Abstract
Fkbp5 is genetically linked to stress-related diseases. Fkbp5 knockout mice are available and widely used to explore the role of Fkbp5 in health and disease. We found that these mice carry a gene duplication of glyoxylase-1, which explains why glyoxylase-1 levels are increased in the Fkbp5 knockout mice.
Keywords: FKBP51, flanking gene problem, glyoxalase-1, knockout mice
In several genetic studies researchers linked FK506 binding protein 5 (Fkbp5) to stress-related diseases and phenotypes such as major depression, posttraumatic stress disorder, and recovery from psychosocial stress (Binder et al. 2004; Zimmermann et al. 2011; Klengel et al. 2013). In addition, Fkbp5 is also linked to treatment response in depression (Binder et al. 2004; Lekman et al. 2008). To elucidate the role of FKBP5 in an animal model, a conventional knockout mouse has been constructed and made available to the scientific community (Tranguch et al. 2005; Touma et al. 2011). These Fkbp5-deficient mice show no overt phenotype unless they are older than 10 months of age (O’Leary et al. 2011) or exposed to stress (Touma et al. 2011; Hartmann et al. 2012).
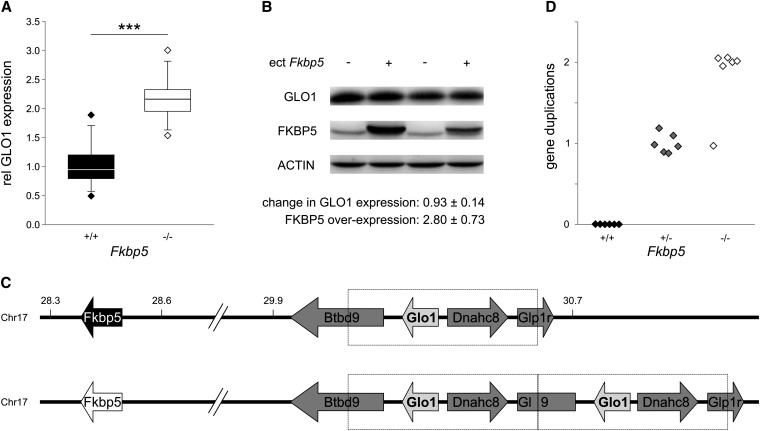
To elucidate the effects of Fkbp5-deletion on molecular pathways, we compared the expression profile of Fkbp5+/+ and Fkbp5−/− litter mates. A marked difference in glyoxalase-1 (Glo1) mRNA was observed with Fkbp5−/− mice expressing greater levels (not shown). Consistent with this observation, about 2-fold more GLO1 protein was found in Fkbp5−/− mice (Figure 1A). For more detailed molecular analyses, we sought to establish a cellular model. Therefore, we overexpressed FKBP5 by transient transfection in either primary rat astrocytes or HEK293 cells. However, overexpression of FKBP5 did not change Glo1 mRNA (not shown) and also not alter protein levels of GLO1 (Figure 1B).
Figure 1.
(A) Comparison of GLO1 protein expression in hippocampi from Fkbp5−/− and Fkbp5+/+ mice. Hippocampi were prepared, and GLO1 expression was determined after protein extraction by Western blotting (polyclonal antibody; Santa Cruz Biotechnologies); signals were normalized to ACTIN (polyclonal antibody; Santa Cruz Biotechnologies). Expression difference was analyzed by Tukey’s test (n = 12 per genotype; P < 0.001). (B) Overexpression of Fkbp5 in HEK-293 cells by transient transfection did not affect GLO1 levels. Cells were transfected with Fkbp5 expressing or control vector, and protein levels were determined in cell extracts by Western blotting 3 d later. Mean protein levels ± SEM of GLO1 and FKBP5 (polyclonal antibody; Bethyl Laboratories) normalized to ACTIN are indicated (n = 5). (C) Scheme of genomic arrangement of Fkbp5 (28.5−28.4 Mb) and Glo1 (30.6−30.6 Mb) on chromosome 17 (28.3−30.7 Mb), without (upper) and with (lower) Glo1 gene duplication. The wild-type Fkbp5 allele (originating from C57BL/6 mice) is usually coinherited with a single copy of Glo1, whereas the knockout Fkbp5 allele (originating from 129SvJ mice) is coinherited with two copies of Glo1. (D) Verification of coinheritance of the Fkbp5 knockout allele with Glo1 duplication. Genomic duplications of the Glo1 spanning region were determined by quantitative reverse-transcription PCR (two independent PCRs per mouse) with primers against the duplication transition region [fw 5′-CTCTGCCCCAGAGAACAGTC and rv 5′-TGATAGAGGCCACACAGCAG (Williams et al. 2009)] and normalized to genomic levels of Npsr1 (determined by quantitative reverse-transcription PCR with the following primers: fw 5′-CAGCTGCTGCCCCGGCTAAC and rv 5′-GGTTGGCTGGCATGGCTCAGG).
We noted that the genes Fkbp5 and Glo1 are only approximately 2 Mb apart from each other on chromosome 17 of the mouse (Figure 1C). In addition, gene duplication around Glo1 was observed in several mouse strains (Egan et al. 2007; Williams et al. 2009). The Fkbp5 deletion was constructed in 129SvJ ES cells, and the resulting mice were then crossed with C57BL/6 animals; 129SvJ mice carry the Glo1 gene duplication but C57BL/6 mice do not (Williams et al. 2009).
Therefore, it appeared likely that through selection of Fkbp5+/+ and Fkbp5−/− alleles in the subsequent crossings the Glo1 gene duplication originating from 129SvJ mice was coselected with the Fkbp5−/− allele, whereas the unduplicated Glo1 cosegregated with the Fkbp5+/+ allele. To test this hypothesis, we used polymerase chain reaction (PCR) primers designed for monitoring the Glo1 gene duplication (Williams et al. 2009). DNA samples from Fkbp5−/−, Fkbp5-/+ and Fkbp5+/+ mice were probed. No Glo1 gene duplication was detectable in Fkbp5+/+ mice, whereas the PCR signal in Fkbp5−/− mice was clearly detectable and twice as high as in Fkbp5-/+ mice (Figure 1D). Therefore, the greater levels of mRNA and protein of GLO1 in Fkbp5−/− mice compared with wild-type mice are likely due to the double Glo1 gene dose in these mice. In general, this so-called “flanking allele” problem is a well-known and likely common phenomenon in gene knockout via homologous recombination (Gerlai 1996; Crusio et al. 2009). It could be avoided, for example, by genome editing with engineered nucleases or by using inducible gene knock out techniques (Sauer 1998; Carbery et al. 2010).
GLO1 is a ubiquitously expressed enzyme involved in the detoxification of methylglyoxal (Thornalley 2008). Methylglyoxal is a toxic byproduct of glycolysis that leads to protein modification and apoptosis (Thornalley 2008) and influences behavior when acting as GABAA receptor agonist (Distler et al. 2012). GLO1 has been linked to diabetic complications, anxiety disorders, schizophrenia, seizure susceptibility, pain, cancer, and aging (Thornalley 2008; Distler and Palmer 2012). At least some of these diseases and phenotypes also have been associated with Fkbp5, making Fkbp5−/− mice potentially very useful genetic model for further investigation. Our observation of Glo1 gene duplication in Fkbp5−/− mice suggests that the Glo1 status should be taken into consideration when interpreting data. Studies on neuroendocrine and stress effects of Fkbp5 gene deletion published so far are likely not biased by the Glo1 gene duplication, in particular because no differences between Fkbp5+/+ and Fkbp5−/− mice have been observed under basal conditions when neuroendocrine parameters or behavior, including anxiety-like behavior, is assessed (Touma et al. 2011; Hartmann et al. 2012).
Footnotes
Communicating editor: I. M. Hall
Literature Cited
- Binder E. B., Salyakina D., Lichtner P., Wochnik G. M., Ising M., et al. , 2004. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36: 1319–1325 [DOI] [PubMed] [Google Scholar]
- Carbery I. D., Ji D., Harrington A., Brown V., Weinstein E. J., et al. , 2010. Targeted genome modification in mice using zinc-finger nucleases. Genetics 186: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio W. E., Goldowitz D., Holmes A., Wolfer D., 2009. Standards for the publication of mouse mutant studies. Genes Brain Behav. 8: 1–4 [DOI] [PubMed] [Google Scholar]
- Distler M. G., Palmer A. A., 2012. Role of Glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: recent advances and mechanistic insights. Front Genet. 3: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler M. G., Plant L. D., Sokoloff G., Hawk A. J., Aneas I., et al. , 2012. Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J. Clin. Invest. 122: 2306–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C. M., Sridhar S., Wigler M., Hall I. M., 2007. Recurrent DNA copy number variation in the laboratory mouse. Nat. Genet. 39: 1384–1389 [DOI] [PubMed] [Google Scholar]
- Gerlai R., 1996. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 19: 177–181 [DOI] [PubMed] [Google Scholar]
- Hartmann J., Wagner K. V., Liebl C., Scharf S. H., Wang X. D., et al. , 2012. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 62: 332–339 [DOI] [PubMed] [Google Scholar]
- Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J. C., et al. , 2013. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16: 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekman M., Laje G., Charney D., Rush A. J., Wilson A. F., et al. , 2008. The FKBP5-gene in depression and treatment response–an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol. Psychiatry 63: 1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary J. C., III, Dharia S., Blair L. J., Brady S., Johnson A. G., et al. , 2011. A new anti-depressive strategy for the elderly: ablation of FKBP5/FKBP51. PLoS ONE 6: e24840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., 1998. Inducible gene targeting in mice using the Cre/lox system. Methods 14: 381–392 [DOI] [PubMed] [Google Scholar]
- Thornalley P. J., 2008. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—role in ageing and disease. Drug Metabol. Drug Interact. 23: 125–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C., Gassen N. C., Herrmann L., Cheung-Flynn J., Bull D. R., et al. , 2011. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry 70: 928–936 [DOI] [PubMed] [Google Scholar]
- Tranguch S., Cheung-Flynn J., Daikoku T., Prapapanich V., Cox M. B., et al. , 2005. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 102: 14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R., Lim J. E., Harr B., Wing C., Walters R., et al. , 2009. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS ONE 4: e4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Brückl T., Nocon A., Pfister H., Binder E. B., et al. , 2011. Interaction of variants in the FKBP5 gene and adverse life events in predicting the first depression onset: results from a ten-year prospective community study. Am. J. Psychiatry 168: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]