Abstract
Intraspecific variation in body pigmentation is an ecologically and evolutionary important trait; however, the pigmentation related trade-offs in marine zooplankton are poorly understood. We tested the effects of intrapopulation phenotypic variation in the pigmentation of the copepod Eurytemora affinis on predation risk, foraging, growth, metabolic activity and antioxidant capacity. Using pigmented and unpigmented specimens, we compared (1) predation and selectivity by the invertebrate predator Cercopagis pengoi, (2) feeding activity of the copepods measured as grazing rate in experiments and gut fluorescence in situ, (3) metabolic activity assayed as RNA:DNA ratio in both experimental and field-collected copepods, (4) reproductive output estimated as egg ratio in the population, and (5) total antioxidant capacity. Moreover, mitochondrial DNA (mtDNA) COI gene variation was analysed. The pigmented individuals were at higher predation risk as evidenced by significantly higher predation rate by C. pengoi on pigmented individuals and positive selection by the predator fed pigmented and unpigmented copepods in a mixture. However, the antioxidant capacity, RNA:DNA and egg ratio values were significantly higher in the pigmented copepods, whereas neither feeding rate nor gut fluorescence differed between the pigmented and unpigmented copepods. The phenotypic variation in pigmentation was not associated with any specific mtDNA genotype. Together, these results support the metabolic stimulation hypothesis to explain variation in E. affinis pigmentation, which translates into beneficial increase in growth via enhanced metabolism and antioxidant protective capacity, together with disadvantageous increase in predation risk. We also suggest an alternative mechanism for the metabolic stimulation via elevated antioxidant levels as a primary means of increasing metabolism without the increase in heat absorbance. The observed trade-offs are relevant to evolutionary mechanisms underlying plasticity and adaptation and have the capacity to modify strength of complex trophic interactions.
Introduction
Variation in pigmentation among individuals, populations and species has been associated with various adaptations to predation, ultraviolet radiation (UVR) and diet, but the ultimate mechanisms and trade-offs regulating the observed patterns in the field are still unclear. Studies on pigment variation and its adaptive function in zooplankton have been focused on carotenoids in calanoid copepods [1], [2] and melanisation of daphniids [3], [4], [5]. Most of these studies attributed pigmentation variability to the trade-offs between protection from UVR and predation [6], [7], with weak pigmentation correlating with lower predation pressure but also with lower UVR tolerance [4]. Deeply colored populations are usually found at high-latitude or high-altitude lakes, but pigmentation is also observed in temperate regions [7], [8]. In temperate marine zooplankton, the evidence for the linkage between pigmentation and UVR tolerance is, however, less compelling. For example, the pigmentation does not appear to play a role in UVR tolerance of marine copepods [9], whereas the opposite was observed in crab larvae [10].
In addition to the photoprotective function of pigments, a metabolic stimulation hypothesis has been advocated to explain the adaptive value of pigmentation by providing warmth through absorption of solar radiation [11], [12]. Any increase in body temperature due to energy absorbance by body pigments under light conditions would thus have primarily positive effects, such as increased enzyme activities, metabolic rates and, ultimately, growth. Laboratory experiments have shown that under sunlight, respiration rate increased in pigmented copepods but not in those lacking visible pigmentation [11], [12], and that average pigmentation intensity was inversely related to temperature [6], [12]. This has important implications for zooplankton performing diel vertical migrations (DVM) as demographic disadvantage to zooplankton that spend a daytime at deeper and cooler waters may actually be similar to or greater than the lethal effects of predation [13]. In fishless lakes, pigmented zooplankters remain in productive near-surface waters during the day and have higher reproduction rates [14]. Therefore, by relaxing DVM and increasing pigmentation, a zooplankter can minimize photodamage, enjoy high food availability and reproductive output in the illuminated trophogenic zone, but at the cost of predation by visual planktivores. Although, fish are commonly considered as the only visual predators in aquatic ecosystems, there are marine and freshwater invertebrates capable of visual predation, such as mysids [15] and onychopod cladocerans [13], [16]. The invasive onychopods Cercopagis pengoi and Bythotrephes longimanus have been implicated in the reduction of zooplankton diversity and abundance in the Laurentian Great Lakes [17] and the Baltic Sea [18] as well as in the forcing zooplankton to migrate below thermocline during daytime [13], [18]. By studying intraspecific and intrapopulation variability in pigmentation in relation to growth and vulnerability to predation, we can better understand mechanisms of these trade-offs in specific populations and environmental conditions.
Many zooplankton pigments are important quenching agents and antioxidants that neutralize reactive oxygen species; they also have other biological functions, such as regulatory effects on cell signaling [19]. The changes in the antioxidative status may thus translate to fitness responses, and increase in investment in the antioxidant system can come at a cost of an investment elsewhere, e. g., it may shorten lifespan, decrease growth and reproductive output, weaken immune system, etc. [19], [20]. However, in different species, elevated growth has been linked with both a reduction and an increase in antioxidants [20]. This is related to a complexity of antioxidant systems and interactions amongst various antioxidants, implying that any specific antioxidative compound may fail to indicate the overall defense state. Thus, responses of individual antioxidants are generally more difficult to interpret than those of overall antioxidant capacity when testing specific relationships between antioxidants, metabolic activity and growth, in order to understand mechanisms of trade-offs.
As far as we know, no studies have addressed pigmentation-mediated trade-offs between fitness parameters and vulnerability to predation by invertebrate predators in marine zooplankton. Moreover, whereas genetic basis and developmental mechanisms generating the diversity of pigmentation and color patterns are well appreciated in vertebrates [21], much less is understood regarding genetic versus environmental influence on pigment variation in invertebrate species under various selection pressures acting on pigmentation-related traits. In Daphnia, both genetic and environmental effects were found to influence pigmentation [14], [22], with differences in melanisation among species, clones and mitochondrial DNA (mtDNA) haplotypes [23], [24]. Similarly, a genetic divergence in mtDNA was found between differently pigmented but otherwise morphologically identical varieties in shrimps [25] and ascidians [26].
In our fieldwork in different areas of the Baltic Sea, we were puzzled by variability in pigmentation in the calanoid copepod Eurytemora affinis Poppe, raising questions about the factors that influence pigmentation in this species. This copepod is one of the few dominant mesozooplankton species in the northern Baltic and a preferred prey for the dominant zooplanktivores [27] as well as for C. pengoi [18]. Interestingly, a recent inventory using mtDNA, revealed a high diversity in the Baltic lineages of E. affinis [28]. This species has been suggested to actually comprise a number of cryptic species [29], two of which were recently reported from the Baltic Sea [30], [31]. Given the observed genetic variation in the Baltic Eurytemora, differences in pigmentation between the cryptic species and association between pigmentation and certain mtDNA haplotypes in E. affinis are possible.
In this study, we investigated whether the observed variation in pigmentation is related to interspecific variation between Eurytemora affinis and a morphologically similar species E. carolleeae recently reported from the Gulf of Finland [31]. When the presence of only original Baltic lineage of E. affinis in our collections was confirmed, we examined the intrapopulation variation of the mitochondrial cytochrome oxidase c subunit 1 (COI) gene in relation to pigmentation, and tested effects of this pigmentation on the predation and selectivity by C. pengoi and on the variations in antioxidant capacity, feeding, metabolic activity and reproduction of the copepods.
Methods
Ethics Statement
The sampling was conducted within national Finnish monitoring in the Gulf of Finland and no specific permissions were required for the sampling locations of this study. Also, we did not require ethical approval to conduct this study as we did not handle or collect animals considered in any animal welfare regulations and no endangered or protected species were involved in the samplings or the experiments.
Defining Pigmented and Unpigmented Forms
The term pigmentation is used here to describe the presence of a dark pigment in the exoskeleton of E. affinis. There was a clear distinction between pigmented and unpigmented individuals, with the latter appearing opaque or transparent in transmitted light, without visible dark coloration of any specific area, apart from the eye, ovaries and egg sacks (in females). By contrast, the pigmented individuals had dark brown coloration on either one side of the body or on the both sides; the pigmentation was particularly strong on antennae, ventro-lateral part of cephalothorax and furcal rami (Figure S1).
Sampling Methods, Locations and Sample Preparation
The experimental animals were collected and the experiments were conducted on board R/V ‘Aranda’ (SYKE, Finland) in August 2011 in the eastern and central Gulf of Finland, the northern Baltic Sea (Table 1). Eurytemora affinis and Cercopagis pengoi were collected from 20 m to the surface, using a WP-2 plankton net (mesh size 100 µm) equipped with a cod end. The samples were used to obtain animals for the experiments and to preserve them for different measurements. For predation and grazing experiments, E. affinis individuals with contrasting pigmentation were sorted with a pipette under a dissecting microscope and kept in groups of 10 individuals in 0.2 µm-filtered seawater (FSW) in 0.1 L vials. For the predation experiments, C. pengoi were carefully picked under a dissecting microscope with forceps and wide-mouth pipettes and transferred individually to FSW in 0.1 L vials; only actively swimming individuals were used in the experiments. All specimens were kept at 16°C approximating the temperature in the mixing layer until the experiments commenced. The remaining zooplankton collections were treated as follows. The water was removed as much as possible with a filter paper and the samples were preserved by: (1) freezing at −80°C for measurements of gut fluorescence and antioxidant capacity; and (2) adding RNAlater 10∶1 (v/v) for genetic analysis and measurements of nucleic acid concentrations; these samples were also used for microscopic analysis to estimate sex- and age-related difference in pigmentation and to calculate egg ratio.
Table 1. Details on sampling dates, locations, sample usage and preservation.
| Date | Station | Latitude | Longitude | Species | Experiments and preservation for further analyses |
| 8 Aug | LL6 | N59°53.01 | E025°11.81 | E. affinis | Exp 1 &2, RNAlater |
| 8 Aug | LL5 | N59°55.01 | E25°35.82 | E. affinis | Exp 1 &2 |
| 9 Aug | LL3a | N 60°04.03 | E 26°20.80 | E. affinis, C. pengoi | Exp 1 &2, RNAlater, –80°C |
| 9 Aug | XVI | N60°15.00 | E27°14.82 | E. affinis, C. pengoi | Exp 1 &2, Exp 3 |
| 9 Aug | XIV3 | N60°12.19 | E26°11.57 | E. affinis, C. pengoi | Exp 1 &2, RNAlater, –80°C |
| 10 Aug | GF1 | N59°42.30 | E024°40.93 | E. affinis, C. pengoi | Exp 1 &2, RNAlater, –80°C |
| 10 Aug | F62 | N59°20.01 | E023°15.81 | E. affinis | Exp 3, RNAlater, –80°C |
| 11 Aug | LL9 | N59°42.010 | E024°01.809 | E. affinis | Exp 3 |
| 11 Aug | LL7 | N59°20.010 | E023°15.810 | E. affinis | RNAlater, –80°C |
Exp 1 & 2– Experiments 1 and 2, predation experiments with Cercopagis pengoi preying on Eurytemora affinis; Exp 3– Experiment 3, grazing experiment with E. affinis feeding on Rhodomonas salina; RNAlater – preservation with RNAlater for genetic analysis, measurements of RNA:DNA ratio, sex- and stage-related differences in pigmentation; –80°C – frozen samples used for gut fluorescence and antioxidant capacity measurements. Note that copepods used in the experiments were collected at several stations and pooled. See Material and Methods for measurement details.
Genetic Analyses
Using a 10% Chelex buffer, genomic DNA was extracted from pigmented and unpigmented individuals preserved in RNAlater (25 ind. group−1; pooled from 6 stations; Table 1) and used for amplification of COI (∼570 bp) gene. Polymerase chain reactions (PCR) were conducted using EuF1 and EuR2 primers [28]. The products were separated in 1.5% (w/v) agarose gel with a 100-bp ladder and visualized by staining with ethidium bromide. The PCR products were purified using the QIAquick PCR Purification Kit (QIAGEN) and sequenced in both directions with an ABI 3730 PRISMH DNA Analyzer at KIGene (Karolinska Institute, Stockholm). The resulting nucleotide sequences were aligned using BioEdit software [32] checking electropherograms to ensure proper base calling. The sequences were compared to all Eurytemora sequences reported for the Baltic Sea (Figure S2) and all unique nucleotide sequences have been submitted to the GenBank (accession numbers JQ822115-JQ822130).
Genetic diversity was compared between the pigmentation phenotypes using estimators implemented in DnaSP version 5.10 [33], including the number of haplotypes (K), haplotype diversity (Hd, i.e., probability that two randomly chosen haplotypes are different in the sample) and nucleotide diversity (π, i.e., average number of nucleotide differences per location between two sequences), and diversity per site based on the number of segregating sites (θ). A statistical parsimony haplotype network was constructed to show genetic relatedness among haplotypes using the software package HapStar [34].
Sex- and Age- related Differences in Pigmentation
To test whether the proportion of pigmented individuals differed between males and females (CIV-VI), 25 individuals of each sex per sample were counted in the 6 field samples preserved with RNAlater and the pigmentation status and developmental stage were noted for each individual. When no sex-related difference was found, we examined the variation between the immature (CIV-V) and mature (CVI) individuals using the same counts.
Predation Experiments
We conducted two predation experiments using parthenogenic females of Cercopagis pengoi (instars II and III) as predators and older copepodites (CIV–VI, males and females without egg sacks) of Eurytemora affinis as a prey. In Experiment 1, predation rates (PR) of C. pengoi offered either pigmented or unpigmented copepods were determined, whereas in Experiment 2, prey selection using a mixed (1∶1) assemblage of pigmented and unpigmented copepods was determined. The experiments consisted of 6 replicate incubations for each treatment and were conducted in either 0.1 (Experiment 1) or 0.2 (Experiment 2) L vials under constant illumination with green light at 15°C and lasted ca. 20–22 h. Incubations were started by adding the known number of prey (10 in Experiment 1or 20 in Experiment 2 to keep the same prey concentration in both experiments) to a single C. pengoi. In each experiment, there were two predator-free control incubations with the same number of prey to correct for prey recovery (100% in all cases). Recovered prey were counted directly upon the experiment termination and the pigmentation status of each copepod was noted. Body length (BL, from the top of the head to the base of the caudal claws) of every C. pengoi and prosome length (PL) in a subsample of 20–25 individuals for each E. affinis group were measured under a dissecting microscope. PR was calculated as the difference between the initial and final numbers of prey and scaled to 24 h.
Grazing Experiment
Experiment 3 was conducted to test for differences in grazing, metabolic activity and antioxidant capacity between the copepods with contrasting pigmentation. As food, we used Rhodomonas salina (Cryptophyceae; CCMP 1319) grown on f/2 medium at 17°C and 8‰ salinity in artificial seawater (Instant Ocean™, Aquarium Systems). To minimize algal growth during the experiment and increase accuracy of the grazing rate (GR) estimates, the algae were pre-treated with γ-radiation to prevent cell division [35]. Groups of pigmented and unpigmented copepods (CIV–VI, males and females without egg sacks; 10 ind. vial−1; 6 replicates per treatment) were incubated during 22–24 h in 0.2 L of FSW with 600 µgC L−1 of food; light regime was the same as in the predation experiments. This food concentration is above the saturation level for E. affinis [36] that ensures no decrease in the feeding rate due to prey depletion. To determine algal concentrations (cells mL–1), 15 mL of the media were preserved with acid Lugol’s solution and analyzed using a laser particle counter Spectrex PC-2000 (Spectrex Corp.). The individual GR was determined as a difference between the initial and final algal concentrations in each vial and scaled to 24 h and individual copepod.
Upon termination of the experiment, the copepods were examined with a dissecting microscope, and dead individuals were noted. In all incubations, survivorship was ≥95%, with no significant difference between the pigmented and unpigmented copepods (Kruskal-Wallis statistic = 0.91, p>0.93). Live copepods recovered from each vial were split in two groups: (1) 5 individuals designated for RNA:DNA ratio assay were transferred to Eppendorf tubes containing 100 µL of RNAlater and stored at 4°C [37], and (2) 4–5 individuals designated for antioxidant capacity measurements were transferred to Eppendorf tubes and stored at –80°C.
Gut Fluorescence
Using the frozen zooplankton samples collected in the field, gut pigment concentration (GPC) in Eurytemora affinis (females, CV-VI) was analyzed fluorometrically. After thawing on ice, the copepods were quickly sorted in groups of 5 individuals per pigmentation type; 2 replicate samples per station, 5 stations in total (Table 1). Gut pigments were extracted in 0.5 mL of 90% acetone for 24 h at 4°C in the dark. After centrifugation for 3 min at 3000 rpm, the fluorescence was measured using a Turner Designs TD700 fluorometer with a detection limit of 0.06 µg L–1 of chlorophyll-a (Chl-a) and 0.08 µg L–1 of phaeophytin-a. Fluorescence was measured before and after acidification with HCl and GPC, and Chl-a was calculated as ng Chl-a eq ind –1 [38]. The duplicate samples were averaged to provide a single value per station for each pigmentation type.
RNA:DNA Ratio
This ratio was used as a proxy for overall metabolic activity in the copepods [39]. The nucleic acid concentrations were measured in both field samples preserved in RNAlater (5 stations, LL6 excluded; Table 1) and those collected upon termination of the grazing experiment (6 replicates). For each replicate, a pooled sample of 5 individuals was used to quantify RNA and DNA concentrations (µg ind.−1) using microplate fluorometric high-range RiboGreen (Molecular Probes, Inc. Eugene, OR) assay [40] optimized for Eurytemora affinis of similar size [41]. Test samples, standards, and the negative controls were measured in duplicates using FLUOstar Optima microplate reader in black solid flat-bottom microplates (Greiner Bio-One GmbH) at excitation/emission wavelengths of 485/590 nm (0.2 s well−1, 10 measurements well−1). The DNA:RNA standard curve slope ratio was 1.76.
Total Antioxidant Capacity
These measurements were done using copepod samples obtained in the Experiment 3 (6 replicates) and frozen samples of field-collected copepods that were also used for the GPC (5 stations; Table 1). The total antioxidant capacity was measured as oxygen radical absorbance capacity (ORAC), with fluorescein as a fluorescent probe, AAPH (2,2-azobis(2-amidinopropane) dihydrochloride as a thermal free radical source and Trolox as a standard [42]; all reagents were purchased from Sigma-Aldrich. The copepods (4–5 individuals sample−1) were homogenized in Tris buffer with ∼50 µg of glass beads (<100 µm) for 2 min at 4°C using FastPrep, centrifuged at 15000×g for 15 min at 4°C and then 10 and 30 µL of the supernatant were taken for measurements of ORAC and protein content, respectively. Protein concentration (mg mL−1) was measured using the bicinchoninic acid assay (BCA, Pierce Ltd.) according to the manufacturer’s instructions. The ORAC values were expressed in Trolox-equivalents, µg mg protein−1. All measurements were conducted in duplicates that were averaged for statistical analysis.
Egg Ratio
Egg ratio was calculated using the field samples preserved in RNAlater (6 stations; Table 1). In each sample, the ratio was calculated using ∼50 females for pigmented and unpigmented E. affinis and dividing a cumulative number of eggs by a total number of mature females in each category.
Data Analysis and Statistics
To assess sex- and age-related differences in pigmentation occurrence, Wilcoxon matched-pairs signed-ranks test was used. Differences in PR (Experiment 1) and GR (Experiment 3) between the pigmentation types were evaluated by an unpaired t-test. To test for differences in prey preference, Manly’s α index [43] was calculated for each prey type in the Experiment 2. This estimator has been shown to be asymptotically distributed as a normal random variate [43]; therefore to assess effects of pigmentation on PR and prey preference, we compared PR observed in the mixed prey incubations and mean α values for pigmented and unpigmented prey using a paired t-test.
Pearson’s product-moment and correlation coefficients were calculated for all pairs of measured physiological and biochemical variables. To further evaluate effect of pigmentation on RNA:DNA ratio and ORAC, 2-way ANOVA was used with pigmentation status (pigmented or unpigmented) and animal origin (field or experiment) as categorical predictors. To compare egg ratio and GPC between the pigmentation types, paired t-test was used with pairing by station. Finally, to establish a relationship between the metabolic status and antioxidant capacity and to evaluate effect of pigmentation on this relationship, we used a general linear model (GLM) with the pigmentation status and the animal origin as the categorical variables and ORAC as continuous variables and RNA:DNA ratio as the dependent variable. The data for the correlation and regression analyses were Box-Cox transformed to improve distribution of the residuals. If not specified otherwise, data are presented as means and standard deviations (SD). Statistica version 8.0 (StatSoft, 2007) was used for statistical analyses; the differences were considered significant at p<0.05.
Results
Species Identity and COI Variability in E. affinis
All 50 sequences had high identity (98–100%) to COI sequences reported for the Baltic lineage of Eurytemora affinis from coastal areas of the Gulf of Finland (e.g., Vyborg Bay: HM473964- HM473975, HM473980- HM473982; Luga Bay: HM473994, HM473996- HM473998), but also from other parts of the Baltic Sea (e.g., Swedish coastal waters: JF727517- JF727523; Gulf of Riga: HM474023, HM474026- HM474027); see Figure S2. By contrast, there were only 87–88% identity to the sequences representing E. carolleeae of North American origin (Luga Bay: HM474011- HM474012, HM474003; Neva Bay: HM474029). Therefore, all species in our collections were assumed to belong to the Baltic lineage of E. affinis.
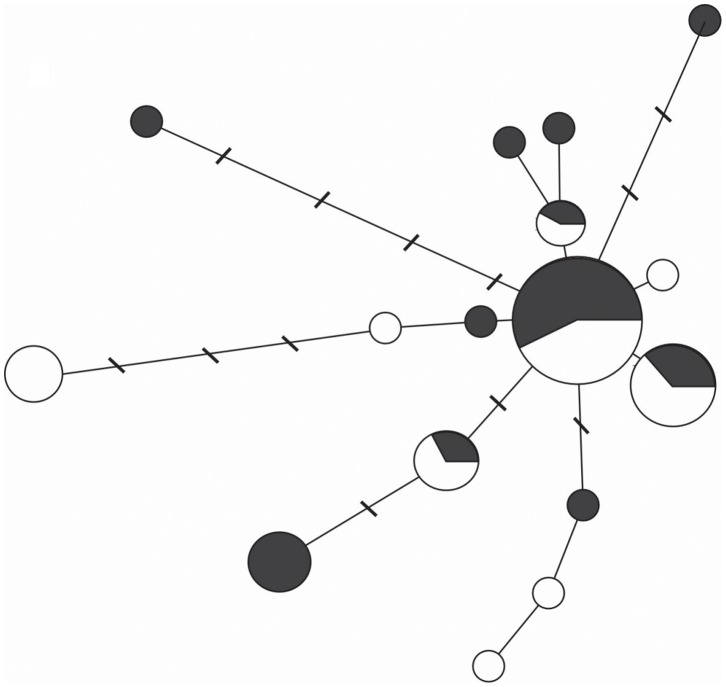
The aligned COI sequence dataset contained 16 unique haplotypes with 20 polymorphic sites sampled across 571–574 bp. In both pigmentation groups, many closely related haplotypes co-existed, with high haplotype diversity and low nucleotide diversity (Table 2). Four haplotypes were shared between the groups, whereas 7 and 5 haplotypes were private for the pigmented and unpigmented copepods, respectively (Fig. 1). There were two prevalent haplotypes, while rare haplotypes were in general one to three mutational steps apart from the prevalent haplotypes.
Table 2. Eurytemora affinis.
| Pigmentation type | K | Hd | π | θ |
| Pigmented | 11 | 0.860 | 0.0042 | 0.0074 |
| Unpigmented | 9 | 0.863 | 0.0039 | 0.0051 |
| Total | 16 | 0.859 | 0.0041 | 0.0077 |
Measures of genetic diversity of the cytochrome c oxidase subunit I (COI) in the copepods with different pigmentation type.
Number of haplotypes (K), haplotype diversity (Hd) and nucleotide diversity (π), and diversity per site (θ).
Figure 1. Minimum spanning tree showing relationships among haplotypes of pigmented (black) and unpigmented (white) Eurytemora affinis.
Size of circle is proportional to the frequency of the haplotype. Slash marks indicate the number of nucleotide substitutions between haplotypes; no slash marks present indicates a single substitution separating haplotypes.
Sex- and Age- related Differences in Pigmentation
Neither sex- (Wilcoxon matched-pairs signed-ranks test: p>0.8) nor age-related (p>0.5) differences in pigmentation were significant. The proportion of the pigmented individuals varied from 25% to 42% among the stations.
Predation on Pigmented and Unpigmented Eurytemora
In the single prey type experiment, the effect of pigmentation on PR was significant (unpaired t-test; t10 = 3.503, p<0.006; Fig. 2A), with the pigmented copepods being consumed at ∼4-fold higher rate than unpigmented ones. Similarly, in the mixed prey experiment, the effect of pigmentation on PR was significant (paired t-test; t5 = 5.000, p<0.003; Fig. 2A), with the pigmented copepods being consumed at ∼2-fold higher rate than unpigmented ones. This was reflected by the significantly higher Manly’s α for the pigmented individuals in the mixed prey incubations (paired t-test; t10 = 2.72, p<0.03; Fig. 2B) indicating that pigmentation increases vulnerability to predation. The observed differences were not related to body size variations in either the predator (C. pengoi BL: t48 = 0.97, p>0.43) or the prey (copepod PL: t48 = 1.01, p>0.32).
Figure 2. Predation rate and prey preference by Cercopagis pengoi offered pigmented and unpigmented Eurytemora affinis in feeding trials (Experiments 1 and 2).
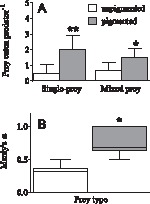
(A) predation rate (PR) in single-prey incubations (Experiment 1; mean ±SD; n = 12), and (B) prey preference index (Manley’s α) estimated from the mixed-prey incubations (Experiment 2; Tukey’s box and whisker plot, n = 12); the index ranges from 0 to 1, with higher values indicating greater preference. Shown are the median value (horizontal line), 25% to 75% response ranges (top and bottom lines of boxes) and minima and maxima (whiskers). Asterisk indicate a significant difference (p<0.05) in preference for the pigmented copepods when compared to those without visible pigmentation.
Copepod Grazing Rate in the Experiment and Gut Fluorescence in situ
There was no significant difference in GR between the pigmented and unpigmented copepods fed Rhodomonas in Experiment 3 (unpaired t-test: t10 = 1.40, p>0.19; Fig. 3A). Similarly, GPC in field collected individuals showed no significant difference between the pigmentation types (paired t-test: t4 = 0.78, p>0.49; Fig. 3A), with mean values varying from 0.02 to 0.07 ng Chl-a eq ind –1.
Figure 3. Feeding, growth, reproduction and antioxidant capacity in pigmented and unpigmented Eurytemora affinis in field and experimental studies.
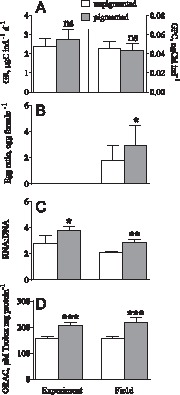
(A) grazing rate (GR; left axis, n = 12) on Rhodomonas salina and gut content fluorescence (GCF; right axis, n = 10) measured in the field collected copepods; (B) egg ratio in the samples collected in the study area (n = 12); (C) RNA:DNA ratio in the copepods (Experiment 3: n = 12; field samples: n = 10), and (D) total antioxidant capacity assayed as ORAC (Experiment 3: n = 12; field samples: n = 10). All data are shown as mean ±SD.
Egg Ratio, Metabolic State and Total Antioxidant Capacity
Pigmentation had significantly positive effects on egg ratio in the field-collected E. affinis (paired t-test: t5 = 3.91, p<0.02; Fig. 3B). Similarly, there were significantly higher values for RNA:DNA ratio (2-way ANOVA; F1,20 = 16.39, p<0.007; Fig. 3C) and ORAC values (F1,18 = 107.3, p<0.0001; Fig. 3D) in the pigmented copepods. Moreover, the experimental conditions with ad libitum feeding affected positively the RNA:DNA ratio compared to the field-collected copepods (F1,20 = 14.02, p<0.002), whereas no significant differences in ORAC values between the animals used in the experiment and those in the field samples were observed (F1,18 = 1.505, p>0.3). In no case the interaction term was significant (p<0.4 in all cases).
There were significant positive correlations between ORAC and growth indices, but not with food intake (Table 3). Finally, there was a significant relationship between the RNA:DNA ratio and ORAC (GLM; F1,19 = 4.98, p<0.04), with a slope being significantly higher in animals used in Experiment 3 compared to the field samples (F1,19 = 15.33, p<0.002), whereas pigmentation did not affect the relationship (F1,19 = 0.93, p>0.8).
Table 3. Eurytemora affinis.
| Variable | RNA:DNA | Egg ratio | GR | GCF |
| ORAC | 0.59 (22) | 0.63 (10) | 0.44 (12) | 0.53 (10) |
| RNA:DNA | 0.61 (10) | 0.46 (12) | 0.51 (10) | |
| Egg ratio | 0.44 (10) |
Correlation matrix with Pearson correlation coefficients among the total antioxidant capacity (ORAC), growth indices (RNA:DNA ratio and egg ratio), and food intake (GR and GCF).
ORAC: oxygen radical absorbance capacity (Experiment 3 and field collected copepods), GR: grazing rate (Experiment 3), egg ratio: number of eggs per female in a sample (field collected copepods), GCF: gut content fluorescence (field collected copepods). Significant correlations (p<0.05) are in bold face. Data were Box-Cox transformed to approach normal distribution. Number of samples is shown in parentheses.
Discussion
In Eurytemora affinis, pigmentation carries both penalty in terms of the greater vulnerability to predation and fitness advantage in terms of enhanced growth, reproduction and metabolism. Three crucial results support this conclusion. First, higher predation rates and positive selectivity for the pigmented copepods were observed in the predation experiments with the visual predator Cercopagis pengoi. Second, growth-related variables (egg ratio and RNA:DNA ratio) were consistently higher in the pigmented copepods, whereas the food intake was similar between the pigmentation types. Consequently, the higher growth output in the pigmented individuals at a given feeding rate implies higher growth efficiency, the capacity to convert ingested energy into biomass. Third, the pigmented copepods had higher antioxidant capacity, suggesting that this phenotype is superior in handling excessive formation of free-radicals, which is often a primary cause or a downstream consequence of tissue damage due to various environmental stressors, including predation pressure [44]. These findings have important implications for food web interactions as increased contribution of the pigmented forms in the population would have positive effects on energy transfer from primary producers to secondary consumers and prey quality, in terms of its antioxidant content, for zooplanktivores.
Our results that pigmented individuals have higher egg production, metabolic activity and antioxidant capacity lend support to the metabolic stimulation hypothesis [12]. Despite experimental evidence that pigmentation enhances survival of zooplankton exposed to high irradiances, field studies often show lack of significant relationships with irradiance, but strong inverse relationships with temperature [6], [12]. In Eurytemora affinis, juvenile development is highly temperature dependent in the range of 7 to 18°C [45], i.e., common summer temperatures in the trophogenic zone in the Baltic Sea. Therefore, increased metabolic stimulation through absorption of solar radiation would translate into increased individual and population growth. More recently, a field study reporting lack of a direct relationship between levels of photoprotective compounds in freshwater copepods and water column radiation provided further support to Byron’s hypothesis [46]. The metabolic stimulation hypothesis was, however, criticized, arguing that the possible temperature gain is insignificant due to the high heat transfer in small-sized planktonic animals [47].
We suggest an alternative mechanism for the metabolic stimulation that could involve elevated antioxidant levels as a primary means of increasing metabolism without temperature increase. As physiological functions of pigments include antioxidant protection, the observed higher total antioxidant capacity (Fig. 3D) in the pigmented copepods is likely to simply reflect higher concentration of pigments contributing to the higher antioxidant defences in this phenotype [48]. Recently, a number of studies have attempted to reveal the relationships between antioxidant capacity and fitness components in various animals [20]. Although the connections appear to be stage- and species-specific, higher antioxidant levels were linked with larger clutches [49], greater reproductive success [50], improved offspring quality [51], and increased life span [52]. The observed positive associations between ORAC levels and growth-related variables (reproductive output and RNA:DNA ratio) with no apparent correlation to the food intake (Table 3) provides further evidence for the positive effects of antioxidant capacity on the copepod fitness. Moreover, the relationship between ORAC and RNA:DNA ratio was not phenotype-specific, indicating common underlying mechanisms.
The apparent lack of costs for pigment synthesis is in disagreement with experimental studies on zooplankton inhabiting high-UVR lakes and suffering growth penalties related to production of photoprotective compounds [4], [23]. In our study, the in situ growth conditions for copepods were suboptimal as indicated by the higher RNA:DNA ratio of the copepods incubated at ad libitum food levels in the grazing experiment compared to the field-collected copepods (Fig. 3B); hence, it is difficult to assess costs because each phenotype is likely to face own challenges. Moreover, our study design might have failed to detect an existing cost: we measured feeding either in the surplus of high quality food in the absence of competition or external stressors (Experiment 3) or using a gut fluorescence (field-collected copepods, pooled samples) without any knowledge on the gut evacuation governed by ambient temperature and food levels. Fitness trade-offs can be obscured in the presence of abundant resources, whereas ambient conditions may differ in multiple variables, such as temperature, food quality and quantity, particularly if the pigmentation phenotypes resided at different depths.
Our study was not designed to address UVR effects on pigmentation in Eurytemora affinis, because it seemed unlikely that these effects would be ecologically relevant in the system studied. Indeed, detrimental UVR effects on copepods were found to be restricted to the first meter of the water column [53], particularly in sea areas with high primary productivity. The actual levels of UVR that summer zooplankton communities in the Baltic Sea are exposed to are relatively low compared to the oligotrophic and/or high elevation shallow lakes in Scandinavia, with high light penetration and a lack of depth refuge from UVB exposure for zooplankton. In the Baltic Sea, plankton communities are more protected from UV exposure, because of the greater depth and high concentrations of colored dissolved organic matter contributing to high attenuation [53]. In the coastal areas, a typical 1% penetration depth for UV-B is ∼0.1 m in inner bays and 0.3 m in outer areas, whereas near infrared wavelength range that contributes most to the temperature-mediated metabolic stimulation reaches deepest in the water column [54]. Moreover, our study was conducted during a filamentous cyanobacteria bloom, which substantially increases water turbidity (Secchi depth <4.5 m) and thus decreases UVR penetration in the water column. Recently, a counter-intuitive contrast in pigmentation of the copepod Arctodiaptomus spinosus in shallow fishless lakes has been reported, with unpigmented copepods inhabiting macrophyte-dominated clear water lakes and pigmented forms occurring in highly turbid and phytoplankton-rich lakes [55]. While the penetration of UV radiation alone failed to explain the differences in copepod pigmentation among these lakes, several possible stressors, such as wind-induced turbulence combined with short-term sunlight exposure and crowding, were advocated in explaining this pattern [55]. In particular, the pigment accumulation in the crowded populations occurring in the turbid lakes was suggested to be a support for immune defense in copepods [55], [56]. Thus, high UVR exposure is not the only factor favoring carotenoid accumulation in zooplankton. Nevertheless, the UVR effects on the copepod pigmentation in the Baltic Sea cannot be ruled out completely. It could be hypothesized, for example, that unpigmented phenotype (∼70% of the population) would be more sensitive to UVR avoiding illuminated waters during daytime, whereas pigmented individuals (∼30%) would stay in the more productive upper part of the water column and produce more offspring – all these at the cost of predation risk. In this context, examining the vertical distribution of copepods with contrasting pigmentation pattern, would help in (1) understanding proximate causes of DVM, (2) establishing linkages between pigment production and vertical position of the copepods in the water column, and (3) revealing energetic costs related to migratory activity, which would allow to compare growth efficiencies between the phenotypes and to evaluate the trade-offs involved.
Whereas defence against predators is usually accompanied by declining rates of growth or development in prey organisms, the underlying physiological responses remain little understood and often explained by reduced feeding and/or increased metabolic costs due to the fight-or-flight response [57]. In our case, the lower egg ratio in the unpigmented E. affinis cannot be explained by reduced foraging under predation risk as indicated by similar gut pigment content. Instead, we suggest that when predation pressure is high, the unpigmented copepods have higher survival rate, but also lower antioxidant capacity and reduced fitness. In line with this, predation-induced decrease in antioxidants has been observed in copepods [56] and damselfly [44], resulting in a weakened immune status and a growth reduction, respectively. It remains to be studied, whether each individual adjust the pigmentation and related antioxidant reserves to the prevailing predation risk according to its individual costs and benefits or, alternatively, whether copepods with different pigmentation suffer differential mortality as a result of selective predation on specific genotypes.
Although, mitochondrial polymorphisms have been found to be linked with pigmentation patterns in various species (e.g., cladocerans [24], shrimps [25], insects [58], fish [59]), there was no association between pigmentation and mtDNA genotypes in the Baltic Eurytemora affinis. This does not, however, imply an absence of genetic differences between the copepods with different pigmentation phenotypes, both with regard to genes responsible for positioning pigments in space and time and those responsible for synthesis of pigments. Recently, the predation and photoprotection concepts have been integrated [60], demonstrating that zooplankters are plastic in their pigmentation, making a trade-off between high and low pigmentation in relation to the prevalent fish/UV threat ratio [2], [8]. What remains to be integrated in this theory is the genetic basis of pigmentation, and this calls for a new approach to study adaptive variation in production of various pigments and their relative importance in different species and systems. Indeed, in some cases, pigmentation polymorphism could be genetically determined, in others, it is a polyphenism, a developmental difference in pigmentation pattern cued by physical or chemical signals. With regard to dietary pigments, such as carotenoids and mycosporine-like amino acids, the latter has been found to be the case [5], [56]. However, both production of the cuticular pigments and pigment-dispersing hormone activities are under genetic control [61], [62] and, therefore, E. affinis pigmentation could be an example of a genetic system that leads to multiple fitness peaks under multiple selection pressures.
In this respect, E. affinis could represent a particularly suitable model, due to its occurrence worldwide and high evolutionary diversification [28], [29], [63]. Together, the observed low nucleotide-diversity and high haplotype-diversity indicate a co-existence of a high number of closely related haplotypes (Fig. 1), which is indicative of a recent population expansion. These findings are in agreement with the results of a pairwise haplotype mismatch analysis [28] suggesting that Baltic E. affinis has undergone a recent expansion. High genetic diversity in such populations is the raw material for natural selection, and therefore, potentially underpins fitness and/or adaptive capacity. Recently, a compelling evidence has been presented on the heritable genetic variation of pigmentation as a fitness-related trait in populations of snakes [64] and isopods [65]. Given the involvement of pigmentation in fitness traits of E. affinis and a likely selection by predation, understanding genetic basis of pigmentation in this species may provide a more comprehensive view on ecological and evolutionary significance of pigmentation patterns in copepods from temperate estuarine systems, which might be different from those in clear lakes exposed to high UVR levels.
Supporting Information
A schematic drawing showing pigmentation pattern in pigmented (A) and unpigmented (B) Eurytemora affinis .
(PDF)
Maximum Likelihood (ML) tree for Eurytemora affinis sequences reported from the Baltic Sea.
(PDF)
Acknowledgments
We thank crew of R/V Aranda for help in sampling, Jon Duberg and Lisa Mattsson (Stockholm University) for sharing their collections and providing laboratory assistance, and Prof. Ragnar Elmgren for fruitful discussions.
Funding Statement
The financial support was provided by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), Walter and Andrée de Nottbeck Foundation, and Stockholm University’s strategic marine environmental research program “Baltic Ecosystem Adaptive Management”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript,
References
- 1. Hairston NG (1979) The adaptive significance of color polymorphism in two species of Diaptomus (Copepoda). Limnol Oceanogr 24: 15–37. [Google Scholar]
- 2. Hylander S, Boeing WJ, Grane’li W, Karlsson J, von Einem J, et al. (2009) Complementary UV protective compounds in zooplankton. Limnol Oceanogr 54: 1883–1893. [Google Scholar]
- 3. Hebert PDN, McWalter DB (1983) Cuticular pigmentation in Arctic Daphnia: Adaptive diversification of asexual lineages? Amer Nat 122: 286–291. [Google Scholar]
- 4. Hessen DO (1996) Competitive trade-off strategies in Arctic Daphnia linked to melanism and UV-B stress. Polar Biol 16: 573–576. [Google Scholar]
- 5. Hansson LA, Hylander S, Sommaruga R (2007) Escape from UV threats in zooplankton: a cocktail of behavior and protective pigmentation. Ecology 88: 1932–1939. [DOI] [PubMed] [Google Scholar]
- 6. Hairston NG (1979) The effect of temperature on carotenoid photoprotection in the copepod Diaptomus nevadensis . Comp Biochem Physiol 62A: 445–448. [Google Scholar]
- 7. Hansson LA (2000) Induced pigmentation in zooplankton: a trade-off between threats from predation and ultraviolet radiation. Proc R Soc Lond B 267: 2327–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansson LA (2004) Plasticity in pigmentation induced by conflicting threats from predation and UV radiation. Ecology 85: 1005–1016. [Google Scholar]
- 9. Speekmann CL, Bollens SM, Avent SR (2000) The effect of ultraviolet radiation on the vertical distribution and mortality of estuarine zooplankton. J Plankton Res 22: 2325–2350. [Google Scholar]
- 10. Morgan SG, Christy JH (1996) Survival of marine larvae under the countervailing selective pressures of photodamage and predation. Limnol Oceanogr 41: 498–504. [Google Scholar]
- 11. Byron ER (1981) Metabolic stimulation by light in a pigmented freshwater invertebrate. Proc Natl Acad Sci USA 78: 1765–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byron ER (1982) The adaptive significance of calanoid copepod pigmentation: a comparative and experimental analysis. Ecology 63: 1871–1886. [Google Scholar]
- 13. Pangle KL, Peacor SD, Johannsson OE (2007) Large nonlethal effects of an invasive invertebrate predator on zooplankton population growth rate. Ecology 88: 402–412. [DOI] [PubMed] [Google Scholar]
- 14. De Meester L, Beenaerts N (1993) Heritable variation in carotenoid content in Daphnia magna . Limnol Oceanogr 38: 1193–1199. [Google Scholar]
- 15. Ramcharan CW, Sprules WG (1986) Visual predation in Mysis relicta Loven. Limnol Oceanogr 31: 414–420. [Google Scholar]
- 16.Rivier IK (1998) The predatory Cladocera (Onychopoda: Podonidae, Polyphemidae, Cercopagidae) and Leptodorida of the world. In Dumont H, editor. Guides to the Identification of the Micro-invertebrates of the Continental Waters of the World 13. Backhuys Publishers, Leiden. 213 p.
- 17. Yan ND, Girard R, Boudreau S (2002) An introduced invertebrate predator (Bythotrephes) reduces zooplankton species richness. Ecol Lett 5: 481–485. [Google Scholar]
- 18. Lehtiniemi M, Gorokhova E (2008) Predation of the introduced cladoceran Cercopagis pengoi on the calanoid copepod Eurytemora affinis in the Gulf of Finland, Baltic Sea. Mar Ecol Prog Ser 362: 193–200. [Google Scholar]
- 19.Sies H, Stahl W (2005) New horizons in carotenoid research. In Packer L, Obermuller-Jevic U, Kraemer K, Sies H, editors. Carotenoids and Retinoids: Molecular aspects and health issues. AOCS Press, Urbana, IL, 315–320.
- 20. Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12: 75–92. [DOI] [PubMed] [Google Scholar]
- 21. Hubbard JK, Uy JAC, Hauber ME, Hoekstra HE, Safran RJ (2010) Vertebrate pigmentation: from underlying genes to adaptive function. Trends Genet 26: 231–239. [DOI] [PubMed] [Google Scholar]
- 22. Gerrish GA, Caceres CE (2003) Genetic versus environmental influence on pigment variation in the ephippia of Daphnia pulicaria . Freshwat Biol 48: 1971–1982. [Google Scholar]
- 23. Hebert PDN, Emery A (1990) The Adaptive Significance of Cuticular Pigmentation in Daphnia . Functional Ecol 4: 703–710. [Google Scholar]
- 24. Van Raay TJ, Crease TJ (1995) Mitochondrial DNA diversity in an apomictic Daphnia complex from the Canadian High Arctic. Mol Ecol 4: 149–161. [DOI] [PubMed] [Google Scholar]
- 25. Tsoi KH, Wang ZY, Chu KH (2005) Genetic divergence between two morphologically similar varieties of the kuruma shrimp Penaeus japonicus . Mar Biol 147: 367–379. [Google Scholar]
- 26. Tarjuelo I, Posada D, Crandall A, Pascual M, Turon X (2004) Phylogeography and speciation of colour morphs in the colonial ascidian Pseudodistoma crucigaster. Mol Ecol 13: 3125–3136. [DOI] [PubMed] [Google Scholar]
- 27. Viherluoto M, Viitasalo M (2001) Effect of light on the feeding rates of pelagic and littoral mysid shrimps: a trade-off between feeding success and predation avoidance. J Exp Mar Biol Ecol 261: 237–244. [DOI] [PubMed] [Google Scholar]
- 28. Winkler G, Souissi S, Poux C, Castric V (2011) Genetic heterogeneity among Eurytemora affinis populations in Western Europe. Mar Biol 158: 1841–1856. [Google Scholar]
- 29. Lee CE, Frost BW (2002) Morphological stasis in the Eurytemora affinis species complex (Copepoda: Temoridae). Hydrobiologia 480: 111–128. [Google Scholar]
- 30. Alekseev VR, Abramson NI, Sukhikh NM (2009) Introduction of sibling species to the ecosystem of the Baltic Sea. Dokl Biol Sci 429: 544–547. [DOI] [PubMed] [Google Scholar]
- 31. Alekseev VR, Souissi A (2011) A new species within the Eurytemora affinis complex (Copepoda: Calanoida) from the Atlantic Coast of USA, with observations on eight morphologically different European populations. Zootaxa 2767: 41–56. [Google Scholar]
- 32. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid Symp Ser 41: 95–98. [Google Scholar]
- 33. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 34. Teacher AGF, Griffiths DJ (2011) HapStar: automated haplotype network layout and visualization. Mol Ecol Res 11: 151–153. [DOI] [PubMed] [Google Scholar]
- 35. Gorokhova E, Mattsson L, Sundström AM (2012) A comparison of TO-PRO-1 iodide and 5-CFDA-AM staining methods for assessing viability of planktonic algae with epifluorescence microscopy. J Microbiol Methods 89: 216–221. [DOI] [PubMed] [Google Scholar]
- 36. Ban S (1994) Effect of temperature and food concentration on post-embryonic development, egg production and adult body size of calanoid copepod Eurytemora affinis . J Plankton Res 16: 721–735. [Google Scholar]
- 37. Gorokhova E (2005) Effects of preservation and storage of microcrustaceans in RNAlater on RNA and DNA degradation. Limnol Oceanogr: Methods 3: 143–148. [Google Scholar]
- 38.Båmstedt U, Gifford DJ, Irigoien X, Atkinson A, Roman M, et al.. (2000) Feeding. In: Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M, editors. ICES Zooplankton Methodology Manual. Academic Press, London, 297–380.
- 39. Holmborn T, Lindell K, Holeton C, Hogfors H, Gorokhova E (2009) Biochemical proxies for growth and metabolism in Acartia bifilosa (Copepoda, Calanoida). Limnol Oceanogr: Methods 7: 785–794. [Google Scholar]
- 40. Gorokhova E, Kyle M (2002) Analysis of nucleic acids in Daphnia: development of methods and ontogenetic variations in RNA-DNA content. J Plankton Res 24: 511–522. [Google Scholar]
- 41. Höök T, Gorokhova E, Hansson S (2008) RNA:DNA ratios of Baltic herring larvae and copepods in embayment and open sea habitats. Estuar Coast Shelf Sci 76: 29–35. [Google Scholar]
- 42. Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, et al. (2003) Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J Agricult Food Chem 51: 3273–3279. [DOI] [PubMed] [Google Scholar]
- 43. Manly BFJ (1974) A model for certain types of selection experiments. Biometrics 30: 281–294. [Google Scholar]
- 44. Slos S, Stoks R (2008) Predation risk induces stress proteins and reduces antioxidant defense. Funct Ecol 22: 637–642. [Google Scholar]
- 45. Vuorinen I (1982) The effect of temperature on the rates of development of Eurytemora hirundoides (Nordquist) in laboratory culture. Ann Zool Fenn 19: 129–134. [Google Scholar]
- 46. Garcia PE, Perez AP, Dieguez MC, Ferraro MA, Zagarese HE (2008) Dual control of the levels of photoprotective compounds by ultraviolet radiation and temperature in the freshwater copepod Boeckella antiqua . J Plankton Res 30: 817–827. [Google Scholar]
- 47. Hairston NG Jr (1981) The interaction of salinity, predators, light and copepod color. Hydrobiologia 81: 151–158. [Google Scholar]
- 48. McGraw KJ (2005) The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colourants? Animal Behaviour 69: 757–764. [Google Scholar]
- 49. Bize P, Devevey G, Monaghan P, Doligez B, Christe P (2008) Fecundity and survival in relation to resistance to oxidative stress in a free living bird. Ecology 89: 2584–2593. [DOI] [PubMed] [Google Scholar]
- 50. Safran RJ, McGraw KJ, Wilkins MR, Hubbard J, Marling J (2010) Positive carotenoid balance over the breeding season predicts reproductive performance in a wild bird. PLoS ONE 5: e9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Biard C, Surai PF, Møller AP (2005) Effects of carotenoid availability during laying on reproduction in the blue tit. Oecologia 144: 32–44. [DOI] [PubMed] [Google Scholar]
- 52. Martin I, Grotewiel MS (2006) Oxidative damage and age-related functional declines. Mech Ageing Dev 127: 411–423. [DOI] [PubMed] [Google Scholar]
- 53. Browman HI, Rodriguez CA, Beland F, Cullen JJ, Davis RF, et al. (2000) Impact of ultraviolet radiation on marine crustacean zooplankton and ichthyoplankton: a synthesis of results from the estuary and Gulf of St. Lawrence, Canada. Mar Ecol Prog Ser 199: 293–311. [Google Scholar]
- 54. Schubert H, Sagert S, Forster RM (2001) Evaluation of the different levels of variability in the underwater light field of a shallow estuary. Helgol Mar Res 55: 12–22. [Google Scholar]
- 55. Schneider T, Herzig A, Koinig KA, Sommaruga R (2012) Copepods in turbid shallow soda lakes accumulate unexpected high levels of carotenoids. PLoS ONE 7: e43063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van der Veen IT (2005) Costly carotenoids: a trade-off between predation and infection risk? J Evol Biol 18: 992–999. [DOI] [PubMed] [Google Scholar]
- 57. Creel S, Christianson D (2008) Relationships between direct predation and risk effects. Trends Ecol Evol 23: 194–201. [DOI] [PubMed] [Google Scholar]
- 58. Brisson JA, De ToniDC, Duncan I, Templeton AR (2005) Abdominal Pigmentation Variation in Drosophila polymorpha: Geographic Variation in the Trait, and Underlying Phylogeography. Evolution 59: 1046–1059. [PubMed] [Google Scholar]
- 59. Rodríguez-Graña L, Herrera G, Herrera L, Castro LR (2004) Divergence of two forms of Triphoturus in the eastern Pacific based on mtDNA cytochrome b gene sequences and larval morphology. J Fish Biol 64: 1455–1461. [Google Scholar]
- 60. Williamson GE, Fischer JM, Bollens SM, Overholt EP, Breckenridge JK (2011) Toward a more comprehensive theory of zooplankton diel vertical migration: Integrating ultraviolet radiation and water transparency into the biotic paradigm. Limnol Oceanogr 56: 1603–1623. [Google Scholar]
- 61.Stevenson J R (1985) Dynamics of the integument. In Bliss DE, Mantel LH, editors. The Biology of Crustacea, Vol. 9, Integument, Pigments, and Hormonal Processes, Academic Press, New York. Pp. 1–42.
- 62. Rao KR, Riehm JP (1988) Pigment-dispersing hormones: A novel family of neuropeptides from arthropods. Peptides 9 Suppl (1) 153–159. [DOI] [PubMed] [Google Scholar]
- 63. Winkler G, Dodson JJ, Lee CE (2008) Heterogeneity within the native range: population genetic analyses of sympatric invasive and noninvasive clades of the freshwater invading copepod Eurytemora affinis . Mol Ecol 17: 415–430. [DOI] [PubMed] [Google Scholar]
- 64. Westphal MF, Massie JL, Bronkema JM, Smith BE, Morgan TJ (2011) Heritable Variation in Garter Snake Color Patterns in Postglacial Populations. PLoS ONE 6(9): e24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Protas ME, Trontelj P, Patel NH (2011) Genetic basis of eye and pigment loss in the cave crustacean, Asellus aquaticus . Proc Natl Acad Sci 108: 5702–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A schematic drawing showing pigmentation pattern in pigmented (A) and unpigmented (B) Eurytemora affinis .
(PDF)
Maximum Likelihood (ML) tree for Eurytemora affinis sequences reported from the Baltic Sea.
(PDF)