Abstract
Background
The CD14 is a key molecule in innate immunity that mediates cell activation and signaling in response to endotoxin and other bacterial wall-derived components. CD14 protein exists in soluble (sCD14) and membrane bound (mCD14) forms. The correlates of sCD14 in persons undergoing long-term hemodialysis (HD) are not known.
Study Design
We hypothesized that elevated sCD14 in hemodialysis patients is associated pro-inflammatory cytokine activation and increased mortality in a 33-month cohort
Subjects
Well defined cohort of 310 long-term HD patients who participated in the Nutritional and Inflammatory Evaluation in Dialysis (NIED) Study.
Predictors and other Measurements
Soluble CD14 in serum.
Predictors and other Measurements
Thirty-three month mortality in the NIED Study cohort.
Results
The mean sCD14 was 7.24±2.45 μg/ml. Tumor necrosis factor-α (TNF-α) was the strongest correlate of sCD14 (r=+0.24, p<0.001) followed by interleukin(IL)-6 (r=+0.18, p=0.002), serum ferritin (r+=0.21, p=<0.001), total iron binding capacity (r=−0.19, p=<0.001), body mass index (r=−0.15, p=0.008), vintage (r=+0.14, p=0.01), low density lipoprotein-C (r=+0.13, p=0.03) and body fat (r=−0.11, p=0.06). Over the 33 months follow-up, 71 (23%) patients died. Multivariable Cox proportional analysis adjusted for case-mix and other nutritional and inflammatory confounders including serum TNF-α, C-Reactive protein, and IL-6 showed that compared to lowest sCD14 tertile, sCD14 levels in the third tertile (>7.8 μg/ml) were associated with higher death risk (hazard ratio, 1.94; 95% confidence interval, 1.01–3.75, p=0.04).
Limitations
Survivor bias in combined incident/prevalent studies.
Conclusions
Elevated sCD14 is positively related to markers of inflammation, negatively related to nutritional status and an independent predictor of mortality in long-term HD patients. Further studies are needed to examine the usefulness of sCD14 in risk stratification and clinical decision-making process in hemodialysis patients.
Keywords: Hemodialysis, mortality, inflammation, endotoxin, soluble CD14, mortality, nutritional status
Introduction
Currently there are ~400,000 end-stage renal disease (ESRD) patients on dialysis in the United States. Despite the magnitude of the resources allotted to the care of ESRD patients, they continue to experience significant mortality and morbidity. Although the cause of increased mortality in ESRD remains a topic of intense debate and intriguing research domain, there is consensus among investigators that inflammation is a key factor. The etiology of unbalanced activation of pro-inflammatory cytokines with decline in renal function, however, remains speculative. One potentially important source of inflammation in ESRD patients is subclinical endotoxemia.[1, 2] Presence of very low levels of endotoxin or endotoxin fragments in the dialysate is capable of inducing the production of cytokines.[3, 4] In vitro studies show that lipopolysaccaride (LPS) concentration as low as 0.01 ng/ml induced upregulation of CD14 expression.[5] CD14 is a key molecule in innate immunity that is constitutively expressed in considerable amounts on the surface (mCD14) of mature monocytes, macrophages and neutrophils.[6] The binding of LPS to the complex of mCD14 and toll-like receptor-4 (TLR4) at the surface of the innate immune cells triggers the secretion of pro-inflammatory cytokines. A soluble form of CD14 (sCD14) is present in serum and is derived both from secretion of CD14 and from enzymatically cleaved glycosyl-phosphatidylinositol-anchored tissue CD14.[7, 8]
Protein-energy wasting (PEW) is present in approximately 40% of patients treated with maintenance dialysis and has been consistently found as a strong predictor of the high morbidity and mortality observed in this population.[9] Inflammation coexists with PEW and cardiovascular disease (CVD) in ESRD.[10] Elevated endotoxin levels and CD14 expression have been linked to inflammation and increased cardiovascular disease in the general population.[11] However, the clinical significance of sCD14 in ESRD patients in North America has not been explored. In order to test the hypothesis that elevated sCD14 is associated with increased mortality in ESRD patients, we measured the plasma sCD14 level in a well defined cohort of 310 patients undergoing thrice weekly in-center hemodialysis (HD) treatment. We hypothesized that sCD14 is an independent predictor of mortality and positively associated with markers of inflammation and negatively with nutritional status in US American ESRD patients.
Methods
Patient Population
We studied long-term (HD) patients who participated in the Nutritional and Inflammatory Evaluation in Dialysis (NIED) Study.[28] The original patient cohort was derived from a pool of over 3,000 HD outpatients over 5 years in eight DaVita chronic dialysis facilities in the South Bay Los Angeles area (see the NIED Study website at www.NIEDStudy.org for more details, as well as previous publications [25, 29, 30]). Inclusion criteria were outpatients who had been undergoing HD treatment for at least 8 weeks, who were 18 years or older and who signed the Institutional Review Board approved consent form. Patients with acute infections or an anticipated life expectancy of less than 6 months (e.g. due to a metastatic malignancy or advanced HIV/AIDS disease) were excluded. A total of 893 long-term HD patients participated in the NIED Study over the course of 5 years (October 2001 to December 2006). From April 1, through September 30, 2004, 310 long-term HD patients who had signed the informed consent form underwent all tests and evaluations for this study. The medical chart of each HD patient was thoroughly reviewed by a collaborating physician, and data pertaining to the underlying kidney disease, cardiovascular history and other comorbid conditions were extracted. A modified version of the Charlson comorbidity index, i.e., without the age and kidney disease components, was used to assess the severity of comorbidities.[31, 32] The 310 HD patients were followed for up to 33 months, i.e., until December 31, 2006.
Anthropometric and Dietary Measures
Body weight assessment and anthropometric measurements were performed while patients underwent a hemodialysis treatment or within 5 to 20 minutes after termination of the treatment. Biceps skinfold (BSF) and triceps skinfold (TSF) thicknesses were measured with a conventional skinfold caliper using standard techniques as previously described.[33, 34]
Near Infra-Red Interactance
To estimate the percentage of body fat and fat-free body mass, near infra-red (NIR) interactance was measured at the same time as the anthropometric measurements.[35] A commercial near-infrared interactance sensor with a coefficient of variation of 0.5% for total body fat measurement (portable Futrex 6100®, Gaithersburg, Maryland, www.futrex.com) was used. NIR measurements were performed by placing, for several seconds on the upper aspect of the arm without a vascular access, a Futrex® sensor, and entering the required data (date of birth, sex, weight and height) of each patient. NIR measurements of body fat appear to correlate significantly with other nutritional measures in HD patients.[25]
Soluble CD-14 measurement
Concentration of sCD14 was measured using commercially available ELISA kit (sCD14 Quantikine ELISA Kit, R&D Systems, MN, USA). The inter- and intra-assay coefficient of variation are <7.5 and <6.5%, respectively. All samples were measured in triplicates and the mean value was reported in μg/mL.
Other Laboratory Tests
Pre-dialysis blood samples and post-dialysis serum urea nitrogen were obtained on a midweek day which coincided chronologically with the drawing of quarterly blood tests in the DaVita facilities. The single-pool Kt/V was used to represent the weekly dialysis dose. All routine laboratory measurements were performed by DaVita® Laboratories (Deland, FL) using automated methods.
Serum high sensitivity CRP was measured by a turbidometric immunoassay (manufacturer: WPCI, Osaka, Japan, unit: mg/L, normal range: <3.0 mg/L).[36, 37] IL-6 and tumor necrosis factor alpha (TNF-α) were measured with immunoassay kits based on a solid phase sandwich ELISA using recombinant human IL-6 and TNF-α (manufacturer: R&D Systems, Minneapolis, MN; units: pg/ml; normal range: IL-6: <9.9 pg/ml, TNF-α: <4.7 pg/ml).[38, 39] CRP, TNF-alpha, and IL-6 were measured in the General Clinical Research Center (GCRC) Laboratories of Harbor-UCLA. Serum transthyretin (prealbumin) was measured using immunoprecipitin analysis. Plasma total homocysteine concentrations were determined by high-performance liquid chromatography in the Harbor-UCLA Clinical Laboratories.
Statistical Methods
Pearson’s correlation coefficient (r) was used for analyses of linear associations. Multivariate regression analyses and analyses of covariance were performed to obtain adjusted p-values controlled for case-mix and other covariates. Restricted cubic splines graphs were utilized as exploratory data analysis (EDA) strategies to illustrate systematic relations between sCD14 with mortality. This method also served to examine the non-linear associations as continuous mortality predictors as an alternative to inappropriate linearity assumptions.[40] Thereafter, to calculate the relative risks of death, hazard ratios (HR) were obtained using Cox proportional hazard models after controlling for the relevant covariates. Plots of log [−log (survival rate)] against log (survival time) were performed to establish the validity of the proportionality assumption. Kaplan-Meier analyses were utilized to assess the differences in surviving proportions between tertiles of sCD14. Stepwise linear regression model including all relevant variables was employed to examine the best predictors of sCD14 as dependent variable. Case-mix covariates included sex, age, race and ethnicity (Hispanics, African Americans, Asians and others), diabetes mellitus, and dialysis vintage; and laboratory measures of the MICS included albumin, creatinine, hemoglobin, total iron binding capacity, normalized protein catabolic rate (nPCR), lymphocyte percent, body mass index; the fully adjusted Cox models included serum CRP, TNF-α, and IL-6 in addition to the case-mix and MICS variables. Fiducial limits are given as mean±SD (standard deviation) or median and inter-quartile range; risk ratios include 95% confidence interval (CI) levels. A p-value <0.05 or a 95% CI that did not span 1.0 was considered to be statistically significant. Descriptive and multivariate statistics were carried out with the statistical software “Stata version 10.0” (Stata Corporation, College Station, Texas).
Results
The 310 subjects of the study were 55.1±14.7 years old (mean ±SD) and included 48% women, 52% Hispanic, 30% African-American and 57% diabetic patients. The mean dialysis vintage was 50±35 months (median: 45, inter-quartile range: 25–68 months). The mean sCD14 was 7.24±2.45 μg/ml. Table 1 shows the relevant demographic, clinical and laboratory measures across the tertiles of sCD14. Age, dialysis vintage, serum alkaline phosphatase, ferritin, homocysteine, and the three pro-inflammatory markers including C-Reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis-α (TNF-α) were higher in patients in the third (highest) sCD14 tertile. However, there was no difference across the tertiles in terms of sex, race/ethnicity, and diabetes mellitus. Anthropometric measures including body mass index (BMI), triceps and biceps skin folds, mid-arm circumference and NIR-measured body fat were lower in the third tertile of sCD14. The same decreasing trend was observed for albumin, prealbumin, and total iron –binding capacity (TIBC). There was no trend for the levels of white blood cell count (WBC), lymphocyte percentage, and iron saturation ratio across the tertiles of sCD14.
Table 1.
Baseline demographic, clinical, and laboratory values in total and according to the tertiles of soluble endotoxin receptor CD14 in 310 maintenance hemodialysis patients 1
| Variable | Tertiles of soluble endotoxin receptor CD14
|
|||
|---|---|---|---|---|
| Tertile 1 <6 (n= 103) |
Tertile 2 6 to 7.84 (n= 104) |
Tertile 3 >7.84 (n= 103) |
P for trend | |
| Demographic | ||||
| Age (years) | 52.7±14.9 | 56.2±14.2 | 56.4±14.9 | 0.08 |
| Women (%) | 52 | 47 | 46 | 0.4 |
| Race: % African-American | 32 | 20 | 37 | 0.5 |
| Ethnicity: % Hispanic | 48 | 63 | 46 | 0.8 |
| Primary insurance: % Medicare | 49 | 55 | 52 | 0.7 |
| Diabetes mellitus (%) | 58 | 63 | 50 | 0.2 |
| Modified Charlson comorbidity score | 1.2±1.2 | 1.7±1.5 | 2.2±1.6 | 0.8 |
| Crude mortality rate (%)3 | 14 | 22 | 33 | 0.001 |
| Body composition | ||||
| Body mass index (kg/m2) | 27.1±7.3 | 26.3±5.9 | 24.9±5.1 | 0.01 |
| Triceps skinfold (mm) | 19.6±11.4 | 17.2±9.9 | 13.9±7.8 | <0.001 |
| Biceps skinfold (mm) | 10.3±8.1 | 9.4±7.3 | 7.9±6.0 | 0.02 |
| Mid-arm circumference (cm) | 31.8±6.4 | 31.0±5.7 | 29.6±5.3 | 0.01 |
| Near infrared measured body fat (%) | 28.4±10.9 | 28.1±10.6 | 25.7±10.6 | 0.08 |
| Hemodialysis treatment measures | ||||
| Dialysis vintage (months) | 43.8±34.3 | 51.9±35.3 | 54.4±35.3 | 0.006 |
| Dialysis dose (Kt/V single pool) | 1.67±0.29 | 1.71±0.30 | 1.71±0.25 | 0.3 |
| nPNA (nPCR) (g.kg−1.day−1) | 1.10±0.22 | 1.10±0.28 | 1.07±0.24 | 0.3 |
| Erythropoietin dose (1,000 u/week) | 15.0±16.2 | 12.7±10.3 | 15.1±15.5 | 0.6 |
| Biochemical measurements | ||||
| Serum albumin (g/dl) | 4.0±0.3 | 4.0±0.4 | 3.9±0.4 | 0.05 |
| prealbumin (transthyretin) (mg/dl) | 30.3±8.4 | 29.4±8.9 | 28.3±8.3 | 0.1 |
| creatinine (mg/dl) | 10.3±2.7 | 9.6±2.7 | 9.9±2.8 | 0.3 |
| total iron binding capacity (mg/dl) | 213±35 | 208±37 | 198±33 | 0.003 |
| calcium (mg/dl) | 9.7±0.7 | 9.6±0.6 | 9.6±0.6 | 0.2 |
| phosphorus (mg/dl) | 5.7±1.2 | 5.4±1.3 | 5.6±1.5 | 0.4 |
| Alkaline phosphatase (U/L) | 115±56 | 126±66 | 140±102 | 0.02 |
| iron saturation ratio | 33.3±10.4 | 35.0±12.1 | 35.0±12.5 | 0.3 |
| ferritin (ng/ml) | 500±332 | 656±422 | 728±353 | <0.001 |
| total homocysteine (μmol/l) | 24.2±7.1 | 25.5±8.1 | 27.2±8.8 | 0.008 |
| LDL-C | 75.6±30.2 | 78.9±31.1 | 83.3±35.4 | 0.1 |
| HDL-C | 39.1±13.1 | 35.5±12.8 | 35.2±12.4 | 0.04 |
| total cholesterol | 142±40 | 148±41 | 147±47 | 0.4 |
| triglycerides | 141±108 | 176±165 | 143±92 | 0.9 |
| C-reactive protein (mg/l) | 4.1±5.1 | 4.9±5.4 | 5.8±6.2 | 0.01 |
| IL-6 (pg/ml) | 8.8±12.5 | 9.7±10.3 | 13.0±16.9 | 0.006 |
| TNF-α (pg/ml) | 2.2±0.8 | 2.5±0.8 | 2.8±1.2 | <0.001 |
| Blood hemoglobin (g/dl) | 12.2±0.8 | 12.1±0.8 | 12.1±0.9 | 0.5 |
| WBC (×1000 cell/μl) | 7.0±2.2 | 6.9±1.7 | 6.7±2.0 | 0.3 |
| lymphocyte (% of total WBC) | 23.8±8.1 | 23.4±6.5 | 23.5±8.1 | 0.7 |
Kt/V, dialysis dose; nPCR, normalized protein catabolic rate; IL-6, Interleukin 6; TNF-α, Tumor necrosis factor α; LDL, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol
All values are presented as mean ± SD or percentages
Mortality pertains to maximum of 33 months
P-values for dialysis dose (vintage), ferritin, vitamin D dose, CRP, IL-6, and TNF- α are based on the logarithmic values of these measures.
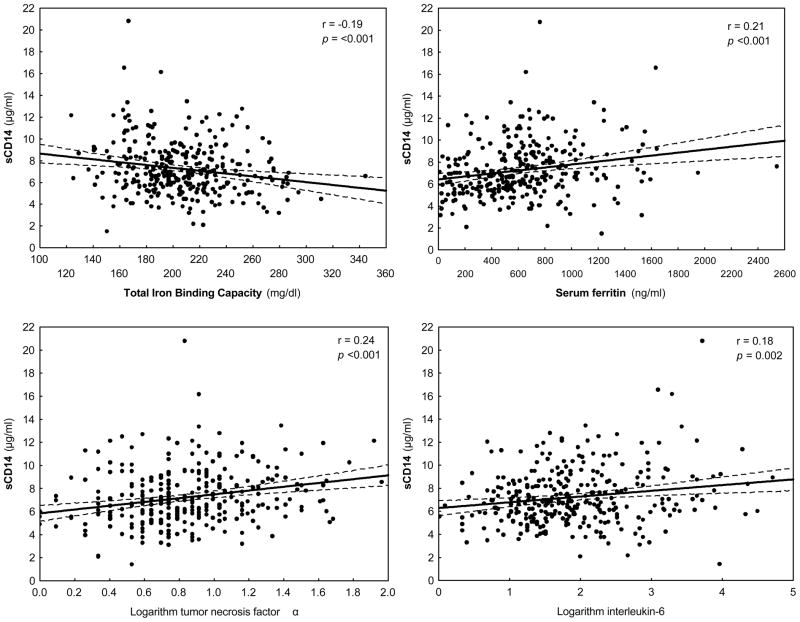
Correlates of sCD14
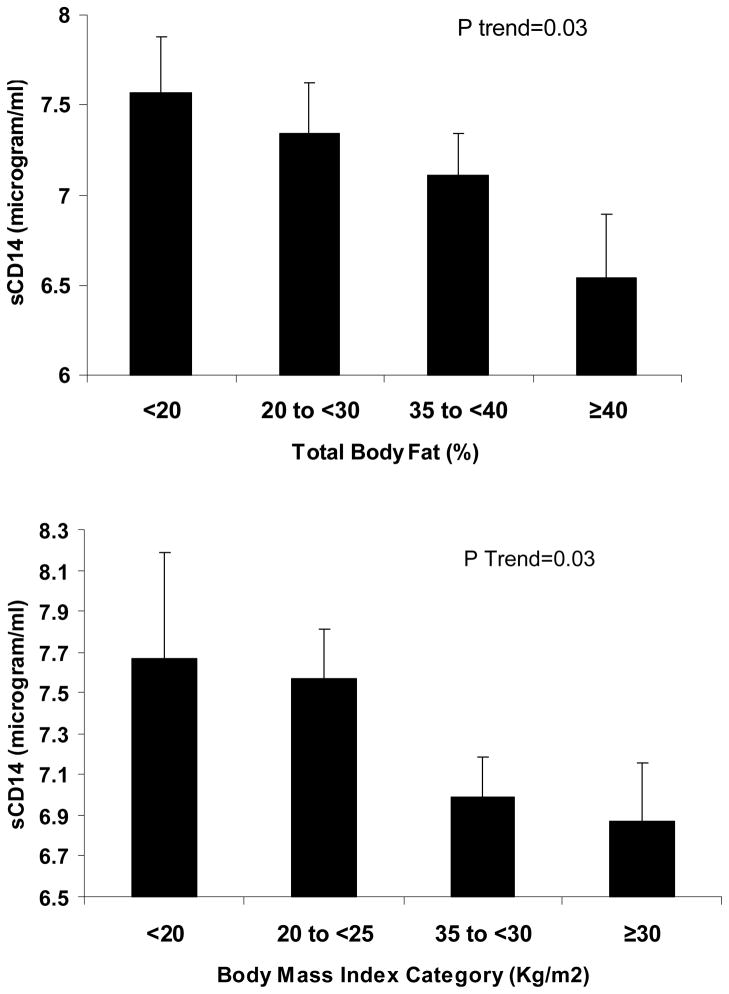
Table 2 shows the correlations between sCD14 and some relevant nutritional, inflammatory and other biochemical variables. Among all variables, patient age, dialysis treatment age (vintage), serum ferritin, TNF-α and IL-6, and low density lipoprotein-C (LDL-C) were positively correlated with sCD14, and these correlations were robust to multivariate adjustment. TNF-α had the strongest correlation with sCD14 (Figure 1). Other variables including BMI, and serum TIBC were negatively correlated with sCD14. Although sCD14 was initially appeared weakly correlated with serum creatinine (r=−0.09, p= 0.11) and logarithm (Log) of parathyroid hormone (PTH) (r=−0.07, p=0.2), the multivariate adjusted associations were stronger after controlling for age, sex, diabetes mellitus, Log vintage, Log IL-6, and Log TNF-α (creatinine: r=-0.13, 0.03; Log PTH: r=−0.13, p=0.02). Figure 2 also shows significant declining trends of sCD14 across categories of body fat (upper panel) and BMI (lower panel).
Table 2.
Bivariate (unadjusted) and partial (adjusted) correlation coefficients between soluble endotoxin receptor CD14 and relevant variables in 310 MHD patients
| Variable | Bivariate correlation | P | Adjusted correlation1 | P |
|---|---|---|---|---|
| Age | 0.07 | 0.2 | 0.12 | 0.04 |
| Dialysis vintage (log scale) | 0.14 | 0.01 | 0.11 | 0.07 |
| Body mass index | −0.15 | 0.008 | −0.15 | 0.009 |
| nPCR (nPNA) | −0.06 | 0.3 | −0.01 | 0.9 |
| NIR body fat percentage | −0.11 | 0.06 | −0.14 | 0.02 |
| Serum calcium | −0.04 | 0.5 | −0.03 | 0.6 |
| phosphorous | −0.05 | 0.4 | −0.04 | 0.5 |
| Intact PTH (log scale) | −0.07 | 0.2 | −0.13 | 0.02 |
| alkaline phosphatase | 0.05 | 0.4 | −0.01 | 0.9 |
| albumin | −0.13 | 0.02 | −0.08 | 0.2 |
| transthyretin | −0.09 | 0.1 | −0.04 | 0.5 |
| TIBC | −0.19 | <0.001 | −0.12 | 0.04 |
| ferritin | 0.21 | <0.001 | 0.19 | 0.001 |
| creatinine | −0.09 | 0.1 | −0.13 | 0.03 |
| interleukin-6 (log scale) | 0.18 | 0.002 | 0.14 | 0.02 |
| TNF-a (log scale) | 0.24 | <0.001 | 0.22 | <0.001 |
| CRP (log scale) | 0.14 | 0.02 | 0.06 | 0.3 |
| LDL-C | 0.13 | 0.03 | 0.13 | 0.03 |
| HDL-C | −0.06 | 0.3 | −0.02 | 0.7 |
| total cholesterol | 0.10 | 0.09 | 0.11 | 0.06 |
| triglycerides | 0.01 | 0.9 | 0.02 | 0.8 |
| Blood hemoglobin | −0.05 | 0.4 | −0.02 | 0.7 |
| WBC | −0.04 | 0.5 | −0.05 | 0.4 |
| lymphocytes % | −0.05 | 0.4 | −0.06 | 0.3 |
TIBC, total iron binding capacity; WBC, White blood cell count; iPTH, intact parathyroid hormone; CRP, C-reactive protein; TNF-a, tumor necrosis factor alpha; LDL, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol;
In the adjusted analysis, age, sex, diabetes, Log IL-6, Log TNF-a, and Log vintage were included as covariate.
Figure 1.
Scatter plots, regression line and 95% confidence intervals, reflecting the correlation between serum level of soluble endotoxine receptor CD14 and serum ferritin, total binding capacity, log for interleukin-6 (IL-6), and log for tumor necrosis factor alpha (TNF-alpha)
Figure 2.
Mean (and SE) of soluble endotoxine receptor CD14 across categories of total body fat and body mass index.
Serum sCD14 and Survival
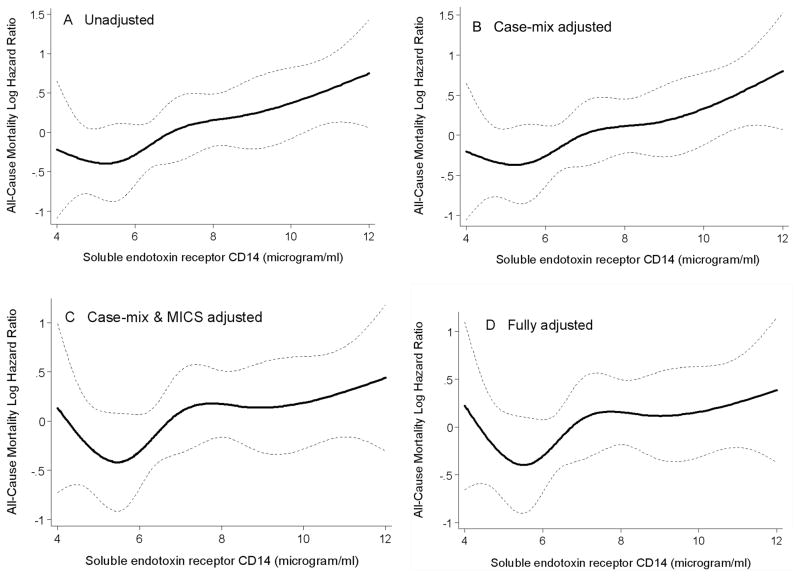
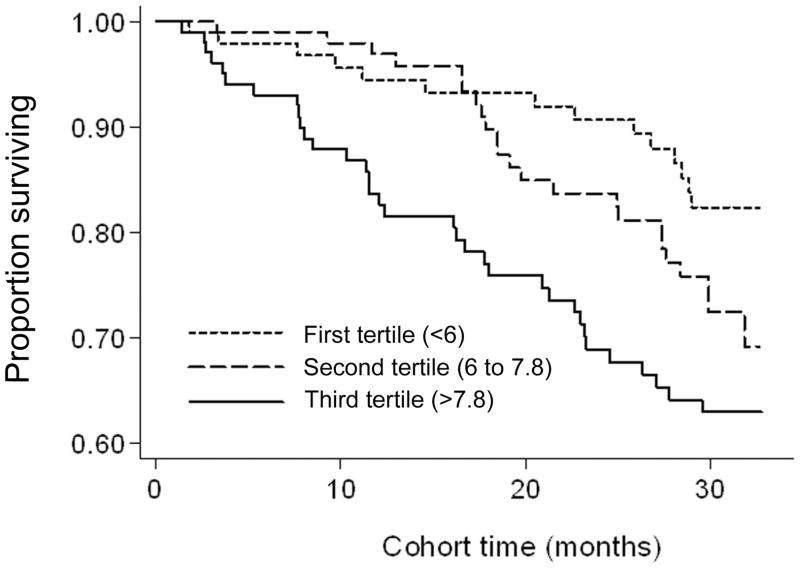
Over the 33 months follow-up, 71 (23%) patients died, 33 (11%) underwent transplantation, and 26 (8%) left the cohort. Figure 3 shows the cubic splines illustrating the associations between baseline sCD14 and mortality in the 33-month cohort of 310 HD patients. A consistent trend towards increased death risk was observed in the patients with higher sCD14 even after multivariate adjustment for other makers of nutrition and inflammation including CRP, IL-6 and TNF-α (Panel D). As depicted in Figure 4, the Kaplan-Meier survival plots showed incrementally worsening survival across increasing sCD14 tertiles. The calculated death hazard ratios listed in Table 3 indicated that MHD patients in the third tertile of sCD14 had a two-fold greater death risk vs. those in the first tertile [HR: 2.59, 95% confidence interval (CI): 1.39–4.83, p=0.003] and that this trend was robust to multivariate adjustment for other measures of MICS including several inflammatory markers and cytokines (HR: 1.94, 95% CI: 1.01–3.75, p<0.05).
Figure 3.
Mortality predictability of soluble endoctoxin receptor CD14 in 310 maintenance hemodialysis patients (April 2004–Jan 2007)
- Case-mix variables: age, sex, race/ethnicity, diabetes mellitus, log vintage
- MICS variables: albumin, creatinine, hemoglobin, normalized protein catabolic rate (nPCR), lymphocyte percentage, and body mass index
- Inflammatory variables: Log C-reactive protein, Log Interleukin-6, Log Tumor necrosis factor-α
Figure 4.
Kaplan-Meier proportion of surviving after 2.8 years of observation according to the tertiles of soluble endoctoxin receptor CD14 in 310 HD patients (April 2004–Jan 2007)
Table 3.
Hazard ratios (HRs) of 33-month mortality according to the tertiles soluble endotoxin receptor CD14 of in 310 maintenance hemodialysis patients
| Unadjusted | Case-mix1 adjusted | Case-mix + MICS2 adjusted | Case-mix + MICS + inflammation adjusted (Full model)3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| CD14 Tertiles | death | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Tertile 1: <6 (n=103) | (n=14) | 1.0 (Reference) | 1.0 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||
| Tertile 2: 6 to 7.84 (n=104) | (n=23) | 1.52 (0.78–2.98) | 0.2 | 1.46 (0.74–2.89) | 0.2 | 1.37 (0.68–2.74) | 0.3 | 1.26 (0.62–2.53) | 0.5 |
| Tertile 3: >7.8 (n=103) | (n=34) | 2.59 (1.39–4.83) | 0.003 | 2.49 (1.33–4.68) | 0.004 | 2.09 (1.09–4.00) | 0.02 | 1.94 (1.01–3.75) | 0.04 |
Case-mix variables include age, sex, race/ethnicity, diabetes, Log vintage
MICS variables include albumin, creatinine, hemoglobin, total iron binding capacity, normalized protein catabolic rate (nPCR), lymphocyte percent, and body mass index
Full model consist of case-mix and MICS variables, and logarithm of three inflammatory markers: C-reactive protein, Interleukin-6, Tumor necrosis factor-α
Discussion
In this cohort of 310 long-term HD outpatients in Southern California, we found that the higher circulating sCD14 level is associated with higher 33-month death risk. The increasing trend of mortality associated with higher sCD14 was robust to controlling for case-mix and other nutritional and inflammatory measures including serum IL-6 and TNF-α. MHD patients in the third tertile of sCD14 had an almost-2 fold increased death risk (hazard ratio of 1.94) after adjustment for other confounders. Lower BMI and WBC and higher LDL-C, ferritin, IL-6, and TNF-α were the strongest correlates of sCD14 variability. These findings imply that sCD14 may be a useful and robust marker of inflammatory and nutritional status with strong association with survival.
Endotoxin, the hydrophobic anchor of lipopolysaccharide (LPS), is a glucosamine-based phospholipid that makes up the outer monolayer of the outer membranes of most Gram-negative bacteria.[12] Upon entry into the blood stream LPS is complexed to LPS-binding protein and the resulting complex then recognizes the cell surface CD14 antigen on monocytes/macrophages leading to an enhanced cytokine production.[13] The mean sCD14 was 7.24±2.45 μg/ml in our study population. Nockher et al[5] reported that the serum levels of sCD14 is about 2.5-fold higher in ESRD patients compared to healthy controls. Using in vitro studies they showed that enhanced expression of CD14 by monocytes after HD and increased sCD14 serum levels are due to chronic exposure to trace amounts of endotoxin. Thus, the elevated sCD14 serum concentrations in HD patients is possibly due to repeated stimulation of CD14 expression by subclinical endotoxemia with subsequent release of sCD14 during each dialysis session.[5] The physiological importance of elevated sCD14 serum levels in hemodialysis is not yet clear, but is complex and cell specific. sCD14 is believed to play a key role in the neutralization of LPS under physiological conditions and attenuate the inflammatory response.[14] During endotoxemia sCD14 partially inhibits the immune response of macrophages that constitutively express mCD14,[15] but it promotes the response of cells that do not express mCD14.[16] Endotoxemia and elevated plasma sCD14 level are associated with inflammation, insulin resistance,[17, 18] muscle wasting,[19] atherosclerosis, and CVD.[11, 20] These results indicate some of the clinical consequences of endotoxemia are mediated by elevated sCD14.
Szeto et al[1] reported that subclinical endotoxemia is common in peritoneal dialysis patients, and the degree of circulating endotoxemia is associated with systemic inflammation and atherosclerosis. Heine et al[21] observed that the number of CD14++ CD16+ monocytes was independently associated with cardiovascular events and death in dialysis patients. We observed that the sCD14 level was positively related to markers of inflammation and negatively related to athropometric measures. Stepwise linear model showed that BMI, LDL-C, ferritin, WBC, IL-6, and TNF-α could account for up to 20% of the variance of sCD14 in the study population. In healthy men and type 2 diabetic patients serum sCD14 concentration is not related to BMI.[17] In our study population, however, we noted that BMI and body fat determined by NIR were negatively and significantly related to sCD14. In contrast to reports in the general population,[22] higher BMI is associated with better outcomes in ESRD patients.[23] The superiority of muscle mass over fat mass in conferring survival advantage is disputed,[24, 25] but depletion of both could have adverse consequences.[26, 27] Elevated sCD14 level was associated with an increased death risk, which persisted even after multivariate adjustment for other makers of nutrition and inflammation including CRP, IL-6 and TNF-α.
It is important to note that one of the coauthors of this manuscript recently examined correlates of sCD14 in a Swedish cohort of HD patients and reported similar findings.[41] The median value of sCD14 in the Swedish cohort was 3.2 μg/mL (25th to 75th percentile, 2.7 to 3.9), whereas in our cohort it is 6.8 μg/mL (25th to 75th percentile, 5.7 to 8.6). This difference may be due to demographic distinctions, in that the HD patients in the current study are representative of the US dialysis population and consist of persons of diverse ethnicity; larger proportion of diabetics, and larger percentage of tunneled catheter or ateriovenous graft as the vascular access; whereas the European cohort study [41] consists of Swedish Caucasians with fewer diabetic patients, in that most subjects have ateriovenous fistula and better nutritional status. Furthermore, differences in dialysis membrane flux or reuse practice may have played a role, esp. since during the NIED study era dialysis members were reused with a median of 12 times (inter quartile range 8 to 17 times).
Our study should be qualified for a number of limitations including selection bias during enrollment leading to younger MHD patients. Furthermore, we do not have measurements of circulating endotoxin levels. However, since the mortality in the original NIED Study cohort was less than the base population[28], it might be argued that a selection bias with such a direction generally would lead to a bias toward the null, so that without this bias, our positive results might have been even stronger. One strength of our cohort is that the subjects were selected randomly without having any prior knowledge of their inflammation status. Moreover, our study include the moderate sample size, the comprehensive clinical and laboratory evaluations including body composition measures, detailed evaluation of comorbid states by study physicians, and measuring pro-inflammatory cytokines. Finally, the same blood specimens that were utilized to measure serum markers of nutrition and inflammation were also used for the sCD14 measurements.
To summarize, this study demonstrates that endotoxin receptor sCD14 is correlated with several surrogates of body composition and inflammation. We found that 1 μg/ml increase in serum level of sCD14 is accompanied with virtually 2 folds increased death risk in chronic HD patient. In addition, TNF-α was the strongest correlate of sCD14 followed by IL-6, serum ferritin, TIBC, BMI, vintage, and LDL-C. Understanding the role of sCD14 and its contribution in morbidity and mortality in the chronic HD population may lead to more useful strategies to risk stratification of chronic HD patients based on inflammatory status and death risk. Hence, future investigations are needed to elucidate the role of sCD14 in inflammatory cascade of chronic HD.
Acknowledgments
Funding Sources: This study was supported by National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Disease grants R21-DK61162 and K23-DK061162 (for KKZ). Additional sources of funding include a research grants from Watson Pharmaceuticals, DaVita Clinical Research (DCR), philanthropist grant by Mr. Harold Simmons (for KKZ), and a General Clinical Research Center (GCRC) grant # M01-RR00425 from the National Centers for Research Resources, National Institutes of Health. The sCD14 analysis was supported by NIH grant R01DK073665 and Norman Coplon Research grant (DSCR).
The authors are thankful to Ms. Stephanie Griffith and Dr. Victor Goh, at Harbor-UCLA GCRC Core Laboratories for the management of blood samples and measuring inflammatory markers. They are also indebted to hard-working collaborating dietitians in 10 DaVita dialysis facilities in Los Angeles South Bay area and DaVita teammates in these facilities.
Some of the data were presented during the Spring Clinical Meeting of the National Kidney Foundation in March 2009 in Nashville, TN.
Footnotes
Potential Conflict of Interests: None to declare
References
- 1.Szeto CC, Kwan BC, Chow KM, et al. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamagami S, Adachi T, Sugimura T, et al. Detection of endotoxin antibody in long-term dialysis patients. Int J Artif Organs. 1990;13:205–210. [PubMed] [Google Scholar]
- 3.Lonnemann G, Behme TC, Lenzner B, et al. Permeability of dialyzer membranes to TNF alpha-inducing substances derived from water bacteria. Kidney Int. 1992;42:61–68. doi: 10.1038/ki.1992.261. [DOI] [PubMed] [Google Scholar]
- 4.Pereira BJ, Sundaram S, Barrett TW, et al. Transfer of cytokine-inducing bacterial products across hemodialyzer membranes in the presence of plasma or whole blood. Clin Nephrol. 1996;46:394–401. [PubMed] [Google Scholar]
- 5.Nockher WA, Scherberich JE. Monocyte cell-surface CD14 expression and soluble CD14 antigen in hemodialysis: evidence for chronic exposure to LPS. Kidney Int. 1995;48:1469–1476. doi: 10.1038/ki.1995.436. [DOI] [PubMed] [Google Scholar]
- 6.Goyert SM, Ferrero E, Rettig WJ, et al. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988;239:497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- 7.Pugin J, Heumann ID, Tomasz A, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 8.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 9.Agodoa L, Briggs J, Mitch W, et al. Nutrition in ESRD patients: rationale and plan for an initiative. J Ren Nutr. 1999;9:116–118. doi: 10.1016/s1051-2276(99)90046-8. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38:1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 11.Wiedermann CJ, Kiechl S, Dunzendorfer S, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 12.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heumann D, Gallay P, Barras C, et al. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–3512. [PubMed] [Google Scholar]
- 14.Wurfel MM, Hailman E, Wright SD. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitchens RL, Thompson PA, Viriyakosol S, et al. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugin J, Schurer-Maly CC, Leturcq D, et al. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Real JM, Broch M, Richart C, et al. CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1780–1784. doi: 10.1210/jc.2002-020173. [DOI] [PubMed] [Google Scholar]
- 18.Raymond RM. Skeletal muscle metabolism and insulin resistance during endotoxin shock in the dog. Am J Emerg Med. 1984;2:45–59. doi: 10.1016/0735-6757(84)90109-8. [DOI] [PubMed] [Google Scholar]
- 19.Lang CH, Frost RA, Jefferson LS, et al. Endotoxin-induced decrease in muscle protein synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am J Physiol Endocrinol Metab. 2000;278:E1133–1143. doi: 10.1152/ajpendo.2000.278.6.E1133. [DOI] [PubMed] [Google Scholar]
- 20.Anker SD, Egerer KR, Volk HD, et al. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol. 1997;79:1426–1430. doi: 10.1016/s0002-9149(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 21.Heine GH, Ulrich C, Seibert E, et al. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 22.Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 24.Beddhu S, Pappas LM, Ramkumar N, et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 26.Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial. 2004;17:229–232. doi: 10.1111/j.0894-0959.2004.17311.x. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Colman S, Bross R, Benner D, et al. The Nutritional and Inflammatory Evaluation in Dialysis patients (NIED) study: overview of the NIED study and the role of dietitians. J Ren Nutr. 2005;15:231–243. doi: 10.1053/j.jrn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Kopple JD, Kamranpour N, et al. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra R, Kermah D, Fried L, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18:2781–2788. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 32.Beddhu S, Bruns FJ, Saul M, et al. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609–613. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 33.Nelson EE, Hong CD, Pesce AL, et al. Anthropometric norms for the dialysis population. Am J Kidney Dis. 1990;16:32–37. doi: 10.1016/s0272-6386(12)80782-7. [DOI] [PubMed] [Google Scholar]
- 34.Williams AJ, McArley A. Body composition, treatment time, and outcome in hemodialysis patients. J Ren Nutr. 1999;9:157–162. doi: 10.1016/s1051-2276(99)90056-0. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Dunne E, Nixon K, et al. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant. 1999;14:169–175. doi: 10.1093/ndt/14.1.169. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 37.Erbagci AB, Tarakcioglu M, Aksoy M, et al. Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta Cardiol. 2002;57:197–204. doi: 10.2143/AC.57.3.2005389. [DOI] [PubMed] [Google Scholar]
- 38.Pecoits-Filho R, Barany P, Lindholm B, et al. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–1688. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 39.Beutler B, Cerami A. The biology of cachectin/TNF - a primary mediator of host response. Ann Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 40.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 41.Raj DS, Carrero JJ, Shah VO, Qureshi AR, Barany P, Heimburger O, Lindholm B, Ferguson J, Moseley P, Stenvinkel P. Soluble CD14 Levels, Interleukin-6 and Mortality Among Prevalent Hemodialysis Patients. Am J Kidney Dis. 2009 doi: 10.1053/j.ajkd.2009.06.022. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]