Abstract
Background
We aimed to determine the brain areas related to food motivation and to examine individual variability using magnetoencephalography (MEG) during a fasted state. Correlation analysis was performed between MEG responses and the subscale and aggregated scores of the Power of Food Scale (PFS) and body mass index (BMI).
Material/Methods
Eight healthy, right-handed males [age, 29.0±10.4 years; BMI, 22.7±2.4 kg/m2 (mean ±SD)] were enrolled. The MEG experiment consisted of 2 food sessions and 2 control sessions in an alternating and counterbalanced order. During the MEG recordings, participants viewed a set of food pictures (food session) or mosaic pictures (control session) projected on a screen.
Results
When participants viewed pictures of food items, we were able to estimate equivalent current dipoles (ECDs) in the insular cortex in all participants peaked approximately 300 ms after the onset of each picture presentation. When they viewed mosaic pictures, 1 of 8 participants exhibited corresponding ECDs. Of note, significant correlations were observed between the intensities of the MEG responses and the subscale scores of Factor 1 (food available) (r=0.846, P=0.008) and those of Factor 2 (food present) (r=0.875, P=0.004), the aggregated scores of PFS (r=0.820, P=0.013), and BMI (r=0.898, P=0.002).
Conclusions
We demonstrated the involvement of the immediate neural responses of the insular cortex in individual differences in appetitive motivation. The signal intensities of the insular cortex were associated with self-awareness of appetitive motive.
Keywords: appetitive motives, immediate neural responses, individual variability, magnetoencephalography (MEG), Power of Food Scale (PFS), insular cortex
Background
Obesity constitutes a considerable health menace to modern adults and children worldwide [1], and is known to be an independent risk factor for various diseases such as diabetes mellitus, hypertension, heart disease, fatty liver, sleep apnea, and certain forms of cancer [2]. Excessive weight gain commonly originates from an imbalance between expenditure versus intake of energy, which depends, at least in part, on distorted appetite [3]. Accordingly, it is essential from a public health perspective to clarify the mechanisms of appetite control. It is known that appetite is regulated not only by homeostatic requirements such as nutritional deficit, but also by other factors, including genetic, biological, and environmental influences, as well as perception, cognition, emotions, and behavior patterns [4]. For instance, the increased availability of highly palatable, energy-dense food, and the increased presence of powerful food stimuli (e.g., commercials and vending machines) substantially contribute to a shift away from eating according to physiological need towards eating by hedonic motives, leading to a positive energy balance and obesity [5,6]. Interestingly, such appetitive motives differ from person to person, partly due to individual differences in reinforcing the value of food or food-related cues [7,8]. Thus, in some susceptible individuals, frequent exposure to food cues may enhance psychological processes even when food intake is not imminent, leading to overeating and weight gain. The reasons for this vulnerability are still unclear. Considering the mechanisms responsible for the enhanced appetitive motives in susceptible individuals, the sensory and emotional framework for decision making to take action including eating should be emphasized. Previous studies demonstrated that human reasoning and decision making to take action depend on many levels of neural operation; most of which proceed unconsciously and the operation is supported by immediate neural activities [9,10]. Therefore, it seems valuable to cast a spotlight on autonomic and immediate neural response to food stimuli, which may be closely related to deciding to eat.
Recently, neuroimaging techniques have been used to elucidate the neurological bases of appetite and have also provided clues to the reasons for such individual vulnerability [11]. To date, positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have been used most frequently in appetite research; hemodynamic changes are assessed as indicators of the changes of neural activation relating to cognition, emotion, and behaviors in various experimental conditions, including fed state and stimuli (visual, olfactory, gustatory, and food intakes) [11]. Although PET and fMRI have rapidly gained an important position in neuroscience research owing to their excellent specificity and spatial resolution, these neuroimaging techniques are generally thought to be less suitable for studying the temporal aspects of rapid neural events because the hemodynamic response evolves in seconds rather than milliseconds [12]. Accordingly, these methods are limited in detecting immediate responses to visual presentations of food cues, and the evaluation of such immediate responses might give us a novel perspective on the automatic responses (like inevitable reflexes) of the brain to visual stimuli of food before cognitive interruption. Magnetoencephalography (MEG) monitors electrophysiological activity inside the brain by measuring induced electromagnetic fields using electric or magnetic sensors over the scalp surface [13–15]; it has an intrinsic, high temporal resolution that allows tracking rapid neurophysiologic processes at the neuronal time scale of milliseconds. This high temporal resolution enables us to determine the flow of neural circuitry formed among multiple brain areas and/or to locate a particular brain area related to appetitive motives by capturing immediate patterns of neural activity.
To support the objective neuroimaging findings, the relationship between neural responses observed using MEG and self-reported awareness of actual appetitive motives in individuals who are exposed to visual stimuli of food in daily life is worthy of exploration. The self-reported awareness of appetitive motives seems to be related to the repetitive, accumulated, and conditioned experiences of automatic and unavoidable neural responses after exposure to food stimuli. The Power of Food Scale (PFS) was developed to measure individual differences in self-awareness of the appetitive responsiveness, such as appetite-related thoughts, feelings, and motivations to eat beyond a physiological need [16]. Based on previous factor analyses, the PFS has been shown to contain a 3-factor structure of food proximity consisting of: (1) ‘food available’, which describes the reaction when food is not physically present but is always available; (2) ‘food present’, which characterizes the reactions to palatable foods when they are physically present, but have not yet been tasted; and (3) ‘food tasted’, which characterizes the reactions to palatable foods when first tasted, but not yet consumed. Although an aggregated factor defined as an average of 3 factors is often used for the overall assessment of appetitive motives, the 3-factor model was known to be superior to the 1-factor model [16]. A recent study demonstrated that the PFS is a reliable and valid measure of appetitive motives in diverse populations, including obese and non-obese individuals, and that there is a relationship between body mass index (BMI) and each of the 3 subscales [17,18]. Recently, an fMRI study attempted to identify the neural substrates related to appetitive motives using the PFS [19]. In that study, however, because of the low temporal resolution of fMRI, identified brain regions were limited to those related to other addictive behaviors. So far, no studies have investigated the immediate neural responses to visual food stimuli before the occurrence of cognitive interruption to eat by electric or magnetic signal changes and their association with the intensity of appetitive motives and BMI.
The aim of the present study was to determine the brain areas related to appetitive motives and to examine individual variability of immediate brain activity as assessed by MEG in fasting individuals during presentation of food images. Next, to support the MEG data, we performed correlation analyses between the subscale and aggregated scores of PFS and the intensities of MEG responses, as well as between BMI and the intensities of the MEG responses. We hypothesized that the MEG responses would vary among individuals, and that participants with higher PFS scores, particularly in subscale-2 (food present), and those with higher BMI, would show higher MEG responses in appetite-related brain regions under fasting conditions.
Material and Methods
Participants
Eight healthy, right-handed male volunteers with normal body weight [age, 29.0±10.4 years; height, 170.5±5.1 cm; body weight, 66.3±9.1 kg; BMI, 22.7±2.4 kg/m2 (Mean ±SD)] were enrolled. Current smokers, participants with a history of mental or neurological disorder, and those taking chronic medications that affect the central nervous system were excluded. The study protocol was approved by the Ethics Committee of Osaka City University, and all participants gave written informed consent to participate in the study. All procedures were done according to the research ethics of the Declaration of Helsinki [20].
Experimental design
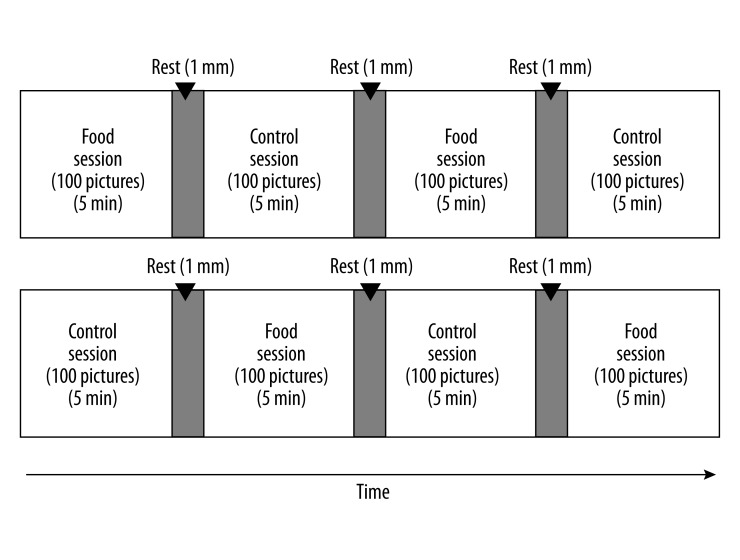
Experiments were conducted in a magnetically shielded room at Osaka City University Hospital. For 1 day before the visit, the participants were instructed to finish dinner by 9:00 p.m. and to fast overnight (they were only allowed to drink water), to avoid intense physical and mental activity, and to maintain normal sleeping hours. After the visit, they were asked to rate their subjective level of hunger on a 5-point Likert-type scale ranging from 1 (Yes, I am very hungry) to 5 (No, I am not hungry at all). The MEG examination consisted of 2 food sessions and 2 control sessions in an alternating and counterbalanced order (Figure 1). Pictures of food items were presented as visual stimuli during the food sessions. In addition, mosaic pictures created from the same pictures of food items were used as visual stimuli in the same sequences during the control sessions. These mosaic images of the original photographs (food items) were used to control for luminance, color, and local features [21,22]. Mosaic pictures were made using commercial software (Adobe Photoshop Elements 6.0, Adobe Systems Inc., San Jose, CA); all of the food pictures were divided into a 30×30 grid and randomly reordered by a constant algorithm. This rearrangement made each picture unrecognizable as food. The original images used to generate the mosaic pictures were not disclosed to the study participants. Participants were instructed to have appetitive motives (without memory recall of past experience and gustatory imagery) as if they brought each food item to their own mouth every time when the food items were presented during the food sessions, and to view the mosaic pictures during the control sessions. The intersession intervals were set at 1 min. While in a supine position on a bed, participants were requested to keep both eyes open and to fixate on a central point throughout the sessions. After the MEG recordings, they were asked to answer yes-or-no questions about their appetitive motives during the recording for each picture of food. The subjective levels of appetitive motives during the MEG recordings were expressed as the number of food items to which they replied “yes”. The study was conducted in a quiet, temperature-controlled room.
Figure 1.
Schematic representation of experimental procedure. The examination consisted of two food sessions and two control sessions in an alternating and counterbalanced order. Pictures of food items were presented as visual stimuli during the food sessions, and mosaic pictures of food items were presented as visual stimuli during the control sessions.
Stimulus presentation
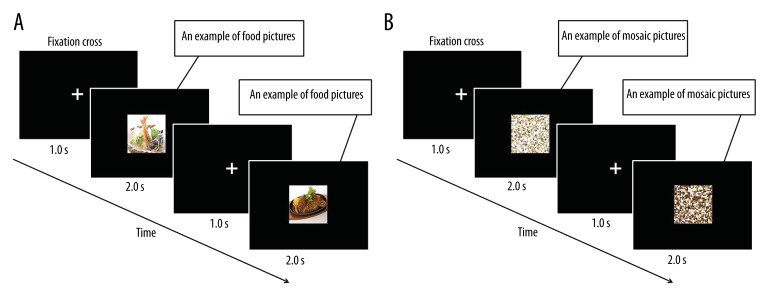
Each session consisted of 100-picture sets of a 2-sec stimulation period followed by a 1-sec inter-stimulus interval (Figure 2A and 2B). Twenty pictures of typical modern Japanese food items were used, including steak, croquette, hamburger, fritter, chicken nugget, French fry, pizza, spaghetti, ice cream, fried dumpling, and fried rice [23]. Each picture was used 5 times to construct the 100-picture set. The sequences of pictures for presentation were randomly assigned for each participant. Before the day of the experiment, each participant was asked to rate each picture for food preference in order to ensure that disliked food items were not presented. These pictures were projected on a screen placed in front of the participants’ eyes using a video projector (PG-B10S; SHARP, Osaka, Japan). The viewing angle of the pictures was 18.4×14.0°.
Figure 2.
Time course of stimulus presentations. A series of 100 color food pictures, consisting of 20 food items (A), and a series of 100 mosaic pictures, consisting of 20 mosaic pictures of food (B), were used. The order of the picture presentation was randomized for each series, and the sequences of pictures for presentation were randomly assigned for each participant. The mosaic pictures created from the same pictures of food items were used as visual stimuli in the same sequences during the control sessions. Each picture was presented for 2.0 s followed by a fixation cross for 1.0 s.
MEG recordings
MEG recordings were performed using a 160-channel whole-head type MEG system (MEG vision; Yokogawa Electric Corporation, Tokyo, Japan) with a magnetic field resolution of 4 fT/Hz1/2 in the white-noise region. The sensor and reference coils were gradiometers 15.5 mm in diameter and 50 mm in baseline, and each pair of sensor coils was separated at a distance of 23 mm. The sampling rate was 1000 Hz with a 0.3 Hz high-pass filter.
MEG data analyses
The MEG signal data corresponding to pictures of food items and mosaic pictures were separately analyzed and each data point was averaged offline after analogue-to-digital conversion with a band-pass filter of 3 to 30 Hz. The mean magnetic signal in the pre-stimulus time period (from 0 to 500 ms before the start of each visual stimulus) was subtracted in each channel to remove potential baseline shifts of MEG data. Epochs including artifacts such as eye blinks and eye movements were excluded from the analyses. To identify the sources of the evoked activities, equivalent current dipole (ECD) analyses were performed using commercial software (MEG 160; Yokogawa Electric Corporation). The ECDs with goodness of fit (GOF) values above 90% were used, based on a previous report [24]. We used a single dipole model in cases where we could detect only 1 ECD in a time window and we used a multiple dipole model in cases where we could detect more than 2 ECDs in all the brain regions and assessed the locations and intensities of the ECDs.
Magnetic resonance imaging overlay
Anatomical magnetic resonance imaging (MRI) was performed using a Philips Achieva 3.0TX (Royal Philips Electronics, Eindhoven, the Netherlands) to permit registration of magnetic source locations with their respective anatomical locations. Before MRI scanning, 5 adhesive markers (Medtronic Surgical Navigation Technologies Inc., Broomfield, CO) were attached to the skin of the participant’s head (the first and second were located 10 mm in front of the left tragus and right tragus, the third 35 mm above the nasion, and the fourth and fifth 40 mm right and left of the third). The MEG data were superimposed on MR images using information obtained from these markers and MEG localization coils.
Power of food scale (PFS)
The PFS is a questionnaire consisting of 15 items scored on a 5-point Likert-type scale ranging from 1 (Do not agree at all) to 5 (Strongly agree), with higher scores indicating greater responsiveness to the food environment [18]. According to previous studies using the PFS [16,17,25], the subscales for PFS are calculated as the average scores of all items included in each subscale (range, 1–5) as well as aggregated factor scores as the average scores of all 15 items (range, 1–5). The participants completed the PFS before the MEG recordings.
Statistical analyses
Data are expressed as mean ±SD unless otherwise stated. Categorical variables were compared using a McNemar test. Association of the intensities of MEG responses with the subscale and aggregated scores of PFS and BMI was evaluated using Pearson’s correlation analyses. Similarly, the relationship between the number of food items to which the participants replied “yes” regarding the subjective levels of appetitive motives and the intensities of MEG responses was also assessed using Pearson’s correlation analyses. All P values were 2-tailed, and values less than 0.05 were considered statistically significant. Statistical analyses were performed using the SPSS 18.0 software package (SPSS, Chicago, IL).
Results
Rating scores of hunger before recording of MEG and of appetitive motives during presentation of food pictures
Before the MEG recording, all the participants rated their subjective levels of hunger as moderate to excessive (1.8±0.5 on a 5-point Likert-type scale). During the MEG recording, they replied “yes” to most of the food pictures presented (17.8±3.1 in 20 food items presented).
MEG data
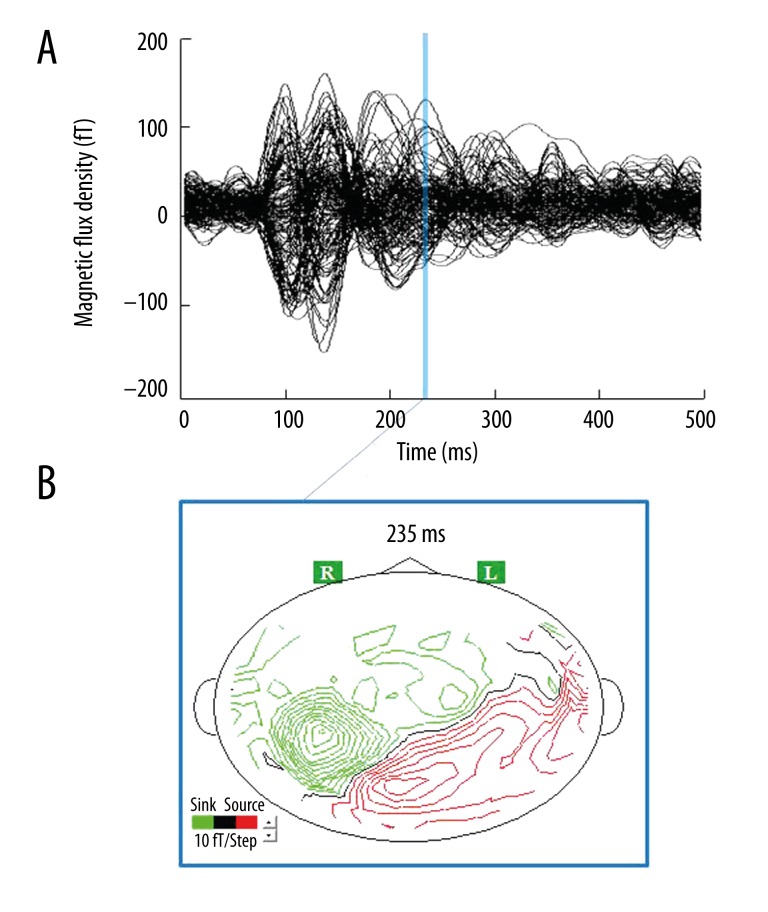
We used a single-dipole model in all participants because we could not detect more than 2 ECDs, with GOF values above 90%, in a time window using a multiple-dipole model. A typical example (participant No. 7) of magnetic fields and isofield contour maps caused by viewing the food pictures is shown in Figure 3. In the ECD analyses, we could identify a magnetic response in the insular cortex for all participants who viewed food pictures (Figure 4), although 1 participant also showed a magnetic response to mosaic pictures in this brain region. The proportion of participants in whom ECDs in the insular cortex were identified by viewing the food pictures was statistically significant relative to the proportion with ECDs identified in response to the mosaic pictures (P=0.016, McNemar test). Because the timing and location of evoked responses varied between 2 conditions (food stimuli vs. control stimuli) in each participant, the comparison was not made in intensities of MEG responses between these 2 conditions. Some individuals exhibited multiple latencies and loci of activity in the insular cortex; for these subjects, the MEG response with the maximal intensity of ECDs was defined as the primary MEG response. The peak latencies of the primary magnetic responses after the onset of food picture presentation ranged from 159 ms to 490 ms (307.3±105.3 ms) in the insular cortex. One of 8 participants (participant No. 2) had the magnetic responses in the left insular cortex when viewing food pictures and the other 7 participants had the responses in the right side. One participant (participant No. 8) had magnetic responses in the left insular cortex when viewing mosaic pictures. The mean latency, GOF value, and intensity of each ECD are summarized in Table 1.
Figure 3.
Example of magnetic fields (A) and isofield contour map (B) caused by viewing food pictures. There was one major magnetic response in the insular cortex (example shown: participant No. 7). The peak latency of the magnetic response after the onset of picture presentation was 235 ms.
Figure 4.
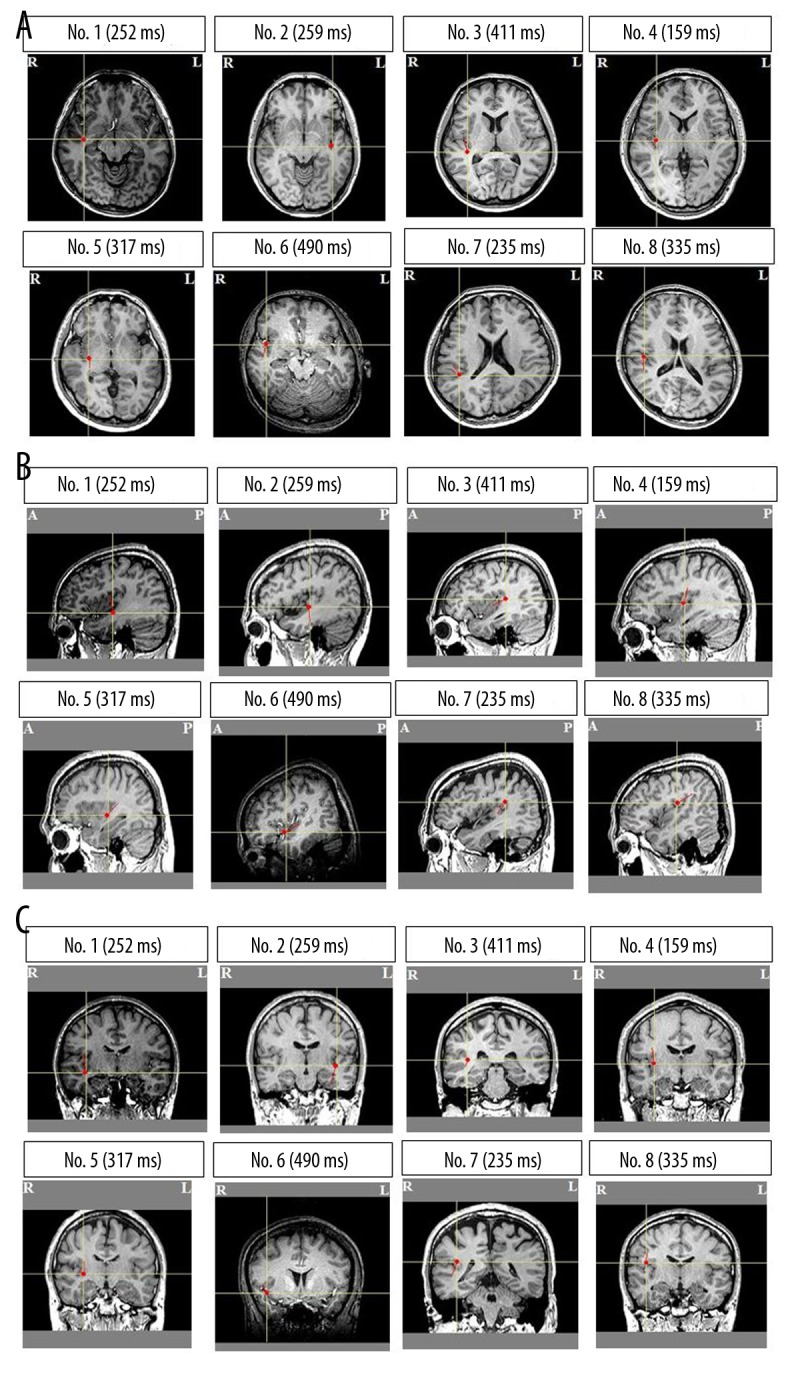
Locations of the equivalent current dipoles (ECDs) by viewing food pictures. Magnetic responses with latencies approximately 300 ms were localized in the insular cortex in all participants (A): axial view; (B): sagittal view; (C): coronal view) (R: right side; L: left side; A: anterior side; P: posterior side). Closed red circles indicate the location of ECDs; short red lines indicate orientation of the ECDs. ECDs are superimposed on individual magnetic resonance images. Peak latencies are indicated in parentheses.
Table 1.
Properties of the equivalent current dipoles (ECDs) related to food pictures in the insular cortex.
| No. of subjects | 8 |
| Peak latency (ms) | 307.3±105.3 |
| GOF (%) | 95.0±4.0 |
| Intensity (nA·m) | 19.5±15.3 |
Data are mean ±SD (n=8). GOF – goodness of fit.
Relationship among the intensity of primary MEG responses, scores of PFS, BMI and appetitive motives during the MEG recording
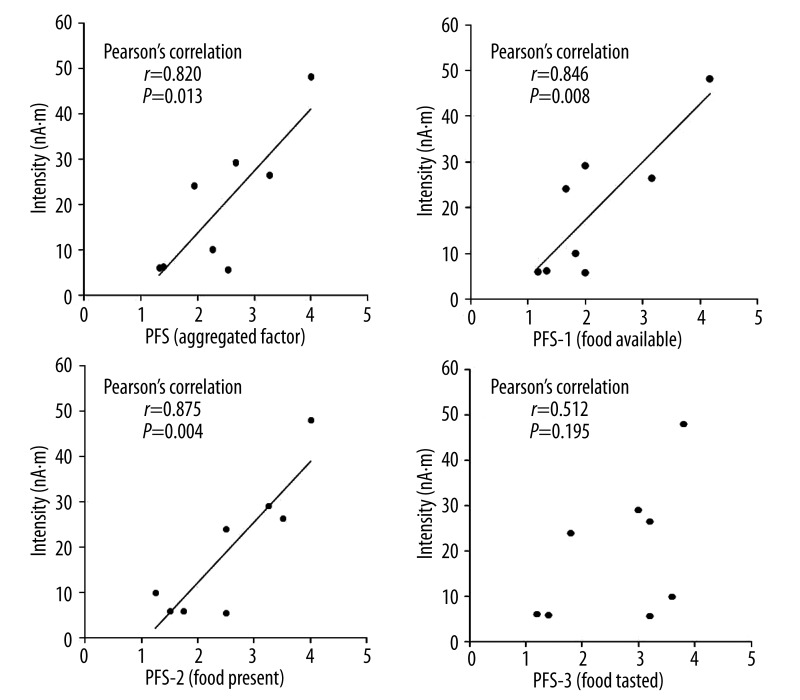
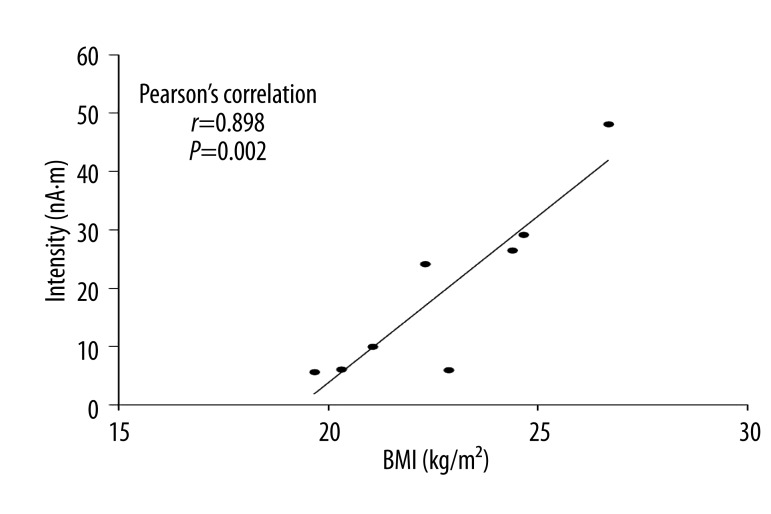
There were no significant correlations between the intensities of the MEG responses and the appetitive motives during the MEG recording expressed as the number of food items. However, significant correlations were observed between the intensities of the MEG responses and the subscale scores of Factor 1 (food available) (r=0.846, P=0.008), and Factor 2 (food present) (r=0.875, P=0.004) and the aggregated scores (r=0.820, P=0.013) of PFS. In contrast, no significant correlation was observed for the subscale scores of Factor 3 (food tasted) (r=0.512, P=0.195) (Figure 5). A significant association was found between the intensities of the MEG responses and BMI (r=0.898, P=0.002) (Figure 6).
Figure 5.
Correlation between scores on power of food scale (PFS) and the intensity of magnetoencephalographic responses. Pearson’s correlation coefficients (r) and P values are shown for all participants.
Figure 6.
Correlation between the body mass index (BMI) and the intensity of magnetoencephalographic responses. Pearson’s correlation coefficients (r) and P values are shown for all participants.
Discussion
In the present study, we identified a brain region associated with appetitive motives activated within 500 ms after viewing food pictures. When the participants viewed food pictures, we were able to estimate ECDs in the insular cortex in all 8 participants approximately 300 ms after the onset of each picture presentation. We could not estimate corresponding ECDs for mosaic pictures except for 1 participant. The intensities of the magnetic responses to food pictures showed a wide variability among the participants, and were significantly correlated with the aggregated scores, the subscale scores of Factor 1 (food available) and Factor 2 (food present) of PFS, as well as BMI, and no significant correlation was observed for the subscale scores of Factor 3 (food tasted).
Recent neuroimaging studies using PET and fMRI have provided a basic understanding of the neurobiological mechanisms underlying individual variation in eating behaviors in humans when various types of food stimuli are presented, particularly in those relating to pleasure, hedonic processing, and addiction to food stimuli. A common framework of these studies is to measure brain responses to sensory stimuli of food (visual, olfactory, or gustatory) in comparison with those to control cues, or to compare brain responses among different palatability or caloric ranges. Another style of studies is to examine the changes in brain activity before and after a caloric load. The participants’ characteristics (age, sex, body size, and diet) and other important features (length of fasting time prior to scan) vary among these studies. These studies commonly have supported the hypothesis that appetite depends on functional crosstalk in a distributed network of areas involved in reward, motivation, and cognitive control. In particular, in an obese population, a pernicious overactivation to food cues was observed in reward/motivation areas such as the insular cortex, paired with reduced activation of brain areas involved in cognitive control, such as the dorsolateral prefrontal cortex [26,27]. In addition, much progress has been made in the elucidation of central and peripheral mechanisms of the gustatory system, in which the insular cortex plays a role in multisensory integration as the central gustatory cortex. The insular cortex is a key intersection of the neural pathway for appetite regulation. Results of previous MEG studies are limited to the identification of cortical responses to food stimuli and the interactions between those cortical responses and higher cognitive functions, such as working memory [28] and the identification of the insulin-mediated modulation of visually evoked magnetic fields [29]. We are the first to have identified the neural substrate related to appetitive motives. Our findings of magnetic activity in the insular cortex detected in all participants are quite consistent with the previous neuroimaging studies [26,27,30–32].
The MEG captured magnetic responses in the insular cortex during the first 500 ms of observation after the onset of presentation of food pictures. One of the interesting findings in our study is the strong linear relationship between the reported appetitive motivation assessed by the PFS that the participants completed and the maximal intensities of MEG signals within the insular cortex. This is the first report to show a linear relationship between subjective level of appetite and cortical activity assessed using the MEG. Particularly of note is that the subscale scores of Factor 2 were the most strongly correlated with the intensities of the MEG signals in the insular cortex. The Factor 2 (food present) of the PFS characterizes the reactions to palatable foods when they are physically (i.e., visibly) presented, but have not yet been tasted. The paradigm of the questionnaire is likely to be similar with the present study design where the food pictures were visually presented to the participants. Thus, the observed correlation seems plausible, and our study adds a novel type of evidence of a quantitative relationship between the subjective motives of appetite and the objective measures of brain activity. In addition, this point proves a novel benefit of the present type of MEG analysis in that MEG signals might represent more specific responses to the appetitive motives to visual stimuli of food cue and produce more quantifiable results than other neuroimaging techniques. As seen in recent studies on appetite and eating behavior, PET and fMRI scanners have become commonplace in neuroimaging approaches. PET measurements provide information about the spatial distribution with expression levels of specific cellular targets such as receptors or enzymes, and recent advances in MRI technology have led to improved prominent spatial specificity of fMRI. However, both approaches have basically been developed for measuring regional blood supply and perfusion as the representatives of brain function and activation on the assumption that these are strongly correlated to each other, which is known as neurovascular coupling [33]. In other words, analyses using PET and fMRI might encounter difficulties because these techniques provide a rough assessment of sum-effects rather than individual responses of the brain. It is also possible that the data from these methods might include other irrelevant signals generated in the region of interest during the data acquisition, different from those relating to appetitive motives. In this regard, our approach using MEG measurements complements previous findings by PET and fMRI and fosters an understanding of the mechanisms of appetite and eating behaviors linked to the involvement of the insular cortex from a new direction.
The superior temporal resolution of the MEG measurements enables us to discuss the timing of the activation in the insular cortex. The present study demonstrates that the insular cortex showed early responses (approximately 300 ms) immediately after food stimuli were presented visually. Several lines of evidence indicate a similar immediate activation of insular cortex with gustatory imagery tasks [34–37]. For example, a previous MEG study revealed that some participants exhibited activation of insular cortex responding to gustatory imagery tasks by the presentation of food pictures, and that the latency of activation ranged widely (312–532 ms), with a mean latency of 420.3±31.7 ms. However, our study was not designed to examine activity with a gustatory imagery task where participants were asked to recall their taste. Instead, our study was designed to examine activity with an appetitive motive task where subjects were instructed to have appetitive motives as if they brought each food to their own mouths every time food items were presented. It is novel to use this instruction of appetitive motives to focus on the motivation to eat rather than simply on aspects of sensation and reward relating to eating.
The present findings demonstrate that participants who are more acutely aware of their own instincts to increase appetitive motives when foods are presented (i.e., high scores on Factor 2) exhibited more intense responses in the insular cortex at an early stage. Although the awareness of the instinct is likely to arise from their own daily experience of exposure to visual food stimuli, these individuals might be manipulated by instinctive responses relating to appetitive motives evoked in the insular cortex. Similarly, but more surprisingly, participants who have a greater awareness of appetitive motives when food is not actually present but is always available (i.e., high scores of Factor 1) also exhibited similar MEG responses in the insular cortex. It is possible that the insular cortex in these individuals tends to be reactive to information about food cues or availability before the presentation of food, possibly leading to individual susceptibility to food cues. In other words, the sensitivity of the insular cortex to visual food stimuli might be transferred genetically or acquired (learned) later in life. In line with this, previous animal studies have demonstrated that the gustatory insular cortex is involved in encoding changes in the incentive value assigned to instrumental outcomes on the basis of prevailing motivational conditions [38], supporting the conditioned theory. Since the insular cortex is a brain region relevant to conditioning, conditioning is a possible mechanism for the observed association of insular cortex activity with scores from Factor 1 and Factor 2 of PFS. This should be taken into account in attempts to better control appetite in clinical settings. Furthermore, from the perspective of the relationship between interoceptive (somatic) sensation and decision-making, Damasio [9] proposed the somatic marker hypothesis that the insular cortex is a critical platform that integrates interoceptive states based on information from sensory nerves (e.g., hungry or satiated, gustatory sensation, and visual information) into conscious feelings and into decision-making processes (e.g., the decision to eat) that involve uncertain risk and reward [39–41]. Based on these neurobehavioral bases of the insular cortex, the present observations could be explained by early processing in the insular cortex, acting as the platform before decision making for eating. Lastly, an association between the intensity of magnetic responses in the insular cortex and BMI was observed. Previous studies using fMRI demonstrated that obese individuals showed a greater difference between high-calorie food pictures and control images than normal weight controls in the insular cortex [42,43]. This point could imply that immediate neural responses in the insular cortex contribute to an increase in body weight by affecting appetitive motives in daily dietary life.
The present study has several potential limitations. First, we examined the brain activity in normal-weight young adults without apparent eating disorders during a fasted state. In order to clarify the neural mechanisms of appetitive motivation in general, further studies using similar MEG analytic methods will be needed in obese subjects and/or during satiety. Second, due to the spatial disadvantages of MEG analyses, we could not examine the involvement of the dorsolateral and orbitofrontal cortices and striatum, which are considered to be associated with appetitive motivation. Third, we cannot draw conclusions about cause-and-effect relationships because of the cross-sectional nature of our data, particularly about the association of the response intensities with BMI. Future prospective cohort studies will be needed to confirm the cause-and-effect relationships. Fourth, the design of the present study focused on brain activity caused by visual food cues. Because appetitive motivation can be evoked through multiple sensory systems, in order to generalize the results of our study, future researches using other sensory modalities are essential.
Conclusions
In summary, the present study revealed that the insular cortex is a core region relating to appetitive motives immediately induced by visual food cues within milliseconds. Even more interestingly, the signal intensities of the insular cortex are associated not only with the self-awareness of appetitive motives after the presentation of the food cues, but also before the food cues are actually presented. These results confirm the involvement of the insular cortex in appetitive networks and may help elucidate the neural basis of the variability of appetite phenotypes among normal individuals and those with abnormal eating behavior. Additionally, these results may aid in the development of evaluation methods of appetite and treatment strategies for patients with disordered appetite.
Acknowledgments
The authors would like to thank Manryoukai Imaging Clinic for MRI scans and Forte Science Communications for editorial help with the manuscript. This work was supported in part by the Grant-in-Aid for Scientific Research C (KAKENHI: 23500848) from Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan and by the grant of Kao Research Council for the Study of Healthcare Science. The funders had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
List of abbreviations
- BMI
body mass index
- ECD
equivalent current dipole
- fMRI
functional magnetic resonance imaging
- GOF
goodness of fit
- MEG
magnetoencephalography
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PFS
Power of Food Scale.
Footnotes
Competing interests
We all declare that we have no competing interests.
Source of support: This work was supported in part by the Grant-in-Aid for Scientific Research C (KAKENHI: 23500848) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and by a grant from the Kao Research Council for the Study of Healthcare Science
References
- 1.World Health Organization (WHO) Obesity: preventing and managing the global epidemic. Report of a WHO consultation (WHO Technical Report Series 894) Geneva. 2000:1–253. [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–29. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Borer KT. Nonhomeostatic control of human appetite and physical activity in regulation of energy balance. Exerc Sport Sci Rev. 2010;38:114–21. doi: 10.1097/JES.0b013e3181e3728f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolls ET. Understanding the mechanisms of food intake and obesity. Obes Rev. 2007;8(Suppl 1):67–72. doi: 10.1111/j.1467-789X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 5.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav. 2007;91:432–39. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 7.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–43. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 10.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 11.Carnell S, Gibson C, Benson L, et al. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–21. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunez PL, Srinivasan R. Electrical fields of the brain: the neurophysics of EEG. 2nd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 14.He B. Modeling and Imaging of Bioelectric Activity: Principles and Applications. New York: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- 15.Hämäläinen MS, Hari R, Ilmoniemi RJ, et al. Magnetoencephalography – theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–97. [Google Scholar]
- 16.Lowe MR, Butryn ML, Didie ER, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–18. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Cappelleri JC, Bushmakin AG, Gerber RA, et al. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes (Lond) 2009;33:913–22. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa T, Orita K, Watanabe Y, Tanaka M. Validation of the Japanese version of the power of food scale in a young adult population. Psychol Rep. 2012;111:253–65. doi: 10.2466/08.02.06.15.PR0.111.4.253-265. [DOI] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Burdette J, Burns M, et al. Power of food moderates food craving, perceived control, and brain networks following a short-term post-absorptive state in older adults. Appetite. 2012;58:806–13. doi: 10.1016/j.appet.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–74. [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura K, Kawashima R, Sato N, et al. Functional delineation of the human occipito-temporal areas related to face and scene processing. A PET study. Brain. 2000;123(Pt 9):1903–12. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- 22.Allison T, McCarthy G, Nobre A, et al. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb Cortex. 1994;4:544–54. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- 23.Science and Technology Agency. Standard Tables of Food Composition in Japan. 5th ed. Tokyo: Printing Bureau of the Ministry of Finance (in Japanese); 2005. [Google Scholar]
- 24.Bowyer SM, Mason K, Tepley N, et al. Magnetoencephalographic validation parameters for clinical evaluation of interictal epileptic activity. J Clin Neurophysiol. 2003;20:87–93. doi: 10.1097/00004691-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Schultes B, Ernst B, Wilms B, et al. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92:277–83. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- 26.Del Parigi A, Chen K, Salbe AD, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–77. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 27.Del Parigi A, Chen K, Salbe AD, et al. Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage. 2005;24:436–43. doi: 10.1016/j.neuroimage.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Stingl KT, Rogić M, Stingl K, et al. The temporal sequence of magnetic brain activity for food categorization and memorization – an exploratory study. Neuroimage. 2010;52:1584–91. doi: 10.1016/j.neuroimage.2010.04.278. [DOI] [PubMed] [Google Scholar]
- 29.Guthoff M, Stingl KT, Tschritter O, et al. The insulin-mediated modulation of visually evoked magnetic fields is reduced in obese subjects. PLoS One. 2011;6:e19482. doi: 10.1371/journal.pone.0019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–71. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 31.Cornier MA, Salzberg AK, Endly DC, et al. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornier MA, Melanson EL, Salzberg AK, et al. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105:1028–34. doi: 10.1016/j.physbeh.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasanoff A. Bloodless FMRI. Trends Neurosci. 2007;30:603–10. doi: 10.1016/j.tins.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi M, Takeda M, Hattori N, et al. Functional imaging of gustatory perception and imagery: “top-down” processing of gustatory signals. Neuroimage. 2004;23:1271–82. doi: 10.1016/j.neuroimage.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi M, Sasabe T, Shigihara Y, et al. Gustatory imagery reveals functional connectivity from the prefrontal to insular cortices traced with magnetoencephalography. PLoS One. 2011;6:e21736. doi: 10.1371/journal.pone.0021736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15:1602–8. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi S, Kubota F, Nisijima K, et al. Cerebral activation focusing on strong tasting food: a functional magnetic resonance imaging study. Neuroreport. 2005;16:281–83. doi: 10.1097/00001756-200502280-00016. [DOI] [PubMed] [Google Scholar]
- 38.Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. J Neurosci. 2000;20:8954–64. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace & Company; 1999. [Google Scholar]
- 40.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Stoeckel LE, Weller RE, Cook EW, III, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]