Abstract
Background and Objective
The γ-secretase inhibitor (GSI) has been shown to inhibit expression of amyloid beta (Aβ), but GSI also has a side effect of reducing cell survival. Since low-power laser irradiation (LLI) has been known to promote cell survival, we examined whether 532 nm LLI can rescue the GSI side effect or not.
Study Design/Materials and Methods
The human-derived glioblastoma cells (A-172) were cultured in 35 mm culture dishes or 96-well plate. The center of dish or selected wells was irradiated with 532 nm laser (Nd:YVO4, CW, 60 mW) for 20, 40 and 60 min, respectively. The irradiated cells were photographed at immediately after, 24 and 48 h later and counted. GSI was supplemented in medium 3 h before LLI. The MTT assay was also used to estimate viable cells at 48 h after irradiation. The expression of phosphorylated Akt (p-Akt) or phosphorylated PTEN (p-PTEN) was examined by immunofluorescent staining and measured by fluorescence intensity using the software (BZ-9000, KEYENCE, Japan).
Results
GSI application depressed cell proliferation as well as cell survival compared to control. GSI down-regulated Aβ but up-regulated p-PTEN and suppressed p-Akt. Application of 532 nm LLI in the presence of GSI significantly recovered the GSI-mediated effects, i.e., LLI could decrease elevated p-PTEN, while increased p-Akt expression with keeping Aβ suppression. The LLI effects had a dose-dependency.
Conclusion
We confirmed that GSI potently suppressed intracellular Aβ and decreased cell survival. We conclude that a combination of GSI application and 532 nm LLI can increase cell proliferation via Akt activation while keeping PTEN and Aβ suppressed.
Introduction
Alzheimer's disease is a serious problem for aged individuals. The number of patients will almost double every 20 years, reaching 65.7 million in 2030 and 115.4 million in 2050 [1]. Amyloid beta (Aβ) is considered as a pathogenic agent of Alzheimer’s disease that is processed from amyloid precursor protein (APP) by γ-secretase (GS) [2]. Intracellular as well as extracellular accumulations of Aβ result in nerve cell toxicity [3]. As GS activity is essential for the release of intact Aβ, γ-secretase inhibitors (GSIs) have been contemplated for the treatment of Alzheimer’s disease. Since GSIs have been shown to decrease Aβ production after administration to transgenic mice overexpressing human APP [4], they were considered as useful drugs to lower Aβ accumulation for long-term treatment in human patients [5], [6].
Despite of the potential benefit, GSIs could have heavy side effects. It is well known that GS-mediated intracellular processes activate Notch signaling pathway, which is associated with cell proliferation and differentiation [7]–[9]. Notch regulates the expression of a phosphatase PTEN (Phosphatase and Tensin homolog deleted from chromosome) via intracellular Notch (ICN), the intracellular moiety of Notch. ICN, when released from Notch by GS, suppresses the expression of PTEN, which dephosphorylates a phosphoinositides that is critical for activation of Akt, a Ser/Thr kinase [10]. Activated Akt plays key roles in mediating cell proliferation, cell survival (anti-apoptotic), cell-cycle progression, differentiation, transcription, translation, and glucose metabolism [11], [12]. Therefore, although GSIs could be effective for treating Alzheimer’s disease with their inhibitory role of Aβ expression and accumulation, they have unwanted side effects of suppressing cell proliferation and survival by inhibiting Akt activation via PTEN elevation. These dual aspects of GSIs await other novel drugs or treatments that ameliorate the side effects.
It has been reported that the low-power laser irradiation (LLI) can promote cell proliferation and survival. Mester et al. first reported such effects on intractable skin ulcer in 1968 [13]. Since then, there are many studies showing LLI-mediated cell proliferation and survival in various fields including wound healing, reumatoid arthritis, tendinopathy, osteoarthritis [14]–[18]. In studies using cell culture systems, it was demonstrated that 532 nm LLI promoted proliferation of B-14 (Chinese hamster ovarian cell line) cells without inducing cell death [19]. Another study showed the 532 nm LLI on blood platelets can trigger signal transduction, leading to platelet activation, as well as the gradual loss of natural platelet reactivity and platelets' ability to respond to activating agents [20]. Mechanisms of these cell proliferating effects of 532 nm LLI are unclear, but recent work using 632.8 nm LLI indicated that Akt activation is involved in prevention of cell apoptosis [21].
Here we examined the effects of 532 nm LLI on cell proliferation in human-derived glioblastoma (A-172) and aimed to reveal mechanisms underlying LLI effects by investigating the involvement of the Notch-Akt signaling pathway.
Materials and Methods
We performed all experiments in accordance with the Declaration of Helsinki and Guide for Animal Experimentation at Soka University.
Laser Irradiation Method
A diode laser apparatus (Nd:YVO4, CW, 532 nm, 0–180 mW) was used. The experiment was conducted in a clean bench under 37°C and 5% CO2. The laser beam was reflected on a mirror and introduced to cells from the top to the bottom. The averaged power was 60 mW and the irradiated area was 7.1 mm2, thus the power density was 845 mW/cm2. In experimental group, the center of dish or well was irradiated for 20, 40 and 60 min with an energy density of 10.1, 20.3, 30.4×102 J/cm2, respectively.
Cell Culture of Glioblastoma (A-172)
The human-derived glioblastoma A-172 cell line was obtained from JCRB (#0228). The cells were cultured in 25 cm2 flasks with DMEM containing 10% fetal bovine serum and incubated in an atmosphere of 5% CO2, 95% air at 37°C. The medium was changed once every three days to maintain cell growth. Trypsinization was performed using 0.1% trypsin-PBS once the cells reached confluency (106 cells). Cells were plated in 35 mm dish, 96-well plates (MS-8096F, Sumitomo Bakelite Co. Ltd., Tokyo, Japan), or LAB-TEK Chamber Slide (Nalge Nunc International, New York, USA) with 10% FBS-DMEM medium at 6×103 cells/ml.
Cell Counting by Microscopic Observations
On the day following plating, culture wells on the clean bench maintained at 37°C and in an atmosphere containing 5% CO2 were photographed with a digital camera before LLI. After taking photographs, the plate was returned to the incubator. Photos were taken immediately after LLI, and 24 and 48 h after LLI. The photos were displayed on a PC monitor and cells were counted per unit area in all areas including the directly irradiated area.
Cell Viability by MTT Assay
Cell viability was measured by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H- tetrazolium bromide) colorimetric assay. The A-172 cells were plated at 0.3 × 104 cells/well in a 96-well plate (200 µl/well) and incubated for 12 hours. At 48 h after LLI, the culture medium was removed, and 200 µl of fresh medium containing 20 µl of MTT (6 mg/ml; Sigma-Aldrich Japan, Tokyo, Japan) was added to each well. The cells were incubated at 37°C for 3 h. Colored precipitates were extracted with 100 µl of acidic isopropanol at room temperature for 1 h, and the absorbance was measured at 570 nm using a plate reader.
Immunofluorescent Staining Method
The cells were rinsed three times with PBS after LLI. They were fixed with 4% PFA, treated with 0.1% Triton X-100 and blocked by 1% BSA-PBS. Primary antibody against phospho-Thr308-Akt (1∶800, Cell Signaling), phospo-Ser473-Akt (1∶800, Promega), phosphor-Ser380/Thr382/383-PTEN (1∶100, Cell Signaling), or Aβ-Amyloid (1∶50, Cell Signaling) was added and left overnight at 4°C. Next day, secondary antibody (Rhodamine, FITC) was added and left for 3 h under room temperature. After washing with PBS, glycerol was added and cover glass was put on. Stained cells were observed by a fluorescent microscope (BZ-9000, KEYENCE, Tokyo, Japan). The center of each well was photographed with same exposure time using a 20x objective lens after focusing on cells under phase contrast. The maximum intensity in irradiated area was measured using an optional software (BZ-analysis application, KEYENCE) without applying any image enhancement. However, the haze reduction was used to make fluorescent images clearer.
Pretreatment Cells with γ-secretase Inhibitor (GSI)
DAPT, a typical agent of GSIs, was cryopreserved at 1 mM in DMSO. DAPT was added directly to FBS-DMEM medium at 25 µM and applied to cells 3 h before LLI. DMSO (1% in the medium) showed harmless effects in preliminary experiments (data not shown).
Analysis of ATP Level in Cell Lysate
An ATP/ADP ratio assay kit (EnzyLightTM, BioAssay Systems, CA, USA) was used to quantify ATP amount. A-172 cells were plated at 0.5×104 cells/well in a 96-well plate (200 µl/well) and incubated for 12 hours. The culture medium was removed after LLI. 90 µl ATP reagent was added to each 96-well and mixed by tapping the plate. After 1 min, luminescence (RLU A) was read on a luminometer (ATTO BIO-INSTRUMENT, Tokyo, Japan). 10 minutes after measuring RLU A, the luminescence of samples was read again (RLU B). This measurement provides the background prior to measuring ADP. Immediately following RLU B measurement, 5 µl ADP reagent was added to each well and mixed by pipetting up and down. After 1 min, luminescence (RLU C) was read for ADP level. The following formula was used to calculate ATP/ADP ratio: (RLU C – RLU B)/RLU A.
Statistical Analysis
All values were presented as mean ± SD. Student’s two-tailed non-paired t-test and one-way ANOVA were used to analyze statistical differences between 2 groups or among multiple groups, respectively.
Results
Effects of 532 nm LLI on the Number of A-172 Cells
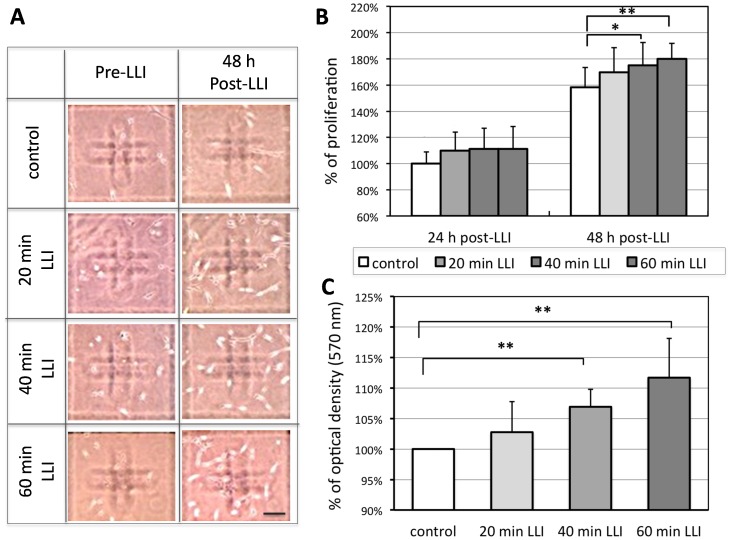
We have examined if LLI affects the proliferation of A-172 cells grown in culture. The cells were plated at 0.3 × 104 cells/well in 96-well plates and were irradiated with 532 nm LLI for 0 (no LLI: control), 20, 40, and 60 min. The number of cells remaining at 24 and 48 hours after LLI was counted and compared to that before LLI (Pre-LLI) (Fig. 1). At 24 h after LLI, the proliferation ratio, the number of remaining cells after LLI normalized to that of untreated control, was 110±14%, 111±16%, or 111±17% for 20 min, 40 min, or 60 min LLI, respectively (Fig. 1B-left bars, no significances among any groups). At 48 h, they were 158±15% for control, 170±19%, 175±18%, or 180±12% for 20 min, 40 min, and 60 min LLI, respectively (Fig. 1B, right bars, n = 12, p<0.05 for 20 and 40 min LLI, p<0.01 for 60 min LLI).
Figure 1. Effects of LLI on the number of A-172 cells.
A: Sample images of A-172 cells under light microscope. The number of cells increased 48 h post-LLI (right column) after 20, 40, 60 min LLI compared to pre-LLI (left column). Cal.: 100 µm. B: Proliferation ratio (the ratio of cell number at 24 or 48 hours following LLI and cell number before LLI) was normalized to control (no LLI) (n = 12 for each group). C: A summary of colorimetric analysis by MTT staining performed at 48 h after LLI (each group: n = 16). The optical density of each group was normalized to the value of control group (no LLI) at 48 h after initial condition. Asterisks: one-way ANOVA, * p<0.05, ** p<0.01.
In addition to cell counts, we also used a calorimetric method to quantify the proliferation and survival in cell culture using the MTT assay. The mean optical density at 570 nm at 48 hours after 40 or 60 min LLI increased significantly over non-irradiated control (Fig. 1C, n = 16, p<0.01 for 40 and 60 min LLI), while little change was observed with 20 min LLI.
Expressions of p-Akt and p-PTEN by 532 nm LLI
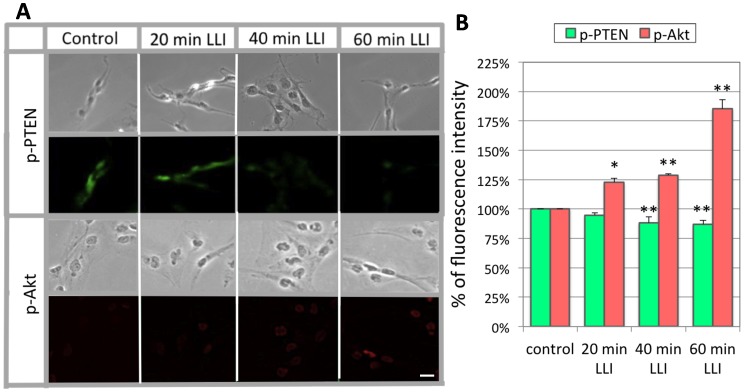
To examine possible involvement of Akt signaling in the LLI-mediated proliferation, we investigated Akt as well as PTEN activation (Fig. 2). Anti-phosphorylated Akt (p-Akt) antibodies detect activated (phosphorylated) Akt molecules, while anti-phosphorylated PTEN (p-PTEN) antibodies detect PTENs that removed the phosphate group from activated phosphoinositides and retained it [22]. With 20 min LLI, the intensity of p-PTEN immunofluorescence did not change significantly (95±2% of control). However, with 40 and 60 min LLI, the p-PTEN immunofluorescence intensity decreased significantly over control (88±5%, p<0.05, for 40 min; 87±3%, p<0.05, for 60 min) (n = 120∶4 experiments and 30 selected cells each). In contrast, p-Akt immunofluorescence intensity significantly increased (122±4%, 129±1%, and 185±8% for 20, 40, and 60 min LLI over control, p<0.05 or p<0.01).
Figure 2. Immunofluorescent staining of Akt and PTEN.
A: The LLI effects on immunofluorescence staining of p-PTEN and p-Akt in cultures cells (bottom). Corresponding phase contrast micrographs are shown on top of each fluorescent image. It should be noted that tumor cells normally proliferate in high proportions and p-Akt is often highly expressed in cancer cells of different natures, whereas our result of control group shows low level of p-Akt expression due to haze reduction. Cal.: 100 µm. B: Average fluorescence intensity for p-PTEN or p-Akt normalized to the control value. Asterisks: one-way ANOVA, * p<0.05, ** p<0.01.
Effects of a Combined Application of GSI and LLI on Cell Proliferation
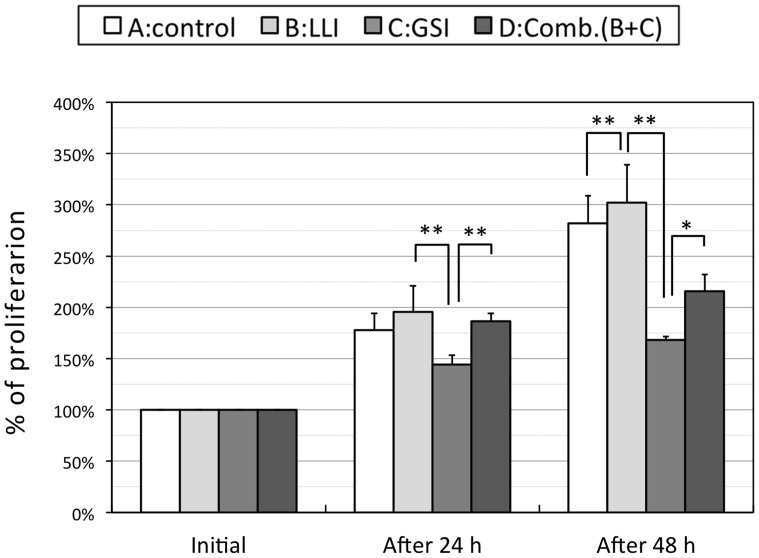
DAPT, a typical agent of GSIs, has been previously shown to retard cell proliferation by reducing generation of ICN [23]. We also tested this agent in A-172 cells in 13 experiments. At 24 h after LLI, the standardized proliferation ratios were 178±16% for control group, 196±26% for experimental group which received 60 min LLI only, 144±9% for pretreated group with GSI, and 186±8% for group received both applications, respectively. At 48 h after LLI, they were 282±27, 302±37, 168±4, and 216±16%, respectively (Fig. 3). There were statistical significances between some pairs of groups as shown in Fig. 3 (p<0.01 or p<0.05). It is worthy of note that the LLI was able to rescue the DAPT-induced reduction of cells by approximately (186–144 = ) 42% at 24 h and (216–168 = ) 48% at 48 h after LLI.
Figure 3. Effects of LLI, DAPT and combined application of both on cell proliferation.
Proliferation ratios for each group at 24 h and 48 h after LLI for control (white bars), 60 min LLI (light grey bars), DAPT (grey bars) or the combination of LLI and DAPT (dark grey bars) are shown. Asterisks: one-way ANOVA, * p<0.05, ** p<0.01.
Effects of LLI on Intracellular Signaling Molecules under GSI
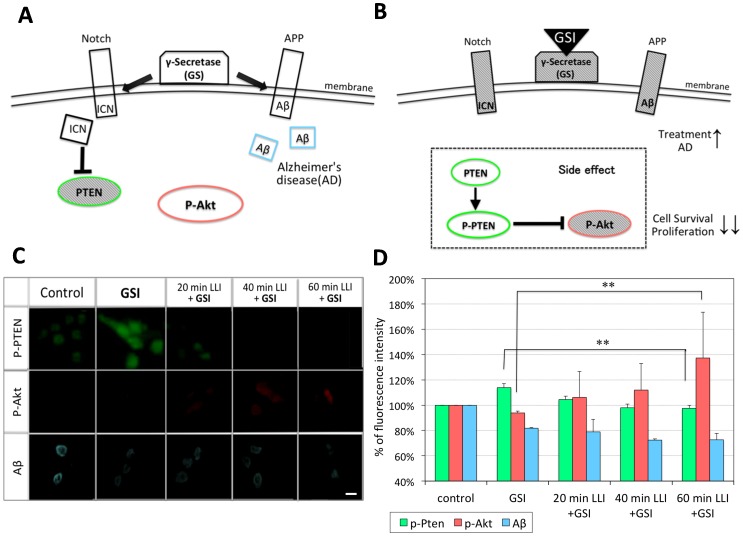
Fig. 4A describes the action of GS in relation to Notch and APP processing. GS cleaves Notch as well as APP at plasma membrane, making ICN and Aβ, respectively. When GS is inhibited by GSI, ICN level will be reduced and PTEN expression will increase, resulting in suppression of Akt signaling pathway and in inhibition of cell proliferation and survival (Fig.4B). Similarly, Aβ level will decrease as more uncleaved APP will remain.
Figure 4. Effects of LLI on the fluorescence intensities of p-Akt, p-PTEN and Aβ.
A, B: Schematic diagrams of Notch and APP signaling pathways. GS can cleave APP and Notch, making Aβ and ICN, respectively (A). GSI inhibits Aβ expression but also inhibits ICN expression (B), a side-effect against cell survival through PTEN activation (dashed box in B). C: LLI effects on immunofluorescence staining of p-Akt, p-PTEN and Aβ in cells pretreated with GSI. Cal.: 100 µm. D: Average fluorescence intensity for p-PTEN (green bars), p-Akt (pink bars) and Aβ (blue bars) was normalized to control. Asterisks: one-way ANOVA, ** p<0.01.
We next tested if the LLI-mediated rescue of GSI-induced reduction in cell number (Fig. 3) involves activation of Akt and the reduction of PTEN. As described above, A-172 cells were treated with DAPT for 3 hours, exposed to LLI for 20, 40, and 60 min, and fixed at 15 min for the immunofluorescent staining of p-Akt and p-PTEN (Fig. 4C). Immunofluorescence intensity was quantified for each protein and normalized to non-DAPT treated control (Fig. 4D) (n = 120∶4 experiments and 30 selected cells each). As expected, DAPT treatment increased the expression of p-PTEN (114±3% when standardizing untreated control as 100%, p<0.01), suggesting that PTEN expression has increased due to inhibition of GS and reduction of ICN. We also examined if soluble Aβ is reduced by immunofluorescent staining using anti-Aβ antibodies. As would be expected for the inhibition of GS, Aβ was reduced in DAPT-treated cells (82±1%, p<0.01). We then tested if LLI could alter the expression of p-PTEN as well as Aβ. Simultaneous application of 20 min LLI reduced p-PTEN to the control level, but did not affect already reduced Aβ level. Effects of 40 and 60 min LLI were similar to 20 min LLI. These data suggest that LLI, within 20 min, acts specifically on the Notch pathway to reduce PTEN expression, but does little to the APP processing and homeostasis of Aβ.
Immunofluorescence intensity of p-Akt was reduced slightly from the control level in DAPT-treated cells (94±1%, p<0.01). This decrease was rescued by LLI. With 20 min LLI, the immnofluorescent staining of p-Akt was recovered to the control level. With 40 and 60 min LLI, the intensity significantly increased 112±21% (p<0.01) and 137±36% (p<0.01) above the control level, respectively. These data suggest that LLI could recover DAPT-induced reduction of p-Akt in a dose-dependent manner.
Effects of LLI on ATP Level in Cell Lysates
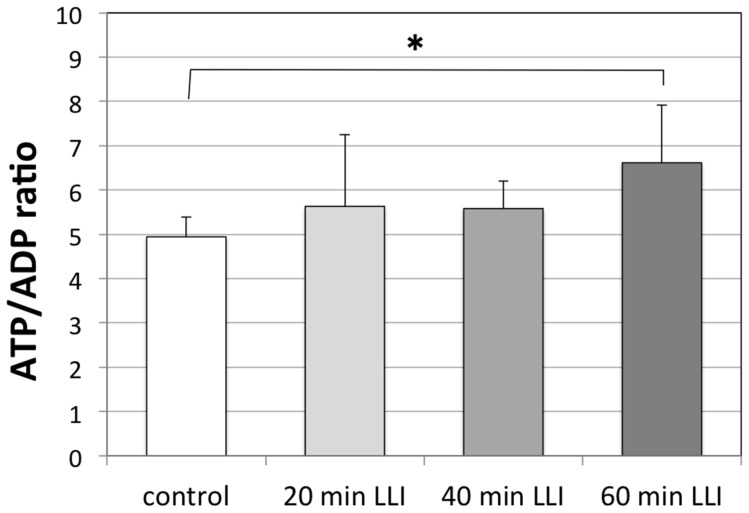
To test the possibility that LLI-induced cell proliferation involves ATP elevation, we examined ATP level in cell lysates using ATP/ADP ratio assay kit (Fig. 5). Mean luminescence after 60 min LLI increased significantly over that of control group (p<0.05, n = 5), while little changes were observed for 20 or 40 min LLI groups.
Figure 5. Effects of LLI on ATP level in cell lysates.
The ATP/ADP ratio measured using a luminescence-based assay for control and for 20, 40 and 60 min LLI. The luminescent densities for ATP and ADP were measured 48 h after LLI treatment. The ATP/ADP ratio was calculated and averaged (n = 5). Asterisks: one-way ANOVA, * p<0.05.
Discussion
In the present study, using conventional cell counting and the colorimetric MTT assay, we demonstrate that 532 nm LLI (60 mW) significantly increased viability of human-derived glioblastoma A-172 cells. The effect showed a LLI dose-dependency with 60 min LLI being the most effective for cell proliferation. These findings contrast to studies using LLI of other wavelengths in the same cell type. The infra-red 808 nm LLI delayed cell cycle and suppressed cell proliferation [24], and the 405 nm LLI promoted the cell death in A-172 cells [25]. Thus, the effect of LLI on the cell proliferation appears to depend on the wavelength. Indeed, LLI wavelength-dependent effects were previously reported in various cell types [26].
Different effects of LLI wavelength on cell proliferation and survival may depend on photoacceptors present in mitochondria, especially those in the mitochondrial respiratory chain [27]. Primary photoacceptors include cytochromes c oxidase (red and near-infrared light region), NADH-dehydrogenase (blue spectral region), and cytochromes b, c1 and c (green light region). Their cofactors are porphyrins (for cytochrome c oxidase and cytochromes b, c1 and c), or flavins (for NADH-dehydrogenase), respectively [28]. These photoreceptors and cofactors contribute to the generation of ATP, a critical source of chemical energy in cells. Mitochondria are the center of many diverse cellular functions such as signaling, cellular differentiation, cell death, as well as the control of the cell cycle and cell growth [29]. Thus photoacceptors in mitochondria upon light absorption may regulate the level of ATP to support cell survival. However, considering that LLI has different biological effects on different cells, it is difficult to conceive that the regulation of mitochondrial photoacceptors is the only mechanism underlying LLI-mediated cell proliferation. It is likely that final consequences of specific photobiological effects are determined not at the level of primary reactions in the mitochondrial respiratory chain but based on secondary cellular signaling [27]. It will be important to characterize how proteins and enzymes expressed in each cell type contribute to photobiological effects.
The present study focused on the effect of LLI on the Notch-PTEN-Akt pathway in glioblastoma. We showed a reciprocal expression of p-PTEN inactivation and p-Akt activation in Fig. 2. Previous work of others showed the effects of LLI on the mitogen-activated protein kinase (MAPK) pathway [30] and the ROS-Src pathway [31]. To our knowledge, this is the first to report that LLI at any wavelength influenced the Notch pathway. Previously, it was suggested that Akt plays a key role for cell survival and proliferation [32]. Using FRET, it was demonstrated that 632 nm LLI increased cell proliferation via the activation of Akt signaling pathway [33]. It was also reported that 632.8 nm LLI inhibits the expression of p21 [34], a molecule that arrests the cell-cycle, and enhances the cell-cycle progress via its phosphorylation by Akt [35]. Recent investigations implicate an important role of Akt in mitochondria for the regulation of cell growth. The expression level of Akt in mitochondria is dynamically regulated by cellular signaling activities [36], and Akt mediates mitochondrial protection in cardiomyocytes [37]. Importantly, Akt suppresses apoptosis signaling independent of cytosolic Akt in cardiac muscle cells [38]. Considering a number of studies addressing the effects of LLI on mitochondria [39], our study may have revealed Akt as an interesting link between LLI and mitochondria.
GSI has been proposed for the treatment of Alzheimer’s disease because its inhibition of GS will decrease the generation of intracellular Aβ, a potential culprit in Alzheimer’s disease [40]. However, GSI treatment will also cause a side effect in healthy cells in Alzheimer’s disease patients since GSI will prevent the normal processing of Notch, whose cleavage by GS at plasma membrane generates ICN, a PTEN suppressor and an Akt enhancer, promoting cell survival [41], [42]. In this study, we observed that GSI down-regulated Aβ and upregulated PTEN, suppressing Akt activation and depressing cell proliferation and cell survival as predicted from previous studies. We also showed that 532 nm LLI was able to decrease PTEN expression of GSI-pretreated cells and to increase Akt expression of those cells while keeping Aβ suppressed. We further demonstrated that the LLI rescued the depression of cell proliferation and even induced further growth. Thus, LLI may be useful to prevent the side effect in the Alzheimer’s disease treatment using GSI. Future studies will examine the combined administration of GSI and 532 nm LLI in animal models of Alzheimer’s disease in vivo.
Acknowledgments
We thank Ms. Ang Foong Yee, Mr. Hideyuki Murayama and Mr. Kei Sadakane (Graduate School of Engineering, Soka University) for their technical assistance and valuable discussion.
Funding Statement
The authors have received Faculty Research Fees (total: 1,200,000 Yen) from Soka University in 2011 and 2012. Yumi Fukuzaki received 600,000 Yen from the Sasakawa Scientific Research Grant of The Japan Science Society in 2011. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Prince M, Jackson J (2009) Alzheimer’s Disease International World Alzheimer Report. Grobal Action on Aging site. Available: http://www.globalaging.org/health/world/2009/alzheimer.pdf. Accessed 2013 Jan 10.
- 2. Li M, Chen L, Lee DH, Yu LC, Zhang Y (2007) The role of intracellular amyloid β in Alzheimer's disease. Progress in Neurobiology 83: 131–139. [DOI] [PubMed] [Google Scholar]
- 3. Zhao H, Zhu J, Cui K, Xu X, O'Brien M, et al. (2009) Bioluminescence imaging reveals inhibition of tumor cell proliferation by Alzheimer's amyloid β protein. Cancer Cell Int 1: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, et al. (2001) Functional γ-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem 76: 173–181. [DOI] [PubMed] [Google Scholar]
- 5. Wolfe MS, De Los Angeles J, Miller DD, Xia W, Selkoe DJ (1999) Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer's disease. Biochemistry 38: 11223–11230. [DOI] [PubMed] [Google Scholar]
- 6. Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, et al. (2001) Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem 76: 173–81. [DOI] [PubMed] [Google Scholar]
- 7. De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, et al. (1999) A presenilin-1-dependent g-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522. [DOI] [PubMed] [Google Scholar]
- 8. Greenwald I (1998) LIN-12/Notch signaling: lessons from worms and flies. Genes Dev 12: 1751–1762. [DOI] [PubMed] [Google Scholar]
- 9. Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776. [DOI] [PubMed] [Google Scholar]
- 10. Conlon RA, Reaume AG, Rossant J (1995) Notch1 is required for the coordinate segmentation of somites. Development 121: 1533–1545. [DOI] [PubMed] [Google Scholar]
- 11. Gutierrez A, Look AT (2007) Notch and PI3K-Akt Pathways Intertwined. Cancer Cell 12: 411–413. [DOI] [PubMed] [Google Scholar]
- 12. Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, et al. (2007) Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nature Med 13: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mester E, Juhász J, Varga P, Karika G (1968) Lasers in clinical practice. Acta Chir Acad Sci Hung 9: 349–357. [PubMed] [Google Scholar]
- 14. Karu T (1989) Photobiology of low-power laser effect, Health Physics. 56: 691–704. [DOI] [PubMed] [Google Scholar]
- 15. da Silva JP, da Silva MA, Almeida AP, Lombardi Junior I, Matos AP (2010) Laser therapy in the tissue repair process: a literature review. Photomed Laser Surg 28: 17–21. [DOI] [PubMed] [Google Scholar]
- 16. Brosseau L, Robinson V, Wells G, Debie R, Gam A, et al. (2005) Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis. Cochrane Database Syst Rev DOI: 10.1002/14651858.CD002049.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bjordal JM, Lopes-Martins RA, Joensen J, Couppe C, Ljunggren AE, et al. (2008) A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC M Disorders DOI: 10.1186/1471-2474-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jamtvedt G, Dahm KT, Christie A, Moe RH, Haavardsholm E, et al. (2007) Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Phys Ther 88: 123–136. [DOI] [PubMed] [Google Scholar]
- 19. Kassák P, Przygodzki T, Habodászová D, Bryszewska M, Sikurová L (2005) Mitochondrial alterations induced by 532 nm laser irradiation. Gen Physiol Biophys 24: 209–220. [PubMed] [Google Scholar]
- 20. Gresner P, Watała C, Sikurová L (2005) The effect of green laser light irradiation on whole blood platelets. J Photochem Photobiol B 79: 43–50. [DOI] [PubMed] [Google Scholar]
- 21. Liang J, Liu L, Xing D (2012) Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apotosis through the Akt/GSK3β/β-catenin pathway. Free Radic Biol Med 53(7): 1459–1467. [DOI] [PubMed] [Google Scholar]
- 22. Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann, et al (2002) Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 15 295(5562): 2088–91. [DOI] [PubMed] [Google Scholar]
- 23. Pancewicza J, Taylora JM, Dattaa A, Baydouna HH, Waldmann TA, et al. (2010) Notch signaling contributes to proliferation and tumor formation of human T-cell leukemia virus type 1-associated adult T-cell leukemia. Proc Natl Acad Sci USA 107(38): 16619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murayama H, Sadakane K, Yamanoha B, Kogure S (2012) Low-power 808-nm laser irradiation inhibits cell proliferation of a human-derived glioblastoma cell line in vitro. Lasers Med Sci 27: 87–93. [DOI] [PubMed] [Google Scholar]
- 25. Ang FY, Fukuzaki Y, Yamanoha B, Kogure S (2011) Immunocytochemical studies on the effect of 405-nm low-power laser irradiation on human-derived A-172 glioblastoma cell. Lasers Med Sci 27: 935–942. [DOI] [PubMed] [Google Scholar]
- 26. Cavett W, Tucci M, Cason Z, Lemos L, England B, et al. (1997) Comparison of cellular responses induced by low level light in different cell types. Biomed Sci Instrum 33: 155–60. [PubMed] [Google Scholar]
- 27.Karu T (2003) Low Power Laser Therapy. Biomedical Photonics Handbook 48: CRC Press. 48 p.
- 28.Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci DOI:10.1186/1423-0127-16-4. [DOI] [PMC free article] [PubMed]
- 29. McBride HM, Neuspiel M, Wasiak S (2006) Mitochondria: more than just a powerhouse. Curr Biol 16: 551–560. [DOI] [PubMed] [Google Scholar]
- 30. Shefer G, Oron U, Irintchev A, Wernig A, Halevy O (2001) Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway. J Cell Physiol 187: 73–80. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Xing D, Gao X (2008) Low-Power Laser Irradiation Activates Src Tyrosine Kinase Through Reactive Oxygen Species-Mediated Signaling Pathway. J Cell Physiol 217: 518–528. [DOI] [PubMed] [Google Scholar]
- 32. Kim D, Chung J (2002) Akt: Versatile Mediator of Cell Survival and Beyond. Journal of Biochemistry and Molecular Biology 35: 106–115. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Xing D, Gao X, Wu S (2009) Low-power laser irradiation promotes cell proliferation by activating PI3K/Akt pathway. J Cell Physiol 219: 553–562. [DOI] [PubMed] [Google Scholar]
- 34. Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, et al. (2002) Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci 115: 1461–1469. [DOI] [PubMed] [Google Scholar]
- 35.Azevedo LH, de Paula Eduardo F, Moreira MS, de Paula Eduardo C, Marques MM (2006) Influence of different power densities of LILT on cultured human fibroblast growth. Lasers Med Sci DOI 10.1007/s10103-006-0379-9. [DOI] [PubMed]
- 36. Bijur GN, Jope RS (2003) Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem 87: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miyamoto S, Murphy AN, Brown JH (2008) Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ 15: 521–529. [DOI] [PubMed] [Google Scholar]
- 38. Su CC, Yang JY (2012) Mitochondrial Aktregulated mitochondrial apoptosis signaling in cardiac muscle cells. Am J Physiol Heart Circ Physiol 302: 716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karu T (2008) Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem Photobiol 84: 1091–1099. [DOI] [PubMed] [Google Scholar]
- 40. Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, et al. (2003) The role of presenilin cofactors in the gamma-secretase complex. Nature 422: 438–441. [DOI] [PubMed] [Google Scholar]
- 41.Panza F, Solfrizzi V, Frisardi V, Capurso C, D'Introno A, et al.. (2009) Disease-modifying approach to the treatment of Alzheimer's disease: from alpha-secretase activators to gamma-secretase inhibitors and modulators. Drugs Aging DOI: 10.2165/11315770-000000000-00000. [DOI] [PubMed]
- 42. Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ (2007) Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood 110: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]