, et al. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- 21.Laskowitz DT, Fillit H, Yeung N, Toku K, Vitek MP. Apolipoprotein E-derived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta Neurol Scand Suppl. 2006;185:15–20. doi: 10.1111/j.1600-0404.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 22.James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2007;9:139–152. doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg GA, Estrada E, Wesley M, Kyner WT. Autoradiographic patterns of brain interstitial fluid flow after collagenase-induced haemorrhage in rat. Acta Neurochir Suppl (Wien) 1990;51:280–282. doi: 10.1007/978-3-7091-9115-6_95. [DOI] [PubMed] [Google Scholar]
- 24.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4: Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The Rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 26.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Holscher C. Stress impairs performance in spatial water maze learning tasks. Behav Brain Res. 1999;100:225–235. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 28.Lynch JR, Wang H, Mace B, et al. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exp Neurol. 2005;192:109–116. doi: 10.1016/j.expneurol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Gao J, Wang H, Sheng H, et al. A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit Care. 2006;4:25–31. doi: 10.1385/NCC:4:1:025. [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Dziennis S, Hurn PD, Alkayed NJ. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci. 2009;27:163–179. doi: 10.3233/RNN-2009-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27:135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J Neurosci Methods. 2008;171:214–217. doi: 10.1016/j.jneumeth.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller AA. Gender influences cerebral vascular responses to angiotensin II through Nox2-derived reactive oxygen species. Stroke. 2009;40:1091–1097. doi: 10.1161/STROKEAHA.108.531707. [DOI] [PubMed] [Google Scholar]
- 34.Brait VH, Jackman KA, Walduck AK, et al. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi AI, Bliwise DL, Bliwise NG, Akbar MS, Uzen G, Frankel MR. Rate of 24-hour blood pressure decline and mortality after spontaneous intracerebral hemorrhage: a retrospective analysis with a random effects regression model. Crit Care Med. 1999;27:480–485. doi: 10.1097/00003246-199903000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Vaartjes I, Reitsma JB, Berger-van Sijl M, Bots ML. Gender differences in mortality after hospital admission for stroke. Cere-brovasc Dis. 2009;28:564–571. doi: 10.1159/000247600. [DOI] [PubMed] [Google Scholar]
- 37.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 38.Kimura Y, Takishita S, Muratani H, et al. Demographic study of first-ever stroke and acute myocardial infarction in Okinawa, Japan. Intern Med. 1998;37:736–745. doi: 10.2169/internalmedicine.37.736. [DOI] [PubMed] [Google Scholar]
- 39.Vemmos KN, Bots ML, Tsibouris PK, et al. Stroke incidence and case fatality in southern Greece: the Arcadia stroke registry. Stroke; a journal of cerebral circulation. 1999;30:363–370. doi: 10.1161/01.str.30.2.363. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LF, Yang J, Hong Z, et al. Proportion of different subtypes of stroke in China. Stroke; a journal of cerebral circulation. 2003;34:2091–2096. doi: 10.1161/01.STR.0000087149.42294.8C. [DOI] [PubMed] [Google Scholar]
- 41.Khan FA, Engstrom G, Jerntorp I, Pessah-Rasmussen H, Janzon L. Seasonal patterns of incidence and case fatality of stroke in Malmo, Sweden: the STROMA study. Neuroepidemiology. 2005;24:26–31. doi: 10.1159/000081046. [DOI] [PubMed] [Google Scholar]
- 42.Thrift AG, Dewey HM, Sturm JW, et al. Incidence of stroke subtypes in the North East Melbourne Stroke Incidence Study (NEMESIS): differences between men and women. Neuroepidemiology. 2009;32:11–18. doi: 10.1159/000170086. [DOI] [PubMed] [Google Scholar]
- 43.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 44.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 45.Colton CA, Brown CM, Vitek MP. Sex steroids, APOE genotype and the innate immune system. Neurobiol Aging. 2005;26:363–372. doi: 10.1016/j.neurobiolaging.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Gromadzka G, Baranska-Gieruszczak M, Sarzynska-Dlugosz I, Ciesielska A, Czlonkowska A. The APOE polymorphism and 1-year outcome in ischemic stroke: genotype-gender interaction. Acta Neurol Scand. 2007;116:392–398. doi: 10.1111/j.1600-0404.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 47.Ost M, Nylen K, Csajbok L, Blennow K, Rosengren L, Nellgard B. Apolipoprotein E polymorphism and gender difference in outcome after severe traumatic brain injury. Acta Anaesthesiol Scand. 2008;52:1364–1369. doi: 10.1111/j.1399-6576.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Durham L, Dawson H, et al. An apolipoprotein E-based therapeutic improves outcome and reduces Alzheimer’s disease pathology following closed head injury: evidence of pharmacogenomic interaction. Neuroscience. 2007;144:1324–1333. doi: 10.1016/j.neuroscience.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Laskowitz DT, Song P, Wang H, et al. Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic. J Neurotrauma. 2010;27:1983–1995. doi: 10.1089/neu.2010.1396. [DOI] [PubMed] [Google Scholar]
- 50.Christensen DJ, Ohkubo N, Oddo J, et al. Apolipoprotein-E and peptide mimetics modulate inflammation by binding the set protein and activating protein phosphatase 2A. J Immunol. 2011;186:2535–2542. doi: 10.4049/jimmunol.1002847. [DOI] [PubMed] [Google Scholar]
- 51.Misra UK, Adlakha CL, Gawdi G, McMillian MK, Pizzo SV, Laskowitz DT. Apolipoprotein E and mimetic peptide initiate a calcium-dependent signaling response in macrophages. J Leukoc Biol. 2001;70:677–683. [PubMed] [Google Scholar]