Abstract
Background
Physical activity (PA) is an important modifiable risk factor for both bone mineral density (BMD) and body mass index (BMI). However, BMI is itself strongly predictive of BMD. Our aim was to determine the association between PA and BMD, with consideration of BMI as a potential mediating factor.
Methods
The Canadian Multicentre Osteoporosis Study (CaMos) is a population-based prospective cohort study of Canadian women and men. PA was determined from interviewer-administered questionnaires at baseline and Year 5 and summarized as daily energy expenditure in total metabolic equivalents of the task multiplied by minutes/day (MET*m/d). Height, weight, and total hip and lumbar spine BMD were measured at baseline and Year 5. General linear models assessed relationships between PA and BMD, both cross-sectionally (baseline PA with baseline BMD) and longitudinally (average PA and change in PA with change in BMD). BMI was considered as a mediating factor. Potential confounders included age, center, education, caffeine intake, alcohol exposure, smoking history, history of weight-cycling, age at menarche, past use of oral contraceptives, history of >3 months missed menstruation, menopausal status, and antiresorptive use, as relevant.
Results
The study included 2855 men and 6442 women. PA was inversely associated with BMI at baseline, and an increase in PA between baseline and Year 5 was associated with a decrease in BMI, with 0.41 (95% CI: 0.22, 0.60) kg/m2 loss per 1000 MET*m/d increase (in men) and 0.40 (95% CI: 0.23, 0.57) kg/m2 loss per 1000 MET*m/d increase (in women). BMI was strongly associated with BMD, both cross-sectionally and longitudinally. However, increased PA was associated with a small increase in total hip BMD, 0.004 (95% CI: 0.000–0.008) g/cm2 per 1000 MET*m/d (in men) and 0.003 (95% CI: 0.000–0.007) g/cm2 per 1000 MET*m/d (in women). Average PA was associated with an increase in lumbar spine BMD in women, but not in men; it was not associated with change in total hip BMD in either sex.
Conclusion
Increased PA is associated with an increase in BMD and a concomitant decrease in BMI. These findings suggest that population-level interventions to increase PA would favorably impact bone and other health outcomes.
Keywords: Physical Activity, Body Mass Index, Bone Mineral Density, Walking, Strenuous Exercise, Population-based
INTRODUCTION
Osteoporotic fractures are a major public health concern. Identification of modifiable factors that can reduce fractures is important for healthy aging and reducing the social, medical and personal costs of fracture. Physical activity (PA) is recognized as a potentially important modifiable factor affecting bone mineral density (BMD), bone accrual and loss, and the risk of fracture [1]. PA directly modulates bone remodeling through mechanical stimuli which result in improvements in both material properties (i.e. increased mineralization) and bone geometry (i.e. increased periosteal diameter and cortical thickness) [2], thereby decreasing the risk of fracture.
PA is often quantified using the metabolic equivalents (MET) of a particular task: most commonly as a total of daily energy expenditure over all activities in MET-minute per day (MET*m/d). PA questionnaires may evaluate occupational activity, leisure and sport activity, or activity in daily life, including household chores and walking. Variations in moderate PA, such as walking, have been shown to be more important in determining daily energy expenditure (measured with doubly-labeled water) than high-intensity activities such as competitive sports [3]. Finally, there is increasing interest in moderate-intensity physical activities such as walking, that are more easily adopted by less active people and that are accessible, safe, and can be continued into old age.
The evidence to support a positive role for PA on bone health and fracture prevention comes mainly from studies among postmenopausal women. Cross-sectional studies of middle-aged and older women have found both significant positive [4–6] and non-significant [7–9] associations between PA and BMD. Longitudinal studies have likewise shown mixed results [10, 11]. A meta-analysis of exercise intervention studies has shown that a range of physical activities is associated with at least some reduction in bone loss among postmenopausal women [12]. For men, a review of the literature concluded that PA has bone-positive effects, but this conclusion was largely based on smaller and less representative populations [13]. Thus, evidence is inconsistent on the relationship between PA and longitudinal BMD changes in older women and men, and studies have not included longitudinal measurements in population-based studies of men and women with additional demographic data relevant to public health stakeholders. Demographic information can help to identify groups at risk for which adherence to guidelines can be improved. Another limitation of the existing literature is that only one of the two strongly correlated outcomes of BMI and BMD have typically been considered. Bone loss is frequently concurrent with weight loss [14]. Given the current urgent focus on obesity, it is important to know whether losing weight is necessarily associated with concurrent bone loss, and whether factors such as PA might modify the risk for bone loss with needed weight loss.
We used data from the Canadian Multicentre Osteoporosis Study (CaMos), a population-based cohort study in nine urban Canadian centers, to explore the both the cross-sectional and longitudinal associations between PA and BMD, with consideration of BMI as an important mediating factor.
METHODS
The recruitment, methods and study design for the Canadian Multicentre Osteoporosis Study (CaMos) have been previously published [15], with further information available at www.camos.org. Briefly, CaMos is an ongoing, prospective, age- and sex-stratified population-based cohort study of women and men enrolled from 1995–97, who were at least 25 years old, community-dwelling, and living within a 50 km radius of nine Canadian sites. Households were randomly selected from a list of residential phone numbers and participants were systematically selected from eligible household members to enroll a stratified cohort with greater numbers of older individuals and women. Of all those selected, 42% agreed to fully participate and had baseline interview and testing. Ethics approval was granted through McGill University and the appropriate ethics review boards for each participating center. Signed informed consent was obtained from all study participants in accordance with the Helsinki Declaration.
Data collection
All participants completed a standardized interviewer-administered questionnaire (CaMos questionnaire ©1995) at baseline. The questionnaire covered demographics, health, nutrition, lifestyle (including PA), as well as a detailed history of fracture and of major risk factors for fracture. Participants underwent a baseline clinical assessment that included measurement of height, weight, and BMD. Participants and/or their health care providers were informed of the participants’ baseline BMD status. Follow-up of participants was irrespective of BMD status, initiation of therapy, or any other condition. Follow-up visits were scheduled in the fifth year of the study (2000–02), and included an interview-administered questionnaire, height and weight measurements, and BMD testing. The CaMos cohort had 9423 participants at baseline; 9297 (98.7%) had complete baseline PA measures (no missing responses) for the cross-sectional analysis, and 7523 (79.8%) had complete baseline and Year 5 PA measures for the longitudinal analysis.
Physical activity measure
Self-reported baseline and Year 5 levels of PA were derived from the questionnaire. For the longitudinal analysis we considered both average PA and change in PA over 5 years. The level of PA was based on a series of questions used in the Hawaii-Los Angeles Multiethnic cohort [16, 17]. Participants were queried on the hours per week partaking in specified types or levels of activity: sleeping, sitting (e.g. in car, at work, watching TV, meals, other), walking, moderate activity (e.g. housework, brisk walking, golfing, bowling, bicycling on level ground), vigorous work (e.g. moving furniture, loading and unloading trucks, shoveling, weight-lifting, equivalent manual labor), and strenuous sports (e.g. jogging, bicycling on hills, tennis, racquetball, swimming laps, aerobics). A summary PA variable was derived from time spent on specific activities multiplied by the reference MET and these were then summed over a 24-hour period. MET values for each activity were taken from Ainsworth et al [18]: sleeping (MET=0.91), sitting (MET=1.0), walking (MET=3.3), moderate activity (MET=4.0), vigorous activity (MET=7.0) or strenuous sports (MET=8.8). Any remaining hours that were unaccounted were recorded as light activity (MET=2.4), to make up a 24-hour day. For those with total daily time exceeding 24 hours, all component times were proportionally reduced to yield 24 hours.
Bone mineral density
BMD was measured at the lumbar spine (L1-L4) and total hip by dual x-ray absorptiometry. Seven centers had Hologic densitometers (Hologic Inc, Waltham, MA) and two centers had Lunar densitometers (GE Lunar, Madison, WI). The same machine was used at baseline and Year 5 in all centers. Machines were calibrated daily according to manufacturers’ recommendations, and daily monitoring was used to assess and correct longitudinal drift. Two centers (Hamilton and Toronto) needed a one-time correction. All Lunar measurements were converted to equivalent Hologic values using standard reference formulas [19, 20]. Machines were cross-calibrated using measurements from an anthropomorphic phantom that was circulated and scanned in each center.
Statistical methods
All descriptive and regression analyses were a priori stratified by sex. Descriptive statistics are reported as mean ± SD, and further stratified by high PA (above the median value) versus low PA (below the median value). T-tests were used for continuous variable comparison of the high and low PA groups and chi-squared tests for categorical variables. ANOVA was used to assess variation of PA by center. General linear models were used to assess the relationships between PA and BMD. For the cross-sectional analyses, we considered baseline PA as the main exposure variable and baseline BMD as the main outcome variable. For the longitudinal analyses, we considered both the average PA (mean PA of baseline and Year 5) and the change in PA (difference between baseline and Year 5) as exposure variables and the change in BMD (difference between baseline and Year 5) as the primary outcome variable.
BMI (kg/m2) was considered as a mediator of the relationship between PA and BMD. Thus, we considered BMI and change in BMI as secondary outcomes (i.e. dependent variables) and as independent variables to be included/excluded from the regression analyses with BMD outcomes. Baseline BMI was included in all longitudinal analyses. The regression models included the following a priori specified potential confounders: age, CaMos center, education (<12 years school, high school diploma, postsecondary education), tobacco history (non-smoker, former smoker, current smoker), alcohol use (non-drinker; moderate or <1 drink/day in women, <2 drink/day in men; high meaning ≥1 drink/day in women, ≥2 drinks/day in men), caffeine use (mg/day), weight cycling (lost/regained ≥10 lb), current use of hormone therapy and/or bisphosphonates, age at menarche, any premenopausal history of >3 months skipped menstruation, past or current use of oral contraceptives and menopausal status, if applicable. We looked at additional potential confounders including calcium intake, vitamin D intake, sun exposure, and co-morbidities (heart disease, hypertension, type 2 diabetes, cancer). Although some were predictors, the associations with the main variables were not sufficiently strong to change the overall results, and hence these variables were not included in the final models. Baseline status of the above variables was used in the cross-sectional analysis and the longitudinal analysis. For the longitudinal analysis, we also included duration of bisphosphonate use, duration of hormone therapy, and change in menopausal status over the 5-year period, if applicable.
We hypothesized there were possible between group differences for those who reported strenuous exercise and/or vigorous work at either time point versus those who consistently reported neither. We tested whether there was an interaction between this group variable and the PA variables in all the longitudinal regressions that would result in different parameters in the different groups. If this analysis was significant at the level p=0.05, we reported the associated stratified results.
RESULTS
The characteristics of the CaMos cohort according to sex and overall level of PA are shown in Table 1. Comparison of those with PA above the median vs. those with PA below the median in both men and women showed that most covariates were still quite similar in the two groups with some bivariate associations reaching the nominal level of statistical significance (shown in bold). On average, over all ages, both men and women reported similar total PA, with a mean of 2500 (95% CI: 2484, 2516) MET*m/d in men versus a mean of 2510 (95% CI: 2501, 2518) MET*m/d in women. On average, over all ages, duration of strenuous sports and vigorous work was higher in men than in women, duration of moderate exercise was higher in women than in men, and duration of walking was similar in both sexes. The mean duration of daily activity in men was 32 minutes of walking, 86 minutes of moderate activity, 8 minutes of strenuous sport, and 21 minutes of vigorous work. The mean duration of daily activity in women was 31 minutes of walking, 117 minutes of moderate activity, 3 minutes of strenuous sport, and 4 minutes of vigorous work.
Table 1.
Baseline Characteristics of the Canadian Multicentre Osteoporosis Study (CaMos) Cohort Stratified by Sex and Higher and Lower Physical Activity (PA)1. Those differences with 95% confidence intervals excluding a null effect are shown in bold.
| Men (N=2855) | Women (N=6442) | |||||||
|---|---|---|---|---|---|---|---|---|
| Lower PA2 | Higher PA2 | Lower PA3 | Higher PA3 | |||||
| mean | SD | mean | SD | mean | SD | mean | SD | |
| Age (y) | 61.0 | 14.4 | 59.7 | 14.5 | 64.0 | 13.3 | 63.1 | 12.2 |
| Height (cm) | 173.3 | 7.4 | 173.7 | 7.0 | 159.4 | 6.6 | 159.8 | 6.5 |
| Weight (kg) | 82.1 | 14.8 | 80.9 | 13.3 | 69.7 | 14.9 | 67.5 | 12.8 |
| BMI (kg/m2) | 27.3 | 4.4 | 26.8 | 3.8 | 27.4 | 5.5 | 26.4 | 4.8 |
| Total hip BMD (g/cm2) | 1.003 | 0.154 | 1.018 | 0.143 | 0.853 | 0.150 | 0.855 | 0.139 |
| Lumbar spine BMD (g/cm2) | 1.041 | 0.170 | 1.054 | 0.167 | 0.943 | 0.175 | 0.932 | 0.170 |
| SF-36 physical summary | 48.1 | 9.9 | 49.7 | 8.8 | 45.3 | 10.9 | 47.6 | 10.1 |
| SF-36 mental summary | 53.7 | 8.2 | 54.5 | 7.8 | 52.8 | 9.3 | 53.2 | 8.7 |
| Caffeine intake (mg/d) | 343.9 | 314.3 | 334.9 | 318.9 | 262.5 | 227.3 | 274.8 | 270.1 |
| n | % | n | % | n | % | n | % | |
| University education | 581 | 40.7 | 480 | 33.6 | 799 | 24.8 | 717 | 22.3 |
| Current smoker | 253 | 17.7 | 273 | 19.1 | 495 | 15.4 | 427 | 13.3 |
| High alcohol intake4 | 116 | 8.2 | 93 | 6.5 | 176 | 5.5 | 153 | 4.8 |
| Weight cycling (≥10 lbs) | 496 | 34.8 | 456 | 31.9 | 1383 | 42.9 | 1301 | 40.4 |
| Premenopausal | NA | NA | NA | NA | 539 | 16.7 | 511 | 15.9 |
| Current hormone therapy | NA | NA | NA | NA | 719 | 22.3 | 737 | 22.9 |
| Past amenorrhea (≥3 mo) | NA | NA | NA | NA | 269 | 8.4 | 286 | 8.9 |
| Oral contraceptive use (ever) | NA | NA | NA | NA | 1455 | 45.2 | 1519 | 47.2 |
T-test for continuous variables allowed comparison of the higher PA group versus the lower PA group; the chi-square test was used for categorical variables.
Lower PA: ≤2455 MET*m/d, Higher PA:>2455 MET*m/d
Lower PA: ≤2508 MET*m/d, Higher PA:>2508 MET*m/d
More than 2 units/day in men and 1 unit/day in women. 1 unit=12 oz beer, 4–5 oz wine, 1–1.5 oz hard liquor
We also compared those in the highest quintile of PA change between baseline and Year 5 versus the lowest quintile. Those in the highest quintile increased their activity by an average of 499 MET*m/d. The corresponding mean change in duration of specific activities was an additional 26 minutes of walking, 61 minutes of moderate activity, 4 minutes of strenuous sports, and 11 minutes of vigorous work. Those in the lowest quintile decreased their activity by an average of 558 MET*m/d; with decreased walking (5 minutes), moderate activity (68 minutes), strenuous sports (6 minutes), vigorous work (25 minutes).
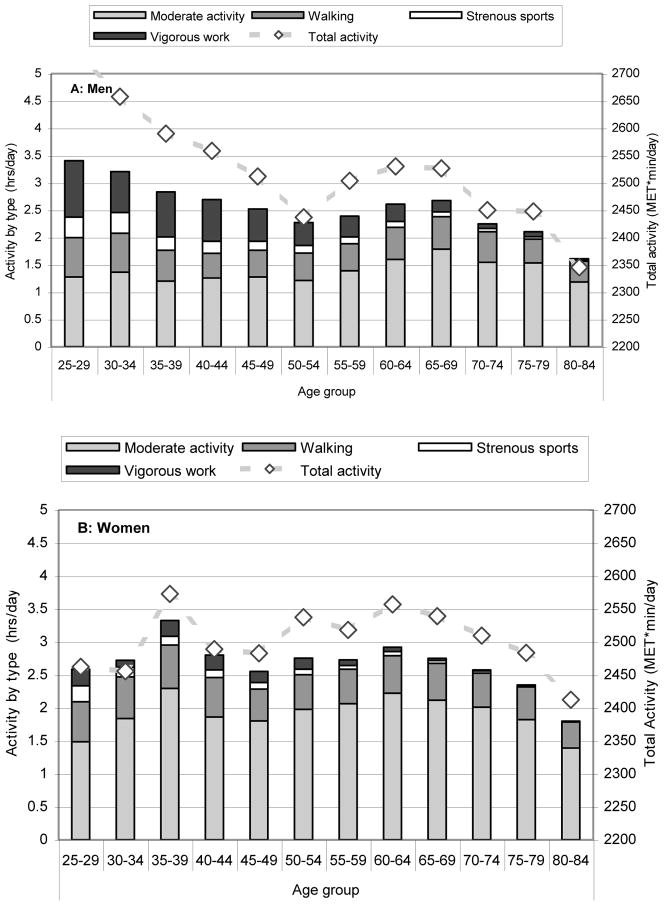
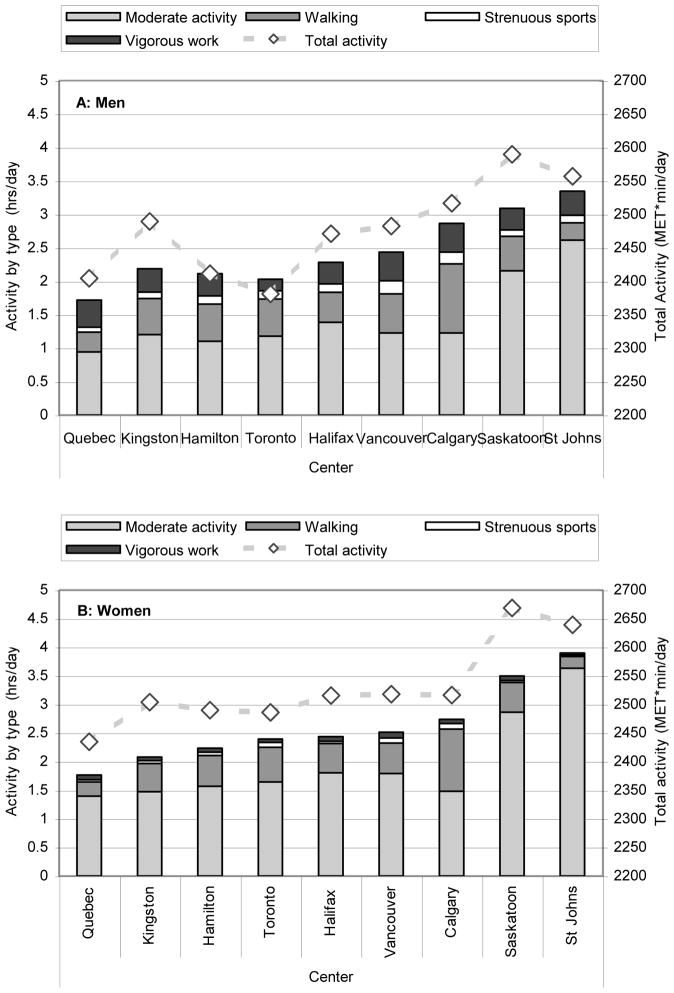
A detailed picture of the variation of PA by sex and age is shown in Figure 1. Strenuous sports and vigorous work, were consistently inversely associated with age (r = −0.25, −0.26 in men, r = −0.18, −0.17 in women; all p < 0.001); the average time spent doing either activity decreased over the entire age range. There were inconsistent associations between age and walking and moderate activity; moreover, there was no uniform age trend for participation in these activities, with women having two peaks around ages 35–39 years and 60–64 years and men having a later peak around ages 65–69 years. A detailed picture of the variation of PA by sex and geographic center is shown in Figure 2. Center was strongly associated with time spent doing moderate exercise and walking, accounting for 10.3% to 21.4% of the variation in these variables, but was not strongly associated with time spent doing strenuous exercise or vigorous work, accounting for less than 1.5% of these overall variations.
Figure 1.
Total Daily Physical Activity (MET min/day, Dashed Line—right Y axis) with Duration by Category (hours/day, Bars---left Y axis) Stratified By Sex (A: Men, B: Women) and Age Group at Baseline (1995–97) in the CaMos Cohort
Figure 2.
Total Daily Physical Activity (MET min/d, Dashed Line—right Y axis) with Duration by Category (hours/day, Bars---left Y axis) Stratified By Sex (A: Men, B: Women) and Center at Baseline (1995–97) in the CaMos Cohort
Table 2 shows the cross-sectional relationships among PA and all outcome variables (lumbar spine BMD, total hip BMD). Higher PA, as measured in MET*m/d, was associated with lower BMI in both men and women after adjustment for age as well as for all covariates, with a slightly stronger association in women than in men. Among men, higher PA was associated with higher total hip BMD, after adjustment for age alone and for all covariates. However, there was no association with lumbar spine BMD in the same analyses. Among women, higher PA was associated with lower lumbar spine BMD, after adjustment for age as well as for all covariates, but there was no association with total hip BMD with the same analyses. For both men and women, the association between PA and BMD was modified by BMI, so that for a given BMI there was a positive association between higher PA and higher BMD in all cases except lumbar spine BMD among women.
Table 2.
Cross-sectional Associations Among Physical Activity, Bone Mineral Density (BMD) and Body Mass Index (BMI) at Baseline (1995–97) in the Canadian Multicentre Osteoporosis Study (CaMos) cohort1.
| Men | |||
|---|---|---|---|
| Exposure | Outcome | Adjustment | Beta (95% CI) |
| Physical activity | BMI | Age | −0.55 (−0.89, −0.21) |
| All covariates | −0.54 (−0.89, −0.20) | ||
| Total hip BMD | Age | 0.014 (0.001, 0.027) | |
| Covariates, w/o BMI | 0.017 (0.004, 0.029) | ||
| Covariates with BMI | 0.022 (0.010, 0.034) | ||
| Lumbar spine BMD | Age | 0.008 (−0.006, 0.023) | |
| Covariates, w/o BMI | 0.012 (−0.003, 0.027) | ||
| Covariates with BMI | 0.016 (0.002, 0.031) | ||
| Women | |||
| Exposure | Outcome | Adjustment | Beta (95% CI) |
| Physical activity | BMI | Age | −1.80 (−2.17, −1.44) |
| All covariates | −1.98 (−2.34, −1.61) | ||
| Total hip BMD | Age | −0.002 (−0.012, 0.008) | |
| Covariates, w/o BMI | −0.001 (−0.011, 0.009) | ||
| Covariates with BMI | 0.021 (0.012, 0.030) | ||
| Lumbar spine BMD | Age | −0.018 (−0.030, −0.006) | |
| Covariates, w/o BMI | −0.014 (−0.027, −0.002) | ||
| Covariates with BMI | 0.003 (−0.009, 0.015) | ||
Associations with 95% Confidence Intervals excluding the null effect are shown in bold.
Table 3 shows the longitudinal relationships among PA and all outcome variables (changes in lumbar spine BMD, total hip BMD). The only statistically significant relationship between 5-year mean PA and BMD change occurred in women, where higher mean PA was associated with an increased lumbar spine BMD. Otherwise, mean level of PA was not associated with longitudinal change in BMI or BMD. However, longitudinal changes in PA were related to changes in BMD. For instance, among men, an increase in PA was associated with an increase in both total hip and lumbar spine BMD after adjustment for covariates. Among women, an increase in PA was associated with an increase in total hip, but not lumbar spine BMD, after adjustment for covariates. Also, an increase in PA was associated with a decrease in BMI (or equivalently, decreases in PA were associated with increases in BMI) among both men and women.
Table 3.
Longitudinal Associations Between Physical Activity (5-year Mean and Change) and 5-year Changes in Bone Mineral Density (BMD) and Body Mass Index (BMI) from Baseline (1995–97) to Year 5 (2000–02) in the Canadian Multicentre Osteoporosis Study (CaMos) Cohort1.
| Men | |||
|---|---|---|---|
| Exposure | Outcome | Adjustment | Beta (95% CI) |
| 5-y mean physical activity | 5-y change in BMI (ΔBMI) | Age | 0.15 (−0.08, 0.38) |
| All covariates | 0.02 (−0.21, 0.26) | ||
| 5-y change in total hip BMD | Age | 0.002 (−0.003, 0.007) | |
| Covariates, w/o ΔBMI | 0.004 (−0.001, 0.009) | ||
| Covariates with ΔBMI | 0.002 (−0.003, 0.007) | ||
| 5-y change in lumbar spine BMD | Age | 0.000 (−0.007, 0.007) | |
| Covariates, w/o ΔBMI | 0.005 (−0.003, 0.012) | ||
| Covariates with ΔBMI | 0.002 (−0.005, 0.011) | ||
| 5-y change in physical activity | 5-y change in BMI (ΔBMI) | Age | −0.47 (−0.68, −0.28) |
| All covariates | −0.41 (−0.60, −0.22) | ||
| 5-y change in total hip BMD | Age | 0.003 (−0.001, 0.007) | |
| Covariates, w/o ΔBMI | 0.004 (0.000, 0.008) | ||
| Covariates with ΔBMI | 0.005 (0.001, 0.009) | ||
| 5-y change in lumbar spine BMD | Age | 0.007 (0.001, 0.012) | |
| Covariates, w/o ΔBMI | 0.009 (0.003, 0.015) | ||
| Covariates with ΔBMI | 0.010 (0.004, 0.017) | ||
| Women | |||
| Exposure | Outcome | Adjustment | Beta (95% CI) |
| 5-y mean physical activity | 5-y change in BMI (ΔBMI) | Age | −0.04 (−0.25, 0.17) |
| All covariates | −0.21 (−0.43, 0.01) | ||
| 5-y change in total hip BMD | Age | −0.001 (−0.006, 0.003) | |
| Covariates, w/o ΔBMI | 0.001 (−0.003, 0.005) | ||
| Covariates with ΔBMI | 0.003 (−0.001, 0.007) | ||
| 5-y change in lumbar spine BMD | Age | 0.004 (−0.002, 0.009) | |
| Covariates, w/o ΔBMI | 0.006 (0.001, 0.012) | ||
| Covariates with ΔBMI | 0.007 (0.001, 0.013) | ||
| 5-y change in physical activity | 5-y change in BMI (ΔBMI) | Age | −0.47 (−0.64, −0.30) |
| All covariates | −0.40 (−0.57, −0.23) | ||
| 5-y change in total hip BMD | Age | 0.004 (0.001, 0.008) | |
| Covariates, w/o ΔBMI | 0.003 (0.000, 0.007) | ||
| Covariates with ΔBMI | 0.006 (0.003, 0.009) | ||
| 5-y change in lumbar spine BMD | Age | 0.001 (−0.003, 0.006) | |
| Covariates, w/o ΔBMI | 0.000 (−0.005, 0.004) | ||
| Covariates with ΔBMI | 0.001 (−0.003, 0.006) | ||
Associations with 95% Confidence Intervals excluding the null effect are shown in bold.
In the longitudinal analysis, we considered grouping those who reported strenuous exercise and/or vigorous work at either baseline or Year 5, versus those who consistently reported neither activity. We tested interaction between this group variable and the two summary variables (mean PA, change in PA) in all longitudinal analysis. There were no statistical interactions at the usual threshold (p=0.05) for any of the BMD regressions. For the BMI regressions we found a statistically significant interaction term for women only (p=0.007) with the estimated between-group difference of 0.46 (95% CI: 0.13–0.80) kg/m2 per 1000 MET*m/d. The resulting subgroup parameters were 0.65 (95% CI: 0.40–0.89) kg/m2 decrease per 1000 MET*m/d increase for those who reported no strenuous exercise or vigorous work at either time point versus 0.19 kg/m2 decrease per 1000 MET*m/d increase for those who reported some strenuous exercise and/or vigorous work.
DISCUSSION
In this population-based five-year prospective study of adult Canadian men and women we found that changes in physical activity (PA) were associated with increases in BMD and decreases in BMI, whereas there was virtually no association between estimated mean levels of PA and changes in either bone or weight parameters. Moreover, an increase in PA was associated with a modest un-coupling of the strong associations between BMD and BMI, so that the increased activity was related to increased total hip BMD despite decreased BMI. In men only, increased physical activity was related to increased lumbar spine BMD as well. Our results complement a recent meta-analysis demonstrating that changes in PA, but not absolute level of PA, lead to changes in BMI [21]. Our results likewise complement a meta-analysis of studies among postmenopausal women showing that a wide range of exercise interventions (walking, aerobic training, and resistance training) improve BMD [12]. Our results showing a similar effect for BMD among those who reported only walking and moderate activity are consistent with this hypothesis. A positive association between PA and BMD in both men and women was noted in a cross-sectional analysis [22]. A long-term prospective study linking PA and bone loss over 15-years noted that bone loss was highest among the most inactive quartile (as measured by average PA over time) [23]. A retrospective study in men showed that long-term participation in activities, rated for their osteogenic potential, is an important determinant of bone size, quality, and strength [24]. Differences in study design preclude direct comparisons, but the implications are similar. Our study adds to these previous findings by demonstrating these associations in a prospective population-based cohort study of both men and women. In addition, our data added the assessment of concurrent changes in BMI, that the link between weight loss and bone loss can be uncoupled if associated with physical activity. This decoupling was most notable for those who reported no strenuous exercise or vigorous work, as the parameters for the inverse relationship between PA and BMI were further from null among those reporting no strenuous exercise or vigorous work, while there was no interaction term with BMD as an outcome. This, at first sight, appears counterintuitive. However, possible explanations are that changes in strenuous and vigorous activity are more strongly linked with appetite, or that BMI is a composite measure, and the off-setting increase in muscle mass varies by level of activity. Alternatively, if PA acts by reducing inactivity, then lower intensity PA, by taking more time per MET, would have a greater impact.
This study demonstrated clear differences in physical activity types and intensities by age and sex with women having more moderate and less vigorous physical activity. These differences may play a role in the development of osteoporosis. The differences in lifetime exercise trajectories of men and women has also been noted in a retrospective study of 4269 men and women, where estimates of PA were divided into three main domains (work, sports, household), with strong sex and age trends present in all domains [25]. Women have consistently lower levels of strenuous exercise and vigorous work over their lifetimes and this, combined with lower peak bone mass, leads to more bone loss and earlier onset of osteoporosis as noted in previous CaMos studies [26, 27]. In the earlier of these papers, Berger et al reported that while there is not a strong relation between bone loss and age in men, the period of lowest total hip bone loss in men is in mid-life. The early period of bone loss in younger men (which has no parallel in women) does correspond with the rapid decrease in physical activity during the same period. Thus, some of the age trends in BMD are natural consequences of aging, but some of these BMD losses are attributable to concurrent declines in PA. Little of the reported PA was of the more intense weight-bearing type that is associated with an osteogenic response; this is especially true in women and the oldest age cohorts, where usual activities are likely insufficient to prevent bone loss.
A somewhat surprising finding of our study is the variation in activity patterns by study center. This variation suggests that the physical and social environment is an important determinant of PA; thus environment is likely to influence multiple health outcomes. Much of the epidemiological research on osteoporosis and PA in older adults has been conducted in Scandinavian countries such as Norway and Finland [4, 11, 23, 28], where the general levels of work and leisure activity are comparatively high, particularly in old age. The relatively modest effect size in this study may be due to the paucity of strenuous exercise and vigorous work among Canadians.
The apparent inconsistencies between the cross-sectional and longitudinal analyses of the effects of PA among women are initially puzzling. However, it is possible that BMD among women depends more on variable accrual of peak bone mass during adolescence, and that these effects overwhelm the relatively smaller longitudinal physical activity effects. A recent study among adolescents demonstrated that the relationship between risk factors and BMD depends on sex, and in particular that PA was a more important predictor of BMD in adolescent boys than in adolescent girls [29]. The sexual dimorphism in bone acquisition is related to endocrine regulators, with testosterone and estrogen having different effects on acquisition of lean mass, fat mass, and bone. The most notable sex-related divergence in this study was at the lumbar spine. The lumbar spine has a larger component of trabecular bone, with different operative mechanical stresses, and different homeostatic regulation than largely cortical bone. Despite this, cancellous spinal bone density is increased in relation to running exercise in regularly cycling, ovulatory premenopausal women [30]. Thus, factors other than PA may well play an important role in bone loss at this site. Finally, a longer follow-up period might reveal effects that were not apparent over five years.
The strengths of this study are its prospective nature, geographically diverse population base, inclusion of both men and women across a wide range of ages, and systematic study of both work- and leisure-related PA of varying intensity levels. Furthermore, quality control and calibration of all BMD values and measured components of BMI improve the reliability of these measures.
The main limitations of this study are those of all large population-based cohort studies that use questionnaires: physical activity was determined by self-report, and so is subject to recall bias. The use of interviewer-administered questionnaires in this instance may have improved the data quality over self-administered questionnaires. The questionnaire addressed broad categories of PA, and we used METs to summarize the data. These MET assessments, however, were designed with aerobic activity and cardiovascular risks in mind rather than the possible effects of activity on bone. Different results might be found with an assessment of PA designed to capture activity with potential to have biomechanical effects on bone. In particular, the questionnaire did not distinguish between weight bearing exercise (jogging) and non-weight bearing exercise (swimming), and consequently might underestimate the effects of strenuous loading exercise. This is a limitation of the present analysis. On the other hand, within this population most non-resting physical activity was light activity and walking—METs are a reasonable measure of inactivity. Areal BMD measured by DXA provides an approximation of bone strength, but is not a specific measure of bone material or geometric properties. Although this study measured height and weight, it would potentially have added to our understanding to have measured waist circumference as well, since metabolic factors in general also relate to bone health.
In summary, this population-based study in adult men and women shows that changes in physical activity are associated with changes in both BMD (increases) and in BMI (decreases). We have shown that increases in physical activity have the potential to decrease the obesity epidemic without increasing the risk of bone loss leading to osteoporosis. This study also highlights the population-wide general decline in PA over a lifetime. There were large variations in largely walking PA by center, thus full ascertainment of the social and environmental determinants of walking behavior could illuminate possible population level interventions. Most CaMos participants were not engaged in strenuous exercise. Notably, the associations between physical activity and BMI were stronger among those who did not report any strenuous exercise or vigorous work. Thus, emphasis should be made on keeping active over the life span to prevent bone loss and to avoid weight gain. CaMos is ongoing, offering the opportunity to examine changes in patterns of physical activity over longer time intervals, and their longer term associations with bone and fracture outcomes in middle-aged and older Canadians.
Acknowledgments
We thank all those participants in CaMos whose careful responses and attendance made this analysis possible. The Canadian Multicentre Osteoporosis Study is funded by the Canadian Institutes of Health Research (CIHR). Over some of its duration, CaMos has also received arms length support from Merck Frosst Canada Ltd., Eli Lilly Canada Inc., Novartis Pharmaceuticals Inc., The Alliance for Better Bone Health: Sanofi-Aventis, Procter & Gamble Pharmaceuticals Canada Inc., Amgen, The Dairy Farmers of Canada and The Arthritis Society.
CAMOS RESEARCH GROUP
David Goltzman (co-principal investigator, McGill University), Nancy Kreiger (co-principal investigator, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto).
CaMos Coordinating Center, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant), Claudie Berger (study statistician).
Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator).
Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator).
Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator).
Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator).
University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie A Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator).
McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator).
University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director), Jola Thingvold (coordinator).
University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator).
University British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Milan Patel (co-director), Yvette Vigna (coordinator); Brian C. Lentle (radiologist).
Reference List
- 1.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004 Nov;36(11):1985–96. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 2.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–98. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 3.Westerterp KR. Pattern and intensity of physical activity. Nature. 2001 Mar 29;410(6828):539. doi: 10.1038/35069142. [DOI] [PubMed] [Google Scholar]
- 4.Augestad LB, Schei B, Forsmo S, Langhammer A, Flanders WD. The association between physical activity and forearm bone mineral density in healthy premenopausal women. J Womens Health (Larchmt ) 2004 Apr;13(3):301–13. doi: 10.1089/154099904323016464. [DOI] [PubMed] [Google Scholar]
- 5.Devine A, Dhaliwal SS, Dick IM, Bollerslev J, Prince RL. Physical activity and calcium consumption are important determinants of lower limb bone mass in older women. J Bone Miner Res. 2004 Oct;19(10):1634–9. doi: 10.1359/JBMR.040804. [DOI] [PubMed] [Google Scholar]
- 6.Lunt M, Masaryk P, Scheidt-Nave C, et al. The effects of lifestyle, dietary dairy intake and diabetes on bone density and vertebral deformity prevalence: the EVOS study. Osteoporos Int. 2001;12(8):688–98. doi: 10.1007/s001980170069. [DOI] [PubMed] [Google Scholar]
- 7.Hawker GA, Forsmo S, Cadarette SM, et al. Correlates of forearm bone mineral density in young Norwegian women: the Nord-Trondelag Health Study. Am J Epidemiol. 2002 Sep 1;156(5):418–27. doi: 10.1093/aje/kwf061. [DOI] [PubMed] [Google Scholar]
- 8.Albrand G, Munoz F, Sornay-Rendu E, Duboeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone. 2003 Jan;32(1):78–85. doi: 10.1016/s8756-3282(02)00919-5. [DOI] [PubMed] [Google Scholar]
- 9.Gerdhem P, Akesson K, Obrant KJ. Effect of previous and present physical activity on bone mass in elderly women. Osteoporos Int. 2003 May;14(3):208–12. doi: 10.1007/s00198-002-1362-3. [DOI] [PubMed] [Google Scholar]
- 10.Puntila E, Kroger H, Lakka T, Tuppurainen M, Jurvelin J, Honkanen R. Leisure-time physical activity and rate of bone loss among peri- and postmenopausal women: a longitudinal study. Bone. 2001 Nov;29(5):442–6. doi: 10.1016/s8756-3282(01)00597-x. [DOI] [PubMed] [Google Scholar]
- 11.Augestad LB, Schei B, Forsmo S, Langhammer A, Flanders WD. Healthy postmenopausal women- physical activity and forearm bone mineral density: the Nord-Trondelag health survey. J Women Aging. 2006;18(1):21–40. doi: 10.1300/J074v18n01_03. [DOI] [PubMed] [Google Scholar]
- 12.Bonaiuti D, Shea B, Iovine R, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;(3):CD000333. doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 13.Papaioannou A, Kennedy CC, Cranney A, et al. Risk factors for low BMD in healthy men age 50 years or older: a systematic review. Osteoporos Int. 2009 Apr;20(4):507–18. doi: 10.1007/s00198-008-0720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005 Nov;16(11):1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 15.Kreiger N, Tenenhouse A, Joseph L, et al. Research notes: the Canadian Multicentre Osteoporosis Study (CaMos) - background, rationale, methods. Can J Aging. 1999;18:376–87. [Google Scholar]
- 16.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000 Feb 15;151(4):346–57. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control. 2007 Mar;18(2):165–75. doi: 10.1007/s10552-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 19.Genant HK. Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res. 1995 Jun;10(6):997–8. doi: 10.1002/jbmr.5650100624. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int. 2001;12(6):438–44. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011 Jun 23;364(25):2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cauley JA, Fullman RL, Stone KL, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005 Dec;16(12):1525–37. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 23.Rikkonen T, Salovaara K, Sirola J, et al. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women: a 15-year follow-up of the OSTPRE study. J Bone Miner Res. 2010 Nov;25(11):2332–40. doi: 10.1002/jbmr.143. [DOI] [PubMed] [Google Scholar]
- 24.Daly RM, Bass SL. Lifetime sport and leisure activity participation is associated with greater bone size, quality and strength in older men. Osteoporos Int. 2006;17(8):1258–67. doi: 10.1007/s00198-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 25.Ratzlaff CR, Doerfling P, Steininger G, et al. Lifetime trajectory of physical activity according to energy expenditure and joint force. Arthritis Care Res (Hoboken ) 2010 Oct;62(10):1452–9. doi: 10.1002/acr.20243. [DOI] [PubMed] [Google Scholar]
- 26.Berger C, Langsetmo L, Joseph L, et al. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Can Med Assoc J. 2008;178(13):1660–8. doi: 10.1503/cmaj.071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger C, Goltzman D, Langsetmo L, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010 doi: 10.1002/jbmr.95. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rikkonen T, Tuppurainen M, Kroger H, Jurvelin J, Honkanen R. Distance of walking in childhood and femoral bone density in perimenopausal women. Eur J Appl Physiol. 2006 Jul;97(5):509–15. doi: 10.1007/s00421-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 29.Weeks BK, Beck BR. The Relationship between Physical Activity and Bone during Adolescence Differs according to Sex and Biological Maturity. J Osteoporos. 2010:546593. doi: 10.4061/2010/546593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petit MA, Prior JC, Barr SI. Running and ovulation positively change cancellous bone in premenopausal women. Med Sci Sports Exerc. 1999 Jun;31(6):780–7. doi: 10.1097/00005768-199906000-00004. [DOI] [PubMed] [Google Scholar]