Abstract
Purpose
To conduct an intervention study designed to assess the effectiveness of using a newsletter to increase medical follow-up in pediatric cancer survivors at risk of selected treatment complications.
Methods
Survivors participating in the Childhood Cancer Survivor Study who were at least 25 years of age and at risk of cardiovascular disease, breast cancer, or osteoporosis related to previous cancer treatment were randomly assigned to receive a newsletter featuring brief health risk information or a newsletter including an insert providing more comprehensive health risk information. A follow-up survey distributed 24 months after the newsletter intervention assessed predictors of medical follow-up.
Results
Overall there were no differences found among the groups in terms of access to a treatment summary, medical follow-up, discussion of childhood cancer health risks, and medical screening for the targeted health behaviors. One exception, indicating borderline significance was that women at risk for osteoporosis who received the newsletter insert were more likely to have discussed their risk with a doctor than those who only received the brief information (10.1% vs. 4.0% p=0.05). Discussion of breast cancer (OR=2.14; 95% CI=1.73–2.65), heart disease (OR=5.54; 95% CI=4.67–6.57) and osteoporosis (OR=7.87; 95% CI=6.34–9.78) risk with physician significantly predicted report of undergoing screening for targeted behavior in previous 2 years as did physician access to treatment summary.
Conclusions
More detailed content in a newsletter had minimal effect on recommended screening. However, survivor’s discussion of cancer-related risks with one’s doctor significantly influenced participation in health screening. These results highlight the integral role of communication in health behavior.
Keywords: pediatric cancer, survivorship, late effects, communication
INTRODUCTION
Treatment for cancer during childhood results in substantial risk of morbidity that has been shown to predispose some long-term survivors to premature mortality [1]. The Childhood Cancer Survivor Study (CCSS), a North American multi-institutional cohort study, estimated that almost 75% of their 10,397 adult participants (mean age, 26.6 years) will develop at least one chronic health problem by 40 years of age and over 40% will experience a chronic condition that is severe, life-threatening or fatal [2]. Because transition of care from pediatric oncology programs is the norm for the majority of childhood cancer survivors, cancer centers are challenged with identifying methods to effectively communicate health risk information to long-term survivors who have been discharged to the care of community physicians. Achieving this goal requires that both survivors and their physicians are knowledgeable about potential treatment-related health problems and interventions to reduce risk or facilitate early detection. However, several studies have demonstrated that childhood cancer survivors are often not informed about the details of their cancer treatment and its associated health risks [3,4,5]. Moreover, because of the relative rarity of childhood cancer and its diverse histological subtypes and treatments, primary care providers are also not likely to be familiar with the health risks unique to childhood cancer [6,7]. These data underscore the importance of educating survivors about health risks associated with childhood cancer so they may advocate for appropriate surveillance and follow-up care [8,9,10].
One of the goals of the CCSS is to educate its participants about potential cancer-health risks, health surveillance measures appropriate to that risk, and methods to preserve health. Since the project began, periodic newsletters have served as the primary educational tool to keep cohort members informed about CCSS research findings, emerging health risks associated with specific therapeutic exposures, and health-promoting resources. In addition, subject matter in survey questionnaires, the study Web site (http://www.stjude.org/ccss), and cancer-related content in the media also have provided study participants with health-related information.
Against this backdrop, an intervention study was designed to assess the utility of the CCSS newsletter to inform survivors about their risk for treatment-related late effects. The researchers’ goal was to determine which of two risk communication approaches— brief or detailed information about cancer-related health risks and health surveillance recommendations — would be more effective at motivating study participants to see their doctor, discuss their risk for treatment-related effects, and receive appropriate medical screening tests. The study involved CCSS cohort members at high risk for developing breast cancer, cardiovascular disease and osteoporosis because of the cancer treatments they received as children, along with a control group of survivors not specifically at risk for any of these conditions. Three core questions guided the study. The first question relates specifically to the impact of the newsletter intervention; the next two focus on associations hypothesized by a conceptual model that relates contextual factors, information seeking, and knowledge gaining health behaviors to cancer survivors getting recommended screening tests.
Does a detailed message targeted to a patient’s cancer history have a greater influence on recommended health behaviors than a more generalized message?
What are the key, potentially modifiable, mediating health behaviors that lead to recommended screening tests?
Are there individual characteristics that are predictive of certain knowledge seeking health behaviors?
METHODS
Subject selection
Study participants were drawn from the CCSS cohort, for which enrollment criteria consisted of: a) diagnosis of leukemia, central nervous system (CNS) tumors (all histologies), Hodgkin lymphoma, non-Hodgkin lymphoma, kidney tumor, neuroblastoma, soft tissue sarcoma, or bone tumor; b) diagnosis and initial treatment at one of the collaborating CCSS institutions; c) diagnosis date between January 1, 1970 and December 31, 1986; d) age less than 21 years at diagnosis; e) survival five or more years from diagnosis [11]. The CCSS protocol and contact documents were reviewed and approved by the Human Subjects Committee at each participating institution.
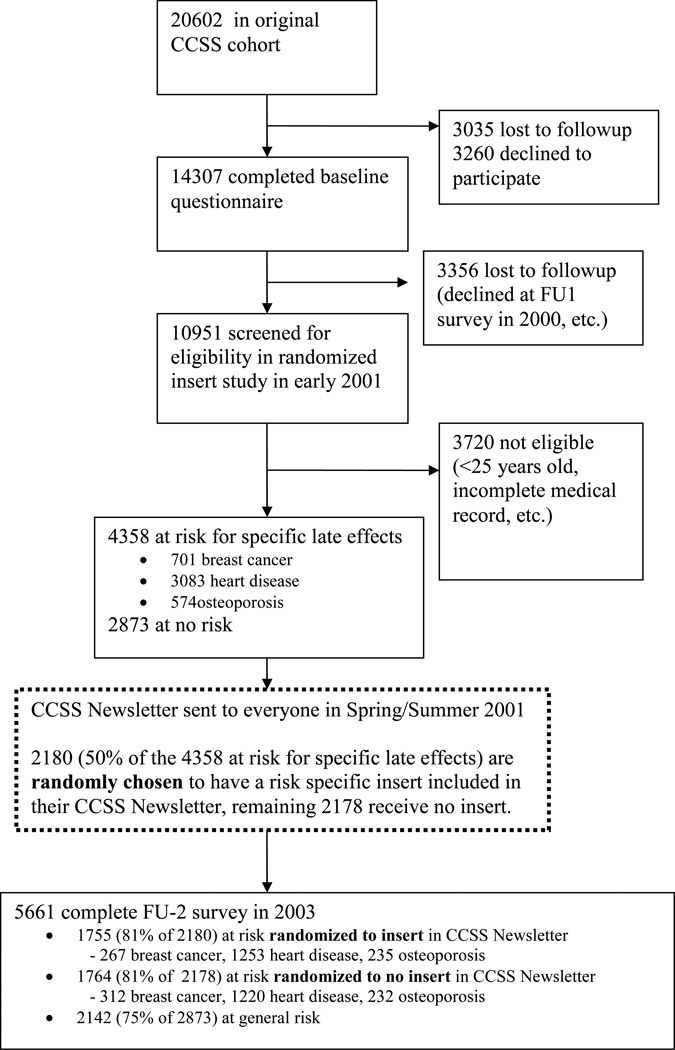
Baseline data for members of the study cohort were collected using a 24-page baseline questionnaire. Of the 20,602 eligible five-year survivors, 3,035 were lost to follow-up, 3,260 declined participation, and 14,307 agreed to participation and completed a baseline questionnaire (Figure 1). Information concerning primary cancer therapy was subsequently abstracted from the medical records of all CCSS participants who returned a signed medical release. In addition to surgical information, abstracted data included specific chemotherapeutic agents administered and site specific radiation exposure (dichotomous yes/no variable). Anthracyclines were defined as Adriamycin (doxorubicin), daunorubicin, and idarubicin; steroids were defined as prednisone and dexamethasone.
Figure 1.
A subsequent Follow-Up 2 (FU-2) survey was distributed to CCSS participants, beginning in 2003. Of the 14,307 survivors who completed the baseline questionnaire, 10,951 survivors were alive and eligible to receive the FU-2 survey, and 9,308 (85%) returned this survey. The current study including the intervention reported here was conducted with data from 5,661 survivors who were 25 years of age or older as of July 2, 2001, had returned both the baseline and FU-2 surveys, and completed a medical release request (see Figure 1).
Design of Newsletter Intervention
Groups selected for inclusion in this newsletter intervention study were categorized by risk for late effects [12] as follows:
At risk for breast cancer—Females who received radiation impacting breast
At risk for heart disease—Survivors who were treated with anthracyclines and/or received radiation impacting the heart
At risk for osteoporosis—Participants who were treated with methotrexate or corticosteroids and/or received cranial or TBI radiation
No risk—Cohort members not at increased risk for above late effects, by virtue of diagnosis and treatment, for any of these health problems.
Risk classification was based on data previously abstracted from medical records. Risk factors were prioritized to assure assignment of survivors into one group only, regardless of exposures. The rank order for risk group assignment was breast cancer, first; cardiovascular disease, second; and osteoporosis, third. One exception to this rank order were female survivors who received chest radiation - this group was at risk for both breast cancer and cardiovascular disease, therefore half were assigned to the breast cancer pool, half to the cardiovascular disease pool. Based on these criteria, 701 female survivors were classified at risk for breast cancer, 3,083 male and female survivors at risk for cardiovascular disease, 574 for osteoporosis, and 2,873 not specifically at risk for any of these conditions.
The intervention component of the study was implemented in Spring/Summer, 2001. Within each risk group, eligible survivors were randomly assigned to either receive newsletter featuring brief or detailed information about cancer-related health risks and health surveillance recommendations. Survivors randomized to the intervention arm providing more comprehensive health risk information received an insert providing a detailed description of childhood cancer treatment predisposing to the medical condition, other factors that increase an individual’s risk, how to reduce that risk, the type of information a doctor should know to evaluate a survivor’s risk, and recommended medical follow-up based on risk. The newsletter and inserts are available at http://ccss.stjude.org/documents/newsletters.
Measures
Intervention targeted variables
Approximately two years after distribution of the newsletter, the FU-2 survey was sent to all CCSS participants as part of the ongoing cohort study; responses to this survey constitute the outcomes for this study. The primary outcome of interest was screening behavior. As recommended by the Children's Oncology Group Long-Term Follow-Up Guidelines, screening was measured as: 1) has had a mammogram in the past 2 years (women only); 2) has had an echocardiogram —“ultrasound of the heart to look at the heart muscle and heart valves” in the past 2 years; and 3) “has had a test to measure bone strength or bone mineral density (such as a DEXA, quantitative CT scan, or ultrasound) in the past 2 years.” In addition to examining each screening behavior separately, a dichotomous measure of received appropriate screening was created based on the specific risk group (breast cancer, heart disease, osteoporosis) to which a survivor was categorized based on treatment history.
The intervention further targeted three mediating variables that were expected to lead to appropriate screening, measured in the FU-2 survey as: seeing a doctor (ever versus never in the previous two years); having access to a cancer treatment summary (participants reported separately about whether they or their doctor had the summary, yes/no/don’t know); and discussing cancer-related health risk with a doctor (heart disease, osteoporosis and cancer in general).
Exposure to intervention - reading the newsletter
The detailed information insert was included in the CCSS-mailed newsletter. Hence, to be exposed to the intervention, the survivor presumably had to read or at the very least look through the newsletter. A simple indicator of whether a survivor exhibited this information-seeking behavior was measured by the question “In the past 2 years, did you read a newsletter from the CCSS study?” (yes vs. no).
Conceptual framework
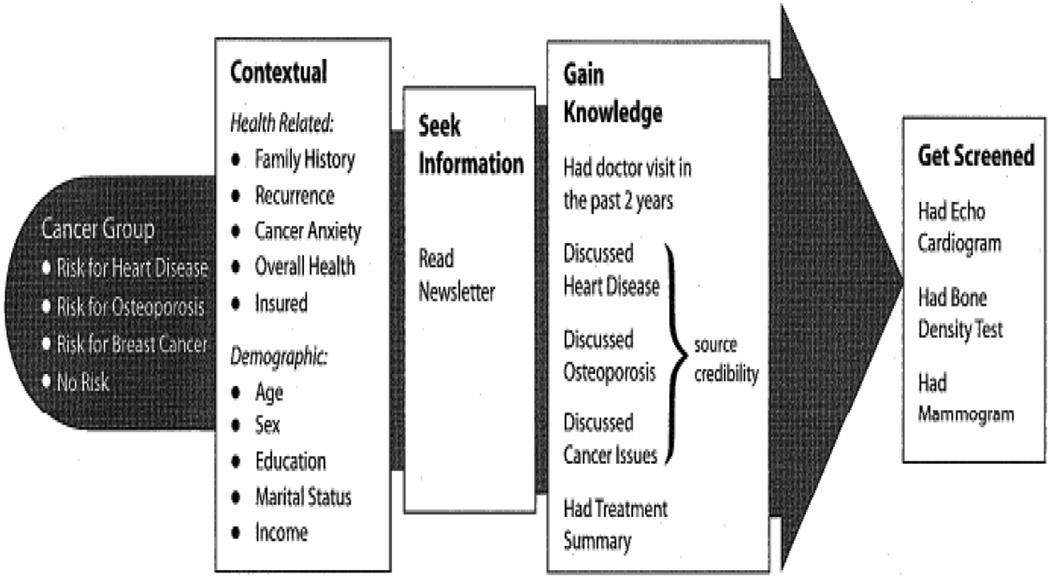
Drawing on communications theory and previous research on childhood and adult cancer survivors [13–22] a conceptual model (Figure 2) was developed to describe expected associations between a survivor’s actual health risk (Cancer Group classification) and receiving screening tests recommended for maintaining health (Get Screened outcomes). Because individual differences, both health-related and demographic, need to be considered, the model hypothesized a path that factored in situational variables as the context for behavior (Context[ual] variables). Information-seeking (Seek Information) plays two roles. Conceptually, it was included as a step to increased knowledge about cancer risk and recommended surveillance [23]. Operationally, it serves as our measure of “exposure to intervention.” In the interest of parsimony, for this analysis, newsletter readership serves as a proxy for this complex construct. Source credibility, a moderator of knowledge gain, was measured by the question, “Your doctor was familiar with health problems that develop after childhood cancer and similar illnesses” (yes vs. no). Discussing one’s risk with a doctor (interpersonal communication) and having access (directly or through a doctor) to one’s own cancer diagnosis and treatment summary information, key ingredients of a Survivor Care Plan [15], were included as components of knowledge transfer (Gain Knowledge).
Figure 2.
Pathways to screening
Health-related variables from follow-up survey selected for consideration included: family history of cancer (yes vs. no), secondary malignancy or recurrence of the primary cancer (yes vs. no), cancer-related anxiety (coded from a Likert item with response anchors: none, some, more than some), self-reported health status (measured on a five-point Likert scale ranging from poor to excellent), and health insurance coverage (yes vs. no).
Standard socio-demographic variables including age at time of newsletter intervention, sex, education, marital status, and income also were measured based on self-report to the FU-2 survey. Education was dichotomized as post-college (yes vs. no) in multivariable logistic regression models, and income was split into three categories: less than $40,000, $40,000–$80,000, and greater than $80,000.
Data Analysis
Descriptive summaries of cancer diagnoses and treatment based on CCSS medical records were calculated for each of the predefined risk groups. Demographic and health-related variables were also summarized by predefined risk groups. To examine balance of randomization, descriptive summaries of study population were additionally stratified by randomization group (insert or no insert).
The rates of the targeted outcomes: (1) visited doctor in last 2 years, (2) survivor or MD has childhood cancer treatment summary, (3) discussed with MD about risks associated with childhood cancer, and (4) appropriate screening behavior were compared between the two randomized intervention arms (newsletter with insert vs. newsletter without insert). Differences in the rates were tested separately within each of the at risk groups, and stratified by gender using one-sided Fisher’s exact tests hypothesizing the rate to be higher in the group who received the insert. Intent-to-treat analyses were conducted including all individuals who were randomized to each intervention arm.
To identify potential predictors of the three different types of screening (mammogram in last 2 years, echocardiogram in last 2 years, and bone density in last 2 years), separate multivariate logistic regression models were used including all survivors who had seen a physician in the last 2 years. Predictors included the expected intervention-targeted mediators of availability of cancer treatment summary and discussions with doctor about risks as well as the more distal mediator of having read the newsletter (or information seeking) and all health-related and demographic variables. Additional logistic regression models were used to identify potential predictors of the intervention-targeted mediators themselves (i.e. discussion about cancer, heart disease and osteoporosis risks, and availability of cancer treatment summary). Of primary interest, these models included the distal mediator of having read the newsletter and also controlled for health-related and demographic variables. Odds ratios, 95% confidence intervals and p-values are reported. Because of the study’s focus on the use of a newsletter for transmitting information, additional logistic regressions were performed taking reading the newsletter as the outcomes.
Missing data for the following categorical predictor variables was minimal (<5%) and dealt with by including a separate missing category for each of them in the logistic regressions: family history of cancer, health insurance, read newsletter, post college, currently married, and income. Since a larger portion of the cancer anxiety variable was missing (12%), multiple imputation (100 datasets) was performed using PROC MI in SAS to impute the cancer anxiety measure. All logistic regressions were performed across all imputed datasets and results were combined and summarized using PROC MIANALYZE in SAS which incorporates uncertainty due to the missing values. SAS Version 9.2 was used for all analyses.
RESULTS
Study Population
Tables 1 and 2 describe the distribution among participants by risk group and random assignment to newsletter intervention (Insert vs. No Insert) along three dimensions: cancer treatment, health related factors and demographics. No appreciable differences were noted between randomized groups within each risk group. The number of survivors at risk for each health outcome differed by age at diagnosis, diagnosis, and treatment. Those that were at risk for osteoporosis were diagnosed at a younger age, whereas, the survivors at risk for breast cancer were diagnosed older. Respondents with an original cancer diagnosis of Hodgkin lymphoma were predominant in the breast cancer risk group; individuals with a diagnosis of leukemia, Hodgkin lymphoma, and bone tumors were predominantly at risk for heart disease; and a leukemia diagnosis was predominant for respondents in the osteoporosis risk group. As expected, respondents in the no risk group were less likely than the at risk groups to be exposed to chemotherapy and radiation treatment fields predisposing to the targeted health risks.
Table 1.
Description of diagnosis and treatments (based on medical records) for the 4 different defined risk groups: At risk for Breast Cancer, Cardiac Disease, Osteoporosis or No Risk.
| At Risk for Breast Cancer (n=579) |
At Risk for Heart Disease (n=2473) |
At Risk for Osteoporosis (n=467) |
No Risk (n=2142) |
|||||
|---|---|---|---|---|---|---|---|---|
| Insert N=267 Count (%) |
No Insert N=312 Count (%) |
Insert N=1253 Count (%) |
No Insert N=1220 Count (%) |
Insert N=235 Count (%) |
No Insert N=232 Count (%) |
Count (%) | ||
| Age at diagnosis | ||||||||
| 0–4 | 25 (9) | 22 (7) | 205 (16) | 190 (16) | 92 (39) | 102 (44) | 586 (27) | |
| 5–9 | 43 (16) | 25 (8) | 292 (23) | 267 (22) | 82 (35) | 68 (29) | 575 (27) | |
| 10–14 | 72 (27) | 108 (35) | 393 (31) | 423 (35) | 44 (19) | 49 (21) | 581 (27) | |
| 15–20 | 127 (48) | 157 (50) | 363 (29) | 340 (28) | 17 (7) | 13 (6) | 400 (19) | |
| Diagnosis | ||||||||
| Leukemia | 13 (5) | 17 (5) | 409 (33) | 430 (35) | 218 (93) | 209 (90) | 372 (17) | |
| CNS tumor | 2 (1) | 1 (0) | 70 (6) | 81 (7) | 10 (4) | 10 (4) | 512 (24) | |
| Hodgkin lymphoma | 179 (67) | 224 (72) | 217 (17) | 203 (17) | -- | -- | 223 (10) | |
| NHL | 18 (7) | 23 (7) | 152 (12) | 109 (9) | 5 (2) | 13 (6) | 195 (9) | |
| Kidney (Wilms) | 22 (8) | 13 (4) | 55 (4) | 45 (4) | -- | -- | 200 (9) | |
| Neuroblastoma | 11 (4) | 8 (3) | 21 (2) | 26 (2) | -- | -- | 130 (6) | |
| Soft tissue sarcoma | 10 (4) | 11 (4) | 95 (8) | 108 (9) | 2 (1) | -- | 346 (16) | |
| Bone tumor | 12 (4) | 15 (5) | 234 (19) | 218 (18) | -- | -- | 164 (8) | |
| Chemotherapy | ||||||||
| Any chemotherapy | 185 (69) | 204 (65) | 1135 (91) | 1103 (90) | 235 (100) | 231 (100) | 926 (43) | |
| No chemotherapy | 80 (30) | 107 (34) | 116 (9) | 114 (9) | -- | 1(0) | 757 (35) | |
| Unknown | -- | -- | -- | -- | -- | -- | 459 (21) | |
| Type of Chemotherapy | ||||||||
| Anthracyclinesa | 41 (22) | 81 (40) | 925 (82) | 874 (79) | -- | -- | 19 (2) | |
| Steroidsa | 98 (53) | 128 (63) | 704 (62) | 677 (61) | 226 (96) | 224 (97) | 434 (47) | |
| Methotrexatea | 31 (17) | 44 (22) | 693 (61) | 684 (62) | 229 (97) | 226 (98) | 364 (39) | |
| Other chemotherapya | 68 (37) | 44 (22) | 52 (5) | 50 (5) | -- | -- | 461 (50) | |
| Radiation | ||||||||
| Any radiation | 267(100) | 312(100) | 930 (74) | 900 (74) | 235(100) | 232(100) | 992(43) | |
| Radiation site | ||||||||
| Chestb | 133 (50) | 165 (53) | 184 (20) | 166 (18) | -- | -- | 19 (2) | |
| Spineb | 1 (0) | -- | 71 (8) | 67 (7) | -- | -- | 2 (0) | |
| Cranialb | 1 (0) | 1 (0) | 309 (33) | 302 (34) | 219 (93) | 218 (94) | 271 (29) | |
| TBIb | 8 (3) | 12 (4) | 14 (2) | 5 (1) | -- | -- | -- | |
| Other siteb | 126 (47) | 135 (43) | 416 (45) | 423 (47) | 16 (7) | 14 (6) | 583 (63) | |
NHL - Non Hodgkin lymphoma
TBI - Total Body Irradiation
Chemotherapy types of drugs can add up to more than total number with any chemotherapy. Percents by types are based only on those with any chemotherapy
Radiation site counts can add up to more than total number with any radiation. Percents by sites are based only on those with any radiation
Table 2.
Descriptive summaries of demographic and health related contextual variables by risk group and intervention randomization group.
| At Risk for Breast Cancer (n=579) |
At Risk for Heart Disease (n=2473) |
At Risk for Osteoporosis(n=467) |
No Risk (n=2142) |
|||||
|---|---|---|---|---|---|---|---|---|
| Insert N=267 Count (%) |
No Insert N=312 Count (%) |
Insert N=1253 Count (%) |
No Insert N=1220 Count (%) |
Insert N=235 Count (%) |
No Insert N=232 Count (%) |
Count (%) | ||
| DEMOGRAPHIC VARIABLES | ||||||||
| Sex | ||||||||
| Female | 267 (100) | 312 (100) | 494 (39) | 419 (34) | 119 (51) | 124 (53) | 1001 (47) | |
| Male | -- | -- | 759 (61) | 801 (66) | 116 (49) | 108 (47) | 1141 (53) | |
| Age at 7/1/2001 | ||||||||
| 25–29 | 39 (15) | 47 (15) | 377 (30) | 357 (29) | 110 (47) | 100 (43) | 728 (34) | |
| 30–39 | 142 (53) | 162 (52) | 693 (55) | 681 (56) | 114 (49) | 120 (52) | 1120 (52) | |
| 40+ | 86 (32) | 104 (33) | 183 (14) | 182 (15) | 11 (5) | 12 (5) | 294 (14) | |
| Education | ||||||||
| HS or less | 37 (14) | 38 (12) | 190 (15) | 187 (15) | 59 (26) | 58 (25) | 427 (20) | |
| Post HS | 76 (29) | 104 (34) | 397 (32) | 378 (31) | 75 (32) | 95 (41) | 700 (33) | |
| College grad + | 152 (57) | 168 (54) | 657 (53) | 652 (54) | 97 (42) | 77 (33) | 988 (47) | |
| Household Income | ||||||||
| <$40,000 | 59 (22) | 71 (23) | 355 (28) | 370 (30) | 88 (37) | 87 (38) | 662 (31) | |
| $40,000–$80,000 | 96 (36) | 116 (37) | 441 (35) | 425 (35) | 77 (33) | 77 (33) | 743 (35) | |
| >$80,000 | 100 (37) | 111 (36) | 339 (27) | 320 (26) | 33 (14) | 29 (13) | 504 (24) | |
| Not reported | 12 (4) | 14 (4) | 118 (9) | 105 (9) | 37 (16) | 39 (17) | 233 (11) | |
| Marital status | ||||||||
| Currently married | 199 (75) | 228 (74) | 760 (61) | 716 (59) | 109 (47) | 110 (48) | 1193 (57) | |
| Not currently married | 68 (25) | 84(26) | 493 (39) | 504 (41) | 126 (53) | 122 (52) | 949 (43) | |
| HEALTH RELATED VARIABLES | ||||||||
| Has Health Insurance | 253 (95) | 296 (95) | 1143 (92) | 1091 (90) | 196 (84) | 194 (86) | 1907 (90) | |
| Any family history of cancer | 62 (23) | 81 (26) | 247 (20) | 225 (19) | 33 (14) | 24 (10) | 438 (21) | |
| Second cancer or recurrence or relapse | 54 (20) | 66 (21) | 109 (9) | 68 (6) | 10 (4) | 19 (8) | 120 (6) | |
| Cancer related anxiety | ||||||||
| None | 135(51) | 138 (44) | 724(58) | 739 (61) | 153 (65) | 145 (63) | 1307(61) | |
| A little | 89(33) | 112(36) | 377 (30) | 329(27) | 60 (26) | 59 (25) | 603(28) | |
| More than a little | 43(16) | 62(20) | 152 (12) | 152 (12) | 22 (9) | 28 (12) | 232(11) | |
| Quality of Overall Health | 3.4 (0.9) | 3.3 (1.0) | 3.5 (1.0) | 3.5 (1.0) | 3.4 (1.0) | 3.6 (1.0) | 3.5 (1.0) | |
| (range 1–5) Mean (SD) | ||||||||
In terms of health-related variables, when compared to other risk groups, respondents at risk for breast cancer were most likely to have insurance, a family history of cancer, a second malignant neoplasm (SMN) or recurrence, and higher cancer-related anxiety. Self-assessed health status was similar among all risk groups. Demographically, differences in age correspond with variations among risk groups in education, marital status, and income. Respondents at risk for breast cancer were older with a mean age of 36.6 years when the study was launched, whereas those at risk for osteoporosis were younger with a mean age of 31.
Newsletter Intervention
Table 3 displays the effect of the newsletter insert on intervention-targeted behaviors. Women at risk for osteoporosis who received a newsletter insert were more likely to have discussed their risk with a doctor than women who didn’t receive the insert, but the percentage was still very low (10.1% vs. 4.0% - borderline statistically significant). With the exception of women at risk for osteoporosis discussing their risk with a doctor, the intervention insert did not significantly impact respondents reporting a visit to a doctor in the past two years, access to a treatment summary (either directly or through his/her MD), discussion of risks associated with childhood cancer with doctor, or receipt of screening for targeted health risks.
Table 3.
Effect of randomized Newsletter Insert on Intervention Targeted Behaviors by risk group
| Visited doctor in last 2 years |
Survivor or MD has childhood cancer treatment summarya |
Discussed with MD about risks associated with childhood cancerb |
Appropriate screening behaviorc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insert | No Insert |
p-valued | Insert | No Insert |
p-valued | Insert | No Insert |
p-valued | Insert | No Insert |
p-valued | |
| At risk Group | ||||||||||||
| Females | ||||||||||||
| Breast Cancer | 95.9% | 96.2% | 0.65 | 47.1% | 43.8% | 0.24 | 47.9% | 49.0% | 0.64 | 57.1% | 56.5% | 0.48 |
| Heart Disease | 91.9% | 92.6% | 0.70 | 42.4% | 39.6% | 0.22 | 19.2% | 15.5% | 0.08 | 31.1% | 26.4% | 0.08 |
| Osteoporosis | 93.3% | 91.1% | 0.35 | 38.1% | 42.2% | 0.78 | 10.1% | 4.0% | 0.05 | 12.4% | 7.8% | 0.18 |
| Males | ||||||||||||
| Heart Disease | 86.8% | 85.0% | 0.17 | 44.9% | 41.4% | 0.10 | 22.0% | 22.5% | 0.62 | 25.2% | 24.8% | 0.46 |
| Osteoporosis | 75.9% | 83.3% | 0.94 | 41.6% | 42.5% | 0.60 | 1.7% | 3.7% | 0.91 | 5.9% | 5.9% | 0.62 |
Survivors’ reporting of whether they or their MD had a summary of their childhood cancer treatment used a dichotomous outcome of yes versus no or don’t know, based upon the view that the intervention had not produced the preferred outcome if the survivor reported they did not know.
Risk discussion depends on at risk group: discussed cancer related issues, discussed heart disease, or discussed osteoporosis
appropriate screening behavior depends on at risk group: mammogram within last 2 years, echocardiogram within last two years, bone density within last 2 years.
p-value based on one sided Fisher’s exact test that outcome has higher rate in insert group versus no insert group.
The majority of survivors had visited a doctor in the previous two years (range: 75.9–96.2%); however, fewer than 50% reported that either they or their physician had a cancer treatment summary. Risk-specific discussions varied according to risk category and sex, with the risk of breast cancer being discussed most frequently and osteoporosis least frequently.
Pathways to appropriate screening
The lack of a strong or consistent intervention effect motivated the expanded aims to identify factors that influence adherence to screening. In particular, we determined the strength of the association between screening and the intervention-targeted mediators, i.e., discussing cancer risk with a doctor and having access to one’s cancer treatment summary. Table 4 indicates that discussing one’s cancer-related risk with a doctor is the strongest predictor of getting screened for late effects. Discussing cancer-related risks with a doctor significantly influenced the odds of reporting screening by mammography (OR 2.15, 95% CI=1.74–2.66), echocardiography (OR 5.54, 95% CI=4.67–6.57), and bone density testing (OR 10.6, 95% CI=8.34–13.47). A physician’s access to the survivor’s cancer treatment summary also significantly predicted the respondent’s receipt of screening for the targeted health risks. Odds ratios for receiving a recommended mammogram, echocardiogram or bone density test were, respectively, OR 1.31, 95% CI=1.02–1.67; OR 1.48, 95% CI=1.23–1.78; and OR 1.32, 95% CI=1.02–1.71.
Table 4.
| Mammogram in last 2 years | Echocardiogram in last 2 years | Bone Density Test in last 2 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |||||
| INTERVENTION RELATED MEDIATORS | ||||||||||
| Childhood Cancer Treatment summary | ||||||||||
| MD has (yes vs. no) | 1.31 | (1.02– 1.67) | 0.03 | 1.48 | (1.23 – 1.78) | < 0.01 | 1.32 | (1.02 – 1.71) | 0.04 | |
| MD has (don’t know vs. no) | 1.02 | (0.78 – 1.33) | 0.88 | 1.03 | (0.83 – 1.28) | 0.80 | 1.08 | (0.80 – 1.45) | 0.62 | |
| Survivor has (yes vs. no) | 1.07 | (0.85 – 1.37) | 0.56 | 1.14 | (0.94 – 1.37) | 0.18 | 1.12 | (0.87 – 1.44) | 0.37 | |
| Survivor has (don't know vs.no) | 0.90 | (0.59 – 1.36) | 0.60 | 1.03 | (0.77 – 1.37) | 0.85 | 0.88 | (0.58 – 1.35) | 0.57 | |
| Discussed with MD about | ||||||||||
| Cancer-related issues (yes vs no) | 2.15 | (1.74 – 2.66) | < 0.01 | 0.99 | (0.83 – 1.17) | 0.87 | 1.24 | (0.98 – 1.56) | 0.07 | |
| Heart disease (yes vs no) | 0.97 | (0.75 – 1.26) | 0.81 | 5.54 | (4.67 – 6.57) | < 0.01 | 0.96 | (0.74 – 1.23) | 0.73 | |
| Osteoporosis (yes vs. no) | 1.46 | (1.12 – 1.90) | <0.01 | 1.21 | (0.96 – 1.52) | 0.11 | 10.6 | (8.34 – 13.47) | < 0.01 | |
| Read newsletter(yes vs. no) | 1.04 | (0.81 – 1.33) | 0.79 | 0.94 | (0.78 – 1.12) | 0.48 | 1.11 | (0.85 – 1.44) | 0.44 | |
| HEALTH RELATED VARIABLES | ||||||||||
| At risk group | ||||||||||
| Breast cancer vs no risk | 1.73 | (1.32 – 2.27) | < 0.01 | 1.82 | (1.40 – 2.37) | < 0.01 | 1.42 | (1.02 – 1.97) | 0.04 | |
| Heart disease vs no risk | 1.06 | (0.84 – 1.35) | 0.62 | 1.77 | (1.48 – 2.12) | < 0.01 | 1.03 | (0.80 – 1.33) | 0.79 | |
| Osteoporosis vs no risk | 1.09 | (0.74 – 1.61) | 0.65 | 0.91 | (0.63 – 1.30) | 0.59 | 1.30 | (0.84 – 1.99) | 0.23 | |
| Family history of cancer (yes vs. no) | 1.39 | (1.10 – 1.76) | < 0.01 | 1.03 | (0.85 – 1.24) | 0.79 | 0.91 | (0.70 – 1.17) | 0.46 | |
| 2nd cancer/recurrence (yes vs. no) | 0.86 | (0.63 – 1.18) | 0.35 | 1.45 | (1.12 – 1.87) | < 0.01 | 1.63 | (1.20 – 2.21) | < 0.01 | |
| Cancer Anxiety | ||||||||||
| Some vs none | 1.30 | (1.03 – 1.64) | 0.03 | 1.08 | (0.90 – 1.30) | 0.39 | 0.88 | (0.68 – 1.14) | 0.34 | |
| More than some vs none | 1.33 | (0.97 −1.83) | 0.08 | 1.27 | (0.99, 1.62) | 0.06 | 1.10 | (0.79, 1.53) | 0.56 | |
| Quality of Overall Health | 1.08 | (0.97 – 1.20) | 0.16 | 0.85 | (0.78 – 0.92) | < 0.01 | 0.84 | (0.75 – 0.94) | < 0.01 | |
| Health insurance (yes vs. no) | 2.17 | (1.32 – 3.57) | < 0.01 | 2.16 | (1.46 – 3.21) | < 0.01 | 1.84 | (1.09 – 3.09) | 0.02 | |
| MD unfamiliar with late effects (yes vs. no) | 0.91 | (0.71 – 1.15) | 0.42 | 1.06 | (0.87 – 1.29) | 0.56 | 0.97 | (0.74 – 1.26) | 0.80 | |
| DEMOGRAPHIC VARIABLES | ||||||||||
| Age (in years) | 1.21 | (1.19 – 1.24) | < 0.01 | 1.02 | (1.01 – 1.04) | < 0.01 | 1.04 | (1.02 – 1.06) | < 0.01 | |
| Sex (female vs. male) | -- | -- | -- | 1.23 | (1.03 – 1.47) | 0.02 | 1.48 | (1.15 – 1.90) | < 0.01 | |
| Post college (yes vs. no) | 0.87 | (0.70 – 1.07) | 0.19 | 1.07 | (0.90 – 1.26) | 0.46 | 1.03 | (0.82 – 1.30) | 0.78 | |
| Currently Married (yes vs. no) | 1.10 | (0.87 – 1.38) | 0.43 | 0.86 | (0.72 – 1.02) | 0.09 | 0.74 | (0.58 – 0.94) | 0.01 | |
| Income | ||||||||||
| $40–80K vs Less than $40K | 0.93 | (0.71 – 1.22) | 0.63 | 1.09 | (0.88, 1.34) | 0.42 | 0.90 | (0.68 – 1.19) | 0.45 | |
| Greater $80K vs Less than $40K | 1.02 | (0.75 – 1.37) | 0.92 | 1.35 | (1.07, 1.72) | 0.01 | 0.90 | (0.65 – 1.25) | 0.53 | |
Odds ratios (OR), 95% confidence intervals (95% CI) and p-values based on multivariate logistic regression including all predictors simultaneously.
n=5025 Survivors visited MD in last 2 years but logistic regression analyses are restricted to those who had information available for each type of screening, i.e. n=2511 female Survivors for mammogram, n=4532 and n=4647 Survivors for echocardiogram and bone density test, respectively.
Among contextual variables, risk status was a significant predictor of obtaining screening, with women at risk for breast cancer being significantly more likely than respondents at no risk for late effects to undergo screening for all three of the targeted health risks. Other significant predictors of receiving a mammogram included: having a family history of cancer, having health insurance, and older age. Respondents at risk for heart disease were significantly more likely to receive an echocardiogram. Significant predictors of receiving an echocardiogram included recurrence of the primary cancer or a second malignancy, and having health insurance. Older age, being a female, and having a higher income also were significant predictors. A one unit increase in overall general quality of health (i.e., poor, fair, good, very good, excellent) reduced by 15% the odds of having an echocardiogram. Being at risk for osteoporosis was not a significant predictor for any type of screening test. Predictors for receiving a bone density tests included respondents who report better general health had 16% lower odds of having bone density testing. Conversely, survivors with a second malignancy or recurrence had significantly higher odds to be screened for osteoporosis. Other significant factors for having bone density testing (i.e. screening for osteoporosis) include being insured, older age, female, and not currently married. The logistic regression analyses reported in Table 4were restricted to respondents who provided information for each type of screening.
Table 5, in turn, shows the relationship of factors believed to influence whether survivors would discuss risks associated with childhood cancer with their doctor and whether the doctor and/or survivor has access to the cancer treatment summary (see Figure 2). Within each risk group, reading the newsletter was a significant predictor in receiving the target behavior change. Among the most robust predictors were: risk group, second malignancy or recurrence of the childhood cancer, cancer anxiety, and gender. One mediator that works against having access to a treatment summary is source credibility, operationalized in this study by a question about the doctor’s familiarity with late effects. Survivors who said their doctor was unfamiliar with late effects were 40% less likely to have access to their treatment summary. Currently married was the only other factor to be negatively associated with having access to a treatment summary.
Table 5.
Predictors of Discussing with MD about risks associated with childhood cancer and Survivor or MD having Childhood cancer treatment summary for Survivors who visited a doctor in last 2 yearsab.
| Discuss with MD about risks associated with childhood cancer | Survivor or MD has childhood cancer treatment summary |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discuss Cancer-related Risks | Discuss Heart Disease risks | Discuss Osteoporosis risks | |||||||||||||
| OR (95% CI) | p- value |
OR (95% CI) | p- value |
OR (95% CI) | p- value |
OR (95% CI) | p- value |
||||||||
| INTERVENTION RELATED EDIATORS | |||||||||||||||
| Read newsletter (yes vs. no) | 1.53 | 1.31 – 1.80 | < 0.01 | 1.40 | 1.17 – 1.67 | < 0.01 | 1.40 | 1.10 – 1.78 | < 0.01 | 1.27 | 1.11 – 1.45 | < 0.01 | |||
| HEALTH RELATED VARIABLES | |||||||||||||||
| At risk group | |||||||||||||||
| Breast cancer vs no risk | 1.80 | 1.44 – 2.25 | < 0.01 | 2.77 | 2.15 – 3.56 | < 0.01 | 1.54 | 1.17 – 2.03 | < 0.01 | 1.31 | 1.06 – 1.62 | 0.01 | |||
| Heart disease vs no risk | 1.09 | 0.94 – 1.27 | 0.26 | 1.73 | 1.46 – 2.05 | < 0.01 | 1.18 | 0.94 – 1.47 | 0.15 | 1.19 | 1.05 – 1.36 | 0.01 | |||
| Osteoporosis vs no risk | 0.91 | 0.70 – 1.20 | 0.51 | 0.81 | 0.56 – 1.16 | 0.26 | 0.60 | 0.38 – 0.95 | 0.03 | 1.08 | 0.86 – 1.36 | 0.49 | |||
| Family history of cancer (yes vs. no) | 1.54 | 1.31 – 1.80 | < 0.01 | 1.06 | 0.89 – 1.27 | 0.50 | 1.28 | 1.03 – 1.59 | 0.03 | 1.01 | 0.87 – 1.17 | 0.88 | |||
| 2nd cancer/recurrence (yes vs. no) | 2.73 | 2.20 – 3.39 | < 0.01 | 0.95 | 0.74 – 1.22 | 0.69 | 1.56 | 1.19 – 2.04 | < 0.01 | 1.64 | 1.33 – 2.02 | < 0.01 | |||
| Cancer Anxiety | |||||||||||||||
| Some vs none | 1.73 | 1.47 – 2.02 | < 0.01 | 1.30 | 1.09 – 1.55 | < 0.01 | 1.23 | 0.97 – 1.54 | 0.08 | 1.17 | 1.02 – 1.35 | 0.03 | |||
| More than some vs none | 2.47 | 2.01 – 3.04 | < 0.01 | 1.32 | 1.04 – 1.67 | 0.02 | 1.74 | 1.32 – 2.30 | < 0.01 | 1.37 | 1.12 – 1.66 | < 0.01 | |||
| Quality of Overall Health | 0.88 | 0.82 – 0.95 | < 0.01 | 0.71 | 0.66 – 0.77 | < 0.01 | 0.78 | 0.71 – 0.86 | < 0.01 | 1.04 | 0.98 – 1.10 | 0.23 | |||
| Health insurance (yes vs no) | 1.37 | 1.03 – 1.83 | 0.03 | 1.48 | 1.06 – 2.06 | 0.02 | 1.30 | 0.86 – 1.98 | 0.22 | 1.42 | 1.12 – 1.80 | < 0.01 | |||
| MD unfamiliar with late effects (yes vs. no) | 1.08 | 0.91 – 1.27 | 0.37 | 1.14 | 0.95 – 1.37 | 0.16 | 1.21 | 0.96 – 1.51 | 0.10 | 0.60 | 0.51 – 0.70 | < 0.01 | |||
| DEMOGRAPHIC VARIABLES | |||||||||||||||
| Age (in years) | 1.00 | 0.99 – 1.02 | 0.57 | 1.05 | 1.04 – 1.06 | < 0.01 | 1.05 | 1.04 – 1.07 | < 0.01 | 0.99 | 0.98 – 1.00 | 0.21 | |||
| Sex (female vs. male) | 1.65 | 1.43 – 1.91 | < 0.01 | 0.63 | 0.54 – 0.75 | < 0.01 | 3.95 | 3.11 – 5.02 | < 0.01 | 0.93 | 0.82 – 1.06 | 0.26 | |||
| Post college (yes vs. no) | 1.25 | 1.08 – 1.44 | < 0.01 | 1.18 | 1.00 – 1.38 | 0.05 | 1.19 | 0.97 – 1.46 | 0.10 | 1.01 | 0.89 – 1.14 | 0.89 | |||
| Currently Married (yes vs. no) | 1.04 | 0.90 – 1.21 | 0.58 | 1.20 | 1.01 – 1.42 | 0.04 | 0.80 | 0.64 – 0.99 | 0.04 | 0.76 | 0.67 – 0.86 | < 0.01 | |||
| Income | |||||||||||||||
| $40–80K vs Less than $40K | 0.87 | 0.73 – 1.04 | 0.13 | 0.92 | 0.76 – 1.13 | 0.43 | 0.91 | 0.71 – 1.17 | 0.46 | 1.20 | 1.03 – 1.40 | 0.02 | |||
| Greater $80K vs Less than $40K | 0.98 | 0.80 – 1.20 | 0.86 | 1.06 | 0.85 – 1.33 | 0.61 | 0.82 | 0.61 – 1.10 | 0.18 | 1.14 | 0.96 – 1.36 | 0.15 | |||
Odds ratios (OR), 95% confidence intervals (95% CI) and p-values based on multivariate logistic regression including all predictors simultaneaously
n=5025 Survivors visited MD in last 2 years.
Intermediary steps
Because reading the newsletter – in addition to being a proxy for exposure to intervention – was found to play a significant role in the intervening variables detailed in Table 5, the characteristics of newsletter readers also were of interest. Regardless of risk category, substantially more women than men reported reading a newsletter from the CCSS Study in the past two years. The percentage difference ranged from 12.7% more female (72% readership) than male readers (59%) in the ‘no risk’ group to 6.6% more female (68% readership) than male readers in the osteoporosis risk group. Women at risk for breast cancer were the most avid readers, with 84% reporting they had read a newsletter. Analysis of the sample as a whole indicated that in addition to gender and older age, higher education and being currently married were all moderately but significantly correlated with higher newsletter readership.
DISCUSSION
Motivated by a desire to provide information to selected high-risk groups in CCSS, the primary goal of this study was to evaluate the impact of education strategies on future health practices. In an earlier CCSS study, Kadan-Lottick and her colleagues [3] found that many members of the CCSS cohort lacked important knowledge about their cancer diagnosis and treatment. A supplemental telephone interview with a random sample (n=635) revealed that only 74% of respondents could provide an "accurate general summary" of all of the elements of their cancer history. Notably, not one of the subjects could give an accurate “detailed summary” of diagnosis and treatment therapies. Without such knowledge, it is reasonable to infer that many childhood cancer survivors are unaware of their personal risk for treatment effects as they age. Further, if survivors are unaware of their risk for late effects, they have no reason to share their cancer history with primary care providers or to request recommended screening tests. Also of consequence is physicians’ knowledge about potential late effects in childhood cancer survivors [24]. Most childhood cancer survivors “drift back” to the care of a primary care physician following treatment [25, 26]; however, it is difficult for busy primary care physicians to stay up-to-date on a rare disease diagnosed in only 16 of every 100,000 persons under the age of 21. Such gaps in knowledge pose a serious challenge for both health care practitioners and patients because they preclude anticipatory, preventive follow-up care for survivors of childhood cancer [27].
Evidence underscores the magnitude of the problem. A study by Yeazel et al. [28] found that the timing and frequency of screening tests for adult-onset malignancies reported by the CCSS cohort fell below optimal levels recommended for the general population. The problem of suboptimal screening for disease is not limited to childhood cancer survivors. Several studies have found that adult cancer survivors also may experience deficiencies in medical care [29, 30], suggesting that findings from this study may have broader implications.
This randomized, controlled trial found that providing more detailed content about an individual’s risk for developing health problems as an adjunct to a periodic newsletter had a minimal effect on the likelihood of childhood cancer survivors receiving recommended screening tests. The lack of a statistically significant intervention effect may be explained by a number of factors. Setting aside individual differences in information processing styles, the relatively weak intervention dose – one targeted (addressing characteristics shared by the entire risk group), not tailored (addressing characteristics unique to the recipient) page of information in a two-year period—may explain the null finding [31,32]. In terms of both message and graphic content, the information communicated to intervention subjects via the one-page inserts expanded on the information provided in the newsletter itself. However, insert content was limited to matching generic editorial content to the recipient’s risk category. Although the verdict is still out, tailored information seems to influence desired outcomes more consistently than targeted information [33–36]. However, even with more intensive interventions, effects are persistently small.
Nevertheless, the newsletter (and insert) appeared to increase awareness and interest in risk that is less commonly known in this population, such as cardiac problems and osteoporosis. However, survivors still showed problems in receiving testing, particularly for osteoporosis, for reasons that are not clear. Other factors influencing adherence to recommended screening include survivors’ awareness of their health risks and access to insurance/health care services as well as physicians’ knowledge of cancer-related health risks and appropriate health screening measures. It is also possible that “incidental exposure” [33] to cancer-related information in the media influence participation in screening behaviors. These are questions for future research [18].
Despite these limitations, the study clearly identified some significant associations that should guide clinical practice. First, our results affirm the importance of cancer survivors and their physicians having access to the cancer treatment summary [20]. Second, discussing cancer-related risks with one’s doctor substantially increased the likelihood of undergoing recommended screening tests. These findings are encouraging, as they identify behavioral targets that can be modified through intervention [6]. Oncologists can and should prepare a treatment summary for each and every patient, and they should ensure that the patient understands the importance of sharing that summary with future care providers, regardless of specialty. Survivorship advocates and other organizations such as individual states’ community-based cancer alliances can help to achieve this goal, identified as a priority by the Institute of Medicine. And every doctor should be heartened to know that taking time to talk with patients about their cancer-related risks and tests that may help to mitigate specific late effects really will make a difference. Third, the study underscores the importance of integrating what is known about the integral role of communication in health behavior. Figure 1 points to some of the key variables that merit additional investigation in future studies.
Like other studies that rely on cross-sectional, survey data, this one is subject to limitations of utilizing self-reported data. Further, it is impossible to assert causality, and how specific activities in the screening pathway led to receiving appropriate screening. Detailed information about the time order of events was not available. Additionally, measuring the impact of several communication variables was limited by the a priori wording of survey questions. Hence, measurement of constructs like information-seeking and source credibility were not as precise as they might have been had standardized measures been employed. At the same time, the applicability of what was learned should not be limited to survivors of childhood cancer as similar factors may influence participation in health screening behaviors of survivors of adult-onset malignancies.
Implications of cancer survivors.
This study is designed to assess communication strategies that increase medical follow-up in pediatric cancer survivors at risk of selected treatment complications. The results are of great importance not only to the pediatric oncology community but also the broad range of adult oncology medical specialties who are directly involved in the long-term medical care of this ever increasing population of cancer survivors.
Acknowledgement
This work was supported by the National Cancer Institute (CA55727, L.L. Robison, Principal Investigator).
Appendix
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived five or more years after diagnosis of childhood cancer. CCSS is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant # U24 CA55727) awarded to St. Jude Children’s Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss
| CCSS Institutions and Investigators | |
|---|---|
| St. Jude Children’s Research Hospital, Memphis, TN | Leslie L. Robison, PhD#‡, Melissa Hudson, MD*‡ |
| Greg T. Armstrong, MD, MSCE‡, Daniel M. Green, MD‡ | |
| Kevin R. Krull, PhD‡, Kiri Ness, PhD‡ | |
| Children's Healthcare of Atlanta/Emory University Atlanta, GA | Lillian Meacham, MD*, Ann Mertens, PhD‡ |
| Children's Hospitals and Clinics of Minnesota Minneapolis St. Paul, MN | Joanna Perkins, MD, MS* |
| Seattle Children’s Hospital, Seattle, WA | Scott Baker, MD*, Eric Chow, MD, MPH‡ |
| Children’s Hospital Colorado, Aurora, CO | Brian Greffe, MD* |
| Children’s Hospital Los Angeles, CA | Kathy Ruccione, RN, MPH* |
| Children’s Hospital, Oklahoma City, OK | John Mulvihill, MD*‡ |
| Children’s Hospital of Orange County, Orange, CA | Leonard Sender, MD* |
| Children’s Hospital of Philadelphia, Philadelphia, PA | Jill Ginsberg, MD*, Anna Meadows, MD‡ |
| Children’s Hospital of Pittsburgh, Pittsburgh, PA | Jean Tersak, MD* |
| Children’s National Medical Center, Washington, DC | Sadhna Shankar, MD*, Roger Packer, MD‡ |
| Cincinnati Children’s Hospital Medical Center Cincinnati, OH | Stella Davies, MD, PhD*‡ |
| City of Hope Medical Center, Los Angeles, CA | Smita Bhatia, MD*‡ |
| Cook Children’s Medical Center, Ft. Worth, TX | Paul Bowman, MD, MPH* |
| Dana-Farber Cancer Institute/Children’s Hospital Boston, MA | Lisa Diller, MD*‡ |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScD*‡ |
| Hospital for Sick Children, Toronto, ON | Mark Greenberg, MBChB*, Paul C. Nathan, MD, MSc*‡ |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MD* |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar, MD*‡, Kevin Oeffinger, MD‡ |
| National Cancer Institute, Bethesda, MD | Roy Wu, PhD‡, Nita Seibel, MD‡, Peter Inskip, ScD‡ |
| Julia Rowland, PhD‡ | |
| Nationwide Children's Hospital, Columbus, Ohio | Laura Martin, MD*, Sue Hammond, MD‡ |
| Northwestern University, Chicago, IL | Kimberley Dilley, MD, MPH* |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MD* |
| Roswell Park Cancer Institute, Buffalo, NY | Denise Rokitka, MD, MPH* |
| St. Louis Children’s Hospital, St. Louis, MO | Robert Hayashi, MD* |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MD*, Sarah S. Donaldson, MD‡ |
| Texas Children’s Hospital, Houston, TX | Zoann Dreyer, MD* |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPH* |
| University of Alberta, Edmonton, AB | Yutaka Yasui, PhD*‡ |
| University of California-Los Angeles, CA | Jacqueline Casillas, MD, MSHS*, Lonnie Zeltzer, MD‡ |
| University of California-San Francisco, CA | Robert Goldsby, MD* |
| University of Chicago, Chicago, IL | Tara Henderson, MD, MPH* |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MD* |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, MD, MPH‡* |
| University of Southern California, Los Angeles, CA | Dennis Deapen, DrPH*‡ |
| UT-Southwestern Medical Center, Dallas, TX | Daniel C. Bowers, MD* |
| U.T.M.D. Anderson Cancer Center, Houston, TX | Louise Strong, MD*‡, Marilyn Stovall, MPH, PhD‡ |
Institutional Principal Investigator
Member CCSS Steering Committee
Project Principal Investigator (U24 CA55727)
Footnotes
Conflict of Interest: None
References
- 1.Mertens AC, Liu Q, Neglia JP, Wasilewski-Masker K, Leisenring W, Armstrong GT, Robison LL, Yasui Y. Cause-Specific Late Mortality Among Five-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oeffinger K, Mertens A, Sklar C, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Kadan-Lottick NS, Robison LL, Gurney JG, Neglia JP, Yasui Y, Hayashi R, Hudson M, Greenberg M, Mertens AC. What do Childhood Cancer Survivors Know About Their Past Diagnosis and Treatment?: The Childhood Cancer Survivor Study. JAMA. 2002;287:1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 4.Smith AB, Bashore L. The effect of clinic-based health promotion education on perceived health status and health promotion behaviors of adolescent and young adult cancer survivors. Journal of Pediatric Oncology Nursing. 2006;23(6):326–334. doi: 10.1177/1043454206293266. [DOI] [PubMed] [Google Scholar]
- 5.Hudson MM, Patte C. Education and health promotion in adolescent and young adult cancer survivors. Pediatr Blood Cancer. 2008;50(5 Suppl):1105–1108. doi: 10.1002/pbc.21458. [DOI] [PubMed] [Google Scholar]
- 6.Bober SL, Recklitis CJ, Campbell EG, Park ER, Kutner JS, Najita JS, Diller L. Caring for cancer survivors: a survey of primary care physicians. Cancer. 2009:4409–4418. doi: 10.1002/cncr.24590. [DOI] [PubMed] [Google Scholar]
- 7.Oeffinger K, Mertens A, Hudson M, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Meadows AT. Long-term follow-up of childhood cancer survivors: future directions for clinical care and research. Pediatr Blood Cancer. 2006;46:143–148. doi: 10.1002/pbc.20613. [DOI] [PubMed] [Google Scholar]
- 9.Edwards AG, Evans R, Dundon J, Haigh S, Hook K, Elwyn GJ. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2006;18(4) doi: 10.1002/14651858.CD001865.pub2. CD001865. [DOI] [PubMed] [Google Scholar]
- 10.Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: education, surveillance, and screening. Pediatr Blood Cancer. 2006;46(2):149–158. doi: 10.1002/pbc.20612. [DOI] [PubMed] [Google Scholar]
- 11.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SE, Green DM, Li FP, Meadows AT, Mulvihill JJ, Neglia JP, Nesbit NE, Packer RJ, Potter JD, Sklar CA, Smith MA, Stovall M, Strong LC, Yasui Y, Zeltzer LK. Study Design and Cohort Characteristics of the Childhood Cancer Survivor Study: A Multi-Institutional Collaborative Project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 12.Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 3.0 available at www.survivorshipguidelines.org. [Google Scholar]
- 13.Bliuc D, Eisman JA, Center JR. A randomized study of two different information-based interventions on the management of osteoporosis in minimal and moderate trauma fractures. Osteoporosis International. 2006;17(i9):1309–1318. doi: 10.1007/s00198-006-0078-1. [DOI] [PubMed] [Google Scholar]
- 14.Bottorff JL, Ratner PA, Johnson JL, Lovato CY, Joab SA. Communicating cancer risk information: the challenges of uncertainty. Patient Educ & Counseling. 1998;33:67–81. doi: 10.1016/s0738-3991(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 16.Hill-Kayser CE, Vachani C, Hampshire MK, Jacobs LA, Metz JM. An internet tool for creation of cancer survivorship care plans for survivors and health care providers: design, implementation, use and user satisfaction. J Med Internet Res. 2009;11(3):e39. doi: 10.2196/jmir.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latimer AE, Katulak N, Mowad L, Salovey P. Motivating cancer prevention and early detection behaviors using psychologically tailored messages. J Health Communication. 2005;10:137–155. doi: 10.1080/10810730500263364. [DOI] [PubMed] [Google Scholar]
- 18.Meissner HI, Smith RA, Rimer BK, Wilson KM, Rokowski W, Vernon SW, Briss PA. The future of research that promotes cancer screening. Cancer. 2004;101(5 Suppl):1251–1259. doi: 10.1002/cncr.20510. [DOI] [PubMed] [Google Scholar]
- 19.Miedema B, MacDonald I, Tatemichi S. Cancer follow-up care: patients’ perspectives. Can Fam Physician. 2003;49:890–895. [PMC free article] [PubMed] [Google Scholar]
- 20.Rowland J. Cancer survivorship: rethinking the cancer control continuum. Sem in Onco Nurs. 2008;24(3):145–152. doi: 10.1016/j.soncn.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Rutten L, Arora N, Bakos A, Aziz N, Rowland J. Information needs and sources of information among cancer patients: a systematic review of research (1980–2003) Patient Education and Counseling. 2005;57:250–261. doi: 10.1016/j.pec.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Salovey P, Schneider TR, Apanovitch AM. Persuasion for the purpose of cancer risk reduction: a discussion. J Nat Cancer Inst Monographs. 1999;25:119–122. doi: 10.1093/oxfordjournals.jncimonographs.a024185. [DOI] [PubMed] [Google Scholar]
- 23.Shim M, Kelly B, Hornik R. Cancer information scanning and seeking behavior is associated with knowledge, lifestyle choices, and screening. J Health Commun. 2006;11:157–172. doi: 10.1080/10810730600637475. [DOI] [PubMed] [Google Scholar]
- 24.Henderson TO, Hlubocky FJ, Wroblewski KE, Diller L, Daugherty CK. Physician preferences and knowledge gaps regarding the care of childhood cancer survivors: a mailed survey of pediatric oncologists. J Clin Oncol. 2010;28(5):878–883. doi: 10.1200/JCO.2009.25.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oeffinger K, Nathan P, Kremer L. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Pediatr Clin No Am. 2008;55:251–273. doi: 10.1016/j.pcl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Oeffinger K, Wallace W. Barriers to follow-up care of survivors in the United State and the United Kingdom. Pediatr Blood Cancer. 2006;46(2):135–142. doi: 10.1002/pbc.20614. [DOI] [PubMed] [Google Scholar]
- 27.Zapka J, Puleo E, Taplin S, Goins K, Yood M, Mouchawar J, Somkin C, Manos M. Processes of care in cervical and breast cancer screening and follow-up—the importance of communication. Preventive Medicine. 2004;39:81–90. doi: 10.1016/j.ypmed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Yeazel M, Oeffinger K, Gurney J, Mertens A, Hudson M, Emmons K, Chen H, Robison L. The Cancer Screening Practices of Adult Survivors of Childhood Cancer. Cancer. 2004;100:631–640. doi: 10.1002/cncr.20008. [DOI] [PubMed] [Google Scholar]
- 29.Snyder CF, Earle CC, Herbert RJ, Neville BA, Blackford AL, Frick KD. Trends in follow-up and preventive care for colorectal cancer survivors. J Gen Intern Med. 2008;23:254–259. doi: 10.1007/s11606-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder CF, Frick KD, Kantsiper ME, Peairs KS, Herbert RJ, Blackford AL, Wolff AC, Earle CC. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054–1061. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreuter MW, Skinner CS. Tailoring, What’s in a Name? Health Education Research. 2000;15:1–4. doi: 10.1093/her/15.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Williams-Piehota P, Pizarro J, Schneider TR, Mowad L, Salovey P. Matching health messages to monitor-blunter coping styles to motivated screening mammography. Health Psych. 2005;24(1):58–67. doi: 10.1037/0278-6133.24.1.58. [DOI] [PubMed] [Google Scholar]
- 33.Vernon SW, del Junco DJ, Tiro JA, Coan SP, Perz CA. Promoting regular5 mammography screening II. Results from a randomized controlled trial in US women veterans. J Natl Cancer Inst. 2008;100(5):347–358. doi: 10.1093/jnci/djn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Dib RP, Mathew JL. Interventions to promote the wearing of hearing protection. Cochrane Database of Systematic Reviews. 2009;(issue 4) doi: 10.1002/14651858.CD005234.pub3. Art. No.: CD005234. [DOI] [PubMed] [Google Scholar]
- 35.Glanz K, Schoenfeld ER, Steffen A. A Randomized Trial of Tailored Skin Cancer Prevention Messages for Adults: Project SCAPE. Am J Public Health. 2010;100:735–741. doi: 10.2105/AJPH.2008.155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards A, Unigwe S, Elwyn G, et al. Personalised risk communication for informed decision making about entering screening programs. Cochrane Database Syst Rev. 2003;(1) doi: 10.1002/14651858.CD001865. CD001865. [DOI] [PubMed] [Google Scholar]
- 37.Viswanath K. Science and Society: the communications revolution and cancer control. Nat Rev Cancer. 2005;5:828–835. doi: 10.1038/nrc1718. [DOI] [PubMed] [Google Scholar]