Abstract
Neuropsychological disturbances have been reported in association with use of the recreational drug “ecstasy,” or 3,4-methylenedioxymethamphetamine (MDMA), but findings have been inconsistent. We performed comprehensive neuropsychological testing examining seven ability domains in 21 MDMA users (MDMA+) and 21 matched control participants (MDMA−). Among MDMA+ participants, median [interquartile range] lifetime MDMA use was 186 [111, 516] doses, with 120 [35–365] days of abstinence. There were no significant group differences in neuropsychological performance, with the exception of the motor speed/dexterity domain in which 43% of MDMA+ were impaired compared with 5% of MDMA− participants (p = .004). Motor impairment differences were not explained by use of other substances and were unrelated to length of abstinence or lifetime number of MDMA doses. Findings provide limited evidence for neuropsychological differences between MDMA+ and MDMA− participants with the exception of motor impairments observed in the MDMA+ group. However, replication of this finding in a larger sample is warranted.
Keywords: Ecstasy, N-Methyl-3, 4-methylenedioxyamphetamine, Neurocognitive, Neurotoxicity, Stimulant, Hallucinogen
INTRODUCTION
Abuse of psychoactive substances such as alcohol (Parsons & Nixon, 1998), methamphetamine (Scott et al., 2007), and cocaine (Jovanovski, Erb, & Zakzanis, 2005) has been associated with neuropsychological (NP) deficits. However, the NP harms associated with “ecstasy,” or 3,4-methylenedioxy-methamphetamine (MDMA), an illicit recreational drug with stimulant and hallucinogenic properties (Capela, Caarmo, Remiao, Bastos, Meisel, & Carvalho, 2009), are less clear. A recent comprehensive review and meta-analysis of 110 studies examining the NP consequences of MDMA showed an inconsistent relationship between MDMA and NP deficits (Rogers et al., 2009). Studies that have demonstrated NP consequences associated with MDMA use have primarily showed small negative effects for verbal and working memory; however, these studies did not match MDMA and control participants on, or statistically adjust for, potential confounds such as premorbid functioning and polydrug use, two of the most consistent NP confounds in the MDMA literature (Rogers et al., 2009). In fact, to our knowledge no study examining NP performance in MDMA users has succeeded in matching users and controls on these potential confounds, with the exception of one study that statistically adjusted for these confounds and showed negative effects of MDMA on verbal delayed recall (Schilt et al., 2008).
In the present study, we sought to examine potential NP deficits associated with MDMA use by comparing NP performance of abstinent MDMA users to that of demographically matched polysubstance exposed controls with similar levels of education and reading ability (as a measure of premorbid functioning and quality of education; Manly, Jacobs, Touradji, Small, & Stern, 2002). Additionally, structured drug history interviews were administered to exclude participants who had been dependent on any drug, other than cannabis, in the past 5 years or alcohol in the past year. We proposed that, if MDMA use is associated with NP dysfunction then it should be detectable during abstinence from MDMA and withstand a priori methodological control (rather than post hoc statistical adjustment) of confounds known to affect NP performance.
METHODS
Participants
Study participants were 42 men and women recruited from the San Diego community, including dance clubs and raves. All gave written informed consent according to the requirements of the University of California San Diego Institutional Review Board before the start of their study assessments. Enrolled participants were verified to be human immunodeficiency virus (HIV) and hepatitis C negative by standard antibody testing. The MDMA+ group comprised 21 participants who had reported use of MDMA within the last 18 months, with a minimum of 20 lifetime doses. The MDMA− group consisted of 21 participants who were frequency matched on age, sex, ethnicity, education, and reading ability, and reported no lifetime use of MDMA.
Potential participants were excluded from both groups if they met criteria for lifetime dependence on any substance with the exception of cannabis or alcohol. These were admissible because of the high prevalence of use in the MDMA+ population. Past dependence on any substances was allowed only if it had been episodic, as determined by a clinician or doctoral-level trainee, and occurred more than 5 years ago (12 months ago for alcohol). Likewise, no substance abuse other than cannabis or alcohol was allowed in the past 12 months. Participants were requested to be abstinent from MDMA for at least 10 days before testing and show negative urine toxicology for any non-prescribed substances except cannabis, as well as negative Breathalyzer test for alcohol, on the day of assessment. Irrespective of toxicology results, participants were not allowed to undergo NP testing if they appeared to be intoxicated or experiencing withdrawal symptoms. Additionally, participants were ineligible if English was their second language or if they had psychiatric, developmental, neurologic, or metabolic conditions of sufficient severity to confound the interpretation of NP findings (e.g., stroke, loss of consciousness > 30 min, schizophrenia, developmental disability).
Participants were selected from a larger study cohort (49 MDMA+ and 103 MDMA−) to obtain groups that were comparable with respect to age, sex, and ethnicity as well as years of education and reading ability, two factors that have been consistently associated with NP performance. By accomplishing similarity in these background characteristics, differences in neurocognitive functioning that may be observed between the groups should not be attributable to pre-existing factors such as marginal quality of education or academic achievement. The resulting 21 MDMA+ and 21 MDMA− participants were statistically comparable with regard to age, years of education, reading ability (Wide Range Achievement Test –3 Reading Quotient), sex, and ethnic representation (Table 1). All participant data included in this manuscript were obtained in compliance with regulations of the UCSD Institutional Review Board.
Table 1.
Demographic and MDMA use characteristics by MDMA status
| Demographic and MDMA use characteristics | MDMA+ (n = 21) | MDMA− (n = 21) | p value |
|---|---|---|---|
| Age, median (IQR) | 26 (25, 31) | 25 (23, 35) | NS |
| Education, median (IQR) | 15 (13, 16) | 14 (12, 16) | NS |
| WRAT Reading, median (IQR) | 107 (101, 115) | 109 (105, 114) | NS |
| Sex, % female | 24% | 19% | NS |
| Ethnicity, % Caucasian | 95% | 85% | NS |
| Age 1st MDMA use, median (IQR) | 21 (18, 26) | — | — |
| MDMA years of use, median (IQR) | 5 (4, 7) | — | — |
| MDMA doses, median (IQR) | 186 (111, 516) | — | — |
| MDMA density (doses per year), median (IQR) | 29 (18, 104) | — | — |
| MDMA days abstinence, median (IQR) | 120 (45, 365) | — | — |
NS = Not Significant (p > .05)
Substance Use and Psychiatric Information
A structured substance use interview was administered to obtain a detailed history of quantity, frequency, and duration of use of MDMA and other recreational substances for the last 30 days and 12 months, and in 5-year epochs covering the participant’s lifetime. The variables derived from this interview were age of onset, number of years of use, length of abstinence, lifetime dose of MDMA consumed, and average doses per year of use, or MDMA “density.”
The method for determining cumulative MDMA exposure was as follows: Frequency of use was coded on a 6-point scale where 0 = “No use ever,” 1 = “Less than 1 day per month,” 2 = “Less than 1 day per week,” 3 = “1–3 days per week,” 4 = “4–6 days per week, “ and 5 = “Every day.” The midpoint of each of these ranges was calculated in number of days per month, such that category 1 = .5 days (assigned arbitrarily); 2 has a range of 1 to 3 days, so the midpoint is 2; category 3 = 4 to 12 days per month (midpoint = 8); 4 = 16 to 24 (midpoint = 20), and 5 reflects daily use (30 days per month).
Participants were asked to estimate quantity of MDMA use in terms of doses or tablets. For each epoch of use, the quantity in doses per day was multiplied by the frequency in days per month, yielding doses per month. This figure was then multiplied by the number of months in each epoch (1 for last 30 days, 12 for previous 12 months, and 60 months for each subsequent 5-year epoch) to obtain the number of doses for each epoch. Then the number of doses consumed in each epoch was summed to compute total lifetime doses. MDMA density was calculated as the average consumption per year of use by dividing lifetime doses by the total years of use (Cherner et al., 2010). Table 1 shows the MDMA use characteristics of the MDMA+ group.
Participants also received the Structured Clinical Interview for DSM-IV (SCID-IV) (Spitzer, Williams, Gibbon, First, 1992) to determine substance use disorder diagnoses for study eligibility, as well as to obtain a history of lifetime and current (30-day) prevalence of major depressive disorder, bipolar disorder, and antisocial personality disorder. Depressed mood in the past 7 days was assessed using the Beck Depression Inventory-I (BDI-I) (Beck, 1972). All psychiatric measures were administered by doctoral-level clinicians or trainees.
Neuropsychological Battery
The NP assessment and interpretive methods have been described elsewhere (Cherner et al., 2010). Participants completed a comprehensive neuropsychological evaluation assessing 7 ability domains (i.e., learning, recall, attention/working memory, processing speed, abstraction/executive functioning, verbal fluency, and motor speed/dexterity). Raw test scores were converted to T-scores (standard scores with a mean of 50 and standard deviation of 10) using demographically corrected norms to adjust for age, education, gender, and ethnicity, as available for each measure. The demographically corrected T-scores for each test were then converted into deficit scores using the method developed by Heaton et al. (1995). The deficit scores assign performances within the normal range (T-scores ≥ 1 standard deviation below the mean) a value of zero, thus reflecting only degree of impairment in 0.5 SD increments (i.e., T-scores between 39 and 35 = 1; 34–30 = 2, 29–25 = 3, 24–20 = 4, and < 20 = 5). The individual test deficit scores were then averaged within each domain to derive the domain deficit score that reflects the severity of deficit within each ability area. Previous work has demonstrated that deficit scores achieve good diagnostic agreement with classifications based on blind ratings by clinicians, with a cut point for impairment set at ≥ 0.50 (Carey et al., 2004; Heaton et al., 1995). In addition, this method has the advantage of data reduction to minimize multiple comparisons, and has shown robust relationships with documented brain injury (Cherner et al., 2002; Masliah et al., 1997).
Statistical Analyses
All statistical tests and procedures were conducted using JMP 7.0.2 (SAS Institute Inc, 2007). Group comparisons on mean values for continuous variables were examined with t tests and Cohen’s d effect sizes were calculated. Differences in dichotomous variables were analyzed using contingency tables with χ2 tests. Correlations using Spearman’s rho (ρ) were used to explore the association between NP performance and length of abstinence from MDMA as well as lifetime number of doses.
RESULTS
Prevalence of Substance Use and Other Psychiatric Disorders
As mentioned above, the groups were matched demographically and had comparable levels of education and reading ability. Table 2 shows that the groups were also comparable in lifetime prevalence of substance use and psychiatric disorders.
Table 2.
Lifetime prevalence of substance use and other psychiatric disorders among MDMA+ (n = 21) and MDMA− (n = 21)
| SCID-IV diagnosis | Lifetime prevalence rate
|
||
|---|---|---|---|
| MDMA+% (n) | MDMA−% (n) | 2-Tailed p value | |
| Substance use | |||
| Alcohol abuse | 38% (8) | 24% (5) | NS |
| Alcohol dependence (>1 year ago) | 0% (0) | 5% (1) | NS |
| Cannabis abuse | 9% (2) | 19% (4) | NS |
| Cannabis dependence | 19% (4) | 5% (1) | NS |
| Cocaine abuse | 5% (1) | 0% (0) | NS |
| Cocaine dependence* | 5% (1) | 0% (0) | NS |
| Amphetamine abuse | 5% (1) | 0% (0) | NS |
| Amphetamine dependence | 0% (0) | 0% (0) | — |
| Opioid abuse | 0% (0) | 0% (0) | — |
| Opioid dependence | 0% (0) | 0% (0) | — |
| Sedative abuse | 5% (1) | 0% (0) | NS |
| Sedative dependence | 0% (0) | 0% (0) | — |
| Hallucinogen abuse | 9% (2) | 0% (0) | NS |
| Hallucinogen dependence | 5% (1) | 0% (0) | NS |
| Polysubstance abuse | 0% (0) | 0% (0) | — |
| Polysubstance dependence | 0% (0) | 0% (0) | — |
| Other psychiatric | |||
| Beck Depression Inventory, median (IQR) | 3 (0, 15) | 3 (1, 7) | NS |
| Major depressive disorder | 29% (6) | 14% (3) | NS |
| Bipolar disorder | 0% (0) | 0% (0) | — |
| Antisocial personality disorder | 0% (0) | 0% (0) | — |
NS = Not significant (p ≥ .05).
Note. One participant in the MDMA+ group met criteria for cocaine dependence 2 years before assessment in the current study as a result of light (two lines per day) use over a 3-month period.
Neuropsychological performance
When examining the proportion of participants in each group who fell in the impaired range (domain deficit score > 0.5), the MDMA+ group had comparable rates of impairment to the MDMA− group globally and in verbal, attention, processing speed, learning, recall, and abstraction abilities (Figure 1). However, the MDMA+ group was significantly more likely to evidence motor impairment relative to the MDMA− group (43% vs. 5%, respectively; χ2 = 8.40, p = .004).
Fig. 1.
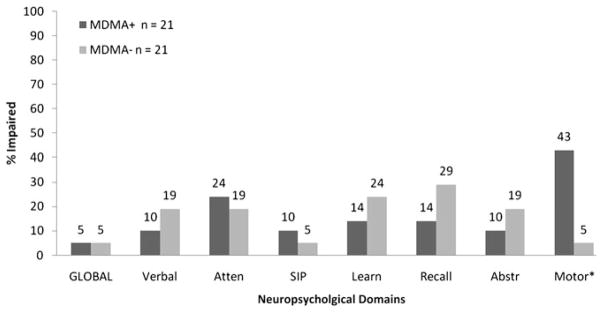
Rates of neuropsychological impairment among MDMA+ and MDMA− groups based on deficit scores from demographically adjusted test T-scores, using a cut point of 0.5. Atten = Attention/Working memory, SIP = Speed of information processing, Abstr = Abstraction/Executive functioning. *p < .01.
Examination of performance for the MDMA+ and MDMA− groups for each of the individual NP tests showed limited statistical differences (Table 3). Both groups were comparable (p > .05) on tests of learning, attention/working memory, processing speed, abstraction/executive, and verbal fluency. Significant differences (not adjusted for multiple comparisons) were observed for tests within the motor (Grooved Pegboard non-dominant hand: p = .03; d = .72) and recall (HVLT-R Delayed Recall: p = .03; d = .71) domains. For Grooved Pegboard non-dominant hand, MDMA+ participants performed significantly worse than their MDMA− counterparts. Conversely, for the HVLT-R Delayed Recall condition, MDMA+ participants performed significantly better than MDMA− participants.
Table 3.
Demographically adjusted performance on individual neuropsychological tests by MDMA status
| Neuropsychological test T-scores | MDMA+ n = 21
|
MDMA− n = 21
|
d | p* | p** | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | % (n) Impaired | Mean (SD) | % (n) Impaired | ||||
| Learning | |||||||
| BVMT-R Learning | 47 (5) | 10 (2) | 51 (9) | 10 (2) | 0.51 | 0.11 | 0.99 |
| HVLT-R Learning | 51 (6) | 0 (0) | 47 (10) | 19 (4) | 0.49 | 0.12 | 0.11 |
| Story Memory Test - Learning | 50 (8) | 5 (1) | 52 (9) | 10 (2) | 0.27 | 0.38 | 0.99 |
| Figure Memory Test - Learning | 44 (6) | 19 (4) | 48 (7) | 19 (4) | 0.51 | 0.11 | 0.99 |
| Recall | |||||||
| BVMT-R Delayed Recall | 48 (8) | 5 (1) | 53 (9) | 10 (2) | 0.56 | 0.07 | 0.99 |
| HVLT-R Delayed Recall | 51 (6) | 0 (0) | 45 (9) | 24 (5) | 0.71 | 0.03 | 0.05 |
| Story Memory Test - Retention | 52 (10) | 10 (2) | 52 (9) | 5 (1) | 0.10 | 0.98 | 0.99 |
| Figure Memory Test - Retention | 47 (7) | 19 (4) | 48 (6) | 10 (2) | 0.20 | 0.53 | 0.66 |
| Attention/Working memory | |||||||
| PASAT Total Correct | 51 (9) | 14 (3) | 49 (10) | 19 (4) | 0.19 | 0.54 | 0.99 |
| WAIS-III Letter-Number Sequencing | 53 (10) | 10 (2) | 54 (9) | 5 (1) | 0.09 | 0.77 | 0.99 |
| Processing speed | |||||||
| Trail Making Test A | 51 (10) | 14 (3) | 53 (10) | 5 (1) | 0.25 | 0.43 | 0.61 |
| WAIS-III Symbol Search | 53 (7) | 0 (0) | 53 (11) | 5 (1) | 0.09 | 0.77 | 0.99 |
| WAIS-III Digit Symbol | 52 (8) | 5 (1) | 53 (9) | 0 (0) | 0.14 | 0.67 | 0.99 |
| Stroop Color Naming | 49 (7) | 0 (0) | 51 (9) | 10 (2) | 0.19 | 0.55 | 0.50 |
| Abstraction/Executive | |||||||
| Trail Making Test B | 49 (9) | 14 (3) | 54 (9) | 5 (1) | 0.62 | 0.06 | 0.61 |
| WCST-64 Perseverations | 50 (10) | 14 (3) | 49 (12) | 24 (5) | 0.06 | 0.85 | 0.70 |
| Stroop Incongruent condition | 51 (9) | 10 (2) | 52 (9) | 5 (1) | 0.11 | 0.72 | 0.99 |
| Stroop Interference Ratio | 52 (9) | 5 (1) | 53 (7) | 10 (2) | 0.07 | 0.82 | 0.99 |
| Halstead Category Test Errors | 47 (10) | 20 (4) | 52 (9) | 10 (2) | 0.55 | 0.09 | 0.41 |
| Verbal Fluency | |||||||
| Letter Fluency (FAS) | 52 (8) | 5 (1) | 46 (9) | 29 (6) | 0.63 | 0.05 | 0.09 |
| Category Fluency (Animals) | 53 (11) | 19 (4) | 48 (9) | 10 (2) | 0.44 | 0.17 | 0.66 |
| Action Fluency (Verbs) | 51 (10) | 10 (2) | 52 (7) | 0 (0) | 0.12 | 0.71 | 0.50 |
| Motor | |||||||
| Grooved Pegboard Dominant Hand | 47 (12) | 24 (5) | 53 (9) | 5 (1) | 0.54 | 0.09 | 0.18 |
| Grooved Pegboard Non-dominant Hand | 43 (10) | 38 (8) | 49 (7) | 5 (1) | 0.72 | 0.02 | 0.02 |
Note. % Impairment was determined using a T-score cut point of T < 40.
d = Cohen’s d for mean performance between groups.
p values are for t-tests comparing mean performance.
p values are for Fisher’s exact test comparing impairment proportions.
BVMT-R = Brief visual memory test-revised; HVLT-R = Hopkins verbal learning test-revised; PASAT = Paced auditory serial addition test; WAIS = Wechsler adult intelligence scale; WCST = Wisconsin card sort test; Bolded values are significant (p < .05).
Notably, moderate effect sizes showing negative NP effects among MDMA users were observed for visual learning (BVMT-R Learning: d = .51; Figure Memory Test Learning: d = .51), visual recall (BVMT-R Delayed Recall: d = .56), abstraction/executive (Trail Making Test B: d = .62; Hal-stead Category Test Errors: d = .55), verbal fluency (Letter Fluency-FAS: d = .63), and motor (Grooved Pegboard Dominant Hand: d = .54) tests. However, it should be noted that the mean T-scores for both groups across these NP tests were in the normal range (T ≥ 40) and impairment rates were comparable. In addition, no findings withstood adjustment for multiple comparisons (Bonferroni alpha = 0.05/24 = 0.002).
Abstinence and Dose Effects on Neuropsychological Performance
Exploration of length of abstinence and dose effects on NP performance among the MDMA+ group are shown in Table 4. Neither number of days abstinent nor lifetime doses of MDMA was associated with any of the domain deficit scores. However, number of lifetime doses of MDMA showed significant correlations with BVMT-R Delayed Recall (rho = 0.45; p = .04), PASAT Total Correct (rho = −0.48; p = .03), and Trail Making Test A (rho = −0.52; p = .02) and B (rho = −0.71; p = .0003). Only the association with Trail Making Test B withstood adjustment for multiple comparisons (Bonferroni alpha = 0.002).
Table 4.
Pairwise correlations (Spearman’s rho) between test T-scores and MDMA use parameters among the MDMA+ Group (n = 21)
| Neuropsychological domain deficit and individual test T-scores | Days abstinentcenter | # of dosescenter | ||
|---|---|---|---|---|
| ρ | p value | ρ | p value | |
| Learning domain deficit** | 0.06 | 0.80 | −0.02 | 0.94 |
| BVMT-R Learning | 0.02 | 0.92 | −0.02 | 0.94 |
| HVLT-R Learning | −0.16 | 0.49 | 0.31 | 0.17 |
| Story Memory Test - Learning | 0.37 | 0.11 | 0.19 | 0.42 |
| Figure Memory Test - Learning | 0.06 | 0.81 | −0.18 | 0.44 |
| Recall domain deficit** | −0.32 | 0.17 | −0.32 | 0.16 |
| BVMT-R Delayed Recall | −0.07 | 0.76 | 0.45 | 0.04 |
| HVLT-R Delayed Recall | −0.14 | 0.55 | 0.13 | 0.58 |
| Story Memory Test - Retention | 0.13 | 0.60 | 0.03 | 0.91 |
| Figure Memory Test - Retention | 0.19 | 0.43 | −0.10 | 0.68 |
| Attention/Working memory domain deficit** | −0.28 | 0.23 | 0.35 | 0.14 |
| PASAT Total Correct | 0.25 | 0.28 | −0.48 | 0.03 |
| WAIS-III Letter-Number Sequencing | 0.24 | 0.34 | −0.14 | 0.54 |
| Processing speed domain deficit** | −0.24 | 0.31 | 0.19 | 0.41 |
| Trail Making Test A | −0.13 | 0.31 | −0.52 | 0.02 |
| WAIS-III Symbol Search | 0.21 | 0.38 | −0.29 | 0.20 |
| WAIS-III Digit Symbol | 0.09 | 0.71 | −0.19 | 0.41 |
| Stroop Color Naming | 0.08 | 0.74 | −0.25 | 0.28 |
| Executive/Abstraction domain deficit** | −0.10 | 0.66 | 0.18 | 0.44 |
| Trail Making Test B | 0.41 | 0.07 | −0.71 | 0.00* |
| WCST-64 Perseverations | 0.27 | 0.26 | −0.18 | 0.43 |
| Stroop Interference Ratio | 0.17 | 0.49 | −0.32 | 0.16 |
| Stroop Incongruent condition | 0.20 | 0.40 | −0.36 | 0.11 |
| Halstead Category Test Errors | 0.17 | 0.50 | −0.24 | 0.31 |
| Verbal Fluency domain deficit** | −0.01 | 0.95 | 0.33 | 0.14 |
| Letter Fluency (FAS) | 0.03 | 0.89 | −0.21 | 0.36 |
| Category Fluency (Animals) | −0.17 | 0.47 | 0.03 | 0.92 |
| Action Fluency (Verb) | −0.05 | 0.85 | −0.21 | 0.38 |
| Motor domain deficit** | 0.01 | 0.98 | 0.33 | 0.34 |
| Grooved Pegboard Dominant Hand | 0.18 | 0.46 | −0.41 | 0.07 |
| Grooved Pegboard Nondominant Hand | 0.01 | 0.98 | −0.16 | 0.49 |
BVMT-R = Brief visual recall test-revised; HVLT-R = Hopkins verbal learning test-revised; PASAT = Paced auditory serial addition test; WAIS = Wechsler adult intelligence scale; WCST = Wisconsin card sort test;
p = 0.0003;
High scores indicate poorer performance.
DISCUSSION
In this study, we sought to determine whether NP performance in MDMA users was worse than that of MDMA− naïve controls matched a priori on key demographic (i.e., age, education, sex, ethnicity), substance use (e.g., alcohol), psychiatric (e.g., depression), medical (i.e., HIV, HCV status), and premorbid (i.e., reading ability) characteristics known to affect NP performance. Our results showed that on the majority of NP tests and domains, the performances of MDMA users were comparable to those of the control group. However, participants in the MDMA+ group had 15 (95% CI = 1.7–133.6) times greater odds of impairment in motor function as measured by the Grooved Pegboard task compared with MDMA− naïve controls.
To our knowledge, only four studies (Croft, Mackay, Mills, & Gruzelier, 2001; Golding, Groome, Rycroft, & Denton, 2007; Hanson & Luciana, 2004, 2010) have examined MDMA associations with motor functioning in humans. Among these studies, two (Croft et al., 2001; Hanson & Luciana, 2004) administered the Grooved Pegboard test only, two used a finger tapping test only (Golding et al., 2007), and one used both (Hanson & Luciana, 2010). Croft and colleagues (Croft et al., 2001) compared MDMA users who also used marijuana with matched control participants who exclusively used marijuana. They found that those who used MDMA performed worse than controls on the Grooved Pegboard test after co-varying for marijuana use. The second study by Hanson and Luciana (2004) showed no differences in performance on the Grooved Pegboard between MDMA users and controls. However, an exploratory comparison of MDMA users who met DSM-IV criteria for abuse/dependence (American Psychiatric Association, 1994) to those who did not showed that those meeting criteria dropped significantly more pegs. In the third study (Golding et al., 2007), no differences were found in finger tapping performance between MDMA users and controls. The fourth study (Hanson & Luciana, 2010) administered both the Grooved Pegboard and finger tapping tests and found no significant differences between MDMA/poly-drug users and nondrug controls. In all four of these studies raw scores were used for statistical analysis rather than demographically adjusted T scores. Thus, it is difficult to compare performance data in these studies with our results. Nevertheless, these previous studies in combination with our observations provide limited preliminary evidence that MDMA may be associated with motor impairment, particularly in relation to performance on the Grooved Pegboard test.
A relationship between MDMA and motor functioning is supported by hundreds of studies in rats and non-human primates that have showed single and multiple doses of MDMA result in neurotoxicity to the serotonergic and to a lesser extent dopaminergic systems (Capela et al., 2009). Serotonin (5-HT) axons arising from the brainstem raphe nuclei innervate motor areas as part of the basal ganglia-thalamocortical circuit (DeVito, Anderson, & Walsh, 1980; Lavoie & Parent, 1990; McQuade & Sharp, 1997; Wilson & Molliver, 1991) and have been shown to have a major role in motor system neurophysiology (Jacobs & Fornal, 1997; Karageorgiou et al., 2009). Additionally, human neuroimaging studies of MDMA users have shown decrease metabolic rate (Obrocki, Schmoldt, Buchert, Andresen, Petersen, & Thomasius, 2002) and structural changes in the 5-HT system (i.e., lower levels of 5-HT reuptake transporters) (Cowan, 2007; McCann et al., 2008) in motor regions of the brain. However, it is unclear whether our findings suggest serotonergic neurotoxicity in that we only observed unilateral (i.e., non-dominant hand only) significant motor differences between MDMA+ and MDMA− groups, although effect sizes for both non-dominant (d = .72) and dominant (d = .54) hands are comparable and would suggest bilateral impairment. Larger studies with greater statistical power will be required to elucidate the nature of the association between MDMA and motor impairment.
Following our identification of the potential association between MDMA and NP impairment, specifically motor impairment, we asked the question of whether the association can be further understood by examination of abstinence and/or dose effects. Abstinence and dose related effects of MDMA have been examined in previous studies and meta-analytically, and a relationship has not been supported (Rogers et al., 2009). In the current study, length of abstinence and number of doses of MDMA was not correlated with the motor speed and dexterity domain deficit score or T-scores for either hand on the Grooved Pegboard test. While dose effects are typically negative in retrospective, self-report studies such as ours, this suggests the possibility that the observed motor impairment in MDMA+ subjects is not attributable to MDMA. Depression can result in motor slowing (Purcell, Maruff, Kyrios, & Pantelis, 1997), so we explored this possible alternative explanation. Among MDMA users, scores on the Beck Depression Inventory were not correlated with performance on the Grooved Pegboard test (dominant: rho = −.10; p = .67; non-dominant: rho = −.01; p = .96) and those with elevated BDI score (≥ 14; n = 6) were no more likely to experience motor deficits than those with BDI scores in the undepressed range (50% vs. 40%). Additionally, only one of the six MDMA+ subjects with a lifetime diagnosis of major depression was classified as impaired on the motor domain and no significant T-score differences (p > .27) on the Grooved Pegboard test were observed when compared with those who never were diagnosed with depression. Another possible explanation is that the observed motor impairment is a subacute and potentially reversible rather than chronic and permanent effect of MDMA, given the relatively short duration of abstinence (1 to 12 months) of the subjects recruited for this study. However, the maximum abstinence of a full year, and the absence of correlations between duration of abstinence and test performance within the MDMA group would tend to argue against this possibility. Future longitudinal investigations examining the course of MDMA related impairments are required to address this current gap in our understanding.
Of interest, we did observed a significant dose (but not abstinence) effect for tests within the recall, attention/working memory, and processing speed domains as well as a strong (Spearman’s rho = −.71; p = .0003) dose effect in the abstraction/executive domain. Examination of MDMA density and each NP tests showed no significant associations with the exception of the Trails B test (Spearman’s rho = −.44; p = .04) (data not shown). Importantly, the proportion of MDMA users impaired in these domains was not associated with MDMA dose. This suggests that individual NP tests, particularly the Trails B test, may be sensitive to MDMA dose effects but when additional tests are administered within these test’s respective domains a more robust effect is not sustained.
Despite our relatively strong methodological approach in which we matched MDMA+ and MDMA− groups on key demographic, substance use, psychiatric, medical, and pre-morbid characteristics, which to date had not been done, several limitations to the current study should be acknowledged. First, it should be noted that the Grooved Pegboard requires perceptual-motor speed, plus close attention to essential visual details of variably oriented notches in the pegboard holes. Thus, the observed effects may not be strictly due to dysfunction of a primary motor pathway. In fact, it has been shown that unilateral stroke can affect performance of both hands on the pegboard, whereas it may cause only unilateral impairment on more simple motor tasks (e.g., grip strength or finger tapping speed) (Haaland & Delaney, 1981). Second, it is becoming more clear that stimulants such as cocaine and methamphetamine can result in motor impairments (Caligiuri & Buitenhuys, 2005; Hanlon, Wesley, Roth, Miller, & Porrino, 2009; Scott et al., 2007). In the current study, among the MDMA+ participants who were found to have impaired motor functioning (n = 9), 66% reported prior use of methamphetamine and 100% reported use of cocaine. Although it is plausible that recreational use of methamphetamine and cocaine could be confounding our results, neither methamphetamine nor cocaine use (i.e., lifetime average daily grams) differed between those who were impaired [median (IQR) grams: meth = 0.05 (0.03, 0.07); cocaine = 0.07 (0.07, 0.10)] and not impaired [median (IQR) grams: meth = 0.06 (0.04, 0.12); cocaine = 0.06 (0.04, 0.07)] on the Grooved Pegboard task (p > .05). In addition, among the motor impaired MDMA+ group, no methamphetamine (Spearman’s rho = −0.25; p = .63) or cocaine (Spearman’s rho = −.48; p = .19) dose effects on motor performance were observed. Third, motor functioning was part of a larger battery of NP tests for which multiple comparisons were conducted between MDMA users and controls. Likewise, our sample was small and our power to detect significant findings was therefore attenuated. Thus, the likelihood of both Type I (false-positive) and II (false-negative) errors is considerable and our findings should be interpreted accordingly. Fourth, only a select group of NP tests for each cognitive domain was used for the current study and we did not include tests of other cognitive processes such as response inhibition and decision-making. However, recent research suggests that significant deficits among MDMA users on NP tests other than those in the current study, as well as reward-related decision-making tests, are likely a result of general heavy drug use rather than specific to MDMA (Hanson & Luciana, 2010; Hanson, Luciana, & Sullwold, 2008). Fifth, our measurement of dose was imprecise and necessarily relied on participant recall. Likewise, we were not able to ascertain the purity of each MDMA dose, which is known to be variable (Rogers et al., 2009); thus, it unknown how much MDMA or common adulterants (e.g., methamphetamine, ketamine) each participant was actually exposed to. Sixth, we did not assess for other psychiatric disorders that might occur among MDMA users, such as anxiety, and did not formally collect information about degree of exposure to other club drugs such as ketamine and gamma-hydroxybutyric acid (GHB). Finally, we used a cross-sectional design to examine NP deficits in MDMA users. Therefore, it is not possible to determine if motor impairments and other performance deficits observed were present before onset of MDMA use. In the only longitudinal (3-year) study of MDMA use in humans (Schilt et al., 2007), motor deficits were not observed, although the median dose of MDMA was only three to six tablets.
In summary, the current results provide limited evidence for an overall deleterious NP effect among abstinent MDMA users when compared with controls matched for co-existing conditions that have confounded interpretation of previous results in the literature. Our results do provide limited preliminary evidence for MDMA associated motor impairment that is not related to dose or length of abstinence. Replication with larger well-matched samples is needed to increase confidence in the present findings. Future work assessing finer grained aspects of motor function are also needed to determine the nature of these impairments (e.g., hypo- or hyper-kinetic) and could assist in distinguishing neuroanatomical loci underlying these dysfunctions as well ascertaining whether these motor impairments are associated with functional and/or quality of life declines that could be targets for future interventions.
Acknowledgments
The information in this manuscript and the manuscript itself has never been published either electronically or in print. Support for this research included National Institutes of Health grant numbers R03-MH64903, P01-DA12065, and P30-MH62512; The authors acknowledge support from the United States National Institutes of Health (grant numbers P01-DA12065 and P30-MH62512) and the contributions of study participants and staff at the HIV Neurobe-havioral Research Center (HNRC), San Diego, CA, USA.
The HNRC Group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, MD; Co-Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and J. Allen McCutchan, MD; Center Manager: Thomas D. Marcotte, PhD; Business Manager: Melanie Sherman; Naval Hospital San Diego: Braden R. Hale, MD, MPH (PI); Neuro-medical Component: Ronald J. Ellis, MD, PhD (PI), J. Allen McCutchan, MD, Scott Letendre, MD, Edmund Capparelli, PharmD, Rachel Schrier, PhD; Jennifer Marquie-Beck; Terry Alexander, RN; Neurobehavioral Component: Robert K. Heaton, PhD (PI), Mariana Cherner, PhD, Steven Paul Woods, PsyD, David J. Moore, PhD; Matthew Dawson, Donald Franklin; Neuroimaging Component: Terry Jernigan, PhD (PI), Christine Fennema-Notestine, PhD, Sarah L. Archibald, MA, John Hesselink, MD, Jacopo Annese, PhD, Michael J. Taylor, PhD, Neurobiology Component: Eliezer Masliah, MD (PI), Ian Everall, FRCPsych, FRCPath, PhD, Cristian Achim, MD, PhD; Neurovirology Component: Douglas Richman, MD, (PI), David M. Smith, MD; International Component: J. Allen McCutchan, MD, (PI); Developmental Component: Ian Everall, FRCPsych, FRCPath, PhD (PI), Stuart Lipton, MD, PhD; Clinical Trials Component: J. Allen McCutchan, MD, J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, Scott Letendre, MD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI), Rodney von Jaeger, MPH; Data Management Unit: Anthony C. Gamst, PhD (PI), Clint Cushman (Data Systems Manager), Daniel R. Masys, MD (Senior Consultant); Statistics Unit: Ian Abramson, PhD (PI), Florin Vaida, PhD, Christopher Ake, PhD
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
- American Psychiatric Association. Diagnostic criteria from DSM-IV. Washington, DC: The American Psychiatric Association; 1994. [Google Scholar]
- Beck AT. Depression: Causes and treatment. Philadelphia: University of Pennsylvania Press; 1972. [Google Scholar]
- Caligiuri MP, Buitenhuys C. Do preclinical findings of methamphetamine-induced motor abnormalities translate to an observable clinical phenotype? Neuropsychopharmacology. 2005;30:2125–2134. doi: 10.1038/sj.npp.1300859. [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: An overview. Molecular Neurobiology. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, et al. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, et al. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug and Alcohol Dependence. 2010;106:154–163. doi: 10.1016/j.drugalcdep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: A review. Psychopharmacology. 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Mackay AJ, Mills AT, Gruzelier JG. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology. 2001;153:373–379. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- DeVito JL, Anderson ME, Walsh KE. A horseradish peroxidase study of afferent connections of the globus pallidus in macaca mulatta. Experimental Brain Research. 1980;38:65–73. doi: 10.1007/BF00237932. [DOI] [PubMed] [Google Scholar]
- Golding JF, Groome DH, Rycroft N, Denton Z. Cognitive performance in light current users and ex-users of ecstasy (MDMA) and controls. The American Journal of Drug and Alcohol Abuse. 2007;33:301–307. doi: 10.1080/00952990601175052. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Delaney HD. Motor deficits after left or right hemisphere damage due to stroke or tumor. Neuropsychologia. 1981;19:17–27. doi: 10.1016/0028-3932(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: An fMRI investigation of sensorimotor control. Psychiatry Research. 2009;181:15–23. doi: 10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Luciana M. Neurocognitive function in users of MDMA: The importance of clinically significant patterns of use. Psychological Medicine. 2004;34:229–246. doi: 10.1017/s0033291703001132. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Luciana M. Neurocognitive impairments in MDMA and other drug users: MDMA alone may not be a cognitive risk factor. Journal of Clinical and Experimental Neuropsychology. 2010;32:337–349. doi: 10.1080/13803390903042361. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug and Alcohol Dependence. 2008;96:99–110. doi: 10.1016/j.drugalcdep.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The HNRC 500–neuropsychology of HIV infection at different disease stages. HIV neurobehavioral research center. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Current Opinion in Neurobiology. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: A quantitative review of the evidence. Journal of Clinical and Experimental Neuropsychology. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Karageorgiou J, Dietrich MS, Charboneau EJ, Woodward ND, Blackford JU, Salomon RM, et al. Prior MDMA (ecstasy) use is associated with increased basal ganglia-thalamocortical circuit activation during motor task performance in humans: An fMRI study. Neuroimage. 2009;46:817–826. doi: 10.1016/j.neuroimage.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Immunohistochemical study of the serotoninergic innervation of the basal ganglia in the squirrel monkey. The Journal of Comparative Neurology. 1990;299:1–16. doi: 10.1002/cne.902990102. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and white elders. Journal of the International Neuropsychological Society. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. the HIV neurobehavioral research center. Annals of Neurology. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, et al. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine (“ecstasy”) users: Relationship to cognitive performance. Psychopharmacology. 2008;200:439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. Journal of Neurochemistry. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- Obrocki J, Schmoldt A, Buchert R, Andresen B, Petersen K, Thomasius R. Specific neurotoxicity of chronic use of ecstasy. Toxicology Letters. 2002;127:285–297. doi: 10.1016/s0378-4274(01)00511-2. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: A review of the research since 1986. Journal of Studies on Alcohol. 1998;59:180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological function in young patients with unipolar major depression. Psychological Medicine. 1997;27:1277–1285. doi: 10.1017/s0033291797005448. [DOI] [PubMed] [Google Scholar]
- Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al. The harmful health effects of recreational ecstasy: A systematic review of observational evidence. Health Technology Assessment. 2009;13:iii–iv. ix–xii, 1–315. doi: 10.3310/hta13050. [DOI] [PubMed] [Google Scholar]
- Schilt T, de Win MM, Jager G, Koeter MW, Ramsey NF, Schmand B, et al. Specific effects of ecstasy and other illicit drugs on cognition in poly-substance users. Psychological Medicine. 2008;38:1309–1317. doi: 10.1017/S0033291707002140. [DOI] [PubMed] [Google Scholar]
- Schilt T, de Win MM, Koeter M, Jager G, Korf DJ, van den Brink W, Schmand B. Cognition in novice ecstasy users with minimal exposure to other drugs: A prospective cohort study. Archives of General Psychiatry. 2007;64:728–736. doi: 10.1001/archpsyc.64.6.728. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: Regional distribution of axon terminals. Neuroscience. 1991;44:537–553. doi: 10.1016/0306-4522(91)90076-z. [DOI] [PubMed] [Google Scholar]


