Abstract
Many stimuli induce short-term increases in the cytosolic concentration of free calcium ions (Ca2+) that encode signaling information about diverse physiological and developmental events. Slow cytosolic Ca2+ oscillations that span an entire day have also been discovered in both plants and animals; it is thought that these daily Ca2+ oscillations may encode circadian clock signaling information. A recent study focusing on the characterization of the extracellular Ca2+-sensing receptor (CAS) has now provided insight into the molecular mechanisms by which the daily Ca2+ oscillation in plants is generated. Here we summarize the major findings regarding daily oscillations of cytosolic Ca2+ concentrations in plants and animals, and discuss hypothetical biological roles for the circadian-clock-regulated physiology in plants.
Information about diverse physiological and developmental events is transmitted through changes in the cytosolic concentration of free calcium ions ([Ca2+]i), which often occur in oscillatory patterns of various amplitudes, frequencies, and durations (1, 2). Most Ca2+ signals are brief, lasting from milliseconds to minutes. However, in both plants and animals, researchers have also found a slow [Ca2+]i oscillation that spans an entire day (3–6). This circadian Ca2+ oscillation is arguably one of the most stable [Ca2+]i oscillations found in nature, in terms of its period and phase (6). Many organisms have a daily internal pacemaker, a circadian clock, that regulates the timing of various physiological processes throughout the day and anticipates the daily and seasonal changes of the surrounding environment. Although the daily [Ca2+]i oscillation is thought to encode circadian clock signaling information, the physiological activities that it regulates and the underlying mechanisms that maintain it remain unknown. A recent study performed by Pei and colleagues has provided insight into the molecular mechanisms by which the daily [Ca2+]i oscillation can be generated in plants (7). Here we summarize the major findings regarding the daily [Ca2+]i oscillation in plants and animals, and discuss its hypothetical biological role in plants.
Circadian [Ca2+]i oscillation in plants and animals
The daily oscillation of [Ca2+]i was first described in plants. Experiments using transgenic plants expressing the Ca2+-sensitive luminescent protein aequorin to monitor [Ca2+]i changes in whole plants showed that [Ca2+]i oscillates with a period close to 24 hours, and that, in tobacco and Arabidopsis, peak Ca2+ concentrations occur just after dawn (3). That this [Ca2+]i oscillation is apparent with bioluminescence imaging of whole plants suggests that it must emanate from numerous cells that exhibit a synchronous [Ca2+]i oscillation. The [Ca2+]i oscillation continues under constant light conditions for at least four days and is reset by light-dark transitions, suggesting that the circadian clock is involved in its regulation. In addition, the amplitude of the oscillation dampens in continuous dark, indicating that light is critical for sustaining this [Ca2+]i rhythm. These findings raised the question of whether the circadian clock and light regulate some physiological responses through changes in cytosolic Ca2+.
Unlike the plant circadian clock, which likely operates independently in different tissues (8, 9), mammals have a master circadian pacemaker located in neurons of the hypothalamic suprachiasmatic nucleus (SCN) that keeps other circadian oscillators throughout the body synchronized (10–12). The SCN neurons exhibit a circadian [Ca2+]i oscillation with a morning peak (5). Circadian-regulated Ca2+ influx could provide an important mechanism for regulating intracellular Ca2+ homeostasis and controlling the rhythmic spontaneous firing rate of SCN neurons (13), which is greater during the day than at night (14) and drives some circadian behaviors (15). Thus, a daily cytosolic Ca2+ oscillation appears to be a conserved feature in both plant and animal cells and may participate in the transduction of circadian information.
The circadian clock is involved not only in the regulation of daily physiological events but also in seasonal photoperiodic responses (16–19). A recent study provided evidence that the daily oscillation in the cytosolic free Ca2+ concentration could encode photoperiodic information in plants (6). Coincidence of light with circadian-regulated key component expression at the end of the day is thought to be crucial to day-length-dependent responses. For instance, in many plants, long-day-specific expression of the clock-regulated CONSTANS protein at the end of the day is essential for photoperiodic flowering regulation (17). Different day-length conditions control the phase and shape of circadian [Ca2+]i oscillation, resulting in a [Ca2+]i that is high at dusk in short days but low in long days: this difference in the [Ca2+]i level at dusk may contribute to the induction of day-length dependent physiological responses such as photoperiodic flowering (6).
Although the above studies have demonstrated that [Ca2+]i fluctuates throughout the day, we do not know the mechanisms that mediate this phenomenon, nor do we fully understand the physiological relevance of the oscillation in plants and animals. One approach to identify which circadian and photoperiodic responses are regulated by the [Ca2+]i rhythm would involve elucidating the underlying molecular mechanisms. Once the mechanisms are unveiled, hypotheses could be tested using mutants and transgenic plants in which the daily [Ca2+]i oscillation patterns have been perturbed. Because Ca2+ is a ubiquitous second messenger, it is unclear how specificity of the response to a particular stimulus is achieved. A recent study in Arabidopsis has led to a model of stimulus-induced modulation of cytosolic Ca2+-sensitive responses (“Ca2+ sensor priming”) (20), in which different physiological stimuli not only induce [Ca2+]i changes with various patterns but also modulate the activity of the appropriate Ca2+ sensors. This model could provide a potential mechanism for mediating Ca2+ specificity among the numerous Ca2+ sensors expressed in plant cells. A recent study has elucidated some of the molecular mechanisms that can mediate the daily rhythmic [Ca2+]i oscillation (7).
Molecular mechanisms generating the daily [Ca2+]i oscillation
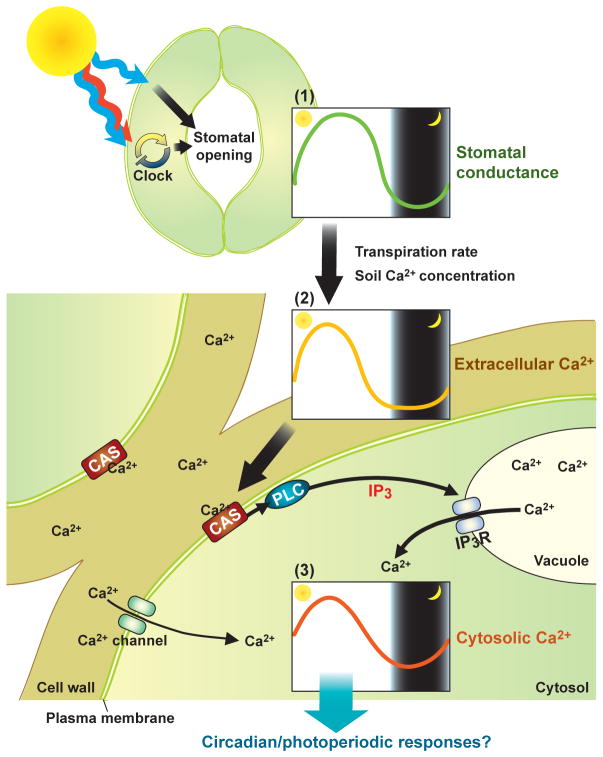
The extracellular Ca2+-sensing receptor (CAS) is a recently identified plant-specific transmembrane Ca2+ receptor with a low-affinity Ca2+ binding site that is involved in monitoring extracellular Ca2+ concentrations ([Ca2+]o) in the cell wall (21). Repression of CAS expression with a CAS antisense (CASas) construct impairs extracellular Ca2+-induced [Ca2+]i increases in guard cells and the ensuing closure of stomata (21). Now, Pei and colleagues report compelling evidence that CAS plays a crucial role in generating circadian [Ca2+]i oscillations as well (7). In CASas transgenic plants, where CAS expression level is severely decreased, the amplitude of the circadian [Ca2+]i oscillation is also reduced (7). The authors demonstrated a consistent correlation between the daily stomatal conductance rhythm, which is regulated by both light and the circadian clock (22–24), and the cytosolic [Ca2+]i oscillation in leaves throughout the day (see Fig. 1). Using a low-affinity aequorin isoform targeted to the extracellular space, they also showed a daily oscillation in the [Ca2+]o of the cell wall. [Ca2+]o began to increase shortly after dawn and peaked at early midday when the stomatal conductance reached its maximum level (Fig. 1). In addition, the [Ca2+]o peak occurred prior to the peak of [Ca2+]i (7). Daily cell wall [Ca2+]o oscillation patterns in the CASas line were the same as in wild type plants. This reinforced the authors’ proposal that CAS senses changes in [Ca2+]o and thus contributes to [Ca2+]o-dependent increases in [Ca2+]i (Fig. 1). Further findings indicate that the Ca2+ concentration in the soil and transpiration rates likely contribute to determining the amplitudes of the oscillations in [Ca2+]o and [Ca2+]i. Moreover, Pei and colleagues provided evidence that the CAS-dependent increase in [Ca2+]i could be controlled through production of inositol 1,4,5-triphosphate (IP3), which in turn stimulates release of Ca2+ from internal stores.
Fig. 1.
A model for extracellular and cytosolic Ca2+ oscillation generation as proposed by Pei and colleagues. (7). This model consists of three modules: 1) daily oscillation of stomatal conductance, 2) resulting extracellular Ca2+ oscillation, and 3) extracellular Ca2+-induced cytosolic Ca2+ oscillation. The daily change in stomatal conductance is regulated by blue light (29) and the circadian clock (30). Red and far-red light and blue light entrain the circadian clock, which comprises interlocking negative feedback loops, to a daily light-dark cycle (25). Analyses of plant circadian clock mutants provide convincing evidence that the circadian clock plays a role in the rhythmic regulation of stomatal apertures (22, 24). The daily change in stomatal conductance results in modulation of the transpiration rate so that it is high during the daytime, accelerating water and solute uptake. This daily oscillation of water and solute uptake efficiency likely regulates the extracellular Ca2+ concentration, which is also high in the daytime (7). Soil Ca2+ concentration affects the amplitude of the extracellular Ca2+ oscillation (7). The more the extracellular Ca2+ concentration increases, the more likely CAS is to bind to Ca2+. CAS is involved in the production of IP3 possibly through the regulation of phospholipase C (PLC) activity (31). IP3 is proposed to stimulate the release of Ca2+ from internal stores, although the gene encoding IP3-gated Ca2+ channel (IP3R) has not been identified in plants. Because the cytosolic Ca2+ oscillation was not totally abolished in the CASas lines (7), other Ca2+ channels may participate in the generation of the cytosolic free Ca2+ oscillation. This daily cytosolic Ca2+ oscillation may encode information that regulates some circadian and photoperiodic responses.
This work enabled the authors to develop the following model for the daily [Ca2+]i rhythm production network. Light-induced increases in stomatal apertures and the ensuing increase in transpiration rates in the daytime result in an increase in water and solute uptake during the day. Subsequently, the higher rate of water and solute transport raises the extracellular Ca2+ concentrations. The [Ca2+]o increase is sensed by CAS, which in turn triggers IP3 production and circadian release of Ca2+ from intracellular stores (Fig. 1). Given that the slow daily Ca2+ oscillation is relatively robust, additional mechanisms may mediate this pattern. As we obtain a more concrete framework for investigating the detailed molecular mechanisms underlying the [Ca2+]i oscillation phenomenon, we will be able to assess its biological role.
Physiological relevance of [Ca2+]i oscillation
For investigation of the biological function of the circadian [Ca2+]i oscillation, the CAS antisense lines are currently the most useful material. Some differences in physiological responses between the CASas lines and wild type plants have already been reported. The CASas lines are defective in external Ca2+-induced stomatal closure (21). However, there is no difference in the daily [Ca2+]o oscillation in the cell wall between the CASas lines and wild type plants (7), suggesting that the CAS dependent [Ca2+]i change is not the primary mechanism of stomatal aperture regulation and that the general daily stomatal conductance oscillations between these two plants might be similar. In other words, [Ca2+]i oscillation information by itself would not be likely to regulate the daytime stomatal opening pattern, consistent with light-induced stomatal opening (23). Because some clock-regulated proteins have been implicated in feedback to the clock or to the input pathway to the clock (25), the CASas lines can be used determine whether [Ca2+]i oscillation also influences the circadian clock.
When the CASas lines and wild type plants were grown on media containing only a small amount of Ca2+, the CASas lines flowered later than wild type plants (21). Taken together with the findings regarding photoperiodic [Ca2+]i oscillation (6), this suggests that the daily [Ca2+]i oscillation may influence flowering behavior. To examine whether [Ca2+]i oscillation affects photoperiodic flowering response, flowering time assays and expression analyses of flowering genes under different day-length conditions and in the presence of different concentrations of Ca2+ in the media must be characterized in the CASas lines. If there is a functional link between daily [Ca2+]i oscillation and flowering time, a major challenge will be to identify the key Ca2+ sensor molecule(s) that decodes the information on changes in [Ca2+]i to regulate the expression of the genes controlling flowering time.
Hypocotyl growth rate is also regulated by the circadian clock. Maximal elongation of the hypocotyl occurs around dusk (26). Extracellular Ca2+ is required for regulating the structural rigidity of the cell wall (27). Decreasing [Ca2+]o might contribute to weakening of the wall and enable shoot growth. [Ca2+]o oscillation reaches a trough around dusk (Fig. 1). Thus, the daily oscillation of [Ca2+]o might be a part of the mechanism by which the circadian clock regulates hypocotyl growth.
The circadian [Ca2+]i oscillation has thus far been measured in whole seedlings using aequorin, suggesting that it emanates from synchronous changes in [Ca2+]i in many cells (3, 6, 7). Thus, more rapid typical [Ca2+]i transients in individual cells are likely filtered during whole organism aequorin imaging (28). Further research should attempt to analyze whether the circadian [Ca2+]i oscillation is indeed global or localized to specific cells or cytoplasmic regions within (specific) cells. Single cell Ca2+ imaging should reveal which cells oscillate, and whether this slow oscillation reflects a uniform gradual baseline adjustment in the cytosolic Ca2+ concentration, or a gradual population change in the numbers of cells that have a different cytosolic Ca2+ concentration. Establishment of single cell Ca2+ imaging will also allow further analysis of the likely complex cell physiological and genetic mechanisms that contribute to this [Ca2+]i oscillation.
We now have a molecular tool that enables us to pose more detailed questions about what information is encoded in the daily cytosolic Ca2+ concentration oscillation. If this oscillation controls some or even many physiological responses, we anticipate that the molecular components involved in each response may vary. The challenge lies in identifying both the physiological functions of the remarkably robust daily Ca2+ oscillation and the further mechanisms and components that mediate this robust rhythm, as well as revealing whether these oscillations mediate diverse functions in plant and animal physiology.
References
- 1.Allen GJ, Schroeder JI. Combining genetics and cell biology to crack the code of plant cell calcium signaling. Sci STKE. 2001;2001:re13. doi: 10.1126/stke.2001.102.re13. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- 4.Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 6.Love J, Dodd AN, Webb AA. Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell. 2004;16:956–966. doi: 10.1105/tpc.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang RH, Han S, Zheng H, Cook CW, Choi CS, Woerner TE, Jackson RB, Pei ZM. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science. 2007;315:1423–1426. doi: 10.1126/science.1134457. [DOI] [PubMed] [Google Scholar]
- 8.Thain SC, Hall A, Millar AJ. Functional independence of circadian clocks that regulate plant gene expression. Curr Biol. 2000;10:951–956. doi: 10.1016/s0960-9822(00)00630-8. [DOI] [PubMed] [Google Scholar]
- 9.Hall A, Kozma-Bognar L, Toth R, Nagy F, Millar AJ. Conditional circadian regulation of PHYTOCHROME A gene expression. Plant Physiol. 2001;127:1808–1818. [PMC free article] [PubMed] [Google Scholar]
- 10.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 12.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 13.Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- 14.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci USA. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanovsky MJ, Kay SA. Living by the calendar: how plants know when to flower. Nat Rev Mol Cell Biol. 2003;4:265–275. doi: 10.1038/nrm1077. [DOI] [PubMed] [Google Scholar]
- 17.Imaizumi T, Kay SA. Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 2006;11:550–558. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Oster H, Maronde E, Albrecht U. The circadian clock as a molecular calendar. Chronobiol Int. 2002;19:507–516. doi: 10.1081/cbi-120004210. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura T. Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:345–350. doi: 10.1016/j.cbpa.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc Natl Acad Sci USA. 2006;103:7506–7511. doi: 10.1073/pnas.0602225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han S, Tang R, Anderson LK, Woerner TE, Pei ZM. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature. 2003;425:196–200. doi: 10.1038/nature01932. [DOI] [PubMed] [Google Scholar]
- 22.Somers DE, Webb AA, Pearson M, Kay SA. The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development. 1998;125:485–494. doi: 10.1242/dev.125.3.485. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 24.Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AA. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 25.McClung CR. Plant circadian rhythms. Plant Cell. 2006;18:792–803. doi: 10.1105/tpc.106.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowson-Day MJ, Millar AJ. Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J. 1999;17:63–71. doi: 10.1046/j.1365-313x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 27.Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodd AN, Jakobsen MK, Baker AJ, Telzerow A, Hou SW, Laplaze L, Barrot L, Poethig RS, Haseloff J, Webb AA. Time of day modulates low-temperature Ca2+ signals in Arabidopsis. Plant J. 2006;48:962–973. doi: 10.1111/j.1365-313X.2006.02933.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimazaki K, Doi M, Assmann SM, Kinoshita T. Light regulation of stomatal movement. Annu Rev Plant Biol. 2007;58:219–247. doi: 10.1146/annurev.arplant.57.032905.105434. [DOI] [PubMed] [Google Scholar]
- 30.Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AA. Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ. 2007;30:333–349. doi: 10.1111/j.1365-3040.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 31.Meijer HJ, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]